Abstract
Cold acclimation is a critical physiological adaptation for coping with seasonal cold. By increasing their cold tolerance individuals can remain active for longer at the onset of winter and can recover more quickly from a cold shock. In insects, despite many physiological studies, little is known about the genetic basis of cold acclimation. Recently, transcriptomic analyses in Drosophila virilis and D. montana revealed candidate genes for cold acclimation by identifying genes upregulated during exposure to cold. Here, we test the role of myo-inositol-1-phosphate synthase (Inos), in cold tolerance in D. montana using an RNAi approach. D. montana has a circumpolar distribution and overwinters as an adult in northern latitudes with extreme cold. We assessed cold tolerance of dsRNA knock-down flies using two metrics: chill-coma recovery time (CCRT) and mortality rate after cold acclimation. Injection of dsRNAInos did not alter CCRT, either overall or in interaction with the cold treatment, however it did induced cold-specific mortality, with high levels of mortality observed in injected flies acclimated at 5°C but not at 19°C. Overall, injection with dsRNAInos induced a temperature-sensitive mortality rate of over 60% in this normally cold-tolerant species. qPCR analysis confirmed that dsRNA injection successfully reduced gene expression of Inos. Thus, our results demonstrate the involvement of Inos in increasing cold tolerance in D. montana. The potential mechanisms involved by which Inos increases cold tolerance are also discussed.
Introduction
Most ectothermic organisms adjust their physiology in response to gradual changes in environmental temperature. Such physiological changes can increase their tolerance to extreme seasonal temperatures allowing them to maintain function under predictable conditions [1–3]. Organisms that adjust their physiology in response to increasing cold (cold acclimation) can maintain function at low temperatures [4]. Therefore, the ability to cold-acclimate has a key role in shaping species distributions, particularly in determining altitudinal or latitudinal limits [5–7]. Strict thermal niches may restrict gene flow among populations adapted to different temperature regimes [8,9]. Consequently, adaptations that protect against temperature extremes may influence patterns of biodiversity and have important evolutionary implications in light of global climate change.
The ability to cold acclimate in insects correlates well with latitudinal distributions, with some high-latitude species exhibiting a greater capacity to acclimate [6,7,10–13]. The ability to cold acclimate is particularly advantageous to species experiencing strong seasonal temperature variation and those which need to overwinter in northern latitudes [14]. Much is known about the physiology and sensory cues involved with successful overwintering. However, our understanding of the genetic basis of cold tolerance is relatively poor. Few genes involved in the perception of cues for seasonal changes, the timing of mechanisms involved and the physiological changes associated with temperature challenges have been identified [15]. Drosophila montana is an ideal species for the study of the genetic basis of cold tolerance. This species belongs to the virilis group of Drosophila, and has a northern circumpolar high latitude distribution. It can survive at high altitude and successfully overwinters as an adult in northern Finland using strategies including reproductive diapause and cold acclimation, i.e. it is frigophilic [16].
A recent analysis of gene expression changes during cold acclimation in D. virilis and D. montana found that a number of differentially expressed genes were common to both species [17]. Although these species are relatively closely related, they have different cold tolerances as measured by chill coma recovery time [18]. This is likely to reflect thermal niche adaptation as D. virilis is typically found at lower latitudes (south from 35°N) than D. montana (30–70°N) [16]. Despite differences in baseline cold tolerance, both species are able to increase their cold tolerance after cold acclimation by a similar level [18].
Among the list of candidate genes obtained by Parker et al. [17] myo-inositol-1-phosphate synthase (Inos) stands out as a plausible candidate given what is known about its function. Inos encodes the enzyme myo-inositol-1-phosphate synthase which is the rate-limiting step in myo-inositol biosynthesis [19], the major metabolite produced during overwintering by D. montana [20]. Since D. montana is not a model species, studying the genetic basis of traits is relatively difficult as available genetic tools are limited. Here, we adopt an RNA interference (RNAi) approach to test the role of Inos in cold acclimation. By altering the expression of this gene, we successfully increased cold sensitivity in this normally cold hardy species and thus confirm its role in cold tolerance.
Material and Methods
Fly rearing
42 isofemale lines from Oulanka, Finland were established by Veltsos et al. [21]. Individuals from all these lines were isolated and intercrossed to produce a line with greater genetic variation in order to avoid potential issues of dealing with inbred lines such as differential susceptibility to RNAi. Lines were collected in 2009 and subsequently maintained at 19°C and constant light. Approximately 5 pairs from each line were collected and mated at random to form 20 new lines. Pairs from the F1 were then mixed to produce genetically diverse lines (essentially producing one mass bred line) for experimentation. Experimental stock flies were then reared in standard malt medium at 19°C and maintained under a 22:2 Light: Dark (LD) light cycle. Only female flies were used in cold-tolerance trials and for micro-injection. Females were collected under light CO2 anaesthesia within 24 hours of emergence to ensure virginity and kept in vials containing 20–25 flies for 14 days prior to experimental procedures to become sexually mature. Note the methodology decribed above is similar to that used by Parker et al. [17] to allow our results to be easily compared.
Synthesis of double-stranded RNA
For both the target gene Inos and the control gene LacZ, (see below), fragments of approximately 800 bp in length were produced using a standard PCR protocol. Primers were designed to amplify regions avoiding intron/exon boundaries. Fragments were subsequently cloned into a pGEM-T Easy vector (Promega, Southampton, UK) according to the manufacturer’s instructions. This plasmid was then used as the template in a second round of PCR. The second set of primers contained a T7 promoter sequence at the 5’ end of both the forward and reverse primer. The resulting PCR products were approximately 500bp in length and contained the T7 promoter region to facilitate transcription of the double-stranded RNA (dsRNA). Synthesis of dsRNA, using T7 PCR products as a template, was carried out using the MEGAscript T7 Transcription Kit (Life Technologies Ltd., Paisley, UK) according to the manufacturer’s instructions. Double-stranded RNA was purified using the MEGAClear Kit (Life Technologies Ltd., Paisley, UK), eluted in a low-salt buffer, and quantified using a Nanodrop Spectrophotometer (Thermo Fisher Scientific, Loughborough, UK). We produced dsRNA for Inos and also the bacterial gene lacZ which was used as a control. The set of primers used for the first and second rounds of PCR are shown in S1 Table.
Microinjection procedure
Prior to micro-injection, flies were anaesthetised under light CO2. For each target gene, three experimental blocks of micro-injection were carried out with approximately 200 flies injected per block. In each block, 100 flies were injected in the thorax with a total of 207 nl of dsRNA (4 μg/μl), of the target gene. The remaining 100 flies were injected with lacZ dsRNA. Microinjection was performed using a Drummond Nanoject II microinjector (Drummond Scientific Company, Broomall, USA). After injection, individuals were separated into small glass vials containing malt food and transferred to the appropriate incubator to assess their capacity to cold acclimate (see below).
Cold acclimation trials
Injected flies were divided into two groups, each containing approximately 70 target and 70 control flies. One group was maintained at the control temperature of 19°C and the second at 5°C (22:2 L:D) for cold treatment. After 3 days all flies were transferred to fresh vials containing agar (10%) for moisture and exposed to a cold shock: -7°C for 16 hours in constant light. Flies were then transferred immediately to individual plastic containers for observation. Chill-coma recover time (CCRT) was recorded as a measure of cold tolerance (see Vesala et al. 2012a). A fly was considered to have “recovered” once standing on all six legs. This experiment was scored blindly to minimise observer bias. Mortality rate after the 3 days of acclimation before the cold shock was also recorded. A total of 385 flies were injected for the experiment divided equally in four groups (see below).
Expression analyses
Real-time PCR was performed to confirm that dsRNA injections produced a change in the expression of the target gene. Expression analyses were performed only on flies maintained at 19°C due to high mortality in the 5°C treatment groups (see results). Flies were maintained at 19°C for two weeks as per the standard fly rearing protocol. Approximately 40 females were then injected with target dsRNA and another 40 with lacZ dsRNA. These females were transferred to new vials containing malt food and incubated at 19°C for 24 hours. Total RNA was extracted from 3 pools of 10 females per injection group (target and control) for each of 3 experimental blocks. RNA extraction was performed using the TRIzol Plus RNA Purification Kit (Life Technologies Ltd., Paisley, UK) and cDNA synthesized using TaqMan Reverse Transcription Reagents (Life Technologies Ltd., Paisley, UK) according to the manufacturer’s instructions
Quantitative real-time PCR was performed with a ABI Prism 7000 Sequence Detection System (Applied Biosystems) using Maxima SYBR Green/Fluorescein Master Mix (Life Technologies) according to the manufacturer’s instructions. The fluorescein acted as the passive reference dye, normalising the SYBR green signal between wells. Reactions were carried out in a final volume of 20 μl with oligonucleotides at a final concentration of 0.6 μM and 1 μl of cDNA template. We used the ΔΔCt method to convert raw expression data to normalised relative expression values, using the control (LacZ injected flies) treatment as the comparison group [22] and RP49 as the reference gene. Log2-transformed relative expression values were analysed using ANOVA in the statistical package R.
Statistical analysis
All statistical analyses were performed with the statistical package R [23]. Data collected from the 3 separate trials in the cold acclimation experiments were analysed using generalised linear mixed models in the package lme4 [24]. The full model fitted temperature, injection and a temperature by injection interaction term as fixed effects, and experimental batch and “observer” were fitted as random effects. Both had significant effects on CCRT (p<0.001), and were therefore included in all statistical models. The statistical significance of random effects was determined by comparing the log-likelihood of the full model to one in which a random effect was omitted using a log-likelihood ratio test. The statistical significance of fixed effects was determined using Wald chi-square tests. If the interaction term was found to be non-significant (p>0.05), a reduced model without the interaction was used to determine significance of the other terms in the model. Note both full and reduced models are reported in S2 and S3 Tables. Mortality rate after the acclimation trials were compared pairwise using a Fisher’s exact test.
Data Archive
All data obtained are presented in S4 Table.
Results
Cold acclimation phenotype
Flies injected with dsRNA showed strong evidence of cold acclimation, with shorter CCRT after acclimation at 5°C (p<0.001 (Fig 1A, S3 Table) similar to what is observed in wild type flies [18]. Injection of dsRNAInos however did not significantly affect CCRT (p = 0.258, Fig 1A, S3 Table). The interaction between temperature and injection was also non-significant (p = 0.755, S2 Table). However, flies injected with dsRNAInos displayed a substantial increase in mortality rate (66%) when acclimated at 5°C (INOS-05°C: Fig 1B). The difference in mortality was significant in all pair-wise comparisons to the other 3 Inos groups (p<0.001 in all cases). However, 19°C dsRNAInos injected flies (INOS-19°C) did not show any difference in mortality to the LACZ control groups (p = 0.387 to the LACZ-19°C and p = 0.379 to the LACZ-05°C). Such a high mortality rate in the INOS-05°C, but not in the INOS-19°C, group points to an important effect of Inos expression in altering cold tolerance.
Fig 1.
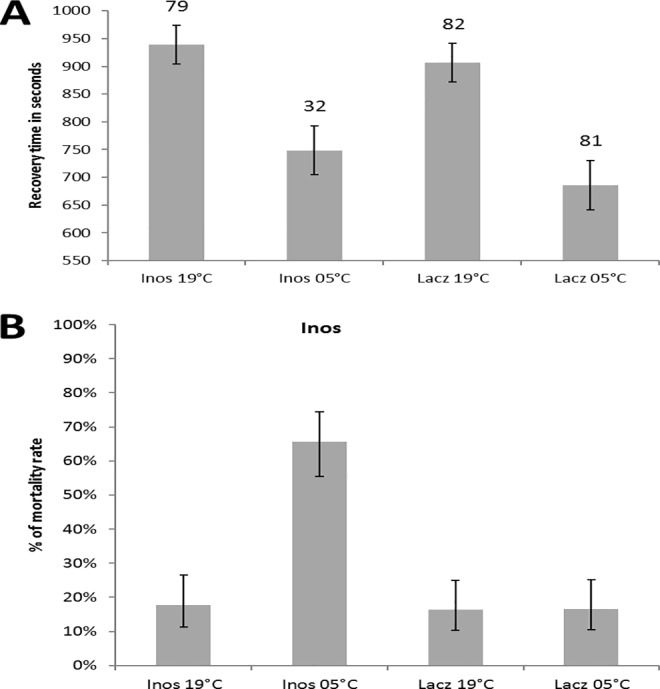
(A) Mean recovery time of females injected with either dsRNAInos (target group) or dsRNAlacZ (control group) after 3 days of acclimation to either 19°C or 5°C followed by exposure to a cold shock. Numbers above bars represent sample size for each group and error bars represent the standard error. (B) Mortality rates of females injected with either dsRNAInos (target group) or dsRNAlacZ (control group) after 3 days of cold acclimation at either 19°C or 5°C. The error bars represent the 95% binomial confidence interval.
Gene expression
Inos expression was reduced following injection of dsRNAInos when examined 24 hours after injection. The reduction was approximately 40% compared to control flies injected with dsRNAlacZ (p = 0.001, Fig 2).
Fig 2. Expression of Inos relative to the expression of RP49 in flies injected with the target dsRNA (solid grey bars) and flies injected with the control dsRNA (dashed bars).
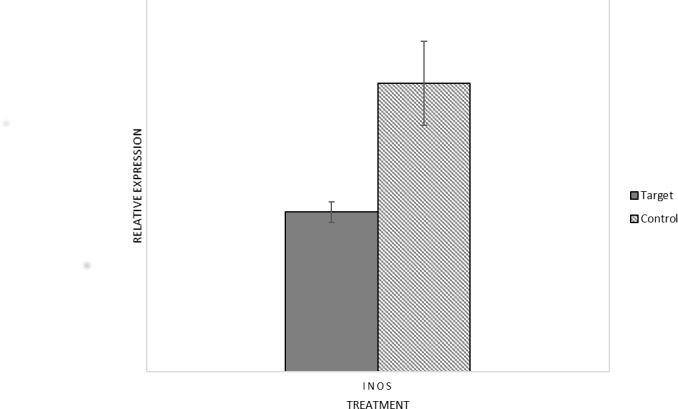
Error bars represent the standard error.
Discussion
Transcriptomics has provided a powerful method to identify candidate genes underlying the evolution and function of traits in non-model species lacking advanced genetic tools [25]. However, following up on transcriptomics can be challenging. Many variables can produce changes in gene expression so it is important to experimentally validate a role of potential candidate genes. Parker et al. [17] used an RNA-seq approach to identify genes which change expression during cold acclimation in D. montana, an extremely cold-adapted species. The ability to cold acclimate has clear fitness consequences and local adaptation to differing thermal regimes is critically important to understanding climate change and species distribution and abundance [9].
By using an RNAi injection technique we were able to examine the effect Inos has on the ability of flies to cope with a cold shock with or without a period of acclimation. Our prediction was that injection of dsRNA complementary to Inos would lead to a reduced ability of flies to acclimate leading to a reduced ability to cope with cold shock.
Even though injection of dsRNAInos did not alter CCRT, either overall or in interaction with the cold treatment, our cold acclimation response results should be considered alongside our finding that flies treated with injection of dsRNAInos showed a large increase in mortality during the cold acclimation treatment. Two thirds of the flies treated with dsRNAInos died during the treatment. This reduced the sample size for these groups, and it is perhaps likely that the surviving flies represent a biased subset of flies less susceptible to RNAi treatment [26] or were otherwise more cold-tolerant.
Our qPCR results showed that injection of dsRNAInos produced a knock-down of Inos expression as expected, reducing gene expression by approximately 40%. The expression levels were measured here only in flies at 19°C as the high mortality rate of flies acclimated at 5°C prevented us from quantifying gene expression in that condition.
Our finding that manipulation of Inos increased cold-induced mortality in this cold tolerant species strongly supports our hypothesis and the results of Parker et al. [17] that Inos is involved in increasing cold tolerance during cold acclimation. Inos encodes the enzyme myo-inositol-1-phosphate synthase, which is part of the inositol biosynthetic pathway, catalysing the conversion of D-glucose-6-phosphate into L-myo-inositol-1-phosphate, the first committed step of de novo inositol synthesis [19]. Inositol compounds are important precursors for structural lipids (phosphatidylinositols) which are important components of eukaryote cell membranes [27,28]. Changes to cell membrane composition are critical for adaptation to temperature as they allow cells to maintain their osmotic balance and function [15,29,30]. We suggest that by increasing expression of Inos D. montana increases the amount of myo-inositol, changing the composition of their cell membrane, which results in an increase in cold tolerance.
In our study we were able to successfully use dsRNA injections to alter gene expression in D. montana, even though this technique has had a very limited effect in D. melanogaster [31,32]. Recently, Scott et al. [33] reviewed the effectiveness of dsRNA injections across several insect groups and found that it varies greatly among taxa, with D. melanogaster representing the extreme end of poor performance while another dipteran Aedes aegypti, performs much more successfully. The reasons for this variation are unknown but may be related to rapid evolution of components of the RNAi anti-viral response amongst species [34]. Our study shows that variation in effectiveness of introducing dsRNA by injection can vary within a single genus. This is an important finding as many other species of Drosophila have now been sequenced, but lack developed functional genetic tools. Finding that dsRNA injections are effective in D. montana opens the door for this relatively simple and inexpensive way of manipulating gene expression in other non-model Drosophila species.
Overall, our study demonstrates that Inos is important for cold tolerance in D. montana. Further studies are necessary to fully understand the molecular mechanism by which Inos affects cold tolerance. For instance, using the CRISPR/CAS9 system [35,36] to produce D. montana transgenic lines should allow for more precise manipulation of gene expression that could provide these answers.
Inos has not been previously implicated in increasing cold tolerance in non D. virilis group species. One implication from this is that the involvement of Inos in cold tolerance is specific to the virilis group flies. This is perhaps unlikely because Inos’ final product, myo-inositol, has been shown to accumulate in response to the onset of winter in several other insect species [37,38], including other dipterans [39]. Taken together these finding suggest that Inos may influence cold tolerance in a wide range of species, but more extensive comparative studies are needed to explore this further.
Supporting Information
(DOCX)
Note experiment batch and recorder were fitted as random effects. Significant values are presented in bold.
(DOCX)
Note experiment batch and recorder were fitted as random effects. Significant values are presented in bold.
(DOCX)
(XLSX)
Acknowledgments
We would like to thank Venera Tyukmaeva, Liam Dougherty, Emily Burdfield-Steel, Claire Stewart, Jade Green, Jonti Siva-Jothy and Lauren Halliwell for help with CCRT observations. We would also like to thank Dr. Rafaela Bruno for providing a plasmid containing a fragment of lacZ. The work was supported by CNPq (Fellowship to FMV) and a NERC Studentship to DJP. Maaria Kankare, Anneli Hoikkala and Paris Veltsos have contributed help and advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Natural Environment Research Council—NE/J020818/1 and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 2003;28: 175–216. [Google Scholar]
- 2.Angilletta MJ, Cooper BS, Schuler MS, Boyles JG. The evolution of thermal physiology in endotherms. Front Biosci (Elite Ed). 2010;2: 861–881. [DOI] [PubMed] [Google Scholar]
- 3.Colinet H, Hoffmann AA. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct Ecol. 2012;26: 84–93. [Google Scholar]
- 4.Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V. Costs and benefits of cold acclimation in field-released Drosophila. Proc Natl Acad Sci U S A. 2008;105: 216–221. d 10.1073/pnas.0708074105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2011;470: 479–485. 10.1038/nature09670 [DOI] [PubMed] [Google Scholar]
- 6.Kellermann V, Loeschcke V, Hoffmann A a., Kristensen TN, Fløjgaard C, David JR, et al. Phylogenetic Constraints In Key Functional Traits Behind Species’ Climate Niches: Patterns Of Desiccation And Cold Resistance Across 95 Drosophila Species. Evolution (N Y). 2012;66: 3377–3389. [DOI] [PubMed] [Google Scholar]
- 7.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am Nat. 2011;178 Suppl: S80–96. [DOI] [PubMed] [Google Scholar]
- 8.Qvarnström A, Ålund M, McFarlane SE, Sirkiä PM. Climate adaptation and speciation: particular focus on reproductive barriers in Ficedula flycatchers. Evol Appl. 2016;9: 119–134. 10.1111/eva.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller I, Seehausen O. Thermal adaptation and ecological speciation. Mol Ecol. 2012;21: 782–799. 10.1111/j.1365-294X.2011.05397.x [DOI] [PubMed] [Google Scholar]
- 10.Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proc Biol Sci. 2000;267: 739–745. 10.1098/rspb.2000.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibert P, Huey RB. Chill-coma temperature in Drosophila: effects of developmental temperature, latitude, and phylogeny. Physiol Biochem Zool. 2001;74: 429–434. 10.1086/320429 [DOI] [PubMed] [Google Scholar]
- 12.Kimura MT. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia. 2004;140: 442–449. 10.1007/s00442-004-1605-4 [DOI] [PubMed] [Google Scholar]
- 13.Calosi P, Bilton DT, Spicer JI, Votier SC, Atfield A. What determines a species’ geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). J Anim Ecol. 2010;79: 194–204. 10.1111/j.1365-2656.2009.01611.x [DOI] [PubMed] [Google Scholar]
- 14.Denlinger DL, Lee REJ, editors. Low Temperature Biology of Insects [Internet]. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 15.Overgaard J, Sørensen JG, Loeschcke V. Genetic variability and evolution of cold-tolerance In: Denlinger DL, Lee REJ, editors. Low Temperature Biology of Insects. Cambridge: Cambridge University Press; 2008. pp. 276–296. [Google Scholar]
- 16.Throckmorton LH. The virilis species group. In: Ashburner, M., Carson, H.L. and Thompson JNJ, editor. The Genetics and Biology of Drosophila. 1982. pp. 227–295.
- 17.Parker DJ, Vesala L, Ritchie MG, Laiho A, Hoikkala A, Kankare M. How consistent are the transcriptome changes associated with cold acclimation in two species of the Drosophila virilis group? Heredity (Edinb). Nature Publishing Group; 2015;115: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vesala L, Salminen TS, Laiho A, Hoikkala A, Kankare M. Cold tolerance and cold-induced modulation of gene expression in two Drosophila virilis group species with different distributions. Insect Mol Biol. 2012;21: 107–118. 10.1111/j.1365-2583.2011.01119.x [DOI] [PubMed] [Google Scholar]
- 19.Majumder AL, Johnson MD, Henry SA. 1L-myo-inositol-1-phosphate synthase. Biochim Biophys Acta. 1997;1348: 245–256. [DOI] [PubMed] [Google Scholar]
- 20.Vesala L, Salminen TS, Koštál V, Zahradníčková H, Hoikkala A. Myo-inositol as a main metabolite in overwintering flies: seasonal metabolomic profiles and cold stress tolerance in a northern drosophilid fly. J Exp Biol. 2012;215: 2891–2897. 10.1242/jeb.069948 [DOI] [PubMed] [Google Scholar]
- 21.Veltsos P, Wicker-Thomas C, Butlin RK, Hoikkala A, Ritchie MG. Sexual selection on song and cuticular hydrocarbons in two distinct populations of Drosophila montana. Ecol Evol. 2012;2: 80–94. 10.1002/ece3.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R Core Team [Internet]. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: 2013. [Google Scholar]
- 24.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67. [Google Scholar]
- 25.Fassbinder-Orth CA. Methods for quantifying gene expression in ecoimmunology: from qPCR to RNA-Seq. Integr Comp Biol. 2014;54: 396–406. 10.1093/icb/icu023 [DOI] [PubMed] [Google Scholar]
- 26.Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, et al. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol. 2011;57: 231–245. 10.1016/j.jinsphys.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Rietveld A, Neutz S, Simons K, Eaton S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J Biol Chem. 1999;274: 12049–12054. [DOI] [PubMed] [Google Scholar]
- 28.Henry SA, Gaspar ML, Jesch SA. The response to inositol: regulation of glycerolipid metabolism and stress response signaling in yeast. Chem Phys Lipids. 2014;180: 23–43. 10.1016/j.chemphyslip.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol. 1995;57: 19–42. 10.1146/annurev.ph.57.030195.000315 [DOI] [PubMed] [Google Scholar]
- 30.Kostál V, Berková P, Simek P. Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol B Biochem Mol Biol. 2003;135: 407–419. [DOI] [PubMed] [Google Scholar]
- 31.Roignant J, Carré C, Mugat B, Szymczak D, Lepesant J-A, Antoniewski C. Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA. 2003;9: 299–308. 10.1261/rna.2154103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SC, Brown SJ, Tomoyasu Y. Larval RNAi in Drosophila? Dev Genes Evol. 2008;218: 505–510. 10.1007/s00427-008-0238-8 [DOI] [PubMed] [Google Scholar]
- 33.Scott JG, Michel K, Bartholomay LC, Siegfried BD, Hunter WB, Smagghe G, et al. Towards the elements of successful insect RNAi. J Insect Physiol. Elsevier Ltd; 2013;59: 1212–1221. 10.1016/j.jinsphys.2013.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obbard DJ, Jiggins FM, Halligan DL, Little TJ. Natural selection drives extremely rapid evolution in antiviral RNAi genes. Curr Biol. 2006;16: 580–585. 10.1016/j.cub.2006.01.065 [DOI] [PubMed] [Google Scholar]
- 35.Port F, Chen H-M, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A. National Academy of Sciences; 2014;111: E2967–2976. 10.1073/pnas.1405500111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Port F, Muschalik N, Bullock SL. Systematic Evaluation of Drosophila CRISPR Tools Reveals Safe and Robust Alternatives to Autonomous Gene Drives in Basic Research. G3 (Bethesda). Genetics Society of America; 2015;5: 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Block W, Somme L. Low temperature adaptations in beetles from the Sub-Antarctic Island of South Georgia. Polar Biol. Springer-Verlag; 1983;2: 109–114. [Google Scholar]
- 38.Košťál V, Nedvěd O, Šimek P. Accumulation of high concentrations of myo-inositol in the overwintering ladybird beetle Ceratomegilla undecimnotata. Cryo-Letters. 1996;17: 267–272. [Google Scholar]
- 39.Teets NM, Peyton JT, Ragland GJ, Colinet H, Renault D, Hahn DA, et al. Combined transcriptomic and metabolomic approach uncovers molecular mechanisms of cold tolerance in a temperate flesh fly. Physiol Genomics. 2012;44: 764–777. 10.1152/physiolgenomics.00042.2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Note experiment batch and recorder were fitted as random effects. Significant values are presented in bold.
(DOCX)
Note experiment batch and recorder were fitted as random effects. Significant values are presented in bold.
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


