Abstract
Nociception, the encoding and processing of noxious environmental stimuli by sensory neurons, functions to protect an organism from bodily damage. Activation of the terminal endings of certain sensory neurons, termed nociceptors, triggers a train of impulses to neurons in the spinal cord. Signals are integrated and processed in the dorsal spinal cord and then projected to the brain where they elicit the perception of pain. A number of neuromodulators that can affect nociceptors are released in the periphery during the inflammation that follows an initial injury. Serotonin (5-HT) is a one such proinflammatory mediator. This review discusses our current understanding of the neuromodulatory role of 5-HT, and specifically how this monoamine activates and sensitizes nociceptors. Potential therapeutic targets to treat pain are described.
Keywords: review, TRPV1, pain, 5-HT, serotonin, nociception
Introduction
The peripheral somatosensory nervous system detects thermal, mechanical, and chemical stimuli in the environment. Nociception is a submodality of somatosensation that encodes both quantitative (temporal, spatial, and intensity characteristics) and qualitative (via distinct subsets of nociceptors) information about noxious stimuli to protect against tissue damage and bodily injury. Noxious stimuli activate specialized nerve endings in peripheral tissues that detect and transduce this information into a pattern of action potentials that is propagated to the spinal cord and medullary dorsal horn neurons. These neurons, in turn, excite ascending pain pathways that reach higher cortical centers to elicit the perception of pain.
Peripheral sensory neurons that respond to noxious stimuli are termed nociceptors. Nociceptors terminate as free nerve endings in tissues where the naked processes are exposed to the inflammatory milieu that is generated following tissue damage [1]. A number of inflammatory mediators have been shown to activate and/or sensitize nociceptors and thus modulate pain processing at the level of the periphery [2]. A better understanding of the actions of these inflammatory neuromodulators is critical to guide the development of novel pain therapies that target the periphery. The main focus of this review is on the peripheral actions of one important neuromodulator of nociception, serotonin (5 hydroxytryptamine, 5-HT).
Peripheral Nociceptors
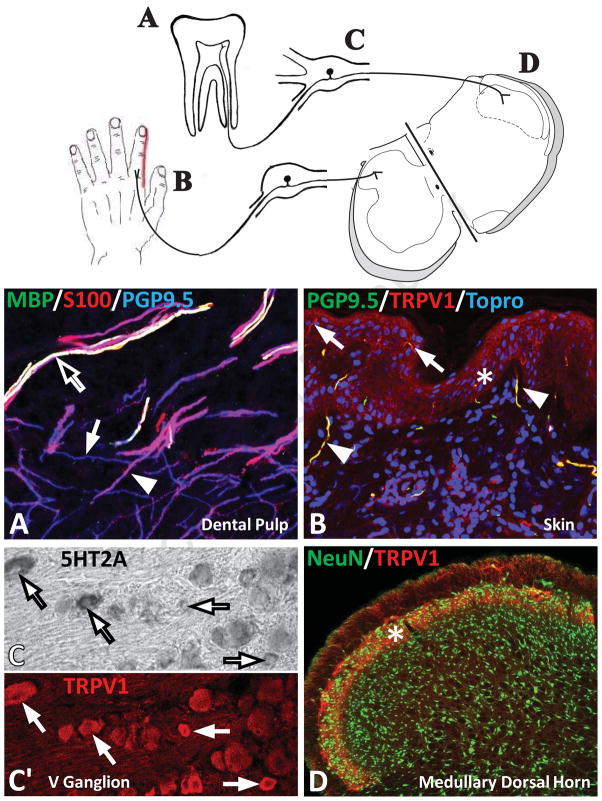
Peripheral tissues are densely innervated by a wide variety of nerve fibers that are specialized for detecting environmental stimuli. Nociceptors that specialize in detecting and transmitting noxious stimuli form a subset of these sensory fibers. Figure 1 illustrates the principal neural components of peripheral nociceptive pathways. The peripheral processes of a subset of sensory ganglion neurons that are dedicated to nociception extend into and terminate in tissues as unmyelinated “free nerve endings”. These peripheral free nerve endings are the sites that are activated by noxious stimuli. For example, human dental pulp, a site that is exquisitely sensitive to pain, possesses abundant nociceptor endings (Fig. 1A). Nociceptive fibers express Protein Gene Product 9.5 (PGP9.5) (Figure 1A, blue; white arrow). These free endings lack associations with Schwann cells (S-100 immunostaining, red; arrowhead) or myelin (myelin basic protein immunostaining; green; black arrow). Many nociceptive fibers also express the transient receptor potential V1 (TRPV1) channel, such as shown here in the skin (Fig 1B, red; PGP9.5, green). TRPVI is a nonselective cation channel involved in detection of noxious chemical and thermal stimuli.
Figure 1. Overview of pain pathways and their cellular components.
Top, Illustration showing the major neural components of the peripheral nociceptive pathway including peripheral nociceptors in human dental pulp (A) and skin (B), sensory neurons located in the dorsal root ganglion or trigeminal ganglion (C) and the termination of the central endings of nociceptors within the rat medullary dorsal horn or spinal dorsal horn (D). A, Confocal micrograph of dental pulp immunostained for Protein Gene Product 9.5 (PGP9.5) to identify nerve fibers (blue; white arrow), S-100 to identify Schwann cells (red; arrowhead), and myelin basic protein (MBP; green-black arrow) to illustrated myelin. Note that in this merged micrograph, blue + red + green immunostaining appear as white, i.e. myelinated axons express all three markers. Nociceptors are identified as unmyelinated free nerve endings (red) that lack association with Schwann cells. B, Confocal micrograph of human skin. Immunostaining for TRPV1 (red) and PGP9.5 (green) identifies nociceptors in the dermis (arrowhead) and epidermis (asterisk; arrow illustrating fine nerve endings expressing TRPV1 and PGP9.5). Cell nuclei are stained with Topro (blue). C, In situ hybridization for 5-HT2A receptors (arrows) in the rat trigeminal ganglion. C′, Corresponding micrograph of same tissue immunostained for TRPV1 (red). D, Confocal micrograph of the sensory trigeminal nuclei immunostained for neuronal nuclei (green) to identify neurons and TRPV1 (red) that identify afferents terminating in the superficial laminae (asterisk).
The cell bodies of the sensory neurons for peripheral nociceptors are located in dorsal root ganglia alongside the spinal cord and in the trigeminal (V) ganglion (for cranial nociceptors). Some of these sensory neurons show a high expression of 5-HT receptors, including 5-HT2A (Figure 1C), and many of these same neurons coexpress TRPV1 (red, arrows in Figure 1C and C′) (discussed in detail below). The central processes of dorsal root sensory neurons terminate within the dorsal horn of the spinal cord and trigeminal neurons within the sensory trigeminal nuclei (and especially the caudal medullary dorsal horn; Figure 1D). The spinal dorsal horn and medullary dorsal horn contain many neurons (Fig 1D, NeuN; green). A subset of these neurons is activated by nociceptor stimulation. The central processes of sensory ganglion cells are segregated to specific laminae of the spinal and medullary dorsal horns. For example, primary afferents that express TRPV1 terminate in the superficial laminae I and II (Fig 1D, red; asterisk). Acute noxious thermal, mechanical or chemical stimuli activate nociceptors in a stimulus-response relationship, but a maintained nociceptive stimulus such as during inflammation evokes increased neuronal discharge (detailed information refer to [2]). This state of heightened neuronal activity is called hyperalgesia and is characterized by hypersensitivity to noxious stimuli. During this barrage of increased neuronal activity, nociceptors may undergo long-term changes that ultimately contribute to chronic pain long after the initial insult has resolved [2] and that is resistant to treatments.
Interactions with Inflammatory Neuromodulators
Typically following an initial noxious insult, a number of substances that activate and sensitize nociceptors are released in injured tissues. Moreover, immune cells, platelets, and other cells are recruited to or activated within the injured area and these cells may synthesize and release additional substances, named proinflammatory mediators, such as histamine, serotonin, and bradykinin [1]. Proinflammatory mediators excite and sensitize sensory neurons [3], further activating pain pathways. For example, in injured tissues bradykinin is synthesized locally from inactive precursors that are normally present, and directly stimulates peripheral nociceptors. Injecting bradykinin into human skin causes pain [4]. Inflammatory mediators can also sensitize nociceptors and lower their threshold for activation by other stimuli.
There are many chemical factors and inflammatory mediators that activate or sensitize nociceptors. For example, peripheral injury leads to the release of oxidized linoleic acid metabolites such as 9-and 13-hydroxyoctadecadienoic acid (HODE). HODE potently activates TRPV1, thereby exciting nociceptors and eliciting pain [5]. Sex hormones may sensitize the peripheral pain system. For instance, injecting estradiol into ovariectomized female rats triggers a massive (>50-fold) upregulation of prolactin in sensory neurons. Prolactin sensitizes TRPV1 and elicits pain responses in female rats [6]. Another example of peripheral modulation is the action of nerve growth factor. Administrating nerve growth factor sensitizes nociceptors as well as upregulates TRPA1, another channel involved in detecting noxious stimuli [7]. This review focuses on the role of another peripheral modulator, serotonin, and its role in the modulation of peripheral nociceptors.
Serotonin (5-HT) as an Inflammatory Neuromodulator
The serotonergic system has gained much attention as a therapeutic target for treating migraine pain [8–9] and is implicated in other pain conditions. While 5-HT is well known for its central nervous system regulation of mood, appetite, and vasoconstriction, it is becoming increasingly clear that 5-HT plays a modulatory role in various acute and persistent pain states [10–15]. The vast majority of 5-HT in the mammalian body is located in peripheral tissues where 5-HT is actively taken up and released with other chemical mediators by platelets, mast cells, and immune cells [14]. Early in the immune response, 5-HT acts as a pro-inflammatory mediator by inducing platelet aggregation and changes in vascular tone [1]. 5-HT is also pronociceptive.
Inflammation in the rat hindpaw induces an increase in peripheral endogenous 5-HT levels indicating an important role for 5-HT in inflammatory pain states [16]. Also, endogenous 5-HT levels increase after thermal injury that results in inflammation in rats [17], and in humans with joint movement pain [18], and in human muscle associated with allodynia [13, 19].
Serotonin evokes inflammation and thermal hyperalgesia
Administering exogenous 5-HT also elicits inflammation and hyperalgesia in humans [12–13, 19–21] and rats [22–25]. Further, injecting 5-HT evokes itching [26–27] and may play an additional role in pain perception [28]. Local injection of 5-HT into the rat hindpaw evokes a transient thermal hyperalgesia that peaks between 10–15 minutes and returns to basal responses within 30 minutes [23, 25, 29]. These effects of 5-HT are likely to be physiologically relevant. Thermal injury induces a local release of 5-HT [17] and peripheral inflammation induces a 4-fold increase in 5-HT [15–16, 30]. Moreover, intraplantar administration of 5-HT also induces significant edema [25]. Interestingly, injecting low concentrations of 5-HT heightens sensitivity to noxious heat but does not induce edema, indicating that distinct mechanisms may be involved in thermal hyperalgesia and edema following 5-HT injection. Further studies identifying these mechanisms may aid in understanding why certain therapeutics treat some pain disorders and not others.
Modulating Pain Thresholds via TRPV1
One mechanism whereby neuromodulators modify nociceptive signals is to alter the threshold for noxious stimuli. TRPV1 channels play a critical role in nociception by transducing thermal and chemical stimuli [31–34] and responding to oxidized linoleic acid metabolites [5, 35] and inflammatory mediators [36–37]. Capsaicin, the pungent ingredient in chili peppers, is an agonist for TRPV1. This compound is often used in functional studies to identify nociceptors and when applied to the skin, elicits itch, pain, and the sensation of heat. During inflammation, activation of TRPV1 by endogenous ligands leads to thermal hyperalgesia [36–37]. Neuromodulators such as 5-HT may regulate the stimulus threshold for sensory neurons by changing the properties of TRPV1 channels [38–39].
Does serotonin modulate TRPV1 channels?
Serotonin may act as a neuromodulator of nociception by altering TRPV1 during inflammation. For example, in sensory neurons 5-HT increases excitability to thermal stimuli and enhances capsaicin- and heat-evoked currents [40–41]. Heat and capsaicin alike stimulate TRPV1. Depleting 5-HT attenuates visceral pain and reduces TRPV1 activation [42]. Repeated application of capsaicin desensitizes TRPV1 and reduces afferent transmission of painful stimuli [43]. Inflammatory mediators may act by altering sensitization and desensitization of TRPV1 channels. For example, 5-HT stimulates nociceptors for a greater duration than inflammation alone [44–45] and this may be mediated by alterations in TRPV1. However, detailed functional studies such as electrophysiology or calcium imaging are required to determine whether 5-HT alters TRPV1 desensitization. Evidence for a role for serotonergic modulation of TRPV1 channels is summarized in Table 1.
Table 1.
Summary of evidence of serotonergic neuromodulation of TRPV1 nociceptors
| Type Sensory Neurons | Methodology | Reported Findings | Citation |
|---|---|---|---|
| rat DRG neurons | ISH/IHC | 13% DRG neurons coexpress 5HT3 mRNA and TRPV1 | Zeitz et al., 2002 |
| rat DRG neurons | microdialysis | 5HT2A/2C agonist induces a 4-fold increase in capsaicin- evoked substance P release in the dorsal horn; 8-fold increase during inflammation; attenuated with 5HT2A antagonist | Bertelsen et al., 2003 |
| rat colonic sensory neurons | whole-cell patch clamp | 1 μM 5HT lowers thermal thresholds and enhances capsaicin-evoked currents via 5HT2 and 4 receptors | Sugiura et al., 2004 |
| rat cutaneous nociceptors | spontaneous pain behaviors | 50 nmol 5HT prolongs capsaicin-evoked flinching and licking/biting of the hindpaw | Sawynok et al., 2006 |
| cultured rat TG neurons | whole-cell patch clamp | 10 μM 5HT exposure upregulates the amplitude of capsaicin-evoked currents | Simonetti et al., 2006 |
| cultured rat DRG neurons | whole-cell patch clamp and calcium imaging | 10 μM 5HT potentiates capsaicin-evoked current and calcium influx via 5HT2A and 7 receptors; more prominent in DRG from inflamed rats | Ohta et al., 2006 |
| rat DRG neurons | IHC | 5HT2A receptors coexpress with TRPV1 in DRG | Van Steenwinckel et al., 2009 |
| rat colon sensory neurons | whole-cell patch clamp and visceral pain behaviors | Endogenous 5HT depletion augments capsaicin- evoked visceral hypersensitivity and capsaicin- evoked currents | Qin et al., 2010 |
| cultured rat TG neurons | calcium imaging and peptide release | 5HT enhances capsaicin-evoked calcium influx and CGRP release via 5HT1B/1D, 2A and 3A receptors which colocalize with TRPV1 | Loyd et al., 2011 |
| rat cutaneous nociceptors | thermal pain behavior | Peripheral 5HT prolongs capsaicin-evoked thermal hypersensitivity via 5HT1B/1D, 2A and 3A receptors | Loyd et al., Neurosci 2012 |
| human dental pulp nociceptors | in vitro superfusion | 5HT enhances capsaicin-evoked CGRP release in dental pulp from female but not male teeth | Loyd et al., Pain 2012 |
Serotonin Activates Capsaicin-sensitive Sensory Neurons
Activating TRPV1 channels by noxious thermal stimuli [31–34], by oxidized linoleic acid metabolites [5, 35], and by inflammatory mediators [36–37] elicits calcium influx into nociceptors. This, in turn, triggers these nociceptors to release inflammatory peptides, primarily calcitonin gene-related peptide (CGRP). One mechanism by which 5-HT enhances peripheral nociception may be by increasing TRPV1-mediated calcium influx and CGRP release. Indeed, treating cultured sensory neurons with 5-HT enhances intracellular calcium accumulation in sensory neurons that respond to the TRPV1 agonist capsaicin [41, 46]. Stimulating TRPV1 increases CGRP release from nociceptive sensory neurons 4-fold. Thus, measuring CGRP release provides a method, albeit indirect, to observe the effect of inflammatory mediators on TRPV1 channels. 5-HT alone has no significant effect on CGRP release from cultured sensory neurons. However, the pretreatment of sensory neurons with 5-HT doubles capsaicin-evoked CGRP release [46].
Serotonin Enhances CGRP Release from Human Dental Pulp
Human dental pulp is composed of many of the same cells, fibers and nociceptors as other peripheral tissues, thus offering a readily available model for the study of human nociceptors [47]. Nociceptors innervating the human dental pulp express TRPV1. These neurons also contain CGRP [48]. Furthermore, 5-HT receptor protein is present in human pulp as reported by western blot [49]. Using a recently-developed in vitro superfusion method to measure CGRP release from human dental pulp from extracted teeth [48], Loyd et al [49] reported that 5-HT significantly enhances capsaicin-evoked CGRP release, similar to reports in rat sensory neurons. Interestingly, this effect only occurred in dental pulp from females with no effect of 5-HT on CGRP release observed in dental pulp from males. Furthermore, the enhancing effect of 5-HT on CGRP release was mostly observed in tissue taken from amenstrual females and females in the last week of menses [49]. This is likely due to changes in hormonal status and may account for differences in pain disorders that are more common in women, such as migraine and irritable bowel syndrome. A recent study reported that peripheral administration of 5-HT evoked thermal hyperalgesia in hindpaws of intact and ovariectomized female rats, comparable to male rats [25]. This finding indicates that there are no sex differences in 5-HT-evoked thermal hyperalgesia in the rat hindpaw. However, studies in models of trigeminal or visceral pain may support a sex difference in 5-HT-evoked nociception. Further investigation may result in treatments that are better designed for pain conditions in women.
Serotonin Interacts with Peripheral Nociceptors via Serotonin Receptors
5-HT1, 5-HT2 and 5-HT3 receptors are expressed in sensory neurons and are involved in nociception. Yet, little is known about their specific expression in the subpopulation of nociceptors that express TRPV1. Providing anatomical evidence of the co-expression of 5-HT receptors and TRPV1 has proven difficult due to the lack of antibodies specific for 5-HT receptor subtypes. Using combined in situ hybridization and fluorescent immunohistochemistry on trigeminal sensory neurons, Loyd et al [46] reported that 5-HT1B, 5-HT1D, 5-HT2A, and 5-HT3A, but not 5-HT2C, receptor mRNAs were detected in sensory neurons that express TRPV1. TRPV1 is predominately expressed in small (<20 μM) to medium diameter (20–40 μM) trigeminal ganglion sensory neurons in the rat. In these TRPV1-positive cells, 5-HT1B, 5-HT1D, 5-HT2A, and 5-HT3A receptors are expressed in medium diameter neurons. Co-expression of TRPV1 with 5-HT2A and 5-HT3A receptors has also been reported in the rat dorsal root ganglion [50–51]. Collectively, these reports provide an anatomical substrate for 5-HT modulation in the subpopulation of nociceptors that respond to capsaicin.
Co-expression of multiple subtypes of 5-HT receptors with TRPV1 is critical for understanding how 5-HT modulates nociceptor activity. Several cell signaling pathways engaged by 5-HT are known to alter TRPV1 activity, possibly by altering phosphorylation of the channel. For example, 5-HT2 receptors are excitatory G protein-coupled receptors that increase PLC/PKC signaling via Gαq coupling. Activating this pathway can sensitize TRPV1. 5-HT3 receptors are ligand-gated cation channels (ionotropic receptors) that directly excite nociceptors [52–53]. In cultured sensory neurons, 5-HT2A and 5-HT3 receptors antagonists attenuate calcium signaling and reduce the ability of 5-HT to enhance capsaicin-evoked CGRP release [46]. In contrast, 5-HT1 receptors are inhibitory G protein-coupled receptors that couple to a decrease in cAMP signaling via Gi/Go. Decreasing cAMP might reduce nociceptive neurotransmission. In support of this notion, 5-HT1B/1D receptor agonists significantly attenuate neurogenic inflammation [54] presumably by inhibiting the release of neuropeptides [55]. The pretreatment of trigeminal ganglion sensory neurons with sumatriptan, a 5-HT1B/1D receptor agonist used to ameliorate migraine headaches, attenuates CGRP release from cultured neurons that are stimulated with 5-HT and capsaicin [46].
5-HT1, 5-HT2 and 5-HT3 receptors also appear to play opposing roles after peripheral administration in models of nociceptive behavior. For example, 5-HT1B/1D receptor agonists attenuate hyperalgesia [25], while 5-HT2A and 5-HT3 agonists induce hyperalgesia in rats [24, 41, 56]. Conversely, 5-HT2A and 5-HT3 antagonists reduce hyperalgesia [17, 24, 56]. Given systemically, ketanserin, a 5-HT2A receptor antagonist, attenuates 5-HT-evoked thermal hyperalgesia; blocking 5-HT3 receptors has no effect [24]. Taken together, these studies support a peripheral role of 5-HT3 receptors in modulating pain, while 5-HT2A receptors are involved in central [57–59] and peripheral control of pain. Attenuating hyperalgesia with anti-serotonergics has also been reported for 5-HT-evoked nocifensive pain [29], formalin-evoked flinch [15, 30], and orofacial nocifensive behavior elicited by Complete Freund’s Adjuvant [60].
Local injection of the 5-HT2A receptor antagonist ketanserin attenuates 5-HT-evoked edema [25, 29], while granisetron, a 5-HT3 receptor antagonist, has no effect on edema [25]. These results indicate that distinct 5-HT receptors control different mechanisms of pain and inflammation. This conclusion is supported by reports of 5-HT2A receptor expression in blood vessels [61] and 5-HT3 receptor expression on nociceptors [50]. Collectively, the studies indicate that the complexity of the peripheral 5-HT system in different pain states may be due, in part, to activation of a broad range of 5-HT receptor subtypes that are co-localized with TRPV1 on nociceptor sensory neurons.
Serotonin Enhances Capsaicin-evoked Thermal Hyperalgesia
Loyd et al [25] evaluated the effect of 5-HT on TRPV1-evoked thermal hyperalgesia and reported that 5-HT enhanced and prolonged capsaicin-evoked thermal sensitivity. Capsaicin alone evokes thermal hyperalgesia peaking between 5–15 minutes [62]. However, after 5-HT pretreatment, the peak thermal sensitivity is prolonged up to 30 minutes. Although behavioral methods do not distinguish a direct from an indirect effect of 5-HT on TRPV1, the findings clearly demonstrate that peripheral 5-HT significantly exacerbates sensitivity to noxious thermal heat. This may be the case with visceral hypersensitivity as well, as it has been reported that 5-HT enhances capsaicin-induced visceral pain [42] and capsaicin-evoked currents in mouse colon sensory neurons [40]. Loyd et al [25] also reported that the 5-HT receptor antagonists ketanserin and granisetron failed to attenuate capsaicin-evoked thermal hyperalgesia, while both antagonists blocked the ability of 5-HT to enhance TRPV1-evoked thermal hyperalgesia, possibly by altering rates of channel phosphorylation, when 5-HT was administered prior to capsaicin. This is important because 5-HT is released when tissues are injured. Consequently, nociceptors that co-express TRPV1 channels and 5-HT receptors will be affected and the pain may be exacerbated.
Targeting Peripheral Nociceptors for Pain Therapy
Therapeutics targeting 5-HT receptors and 5-HT re-uptake are being examined in clinical trials for their ability to treat the pain associated with migraine [8–9], fibromyalgia [63], and irritable bowel syndrome [64]. To date, the triptan class of medications used to treat migraine and cluster headache, including the 5-HT1B/1D agonist sumatriptan, are the only 5-HT selective therapeutics that treat pain successfully in the clinic. Other centrally-acting drugs, such as venlafaxine and duloxetine, that act as dual norepinephrine and 5-HT reuptake inhibitors, have shown some efficacy in the treatment of various pain symptoms, including fibromyalgia [65].
Because 5-HT is pronociceptive in the peripheral nervous system, it may be effective to target the peripheral serotonergic system as a therapeutic option. However, few studies have investigated peripheral administration of serotonergic blocking agents for pain treatment. In preclinical studies, local pretreatment with ketanserin or granisetron significantly blocks 5-HT-evoked thermal hyperalgesia [25]. This appears to be mediated peripherally; administration of these drugs into the contralateral hindpaw has no effect on hyperalgesia in the inflamed paw. Importantly, peripheral granisetron evokes a steady, dose-response attenuation of thermal hyperalgesia [25]. This finding is consistent with its ability to block 5-HT-enhancement of proinflammatory neuropeptide release in vitro [46], indicating that it may serve as a valuable peripheral pain therapeutic [66–67]. In support, clinical studies have reported that local injection of graniestron reduces hyperalgesia and allodynia in humans [66, 68]. Moreover, a topical application of another 5-HT3 receptor antagonist, odansetron, attenuates nociception induced by intradermal capsaicin [69]. Other 5-HT receptors may also be useful therapeutic targets, such as the 5-HT7 receptor. It was recently reported that CGRP release may be reduced by administration of a selective 5-HT7 receptor antagonist in an animal model of experimental migraine [70]. Further studies analyzing the anatomical expression and therapeutic relevance of the 5-HT receptors more recently reported to be involved in peripheral pain processing are warranted.
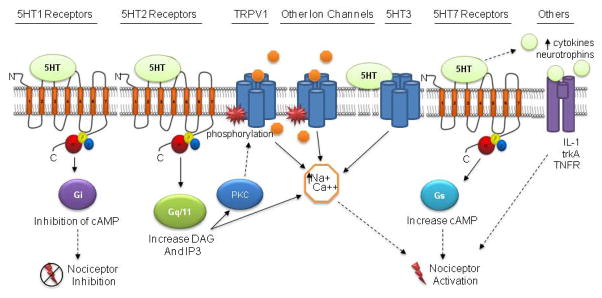
It is critical for drug development to understand the mechanisms by which 5-HT, and other proinflammatory mediators, evoke pain. One hypothesis is that 5-HT may be acting via 5-HT receptors colocalized with TRPV1 on nociceptors to modulate the activation and/or sensitization of sensory neurons and/or upregulating the expression of TRPV1 (Figure 2). Because the different 5-HT receptor subtypes trigger a variety of downstream signaling pathways, 5-HT may excite or depress nociceptors (Figure 2). 5-HT may also alter the function or expression of other ligand-gated or voltage-gated ion channels to modulate peripheral nociceptors, however, this effect in sensory neurons is unclear. Alternatively, 5-HT may excite nociceptors indirectly by augmenting the release of cytokines and neurotrophins (Figure 2). Future studies examining these proposed mechanisms will yield important information for the development of novel pain therapeutics targeting the peripheral 5-HT system. Furthermore, studies utilizing a variety of animal models of pain, such as visceral pain and orofacial pain, will be especially useful for a better understanding of the ability of peripherally administered therapeutics to treat various pain conditions.
Figure 2.
Schematic illustrating 5-HT receptor signaling pathways expressed in sensory neurons. 5-HT1 receptors are inhibitory G protein coupled (Gi/Go) receptors that couple to a decrease in cAMP. A decline in cAMP may reduce nociceptive neurotransmission (dotted line represents hypothetic relationship). 5-HT2 receptors are excitatory G protein coupled (Gαq) that couple to an increase in DAG and IP3, which increases intracellular calcium and PKC signaling. Increasing PKC activity may lead to phosphorylation of TRPV1 or other ion channels. 5-HT3 is a ligand-gated cation channel (i.e., ionotropic receptor) that directly excites nociceptors. Also illustrated is the potential for 5-HT enhancement of the release of other endogenous mediators to indirectly activate nociceptors, i.e. cytokines and neurotrophins.
Summary and Conclusions
Based on the current literature, it can be concluded that peripherally acting 5-HT enhances nociceptor activity as observed by (a) increased calcium influx in nociceptive sensory neurons, (b) increased proinflammatory peptide release from sensory neurons, and (c) enhanced behavioral hyperalgesia. There are a number of possible mechanisms through which 5-HT evokes pain, including recent evidence of a modulatory role of 5-HT on the nociceptors that express TRPV1 (Figure 2). While the specific mechanisms by which 5-HT and other neuromodulators affect nociception remain to be elucidated, it is important to continue to investigate these pathways to aid in the development of new and better therapeutics for pain conditions.
Review of serotonin’s modulatory role in peripheral pain processing
Preclinical evidence of serotonin enhancement of thermal hyperalgesia
Serotonin may evoke hyperalgesia via the TRPV1 population of sensory neurons
Hyperalgesia can be attenuated with local serotonergic blocking agents
Future studies should examine the therapeutic potential of peripheral serotonergics
Acknowledgments
The authors would like to acknowledge Gabriela Weiss and Mei Li for excellent technical assistance in obtaining the confocal microscopy images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75(2):125–31. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 2.Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13(6):2273–86. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores CM, et al. Capsaicin-evoked CGRP release from rat buccal mucosa: development of a model system for studying trigeminal mechanisms of neurogenic inflammation. Eur J Neurosci. 2001;14(7):1113–20. doi: 10.1046/j.0953-816x.2001.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whalley ET, et al. The effect of kinin agonists and antagonists on the pain response of the human blister base. Naunyn Schmiedebergs Arch Pharmacol. 1987;336(6):652–5. doi: 10.1007/BF00165756. [DOI] [PubMed] [Google Scholar]
- 5.Patwardhan AM, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120(5):1617–26. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diogenes A, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26(31):8126–36. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86(6):550–5. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari MD, et al. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta- analysis of 53 trials. Lancet. 2001;358(9294):1668–75. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen LC, Ashcroft DM. Meta-analysis of the efficacy and safety of zolmitriptan in the acute treatment of migraine. Headache. 2008;48(2):236–47. doi: 10.1111/j.1526-4610.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 10.Herken H, et al. Possible association of temporomandibular joint pain and dysfunction with a polymorphism in the serotonin transporter gene. Am J Orthod Dentofacial Orthop. 2001;120(3):308–13. doi: 10.1067/mod.2001.115307. [DOI] [PubMed] [Google Scholar]
- 11.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26(Suppl 3):S12–9. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 12.Ernberg M, et al. Effects of local serotonin administration on pain and microcirculation in the human masseter muscle. J Orofac Pain. 2006;20(3):241–8. [PubMed] [Google Scholar]
- 13.Ernberg M, Lundeberg T, Kopp S. Pain and allodynia/hyperalgesia induced by intramuscular injection of serotonin in patients with fibromyalgia and healthy individuals. Pain. 2000;85(1–2):31–9. doi: 10.1016/s0304-3959(99)00233-x. [DOI] [PubMed] [Google Scholar]
- 14.Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol Neurobiol. 2004;30(2):117–25. doi: 10.1385/MN:30:2:117. [DOI] [PubMed] [Google Scholar]
- 15.Parada CA, et al. The major role of peripheral release of histamine and 5hydroxytryptamine in formalin- induced nociception. Neuroscience. 2001;102(4):937–44. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima K, et al. The nociceptive mechanism of 5-hydroxytryptamine released into the peripheral tissue in acute inflammatory pain in rats. Eur J Pain. 2009;13(5):441–7. doi: 10.1016/j.ejpain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki M, et al. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain. 2006;122(1–2):130–6. doi: 10.1016/j.pain.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Kopp S. The influence of neuropeptides, serotonin, and interleukin 1beta on temporomandibular joint pain and inflammation. J Oral Maxillofac Surg. 1998;56(2):189–91. doi: 10.1016/s0278-2391(98)90867-9. [DOI] [PubMed] [Google Scholar]
- 19.Ernberg M, et al. Plasma and serum serotonin levels and their relationship to orofacial pain and anxiety in fibromyalgia. J Orofac Pain. 2000;14(1):37–46. [PubMed] [Google Scholar]
- 20.Schmelz M, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89(5):2441–8. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 21.Babenko V, et al. Duration and distribution of experimental muscle hyperalgesia in humans following combined infusions of serotonin and bradykinin. Brain Res. 2000;853(2):275–81. doi: 10.1016/s0006-8993(99)02270-2. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto K, et al. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99(1–2):133–43. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- 23.Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48(2):485–90. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga A, Saika M, Senba E. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. 1998;76(3):349–55. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 25.Loyd DR, Chen PB, Hargreaves KM. Antihyperalgesic effects of anti-serotonergic compounds on serotonin- and capsaicin-evoked thermal hyperalgesia in the rat. Neuroscience. 2012;203:207–15. doi: 10.1016/j.neuroscience.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol. 2010;104(5):2442–50. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87(3):1280–9. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- 28.Ringkamp M, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31(42):14841–9. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sufka KJ, Schomburg FM, Giordano J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol Biochem Behav. 1992;41(1):536. doi: 10.1016/0091-3057(92)90058-n. [DOI] [PubMed] [Google Scholar]
- 30.Doak GJ, Sawynok J. Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience. 1997;80(3):939–49. doi: 10.1016/s0306-4522(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 31.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 32.Voets T, et al. The principle of temperature-dependent gating in cold-and heat-sensitive TRP channels. Nature. 2004;430(7001):748–54. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 33.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 34.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–7. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 35.Patwardhan AM, et al. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106(44):18820–4. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;(179):155–71. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 37.Cesare P, et al. Ion channels gated by heat. Proc Natl Acad Sci U S A. 1999;96(14):7658–63. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 39.Chuang HH, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 40.Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24(43):9521–30. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohta T, et al. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol. 2006;576(Pt 3):809–22. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin HY, et al. Visceral hypersensitivity induced by activation of transient receptor potential vanilloid type 1 is mediated through the serotonin pathway in rat colon. Eur J Pharmacol. 2010;647(1–3):75–83. doi: 10.1016/j.ejphar.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Simon SA. The influence of removing extracellular Ca2+ in the desensitization responses to capsaicin, zingerone and olvanil in rat trigeminal ganglion neurons. Brain Res. 1998;809(2):246–52. doi: 10.1016/s0006-8993(98)00853-1. [DOI] [PubMed] [Google Scholar]
- 44.Aley KO, et al. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–5. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbert MK, Schmidt RF. Activation of normal and inflamed fine articular afferent units by serotonin. Pain. 1992;50(1):79–88. doi: 10.1016/0304-3959(92)90115-R. [DOI] [PubMed] [Google Scholar]
- 46.Loyd DR, et al. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain. 2011;152(10):2267–76. doi: 10.1016/j.pain.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry MA, Hargreaves KM. Peripheral mechanisms of odontogenic pain. Dent Clin North Am. 2007;51(1):19–44. v. doi: 10.1016/j.cden.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Fehrenbacher JC, et al. Capsaicin-evoked iCGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain. 2009;144(3):253–61. doi: 10.1016/j.pain.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loyd DR, et al. Sex differences in serotonin enhancement of capsaicin-evoked calcitonin gene-related peptide release from human dental pulp. Pain. 2012 doi: 10.1016/j.pain.2012.06.018. doi: http://dx.doi.org/10.1016/j.pain.2012.06.018. [DOI] [PMC free article] [PubMed]
- 50.Zeitz KP, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22(3):1010–9. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Steenwinckel J, et al. The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience. 2009;161(3):838–46. doi: 10.1016/j.neuroscience.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto K, et al. Blockade of peripheral 5-HT3 receptor attenuates the formalininduced nocifensive behavior in persistent temporomandibular joint inflammation of rat. Neurosci Lett. 2004;367(2):259–63. doi: 10.1016/j.neulet.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Fozard JR. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984;23(12B):1473–86. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- 54.Carmichael NM, Charlton MP, Dostrovsky JO. Activation of the 5-HT1B/D receptor reduces hindlimb neurogenic inflammation caused by sensory nerve stimulation and capsaicin. Pain. 2008;134(1–2):97–105. doi: 10.1016/j.pain.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Moskowitz MA, Buzzi MG. Neuroeffector functions of sensory fibres: implications for headache mechanisms and drug actions. J Neurol. 1991;238(Suppl 1):S18–22. doi: 10.1007/BF01642901. [DOI] [PubMed] [Google Scholar]
- 56.Obata H, et al. Antinociception in rat by sarpogrelate, a selective 5-HT(2A) receptor antagonist, is peripheral. Eur J Pharmacol. 2000;404(1–2):95–102. doi: 10.1016/s0014-2999(00)00522-7. [DOI] [PubMed] [Google Scholar]
- 57.Bardin L, Lavarenne J, Eschalier A. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain. 2000;86(1–2):11–8. doi: 10.1016/s0304-3959(99)00307-3. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki M, et al. Antinociception with intrathecal alpha-methyl-5-hydroxytryptamine, a 5-hydroxytryptamine 2A/2C receptor agonist, in two rat models of sustained pain. Anesth Analg. 2003;96(4):1072–8. doi: 10.1213/01.ANE.0000050560.15341.A8. [DOI] [PubMed] [Google Scholar]
- 59.Schmauss C, et al. Pharmacological antagonism of the antinociceptive effects of serotonin in the rat spinal cord. Eur J Pharmacol. 1983;90(4):349–57. doi: 10.1016/0014-2999(83)90556-3. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto K, et al. The role of peripheral 5-HT2A and 5-HT1A receptors on the orofacial formalin test in rats with persistent temporomandibular joint inflammation. Neuroscience. 2005;130(2):465–74. doi: 10.1016/j.neuroscience.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Ullmer C, et al. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995;370(3):215–21. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- 62.Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67(1):179–88. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- 63.Hauser W, et al. Treatment of fibromyalgia syndrome with antidepressants: a metaanalysis. JAMA. 2009;301(2):198–209. doi: 10.1001/jama.2008.944. [DOI] [PubMed] [Google Scholar]
- 64.Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15(1):79–86. doi: 10.1046/j.1365-2982.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 65.Andrews N, O’Neill MF. It might be a big family but the pain remains-last chance saloon for selective 5-HT receptor ligands? Curr Opin Pharmacol. 2011;11(1):3944. doi: 10.1016/j.coph.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Ernberg M, Lundeberg T, Kopp S. Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain. 2000;84(2–3):339–46. doi: 10.1016/s0304-3959(99)00221-3. [DOI] [PubMed] [Google Scholar]
- 67.Sung D, et al. Serotonin (5-HT) excites rat masticatory muscle afferent fibers through activation of peripheral 5-HT3 receptors. Pain. 2008;134(1–2):41–50. doi: 10.1016/j.pain.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 68.Christidis N, et al. Intramuscular injection of granisetron into the masseter muscle increases the pressure pain threshold in healthy participants and patients with localized myalgia. Clin J Pain. 2007;23(6):467–72. doi: 10.1097/AJP.0b013e318058abb1. [DOI] [PubMed] [Google Scholar]
- 69.Giordano J, Daleo C, Sacks SM. Topical ondansetron attenuates nociceptive and inflammatory effects of intradermal capsaicin in humans. Eur J Pharmacol. 1998;354(1):R13–4. doi: 10.1016/s0014-2999(98)00492-0. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, et al. Selective inhibition of 5-HT7 receptor reduces CGRP release in an experimental model for migraine. Headache. 2010;50(4):579–87. doi: 10.1111/j.1526-4610.2010.01632.x. [DOI] [PubMed] [Google Scholar]