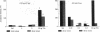Abstract
The neurohypophyseal neuropeptides (Arg8)-vasopressin (AVP) and [pGlu4,Cyt6]AVP-(4-8) (where pGlu is pyroglutamic acid and Cyt is cystine) facilitate the retention of one-trial-learning passive avoidance behavior in rats when administered into the cerebral ventricle immediately after the learning trial. The fragment [pGlu4,Cyt6]AVP-(4-8) was considerably more effective than AVP. Oxytocin (OXT) and [pGlu4,Cyt6]OXT-(4-8) have the opposite effect and attenuate passive avoidance behavior also when administered into the cerebral ventricle after the learning trial. Again the fragment was more active than the parent molecule. The ancient arginine-containing neurohypophyseal hormone vasotocin in "high" doses (10ng) had a vasopressin-like effect and in "low" doses (0.1 ng) had an OXT-like effect on passive avoidance behavior. Because both vasopressinergic (V1) and oxytocinergic receptors have been demonstrated in the central nervous system, we asked whether specific antagonists of the V1, V2, and OXT receptor could antagonize the effects of these neuropeptides on passive avoidance behavior. The three antagonists were approximately equally active in blocking the effect of vasopressin, whereas the fragment [pGlu4]AVP-(4-8) and the high dose of vasotocin were more readily blocked by the OXT antagonist. The attenuating effect of OXT, the fragment [pGlu4,Cyt6]OXT-(4-8), and the low dose of vasotocin was markedly reduced by the OXT antagonist. This effect could also be reduced by pretreatment with the V1 antagonist but not with the V2 antagonist. These results suggest the existence of a separate neurohypophyseal hormone receptor complex in the brain affecting memory processes that differs from the peripheral V1, V2, and OXT receptor.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audigier S., Barberis C. Pharmacological characterization of two specific binding sites for neurohypophyseal hormones in hippocampal synaptic plasma membranes of the rat. EMBO J. 1985 Jun;4(6):1407–1412. doi: 10.1002/j.1460-2075.1985.tb03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C. [3H]vasopressin binding to rat hippocampal synaptic plasma membrane. Kinetic and pharmacological characterization. FEBS Lett. 1983 Oct 17;162(2):400–405. doi: 10.1016/0014-5793(83)80795-9. [DOI] [PubMed] [Google Scholar]
- Bohus B., Urban I., van Wimersma Greidanus T. B., de Wied D. Opposite effects of oxytocin and vasopressin on avoidance behaviour and hippocampal theta rhythm in the rat. Neuropharmacology. 1978 Apr-May;17(4-5):239–247. doi: 10.1016/0028-3908(78)90107-7. [DOI] [PubMed] [Google Scholar]
- Burbach J. P., Kovács G. L., de Wied D., van Nispen J. W., Greven H. M. A major metabolite of arginine vasopressin in the brain is a highly potent neuropeptide. Science. 1983 Sep 23;221(4617):1310–1312. doi: 10.1126/science.6351252. [DOI] [PubMed] [Google Scholar]
- Cheng S. W., North W. G. Vasopressin reduces release from vasopressin-neurons and oxytocin-neurons by acting on V2-like receptors. Brain Res. 1989 Feb 6;479(1):35–39. doi: 10.1016/0006-8993(89)91332-2. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., Rotteveel F., Voorhuis T. A., Terlou M. Topography of binding sites for neurohypophyseal hormones in rat brain. Eur J Pharmacol. 1985 Mar 26;110(1):113–119. doi: 10.1016/0014-2999(85)90036-6. [DOI] [PubMed] [Google Scholar]
- Dorsa D. M., Petracca F. M., Baskin D. G., Cornett L. E. Localization and characterization of vasopressin-binding sites in the amygdala of the rat brain. J Neurosci. 1984 Jul;4(7):1764–1770. doi: 10.1523/JNEUROSCI.04-07-01764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S., Gaillard R. C., Guillon G., Marie J., Schoenenberg P., Muller A. F., Manning M., Sawyer W. H. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol Pharmacol. 1986 Aug;30(2):171–177. [PubMed] [Google Scholar]
- Koob G. F., Le Moal M., Gaffori O., Manning M., Sawyer W. H., Rivier J., Bloom F. E. Arginine vasopressin and a vasopressin antagonist peptide: opposite effects on extinction of active avoidance in rats. Regul Pept. 1981 Jun;2(3):153–163. doi: 10.1016/0167-0115(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Kovács G. L., De Wied D. Hormonally active arginine-vasopressin suppresses endotoxin-induced fever in rats: lack of effect of oxytocin and a behaviorally active vasopressin fragment. Neuroendocrinology. 1983 Oct;37(4):258–261. doi: 10.1159/000123554. [DOI] [PubMed] [Google Scholar]
- Kovács G. L., Szabó G., Sarnyai Z., Telegdy G. Neurohypophyseal hormones and behavior. Prog Brain Res. 1987;72:109–118. doi: 10.1016/s0079-6123(08)60200-9. [DOI] [PubMed] [Google Scholar]
- Kovács G. L., Veldhuis H. D., Versteeg D. H., De Wied D. Facilitation of avoidance behavior by vasopressin fragments microinjected into limbic-midbrain structures. Brain Res. 1986 Apr 16;371(1):17–24. doi: 10.1016/0006-8993(86)90805-x. [DOI] [PubMed] [Google Scholar]
- Kruszynski M., Lammek B., Manning M., Seto J., Haldar J., Sawyer W. H. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine ]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1980 Apr;23(4):364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Le Moal M., Koob G. F., Koda L. Y., Bloom F. E., Manning M., Sawyer W. H., Rivier J. Vasopressor receptor antagonist prevents behavioural effects of vasopressin. Nature. 1981 Jun 11;291(5815):491–493. doi: 10.1038/291491a0. [DOI] [PubMed] [Google Scholar]
- Manning M., Kruszynski M., Bankowski K., Olma A., Lammek B., Cheng L. L., Klis W. A., Seto J., Haldar J., Sawyer W. H. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem. 1989 Feb;32(2):382–391. doi: 10.1021/jm00122a016. [DOI] [PubMed] [Google Scholar]
- Manning M., Nawrocka E., Misicka A., Olma A., Klis W. A., Seto J., Sawyer W. H. Potent and selective antagonists of the antidiuretic responses to arginine-vasopressin based on modifications of [1-(beta-mercapto-beta,beta-pentamethylenepropionic acid),2-D-isoleucine,4- valine]arginine-vasopressin at position 4. J Med Chem. 1984 Apr;27(4):423–429. doi: 10.1021/jm00370a002. [DOI] [PubMed] [Google Scholar]
- de Wied D. Behavioral effects of intraventricularly administered vasopressin and vasopressin fragments. Life Sci. 1976 Sep 1;19(5):685–690. doi: 10.1016/0024-3205(76)90165-x. [DOI] [PubMed] [Google Scholar]
- de Wied D., Gaffori O., van Ree J. M., de Jong W. Central target for the behavioural effects of vasopressin neuropeptides. Nature. 1984 Mar 15;308(5956):276–278. doi: 10.1038/308276a0. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus T. B., van Ree J. M., de Wied D. Vasopressin and memory. Pharmacol Ther. 1983;20(3):437–458. doi: 10.1016/0163-7258(83)90036-0. [DOI] [PubMed] [Google Scholar]