Abstract
Conspicuous asymmetries seen in many animals and plants offer diverse opportunities to test how the development of a similar morphological feature has evolved in wildly different types of organisms. One key question is: do common rules govern how direction of asymmetry is determined (symmetry is broken) during ontogeny to yield an asymmetrical individual? Examples from numerous organisms illustrate how diverse this process is. These examples also provide some surprising answers to related questions. Is direction of asymmetry in an individual determined by genes, environment or chance? Is direction of asymmetry determined locally (structure by structure) or globally (at the level of the whole body)? Does direction of asymmetry persist when an asymmetrical structure regenerates following autotomy? The answers vary greatly for asymmetries as diverse as gastropod coiling direction, flatfish eye side, crossbill finch bill crossing, asymmetrical claws in shrimp, lobsters and crabs, katydid sound-producing structures, earwig penises and various plant asymmetries. Several examples also reveal how stochastic asymmetry in mollusc and crustacean early cleavage, in Drosophila oogenesis, and in Caenorhabditis elegans epidermal blast cell movement, is a normal component of deterministic development. Collectively, these examples shed light on the role of genes as leaders or followers in evolution.
This article is part of the themed issue ‘Provocative questions in left–right asymmetry’.
Keywords: left–right asymmetry, symmetry breaking, random asymmetry, evolution of development, handed behaviour, phenotypic plasticity
1. Introduction: direction of asymmetry
(a). Background
Conspicuous phenotypic differences are usually thought to be associated with genes for alternative phenotypes. In the case of left–right asymmetries, however, the most obvious difference between phenotypes—direction of asymmetry—is sometimes heritable and sometimes not. In some cases it may even be directly induced by the environment. Because left–right asymmetries have evolved independently in a wide array of animals and plants [1–5], they provide an attractive system for studying the contribution of genes, environment and chance to the developmental origins and subsequent evolution of novel phenotypic variation.
Whether they be studies of development, function or evolution of left–right asymmetries, two fundamental questions inevitably arise: (i) why is one side different from the other (i.e. what causes or favours asymmetry in general), and (ii) why is the side that ‘differs’ on the right versus the left (i.e. what causes or favours asymmetry in a particular direction [6])? These questions can apply at either the individual level, or the population or species level. In this review, I will focus primarily on the factors that cause asymmetry to be oriented in a particular direction during development of an individual by examining clues from a variety of model and non-model organisms.
Much has been written about the diversity of developmental routes to left–right asymmetry in animals ([7–10] and references therein) and plants [11–13]. One common theme emerges from studies of molecular mechanisms: asymmetry at the macroscopic level can often be traced back to subcellular (e.g. cytoskeletal) asymmetries, a connection expected on theoretical grounds [10,14,15]. But these studies focus almost exclusively on model taxa with fixed asymmetries (little or no variation within species) where development of asymmetry is deterministic. The random asymmetries seen in many animals and plants [4,5,16] continue to pose a puzzle: if symmetry breaking is not coupled to subcellular asymmetry, how is asymmetry oriented developmentally to the right or left in these organisms?
(b). Types of left–right asymmetries: helical and bilateral
In most organisms, conspicuous left–right asymmetries manifest either as (i) helices or spirals or (ii) alternate bilateral forms where one side of the body is not a mirror image of the other across a midplane. Both have two states, right-handed (dextral = clockwise) or left-handed (sinistral = anticlockwise), and therefore both depend on some mechanism to specify direction of asymmetry during development.
Helices and spirals coil about a single axis. Helix coiling direction is (arbitrarily) called right-handed (dextral) if the motion tracing out the helix from the near end to the far end from the viewer's perspective is clockwise, and left-handed (sinistral) if that motion is anticlockwise (figure 1a-1) [17]. In a true helix, the diameter does not change along its length (figure 1a-1). Gastropod shells coil in a helicospiral, where helix diameter increases progressing from the apex to the aperture. Conveniently, in dextral (right-handed) snails, the aperture is to the right when the apex is up (figure 1a–3).
Figure 1.
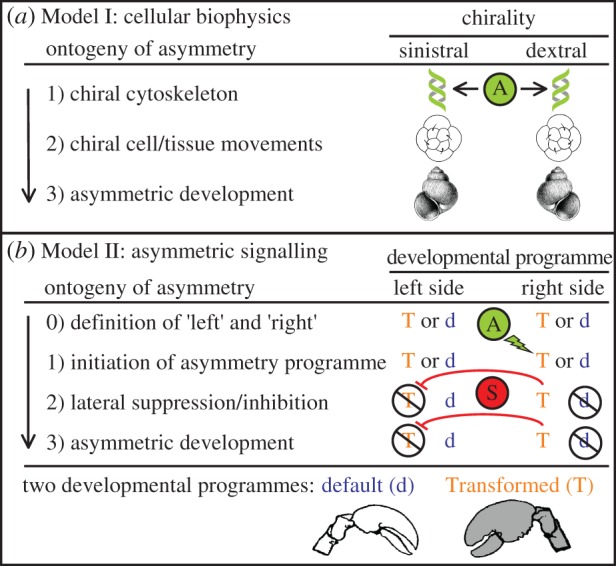
Two alternative models of the developmental origins of symmetry breaking (A) during the ontogeny of left–right asymmetry. (a) Model I, cellular biophysics. Stage 1, subcellular chirality (asymmetry, A) already exists in the early embryo; stage 2, the orientation of early cleavage stages or cell movements are biased by the pre-existing subcellular chirality, stage 3, development proceeds to yield an asymmetric phenotype whose direction of asymmetry was pre-determined in the egg or early embryo. (b) Model II, asymmetric signalling. Stage 0, in the early embryo each side has the potential to develop via either a default (d) or a Transformed (T) developmental programme; stage 1, an asymmetric signal of some type (A) initiates the developmental programme to transform (T) on one side; stage 2, initiation of programme T on one side overrides the default developmental programme (d) on that side and also suppresses (S) the transformation programme (T) on the opposite side; stage 3, development proceeds to yield an asymmetric phenotype, where the transformed phenotype (T) is only fully expressed on one side.
Figure 3.
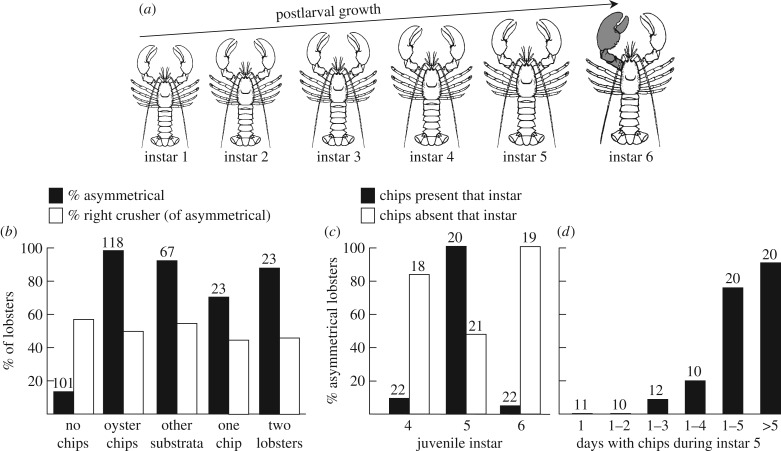
Effect of growth environment on development of claw asymmetry in American lobsters (Homarus americanus): experiments of C. K. Govind. (a) Schematic of early postlarval growth showing the sudden transformation to a crusher claw (grey) starting at instar 6. (b–d) All graphs indicate lobsters scored at instars 8 or 9. (b) Per cent of juvenile lobsters with asymmetrical claws (solid bars), and the per cent of asymmetrical lobsters with a crusher claw on the right side (open bars), as a function of growth conditions experienced during instars 4 and 5: no oyster chips* (pooled results from [54–56]), a few oyster chips* (pooled results from [54–58]), various other substrata (gravel, mud or plastic chips; pooled from [55]), a single oyster chip* [56], or a second juvenile lobster [56]. (c) Per cent of juvenile lobsters with asymmetrical claws as a function of whether oyster chips* were present or absent during a single instar [57]: solid bars, chips* present only for instar 5; open bars, chips* present for all instars except the one indicated (e.g. lobsters lacking chips at instar 5 were reared with chips during all other instars). (d) Per cent of juvenile lobsters with asymmetrical claws after exposure to oyster chips* for different numbers of days during the first 5 days of instar 5 (first 5 columns) or for the remaining days after day 5 (last column) [57]. Numbers above bars are sample sizes. * Oyster chips are small, hard fragments of oyster shell that lobsters manipulate with their claws and therefore stimulate claw activity.
In bilateral asymmetries, right and left sides are on opposite sides of a midplane [6]. In animals, this plane is defined by two body axes, the antero-posterior (AP) axis and the dorsoventral (DV) axis. By convention, the right side is the side to the right of the AP axis when viewed from a dorsal perspective with the anterior end facing forward or up (i.e. it is defined from the perspective of the organism itself) [18]. In plant flowers, however, the naming convention is different: the right side is defined from the perspective of an observer facing a mature flower [19]. Although disorienting from a developmental perspective, this practical convention avoids the considerable confusion that arises where the floral midplane rotates during emergence by 90° (transversely zygomorphic flowers [20]) or 180° or more (resupinate flowers [21]).
Much is now known about how the axes that define the midplane are patterned. Across vertebrates, the two body axes (AP and DV) are patterned by a combination of Nodal and BMP signalling [22,23]. More broadly, although the functions of some components vary, the Chordin–BMP pathway appears to pattern the DV axis in most animals [24]. Improbable as it might seem, the midplane defined by these axes can be remarkably crisp (figure 6a), so it is more than just a hypothetical construct.
Figure 6.
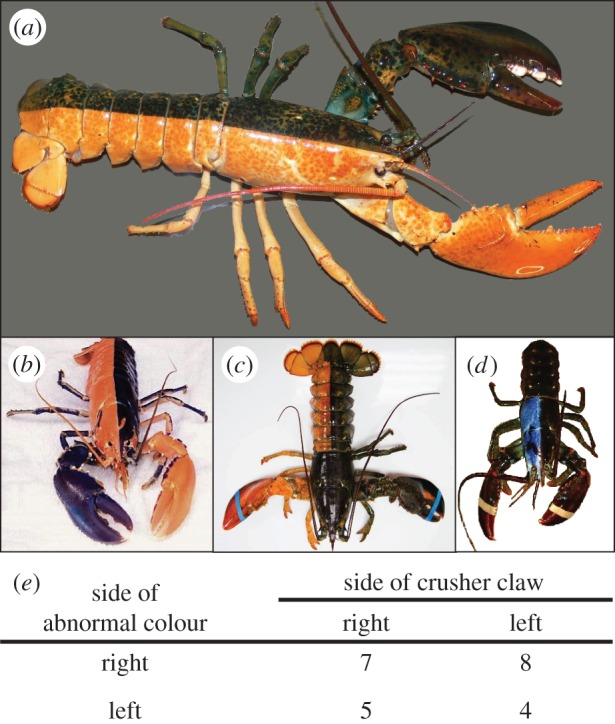
Examples of bi-colour lobsters (Homarus americanus) and a test of concordance between the direction of colour asymmetry and the direction of claw asymmetry. (a) Typical, fully bicoloured form, abnormally coloured (orange) right side, crusher claw on left. (b) Highly unusual harlequin-coloured lobster: right side of the body is abnormally coloured, but the left claw is abnormally coloured, blotchy colour patterns appear on the periopods (crusher claw on right; not scored). (c) Unusual partial bicolour: body legs and claw on the right side abnormally coloured, but the entire carapace is normal colour (crusher claw on right). (d) Unusual partial bicolour: only the right half of the carapace is abnormally coloured (blue) (crusher claw probably on left, not scored). (e) Counts of various combinations of body side colour and side of crusher claw (N = 24, excluding four ambiguous specimens like (b) and (d)) (photos: (a) A. R. Palmer and (b–d) various public-domain sources).
(c). Alternative models of symmetry breaking
Although much still remains to be learned, two fundamentally different models of symmetry breaking have emerged (figure 1). Direction of asymmetry may be determined during development either (i) by subcellular chirality effects on cellular biophysics very early in development (Model I) or (ii) by an asymmetric signal on one side of the body later in development (Model II). Symmetry breaking during development (A in figure 1) is therefore tied directly to subcellular asymmetries in Model I, but the initial asymmetric signal in Model II can arise from at least three sources: asymmetric gene expression (itself possibly biased by subcellular asymmetries), asymmetric effects of environment (can be random but can be biased towards one side experimentally), or chance (purely stochastic, cannot be biased towards one side experimentally).
Where an egg or early embryo is already chiral, no asymmetric signalling is required to determine direction of asymmetry (Model I, figure 1a). In some animal groups, the earliest embryos are already chiral [25] and sometimes even the egg cytoplasm is chirally arranged around the animal–vegetal axis [26] (stage 1). In these cases, direction of adult asymmetry may be considered an emergent property of egg or early embryo chirality. In extreme cases, no developmental ‘decisions’ are made about rightness or leftness at all during development, because direction of asymmetry is preordained in the egg itself.
If the early embryo is not already chiral, then the ontogeny of asymmetry in an individual depends on whether the asymmetry is in a paired bilateral trait or due to lateral displacement or twisting of a medial structure.
Paired, bilateral traits must pass through four developmental stages (Model II, figure 1b), regardless of whether direction of asymmetry is fixed (all individuals within a species asymmetric in the same direction) or random (equal numbers of right- and left-sided individuals within a species). Stage 0: the right and left sides become defined in the early embryo as soon as the AP and DV axes define the midplane (see §1b). At this stage, each side has the potential to develop via either a default developmental programme (d) or a Transformed developmental programme (T), but no asymmetry yet exists. Stage 1: symmetry is broken later in ontogeny when some asymmetric signal (A) initiates the Transformed programme (T) on one side of the body. Stage 2: once initiated on one side, the Transformed programme (T) has two immediate effects. First, it replaces and thereby effectively inactivates the default developmental programme (d) on that side. Second, it must somehow inhibit the Transformed programme (T) on the opposite side, otherwise both sides could potentially begin to transform and yield a symmetrical (doubly transformed) phenotype. Stage 3: one of the two potential developmental programmes on each side of the body (d or T) has been inactivated, so that one side follows the default developmental pathway (d) and the other the Transformed pathway (T), which ultimately yields an asymmetrical phenotype.
The development of asymmetries in medial structures that deviate to one side of the body, or that twist, may not neatly follow either model. Vertebrate visceral asymmetries illustrate this nicely. In chickens, transient leftward tissue migration across the midline determines the direction of heart asymmetry before the Nodal signalling cascade is activated [27]. This leftward tissue migration may very well be biased by subcellular chiralities [28] (more consistent with Model I). The direction of heart asymmetry in mice, however, appears to be initiated by an asymmetric signal (left-sided Nodal expression) that in turn inhibits right-sided Nodal expression via Lefty [29] (more consistent with Model II). Finally, although an asymmetric signal (Model II, stage 1) may initiate bending or twisting of a medial structure, no inhibition may be needed (Model II, stage 2) because once bending or twisting starts, the direction of asymmetry is fixed (i.e. alternate default and Transformed programmes need not exist on opposite sides of the body).
In this review, I will focus primarily on the signal that initiates the Transformed programme (T) on one side of the body. Clearly, some kind of signal (A in figure 1b) is required to induce one side to transform so that it differs from the other. As will become apparent below, the source of that signal during development appears to vary greatly among organisms: it can be a deterministic effect of genes, a deterministic effect of environment or purely stochastic.
2. Genetic determination of direction of asymmetry
To be clear, what is meant by saying that a trait is ‘determined genetically’? Because most phenotypic traits emerge via the expression of many genes during development [30], no single gene can be said to ‘determine’ a particular trait. To say anything at all about whether one or more genes influence the phenotype of an organism, some phenotypic variation must exist among individuals. The statement about genetic determination then becomes: is the phenotypic variation heritable or not? In the present context, this would be: is left-sidedness more common among the offspring of left-sided parents than among the offspring of right-sided parents? In species that are exclusively left-sided, no compelling statement can be made about whether left-sidedness is ‘determined’ genetically or by how many genes. Only when a right-sided variant emerges within a left-sided species can one ask whether, or how many, genes affect direction of asymmetry.
(a). Gastropod shell coiling direction: alternative alleles for right, left and stochastic
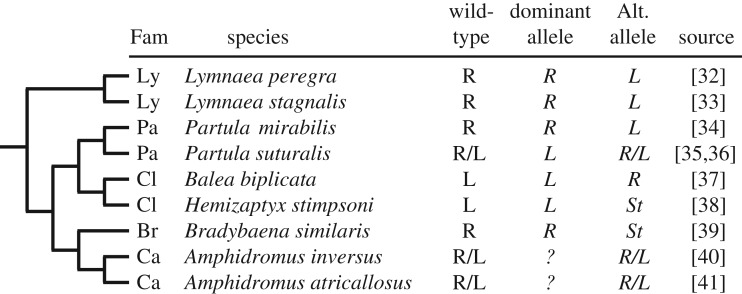
The much beloved story about inheritance of coiling direction in gastropods is rather murkier on closer inspection (figure 2). First, instead of two alleles (right and left) three alleles are now known (right, left and stochastic). Second, dominance relations among these alleles vary: the stochastic allele appears to be consistently recessive regardless of whether the wild-type allele is right or left, but whether the right or left allele is recessive in species with only right and left alleles depends on the group. Having said this, in all six species where polymorphism is rare the dominant allele is the one for the wild-type phenotype. Third, left alleles and stochastic alleles have evolved multiple times, as have dextral alleles in typically sinistral families [42], so the genetic determination of coiling direction in gastropods appears to be quite labile.
Figure 2.
Phylogenetic relations among species of gastropods for which inheritance of coiling direction is known (tree condensed greatly from [31]). Family (Fam) abbreviations: Ly, Lymnaeidae; Pa, Partulidae; Cl, Clausiliidae; Br, Bradybaenidae; Ca, Camaenidae. R, right or dextral; L, left or sinistral; R/L, polymorphic within species, discrete alleles for R and L; St, stochastic: allele where homozygous recessive offspring are phenotypically R or L at random.
The original single-gene model for shell coiling direction in Lymnaea [43] was muddied by the puzzling occurrence of ‘dextral breakthroughs’: occasional spontaneous appearance of dextral offspring in supposedly homozygous recessive sinistral lineages [44]. But careful breeding experiments later revealed that dextral breakthroughs arise from recombination, most probably between two point mutations on the same gene [32].
Some 93 years after its genetic basis was discovered, coiling direction in Lymnaea stagnalis was finally linked to a diaphanous-related formin gene that affects the egg cytoskeleton [26]. Nonetheless, the diversity of alleles (right, left and stochastic) and the variable dominance relations in other gastropod species (figure 2), coupled with the curious observation that sinistral spiral cleavage is not a mirror image of dextral cleavage [45], suggests that no simple one-to-one mapping exists between coiling direction and a particular subcellular chirality (Model I, figure 1) in all gastropods.
(b). Flatfish eye side: weak or polygenic inheritance
The inheritance of whole-body asymmetry in the normally polymorphic starry flounder (Platichthys stellatus) is either polygenic or has a large environmental component [46] (table 1). Off the west coast of the USA, right-eyed and left-eyed forms are roughly equally common, whereas Japanese fish from the western Pacific are almost exclusively left-eyed [47]. Overall, eye side is about 70–80% heritable (table 1f), a result we have repeated in P. stellatus from the British Columbia coast of Canada (C. A. Bergstrom and A. R. Palmer 2005, unpublished data). Even in crosses between two right-eyed parents, 33% of offspring are left-eyed (table 1c). Heritability is bit higher among Japanese parents (85%) compared with US parents (68%) (table 1a,b), but the geographical origin of the female parent has surprisingly little effect on heritability in trans-Pacific crosses: 81% versus 78% (table 1d,e). Because direction of asymmetry is not consistently random throughout the species' range, some heritability of eye-side variation is expected. The rather consistent eye-side frequencies in multiple crosses between parents of the same phenotype, and the similar contribution of male and female parents, suggests either that many loci are involved or that a smaller number of loci experience variable penetrance.
Table 1.
Inheritance of eye-side direction in polymorphic starry flounder (Platichthys stellatus): percentage of left-eyed offspring from various crosses [46]. M, male; F, female; Jap, Japan; US, United States west coast; N, number of offspring; crosses, number of separate crosses.
| cross type (M × F) | eye side of male parent | eye side of female parent |
|||||
|---|---|---|---|---|---|---|---|
| left |
right |
||||||
| % left | N | crosses | % left | N | crosses | ||
| (a) Jap × Jap | left | 85.3 | 150 | 2 | |||
| (b) US × US | left | 67.8 | 435 | 2 | 36.9 | 509 | 3 |
| (c) US × US | right | 58.3 | 609 | 3 | 33.1 | 574 | 3 |
| (d) Jap × US | left | 80.7 | 1446 | 2 | 51.2 | 568 | 2 |
| (e) US × Jap | left | 78.5 | 694 | 1 | |||
| (f) pooled | left | 78.3 | 2031 | 7 | 44.5 | 1077 | 5 |
| right | 58.3 | 609 | 3 | 33.1 | 574 | 3 | |
(c). Bill crossing direction in crossbill finches: not inherited
Crossbill finches (Loxia sp.) are unusual among birds because their upper beak crosses dramatically to one side or the other of the lower beak [48]. In most species, direction of bill crossing is close to random, although the upper bill crosses to the left more frequently in the white-winged crossbill (L. leucoptera) [49]. A modest series of crosses in white-winged (L. leucoptera), red (L. curvirostra) and parrot (L. pytyopsittacus) crossbills, revealed no strong signal that direction of bill crossing was inherited [48], consistent with the general pattern that direction of asymmetry is almost never inherited in cases of random asymmetry [3].
(d). Plant asymmetries: both inherited and not inherited
Many plants exhibit random asymmetry of various characters, including seedling handedness, leaf rolling orientation and spiral orientation of support structures and flowers on inflorescences [2]. In nearly all cases of random asymmetries where crossing has been tried, direction of asymmetry is not inherited [3]. The one known exception is direction of style bend in Heteranthera flowers, where direction of bending is controlled by a single locus with right-bending dominant [50]. This remarkable exception nonetheless provides a powerful test of whether genes are leaders or followers in the evolutionary origin of left–right asymmetry and could offer valuable insights into how genes somehow ‘capture’ pre-existing phenotypic variation [3] (see §6).
Rather fewer studies have been done on whether direction of asymmetry is inherited in plants where asymmetry variation is not random. The most striking are studies of seed-pod coiling direction in Medicago species. In some species, pods coil dextrally and in others sinistrally. Curiously, in dextrally coiled species, occasional sinistral variants are known, whereas dextral variants are virtually unknown in sinistral species [51]. In two dextrally coiled species, M. tuberculata and M. litoralis, coiling direction is controlled by two alleles at single locus with dextral dominant [51]. Therefore, as in snails, the dominant allele corresponds to the predominant phenotype in natural populations.
In laboratory mutants of Arabidopsis, different alleles for α-tubulin, B-tubulin or microtubule-associated proteins yield helical growth in roots, hypocotyls, petioles and petals [11,52]. In these cases, the mutant is spiral but the wild-type is symmetric. Some alleles cause a dextral spiral, others cause a sinistral spiral, although alleles for sinistral growth typically override those for dextral epistatically. Whether these mutants have analogues in natural populations, however, remains to be seen.
3. Environmental determination of direction of asymmetry
Animals rarely find themselves living in a predictably asymmetric environment, so how could environmental effects bias asymmetry in a particular direction? One familiar environmental effect is handed behaviour, where some individuals are right-handed and others left-handed. Handed behaviour occurs when one side is used consistently more than the other and this difference in use level could serve as the asymmetric signal (A in figure 1b) that initiates an asymmetric developmental programme. Significantly, even though the preferred side may vary at random the phenotypic effects of handed behaviour are predictable: greater use of one side initiates transformation of that side. This differs from purely stochastic determination of asymmetry (see §4), where direction of asymmetry cannot be biased towards one side.
(a). Lobsters: handed behaviour and direction of asymmetry
Perhaps the most compelling evidence that handed behaviour, or differential use of one side, can induce morphological asymmetry comes from C. K. Govind's studies of claw asymmetry in American lobsters, Homarus americanus (summarized in [53]). Most lobsters have a large crusher on one side and a smaller cutter on the other (figure 6). However, newly settled lobsters possess two cutter-type claws and retain them through the first five juvenile instars (figure 3a). Transformation of a cutter type into a crusher type on one side starts abruptly at instar 6. This dramatic, qualitative change renders the lobster system ideal for studying the factors that initiate crusher–claw transformation on one side.
Govind's many experiments with juvenile lobsters yield two important conclusions: (i) greater activation of mechanoreceptors (or possibly proprioceptors) in one claw induces it to transform from a cutter type into a crusher type that in turn inhibits transformation of the other claw (Model II, figure 1b) and (ii) initiation of claw transformation is restricted to a narrow time window during (or close to) instar 5.
Differential use of one claw is critical. First—and most significantly—in the absence of sufficient stimulation, most individuals (more than 85%) never develop a crusher claw (‘no chips’, figure 3b and table 2a) and therefore lack a crusher claw for life. The mere addition of oyster chips (a claw-activity stimulus) to culture bowls induces a crusher claw to develop in 95% of individuals (figure 3b and table 2g). Addition of other stimuli (other substrata, a single oyster chip, a second lobster) induces crusher claws at somewhat lower rates (70–90%) but nonetheless at much higher rates than no stimulus at all (figure 3b). Clearly, some kind of stimulus is required to initiate transformation to a crusher even though transformation of the right or left claw occurs at random (open bars, figure 3b).
Table 2.
Effect of chela manipulations on which side develops a crusher claw (figure 6a) in American lobsters Homarus americanus [54]. All lobsters were reared on soft food either without any substrata, or with oyster chips for claw stimulation. N, number of individuals.
| treatment in instar 4 or 5 | phenotypes at instar 8 or 9 |
||
|---|---|---|---|
| % asymmetrical | % left crushera | N | |
| oyster chips absent | |||
| (a) intact claws | 14.3 | 66.7 | 21 |
| (b) left claw exercised | 90.0 | 83.3 | 20 |
| (c) left claw stroked | 21.7 | 40.0 | 23 |
| (d) left claw autotomy | 5.0 | 0.0 | 20 |
| (e) left claw autotomy and handle animal | 0.0 | 0.0 | 21 |
| (f) left claw autotomy and right claw exercised | 78.9 | 0.0 | 19 |
| oyster chips present | |||
| (g) intact claws | 95.7 | 54.5 | 23 |
| (h) right claw dactylotomy | 76.5 | 84.6 | 17 |
| (i) left claw autotomy | 95.8 | 0.0 | 24 |
| (j) left claw autotomy and right claw dactylotomy | 22.7 | 40.0 | 22 |
| (k) left claw autotomy and right closer muscle tenotomy | 5.6 | 100.0a | 18 |
| (l) left claw autotomy and right opener muscle tenotomy | 17.6 | 33.3 | 17 |
a % of asymmetrical individuals.
The timing is also critical: differential claw use must be during or close to instar 5. Adding a claw-activity stimulus (oyster chips) during instar 5 induces asymmetrical claws in nearly 100% of individuals, whereas fewer than 10% develop a crusher claw if the stimuli are presented only during instars 4 or 6 (solid bars, figure 3c). Similarly, removing claw-activity stimuli only during instar 5 yields many fewer lobsters with crusher claws, whereas removing the stimuli only during instar 4 or instar 6 has little effect on the numbers of transforming lobsters (open bars, figure 3c). Finally, claw-activity stimuli must be present for 4 days or more during instar 5 to ensure development of a crusher claw (figure 3d).
Significantly, experimental manipulations of one claw during instar 5 when no other claw-activity stimuli are present can bias crusher transformation to one side of the body. For example, experimental exercise of one claw induces a crusher claw on that side either by itself (table 2b), or when coupled with autotomy of the opposite claw (table 2f) in 80–90% of individuals. Merely removing one claw does not, by itself, induce crusher formation on the other side if no other stimuli are present (table 2d,e).
Experimental manipulations of one claw during instar 5 when claw-activity stimuli are present narrows down the kinds of differential activity that can bias crusher transformation to one side. For example, removal of one claw does induce a crusher claw on the opposite side if claw-activity stimuli (oyster chips) are present (table 2i). In addition, removing the movable finger (dactyl) on one side induces a high proportion (84%) of crusher claws on the other side (table 2h). Impairing the activity of both claws simultaneously, via autotomy of one claw and severing the movable finger or the tendons of the closer and opener muscles of the other, yields a dramatic reduction in the number of crusher claw transformations (table 2j–l).
The inhibition of transformation of the opposite side, once one side has transformed into a crusher claw is nearly perfect: ‘In over 15 years of rearing approximately 2000 lobsters, we have not encountered the double-crusher configuration’ [53]. The lobster claw system therefore illustrates particularly clearly the Model II mode of symmetry breaking (figure 1b).
(b). Katydids: handed behaviour preceded asymmetry in sound-producing structures
Handed behaviour in a symmetrical ancestor may have preceded handed morphology evolutionarily in the sound-producing file-scraper system of katydids (Tettigonoidea) [59,60]. Tettigoniid katydids show a clear morphological asymmetry: the left wing cover with the file overlaps the right wing cover with the scraper. However, in the Haglidae (sister group to tettigoniid katydids) both right and left wing covers of an individual bear a file, but only one file at a time is used to sing [59]. Singing by an individual is therefore necessarily a handed behaviour [59], although it would be interesting to know whether an individual haglid katydid consistently crosses one wing over the other. Finally, file development, at least in locusts, ‘is in part directly due to the stimulus from the friction of one wing over the other’ [61], so handed singing behaviour could easily amplify morphological asymmetry.
(c). Earwigs: right-handed penis use preceded penis asymmetry
Tantalizing evidence suggests that handed penis use preceded the evolutionary reduction and loss of one penis in earwigs (Dermaptera) [62,63]. Males in all five basal earwig families possess two penises, whereas those in the most derived clade (three families in the Eudermaptera) possess only the right penis. In four of the five basal families, the right or left penis is used (or positioned for mating) at random within species [62,64]. This handed behaviour appears not to be heritable [65]. Remarkably, males in the family that is sister group to the right-penis-only Eudermaptera exhibit a pronounced preference to use the right penis, even though two fully functional penises are present [62]. Kamimura's studies provide some of the clearest evidence yet of how a directional handed behaviour may have facilitated evolution of a directional morphological asymmetry.
4. Stochastic determination of direction of asymmetry
In the ongoing quest for deterministic explanations such as asymmetric gene expression or predictable environmental effects, a third hypothesis for how left- or right-sidedness might arise during development is often overlooked: chance. Neither Model I nor Model II modes of development (figure 1) preclude stochastic determination of direction of asymmetry. Unlike environmental effects (see §3), however, direction of asymmetry cannot be biased by manipulating growth conditions during development of these types of asymmetries.
In Model I (figure 1a), cytoskeletal chirality might spontaneously take one of two stable states: dextral or sinistral, like the random orientation of microtubules that rotate Drosophila eggs during development to ensure proper egg shape and orientation of the AP axis [66,67]. Presumably, a similar spontaneous alignment of egg cytoskeleton into alternative orientations predisposes eggs to cleave dextrally or sinistrally in recessive homozygotes of the stochastic allele in two gastropod species (figure 2). In Model II (figure 1b), the initial signal triggering an alternative developmental pathway (A) could arise spontaneously on either the right or left side. Once initiated, lateral inhibition would then stabilize this asymmetry in one direction [53].
(a). Deterministic and stochastic chiral blastomere movements during development
Early cleavage yields asymmetrical embryos at the four- or eight-cell stage in many protostome animals. The most familiar of these is spiral cleavage, seen in many spiralian protostomes (molluscs, annelids, nemerteans, flatworms, gnathostomulids and entoprocts) [68]. Cleavage orientation is typically fixed within species (with occasional reversals), and overwhelmingly clockwise (dextral) going from the four- to eight-cell stage when viewed from the animal pole in fully spirally cleaving groups [69]. Nonetheless, a few examples of invariant sinistral cleavage at the eight-cell stage are known in polychaetes [70], bivalves [71] and sinistrally coiled gastropod species [45,72].
Surprisingly, given the generally determinate cleavage seen in spiralian protostomes [73], the chirality of early cleavage is not fixed in a wide range of taxa (table 3). Most famously, in gastropod species that are polymorphic for coiling direction, the direction of shell coiling is directly tied to cleavage orientation. Species that normally have dextrally coiled shells exhibit dextral cleavage at the eight-cell stage. However, sinistral cleavage at the eight-cell stage, owing either to natural genetic variation [32,85] or to experimental manipulation of blastomere arrangements [45,75], yields a snail with a sinistrally coiled shell. In addition, some polychaete embryos exhibit reversed chirality in up to 10% of single broods (table 3). Curiously, all bivalves examined so far exhibit stochastic cleavage orientation transiting from the two- to four-cell stage even though they overwhelmingly sinistral or overwhelmingly dextral at the eight-cell stage (table 3).
Table 3.
Animals that exhibit dimorphic, mirror image early embryo forms during normal development. Concordant: chiral state of eight-cell embryo depends on chiral state of four-cell embryo.
| taxon | observed chirality |
concordant? | effect on juvenile phenoype | source | |
|---|---|---|---|---|---|
| 4-cell | 8-cell | ||||
| Spiralia | |||||
| Mollusca- Gastropoda | |||||
| Basommatophora | |||||
| Lymnaea peregra (dextral) | 100% sin | 100% dex | yes | dextral shell | [32] |
| L. peregra (sinistral) | 100% dex | 100% sin | yes | sinistral shell | [32] |
| Lymnaea stagnalis (dextral) | mostly sin | 100% dex | mostly | dextral shell | [74] |
| L. stagnalis (sinistral) | mostly dex | 100% sin | mostly | sinistral shell | [75] |
| Mollusca- Bivalvia | |||||
| Heterodonta | |||||
| Dreissena polymorpha | stochastic | 80% sin | no | not evident | [71] |
| Cumingia tellinoides | stochastic | 100% dex | no | not evident | [76] |
| Pholas dactylus | stochastic | 100% dex | no | not evident | [77] |
| Spisula subtruncata | stochastic | 100% dex | no | not evident | [77] |
| Annelida- Polychaeta | |||||
| Platynereis dumerilii | 10% sin | 10% sin | yes | not evident | [78] |
| Ecdysozoa | |||||
| Arthropoda- Crustacea | |||||
| Cladocera | |||||
| Holopedium gibberum | ? | stochastica | ? | not evident | [79]b |
| Daphnia pulex | ? | stochastica | ? | not evident | [79]b |
| Copepoda | |||||
| Cyclops viridis | ? | stochastica | ? | not evident | [80]b |
| Polyphemus pediculus | ? | stochastica | ? | not evident | [80]b |
| Amphipoda | |||||
| Parhyale hawaiensis | stochastic | stochastic | yes | not evident | [81] |
| Orchestia cavimana | stochastic | stochastic | yes | not evident | [79] |
| Cheirimedon femoratus | stochastic | stochastic | yes | not evident | [82] |
| Tryphosella kergueleni | stochastic | stochastic | yes | not evident | [82] |
| Euphausiacea | |||||
| (not identified) | ? | stochastica | ? | not evident | [80]b |
| Meganyctiphanes norvegica | stochastic | stochastic | yes | not evident | [80] |
| Decapoda | |||||
| Sicyonia ingentis | 75% DII | 50% DIIr | no | not evident | [83] |
| Penaeus vannamei | 92% DII | 64% DIIr | partly | not evident | [83] |
| Penaeus monodon | stochastic | stochastic | yes | not evident | [84] |
aAssumed, counts not cited.
bResults cited in tabled reference.
Rather unexpectedly, numerous crustaceans also exhibit embryonic asymmetry at the eight-cell stage (table 3), even though their cleavage is not considered to be spiral [86]. In striking contrast to snails and polychaetes, though, both mirror image forms of eight-cell embryos are equally common within these species, and chiral embryo form is not obviously correlated with asymmetries in juvenile form. Like gastropods, but unlike bivalves, chirality at the eight-cell stage appears to depend on chirality at the four-cell stage.
Clearly, no simple rule governs whether the direction of asymmetrical early cleavages is deterministic or stochastic, or whether these cleavage asymmetries have any downstream phenotypic effects.
(b). Normal versus anomalous random asymmetry: the role of lateral inhibition
In many species, random asymmetry is the rule: the vast majority of individuals are asymmetric (species 1–4, figure 4d), but direction of asymmetry is random [4]. Sadly, little is known about the developmental steps that determine whether an individual becomes right-sided or left-sided in cases of random asymmetry. In some cases, random asymmetry could arise developmentally via a deterministic process like handed behaviour (see §3a). In these cases, experimental biasing of environmental effects to one side should cause symmetry to break in a predictable direction because the stimulus (A in figure 1b) occurs on only one side. If laterally biased environmental effects do not yield a predictable direction of asymmetry in cases of random asymmetry, then clearly right- or left-sidedness is purely stochastic.
Figure 4.
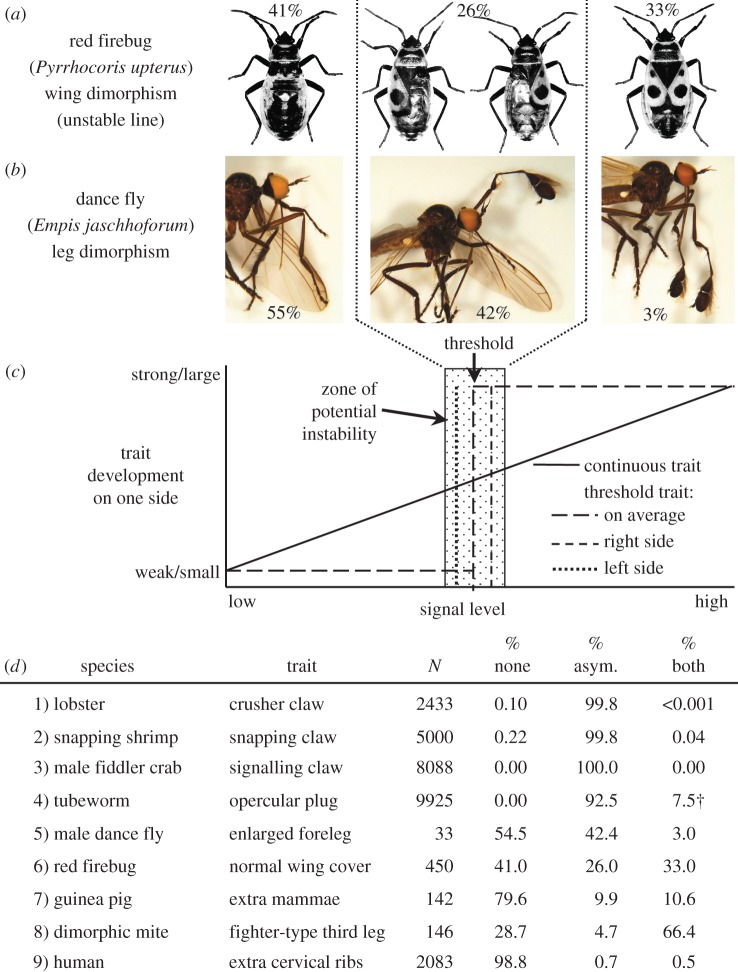
Normal versus anomalous random asymmetry (modified from [87]). (a) A clear example of anomalous random asymmetry in forewings of the red firebug, Pyrrhocoris apterus [88]. (b) A possible example in forelegs of the dance fly, Empis jaschhoforum [89]. (c) Hypothetical model for anomalous random asymmetries that arise due to small, random differences in signal or threshold level between the right and left sides in a threshold trait during development. (d) Examples of the gradation from clearly normal random asymmetries (1–4) to clearly anomalous (6–9), where the enlarged/extra trait may be absent (‘none’), present on either right or left at random (‘asym.’), or present on both sides (‘both’); species and sources: (1) Homarus americanus [90], (2) Synalpheus longicarpus [91], (3) Uca lactea [92], (4) Hydroides ezoensis [93] († Individuals bearing two opercular plugs vary significantly, from 1% to 30% depending on season; those bearing two plugs appeared to be transitioning from right- to left-sided or vice-versa as one was typically ‘more advanced’ and eventually autotomized), (5) Empis jaschhoforum [89], (6) Pyrrhocoris apterus (unstable line) [94], (7) Cavia porcellus [95], (8) Sancassania berlesei [96]. (9) Homo sapiens [97]. Empis jaschhoforum lies ambiguously between ‘normal’ and ‘anomalous’. Original photographs from [88,89], with permission. (Online version in colour.)
In cases of anomalous random asymmetries—where conspicuously asymmetric individuals, like those bearing extra toes, extra mammae or lacking a normally dimorphic bilateral trait on one side, are the exception [87]—direction of asymmetry seems likely to be determined stochastically during development. Thinking of left–right asymmetry as a threshold trait, but where lateral inhibition (S in figure 1b) is lacking [98], helps explain such anomalous asymmetries. During the development of a threshold trait (figure 4c), morphogen levels increase locally on each side at the same rate, on average. By chance, however, morphogen levels cross the threshold that induces transformation on one side before the other, or, alternatively, receptor sensitivity might differ slightly between sides. With no lateral inhibition, if morphogen levels continue to increase on the lagging side, it too will eventually transform. The frequencies of symmetrical (both sides small or both sides large) and asymmetrical (left large or right large) individuals should follow a binomial distribution, with some probability p that one side of the body transforms from small to large (figure 3c).
No one would argue that the asymmetrical wing covers in red firebugs (figure 4a) are anomalous in the ‘unstable’ laboratory line developed by Socha [88], where a striking 26% of individuals show this anomaly (species 6, figure 4d). The story is less obvious for the dimorphic forelegs of dance flies (figure 4b), where much has been made about the possible functional significance of asymmetry [89] and the possible maintenance of left and right forms by frequency-dependent selection [99]. An equally plausible alternative hypothesis is that dance fly foreleg asymmetry simply represents an unusual case of an anomalous random asymmetry in a threshold trait.
Clearly, a continuum exists between normal (functionally significant) random asymmetries (species 1–4, figure 4d)—where conspicuously asymmetrical individuals predominate and lateral inhibition of one side after the other has transformed an essential development step (S in figure 1b)—and anomalous (likely non-functional) random asymmetries (species 7–9, figure 4d)—where conspicuously asymmetrical individuals are relatively rare and lateral inhibition is lacking [87].
(c). Other examples of stochastic developmental pathways
Even in the highly deterministic development of nematodes, some cell fates are stochastic [100]. In Caenorhabditis elegans, six bilaterally symmetrical pairs of epidermal blast cells (P1/2–P11/12) lie on opposite sides of the body near the ventral midline. In the first larval stage, these cell pairs rotate relative to one another to align themselves along the ventral midline. After rotation, cell fate is fixed based on its position along the AP axis. Remarkably, sometimes the right member of a pair rotates anteriorly and sometimes the left member does. For most of the six epidermal blast cell pairs rotation is random, although rotation of the anteriormost (P1/2) and posteriormost (P11/12) pair is biased in some taxa. For example, the posteriormost cell (P12) typically comes from the right side in most Cephalobidae and from the left in Panagrolamidae. In the Rhabditidae, P12 comes from the right side in three species of Caenorhabditis, from the left in seven other species, and from the left or right member at random in Rhabditella axei and Pellioditis typica [100]. Once aligned along the ventral midline, however, fates of cells P1–P12 are fixed, so the end result is deterministic development.
Some curious stochastic developmental steps also occur in Drosophila oogenesis. Long before fertilization, maturing Drosophila egg chambers exhibit a pronounced rotation within their covering of follicle cells. This rotation is driven by lamellipodia of the enveloping follicle cells [101] and the rotation is essential to align actin bundles perpendicular to the long axis of the egg to ensure that the egg chambers elongate along the future AP axis. Without this rotation, eggs remain more or less spherical [67]. Remarkably, egg chambers rotate either clockwise or anticlockwise at random [66] depending on the orientation of microtubules [67].
Stochastic steps can clearly occur as a part of normal and even highly regulated development.
5. Local, global and persistent control of direction of asymmetry
Two other phenomena yield some intriguing insights into the developmental basis of left–right asymmetry. First, where multiple conspicuous asymmetries occur on single individuals, is direction of asymmetry concordant among traits? Second, what happens to direction of asymmetry when one member of an asymmetrical bilateral pair regenerates following loss due to injury? They provide clues about (i) how asymmetry is initiated (Model II, stage 1, figure 1) and (ii) how asymmetry is maintained via inhibition of the Transformed programme in bilaterally paired structures (Model II, stage 2).
(a). Is direction of asymmetry determined ‘locally’ or ‘globally’?
Organisms that possess multiple asymmetrical structures permit a simple, qualitative test of how direction of asymmetry is determined during development of an individual: is it determined (i) locally (structure by structure) or (ii) globally (at the level of the whole body)? Local control of direction would yield discordant asymmetries, because asymmetry in one structure should be independent of that in another. Global control of direction would yield a consistent concordance among individuals (e.g. structures 1 and 2 always asymmetric in the same, or in the opposite, direction).
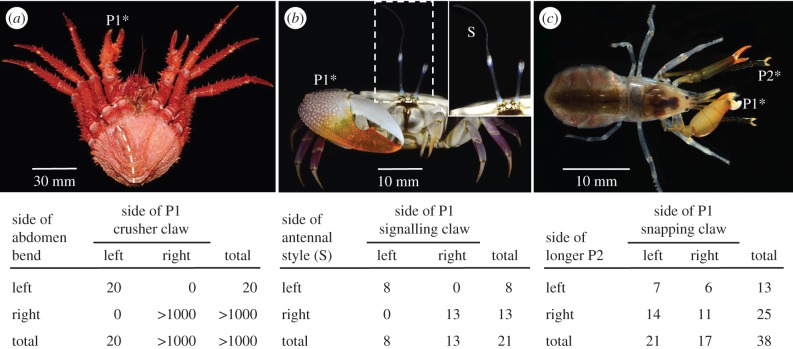
The pattern of concordance of multiple asymmetries in crustaceans is decidedly mixed. In female king crabs (Lithodidae), the development of asymmetry of the claw and the abdomen (figure 5a) is tightly coupled—reversal in one is associated with reversal in the other—but independent of a seemingly invariant mandibular asymmetry [102]. King crabs are a charismatic family of sometimes large and bizarre looking crabs that include several important commercial species [104]. As a likely relic of their evolutionary origin from shell-bearing hermit crabs [105], which have larger right-claws and soft, dextrally coiled abdomens, king crabs exhibit many conspicuous anatomical asymmetries, most notably in the abdomen of females and the claws of both sexes [104]. Like their putative hermit-crab ancestors, asymmetries of king crabs are generally fixed within species: larger (major) P1 chela on the right, female abdomen deflected towards the right (the male abdominal plates are not deflected). Nonetheless, females with reversed abdominal asymmetry occur in nature at low frequency in many species (fewer than 1 in 4000 in one species in one estimate [106]). Curiously, reversed asymmetry can be quite common in laboratory cultures (20–30%; table 4), suggesting a strong environmental component. Direct evidence of a strong genetic component to reversal is also lacking: the incidence of reversed larvae and juveniles did not differ among broods reared from three normal or one reversed female (table 4). In all reported cases of reversed abdominal asymmetry in females, claw asymmetry was also reversed (figure 5a). Mandibular asymmetry, however, did not differ between normal and reversed Lopholithodes foraminatus: a denticulated lobe of the left mandible always fits into an indentation on the right [102]. Significantly, asymmetry did not reverse following autotomy of the larger claw in either normal or reversed individuals [102], so direction of claw asymmetry is clearly fixed and determined globally.
Figure 5.
Concordance of multiple asymmetries on individual crustaceans. (a) Ventral view of adult female king crab, Paralomis formosa, illustrating the enlarged claw on the right (P1*) and a pronounced deflection of the abdomen to the right (dashed curve), both typical of female lithodid crabs. In reversed individuals, the enlarged claw is always on the left, the side to which the abdomen also bends (pooled data from seven field-collected adults of five species and 13 laboratory-reared juveniles of two species; data and citations in [102]). (b) Adult male fiddler crab, U. stylifera, illustrating the large P1 signalling claw on the right side (P1*) and the elongate style (S) on the tip of the right eyestalk (enlarged in inset). Like most Uca species, the side of the signalling claw is random. The style in U. stylifera is always on the same side as the signalling claw (table: A. R. Palmer, original data and [103]). (c) Adult female rock-boring snapping shrimp, Alpheus saxidomus. Both periopod P1 (large claws) and periopod P2 (long, snakelike, multi-segmented chelipeds) are asymmetrical. In this specimen, the P1 snapper claw is on the right side (P1*) and the longest P2 feeding leg is on the left side (P2*). In a sample of individuals, there is no consistent concordance of asymmetry (table: A. R. Palmer, original data) (photos: (a) S. E. Anosov and (b,c) A. Anker).
Table 4.
Numbers of king crab (Lopholithodes foraminatus) offspring with the major claw on the left or right side (figure 2a). When reared in the laboratory, approximately 25% of larvae/juveniles consistently exhibit reversed asymmetry, regardless of the asymmetry of the female parent [102].
| side of major claw of female parent | stage scored | side of major claw (P1) |
||
|---|---|---|---|---|
| left | right | % left | ||
| right | juvenile crabs | 41 | 116 | 26.1 |
| right | glaucothoe larvae | 22 | 75 | 22.7 |
| left | glaucothoe larvae | 73 | 218 | 25.1 |
| right | glaucothoe larvae | 70 | 180 | 28.0 |
Males of the fiddler crab Uca stylifera exhibit two striking asymmetrical structures (figure 5b): a massive signalling claw, typical of all male fiddler crabs [103], and a bizarre, long filamentous style extending from the distal end of one eye whose function is unknown (J. H. Christy 2015, personal communication). This ocular style occurs on all males of U. stylifera, and variably in males of other species in the subgenus Uca [103]. A small sample of crabs reveals that these two extreme traits always occur on the same side of the body, confirming the claim by Crane [103, p. 125] that such styles occur ‘on [the] major side only’. Clearly, direction of asymmetry in these traits is also determined globally here.
The rock-boring snapping shrimp, Alpheus saxidomus, also exhibits two impressive asymmetrical structures (figure 5b): the hypertrophied snapping claw on one side of the first thoracic segment (P1) and snakelike feeding limbs on the second thoracic segment (P2), one of which is more than 25% longer than the other. Although the longer P2 is somewhat more common on the right side, direction of asymmetry of the P1 and P2 limbs is completely uncorrelated. So, in this example, direction of asymmetry is determined locally—independently on each body segment—even though these segments are immediately adjacent to each other.
Finally, a survey of rare bicolour lobsters (figure 6a–d) permits a test of whether the developmental anomaly that results in abnormal colouration of one side of the body has any effect on which side develops the large crusher claw. In most of the 23 bicolour lobsters surveyed, one entire side of the body exhibited an unusual colour (usually bright orange or bright blue). However, some exhibited rather bizarre colouration patterns ranging from a complex mosaic (figure 6b) through varying degrees of partial discolouration on one side (figure 6c,d). Among those with a discolouration clearly restricted to one side, the side of the crusher claw was independent of the side of abnormal colour (figure 6e). Perhaps this independence is not surprising, because abnormal colouration results from some (as yet unknown) developmental error during early cleavage and differential use determines which side develops into the crusher claw (see §3a).
(b). Is asymmetry reversed or induced following loss and regeneration?
Whether asymmetry is reversed or induced following loss and regeneration of a structure on one side of the body depends on both the trait and the taxon (table 5). In some taxa, claw asymmetry does not reverse. In lobsters (Homarus), male fiddler crabs (Uca), and hermit and king crabs (Paguroidea), the large claw always regenerates on the same side of an individual. However, in other taxa, direction of asymmetry does reverse after loss of the larger structure: snapping shrimp claws (Alpheoidea), swimming crab claws (Portunoidea), mud crab claws (Xanthoidea) and the opercula of serpulid worms (Serpulidae). Curiously, whether asymmetry reverses after regeneration or not does not depend on whether asymmetry is random or fixed within species (table 5).
Table 5.
Reversal of asymmetry after regeneration. Data type: E, experimental result; I, inferred.
| taxon | trait | typical state of family and species | trait state post-regeneration |
|||
|---|---|---|---|---|---|---|
| side | stature | data type | source | |||
| Crustacea | ||||||
| Caridea (caridean shrimp) | ||||||
| Alpheoidea (snapping shrimp) | ||||||
| Alpheus armillatus | side of snapper claw | random | reversed | ? | E | [91] |
| Alpheus glaber | side of snapper claw | random | reversed | ? | E | [107] |
| Alpheus heterochelis | side of snapper claw | random | reversed | ? | E | [108] |
| Alpheus macrocheles | side of snapper claw | random | reversed | ? | E | [107] |
| Astacidea, Nephropoidea (lobster) | ||||||
| Homarus americanus | side of crusher claw | random | same | diminished | E | [109] |
| Brachyura (‘true’ crabs) | ||||||
| Ocypodoidea (fiddler crabs) | ||||||
| Uca cumulanta (male) | side of signalling claw | random | same | diminisheda | I | [110] |
| Uca lactea (male) | side of signalling claw | random | same | diminisheda | E | [92] |
| Uca pugilator (male) | side of signalling claw | random | same | diminisheda | E | [111] |
| Uca rapax (male) | side of signalling claw | random | same | diminisheda | E | [112] |
| Portunoidea (swimming crabs) | ||||||
| Callinectes sapidus | side of major claw | right | reversed | diminished | I | [113] |
| Carcinus maenas | side of major claw | right | reversed | diminished | I | [114] |
| Macropipus puber | side of major claw | right | reversed | diminished | E | [115] |
| Portunus corrugatus | side of major claw | right | reversed | diminished | E | [116] |
| Portunus depurator | side of major claw | right | reversed | diminished | E | [116] |
| Portunus trituberculatus | side of major claw | right | reversed | diminished | E | [117] |
| Potamon potamios | side of major claw | right | reversed | ? | I | [118] |
| Xanthoidea (mud crabs) | ||||||
| Eriphia spinifrons | side of major claw | right | reversed | ? | E | [116] |
| Menippe mercenaria | side of major claw | right | reversed | diminished | E | [119] |
| Anomura (‘false’ crabs) | ||||||
| Paguroidea (hermit crabs, king crabs) | ||||||
| Birgus latro | side of major claw | right | same | ? | I | [120] |
| Pagurus prideauxi | side of major claw | right | same | ? | E | [121] |
| Lopholithodes foraminatus | side of major claw | right | same | ? | E | [102] |
| Annelida | ||||||
| Polychaeta, Serpulidae (calcareous tube worm) | ||||||
| Hydroides dianthus | side of operculum | random | reversed | ? | E | [111] |
Most heterochelous brachyuran crabs (excluding fiddler crabs) are typically right-handed, with a small minority of left-handed individuals [114,124,125]. In very young individuals, the larger claw is almost universally on the right side [113,117]. The proportion of left-handers tends to increase with increasing body size in some portunids [113,126] and xanthids [119], but not all [124,127].
Regenerated larger claws usually differ in form from non-regenerated ones. They tend to be smaller, they lack some dentition characteristic of major claws and they are often weaker [117,122,123,128,129].
Loss and regeneration may also induce morphological asymmetry where one did not exist. For example, in normally homochelous species like crayfish, a regenerated claw often differs in size and shape from a normal claw, which renders a previously symmetrical individual asymmetrical after regeneration [130]. Such asymmetries persist even in regenerated first antennae of harpacticoid copepods [131] and regenerated second antennae of cladocerans water fleas [132].
Clearly, if direction of asymmetry does reverse after loss and regeneration of the overdeveloped side, the direction of asymmetry is not inherited.
(c). Bilateral inhibition mechanisms in asymmetrical crustacean claws
Experimental manipulations of the larger (transformed) claw provide some intriguing clues about the mechanism of bilateral inhibition (S, Model II, figure 1) that maintain asymmetry in normally asymmetrical crustaceans. In some remarkable experiments on the swimming crab Portunus trituberculatus, gluing the normally larger right chela closed at the megalopa (last larval) stage yielded a high incidence of young crabs with double-crusher claws [117]. These experiments suggest that inhibition of the ‘major-claw’ programme on the left side depends more on activity of, rather than the presence of, the right major claw. Similar evidence from snapping shrimp points to activity (rather than presence) of the snapper as key component of lateral inhibition: double-snapper snapping shrimp may be induced experimentally either by cutting the nerve that innervates the snapper claw [133] or by removing the movable finger (dactyl) of the snapper [108].
The mechanism of bilateral inhibition in these crustaceans clearly differs from lobsters, where no manipulations ever produced double-crusher individuals [53].
6. Summary and evolutionary significance
In species where direction of asymmetry varies, even where morphological asymmetries are quite conspicuous, modes of inheritance vary enormously. Direction of asymmetry may be determined by alternative alleles at a single locus, by effects of multiple loci, by asymmetric environmental effects, or by chance. While this variation may be troubling for those searching for unifying mechanisms, it permits a powerful test of a fundamental question about how development evolves: are genes leaders or followers in the evolution of novel forms? This question can be answered by mapping the different modes of inheritance—made easier for left–right asymmetries than for most other traits because of the discrete difference between right- and left-sided forms—onto phylogenetic trees for any taxon where mode of inheritance varies among species. If random asymmetry (no genetic basis to direction of asymmetry [3]) is ancestral to fixed, then genetic determination of direction of asymmetry arose after conspicuous asymmetries, both right and left, already existed. The evidence to date is surprising: genes (for direction of asymmetry) are followers almost as often as they are leaders in the evolution of fixed left–right asymmetry [3].
Although the evidence seems strong that genes are followers in a high percentage of origins of fixed asymmetries, one recurring question arises: how could genetic variation capture pre-existing phenotypic variation for direction of asymmetry? A potential answer to this fascinating question lies in enantiostylous Heteranthera multiflora, a species where all the styles on an individual plant bend in the same direction (either right or left, depending on the individual). Two facts render this system highly attractive: (i) in most Heteranthera species, direction of style bend varies at random within a single individual [134], so direction is clearly not inherited. (ii) In Heteranthera multiflora two alleles at a single locus control direction of style bending [50]. Heteranthera multiflora most probably evolved from an ancestor that exhibited monomorphic enantiostyly (styles on flowers of an individual plant bend both right and left), as it has in other monocot clades where dimorphic enantiostyly has evolved [16,134]. The obvious tantalizing questions are: (i) what gene determines style bending H. multiflora, and (ii) what was the inferred function of that gene in ancestors where style bend was not determined genetically? Answers to these questions would provide the first compelling evidence for how genetic variation ‘captures’ pre-existing phenotypic variation over evolutionary time.
Acknowledgements
I thank Arthur Anker for permission to use his lovely photographs, J. Luque, T. Miyashita and two referees for thoughtful comments on the manuscript, and Michael Levin for the invitation to contribute to this special issue.
Competing interests
The author declares no competing interests.
Funding
This study was supported by Natural Sciences and Engineering Research Council of Canada Discovery grant no. RGPIN 2014-04863.
References
- 1.Ludwig W. 1932. Das Rechts-Links Problem im Tierreich und beim Menschen. Berlin, Germany: Springer. [Google Scholar]
- 2.Kihara H. 1972. Right- and left-handedness in plants: a review. Seiken Zihô 23, 1–37. [Google Scholar]
- 3.Palmer AR. 2004. Symmetry breaking and the evolution of development. Science 306, 828–833. ( 10.1126/science.1103707) [DOI] [PubMed] [Google Scholar]
- 4.Palmer AR. 2005. Antisymmetry. In Variation (eds Hallgrímsson B, Hall BK), pp. 359–397. New York, NY: Elsevier. [Google Scholar]
- 5.Palmer AR. 2009. Animal asymmetry. Curr. Biol. 19, R473–R477. ( 10.1016/j.cub.2009.04.006) [DOI] [PubMed] [Google Scholar]
- 6.Brown NA, Wolpert L. 1990. The development of handedness in left-right asymmetry. Development 109, 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Coutelis J-B, González-Morales N, Géminard C, Noselli S. 2014. Diversity and convergence in the mechanisms establishing L/R asymmetry in Metazoa. EMBO Rep. 15, 926–937. ( 10.15252/embr.201438972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenberg LN, Levin M. 2013. A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev. Biol. 379, 1–15. ( 10.1016/j.ydbio.2013.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum M, Feistel K, Thumberger T, Schweickert A. 2014. The evolution and conservation of left-right patterning mechanisms. Development 141, 1603–1613. ( 10.1242/dev.100560) [DOI] [PubMed] [Google Scholar]
- 10.Naganathan SR, Middelkoop TC, Fürthauer S, Grill SW. 2016. Actomyosin-driven left-right asymmetry: from molecular torques to chiral self organization. Curr. Opin. Cell Biol. 38, 24–30. ( 10.1016/j.ceb.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T. 2002. Molecular genetic analysis of left-right handedness in plants. Phil. Trans. R. Soc. Lond. B 357, 799–808. ( 10.1098/rstb.2002.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards W, Moles AT, Franks P. 2007. The global trend in plant twining direction. Glob. Ecol. Biogeogr. 16, 795–800. ( 10.1111/j.1466-8238.2007.00326.x) [DOI] [Google Scholar]
- 13.Endress PK. 2012. The immense diversity of floral monosymmetry and asymmetry across angiosperms. Bot. Rev. 78, 345–397. ( 10.1007/s12229-012-9106-3) [DOI] [Google Scholar]
- 14.Henley CL. 2012. Possible origins of macroscopic left-right asymmetry in organisms. J. Stat. Phys. 148, 741–775. ( 10.1007/s10955-012-0520-z) [DOI] [Google Scholar]
- 15.Pohl C. 2015. Cytoskeletal symmetry breaking and chirality: from reconstituted systems to animal development. Symmetry 7, 2062–2107. ( 10.3390/sym7042062) [DOI] [Google Scholar]
- 16.Levin M, Palmer AR. 2007. Left–right patterning from the inside out: widespread evidence for intracellular control. Bioessays 29, 271–287. ( 10.1002/bies.20545) [DOI] [PubMed] [Google Scholar]
- 17.Galloway J. 1983. Helix through the looking glass. New Sci. 97, 242–245. [Google Scholar]
- 18.Neville AC. 1976. Animal asymmetry. London, UK: Edward Arnold. [Google Scholar]
- 19.Endress PK. 2001. Evolution of floral symmetry. Curr. Opin. Plant Biol. 4, 86–91. ( 10.1016/S1369-5266(00)00140-0) [DOI] [PubMed] [Google Scholar]
- 20.Neal P. 1998. Floral symmetry and its role in plant-pollinator systems: terminology, distribution, and hypotheses. Annu. Rev. Ecol. Syst. 29, 345–373. ( 10.1146/annurev.ecolsys.29.1.345) [DOI] [Google Scholar]
- 21.Hill AW. 1939. Resupination studies of flowers and leaves. Ann. Bot. 3, 871–887. [Google Scholar]
- 22.Thisse B, Thisse C. 2015. Formation of the vertebrate embryo: moving beyond the Spemann organizer. Sem. Cell Dev. Biol. 42, 94–102. ( 10.1016/j.semcdb.2015.05.007) [DOI] [PubMed] [Google Scholar]
- 23.Tuazon FB, Mullins MC. 2015. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Sem. Cell Dev. Biol. 42, 118–133. ( 10.1016/j.semcdb.2015.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinhardt H. 2015. Dorsoventral patterning by the Chordin-BMP pathway: a unified model from a pattern-formation perspective for Drosophila, vertebrates, sea urchins and Nematostella. Dev. Biol. 405, 137–148. ( 10.1016/j.ydbio.2015.05.025) [DOI] [PubMed] [Google Scholar]
- 25.Henry JJ, Martindale MQ. 1999. Conservation and innovation in spiralian development. Hydrobiologia 402, 255–265. ( 10.1023/A:1003756912738) [DOI] [Google Scholar]
- 26.Davison A, et al. 2016. Formin is associated with left-right asymmetry in the pond snail and the frog. Curr. Biol. 26, 654–660. ( 10.1016/j.cub.2015.12.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. 2009. Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science 324, 941–944. ( 10.1126/science.1172478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Hamada H. 2012. Left-right patterning: Conserved and divergent mechanisms. Development 139, 3257–3262. ( 10.1242/dev.061606) [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. 2006. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev. Cell 11, 495–504. ( 10.1016/j.devcel.2006.08.002) [DOI] [PubMed] [Google Scholar]
- 30.Davidson EH, McClay DR, Hood L. 2003. Regulatory gene networks and the properties of the developmental process. Proc. Natl. Acad. Sci. USA 100, 1475–1480. ( 10.1073/pnas.0437746100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wade CM, Mordan PB, Naggs F. 2006. Evolutionary relationships among the Pulmonate land snails and slugs (Pulmonata, Stylommatophora). Biol. J. Linn. Soc. 87, 593–610. ( 10.1111/j.1095-8312.2006.00596.x) [DOI] [Google Scholar]
- 32.Freeman G, Lundelius JW. 1982. The developmental genetics of dextrality and sinistrality in the gastropod Lymnaea peregra. Roux’s Arch. Dev. Biol. 191, 69–83. ( 10.1007/BF00848443) [DOI] [PubMed] [Google Scholar]
- 33.Asami T, Gittenberger E, Falkner G. 2008. Whole-body enantiomorphy and maternal inheritance of chiral reversal in the pond snail Lymnaea stagnalis. J. Hered. 99, 552–557. ( 10.1093/jhered/esn032) [DOI] [PubMed] [Google Scholar]
- 34.Murray J, Clarke BC. 1980. The genus Partula on Moorea: speciation in progress. Proc. R. Soc. Lond. B 211, 83–117. ( 10.1098/rspb.1980.0159) [DOI] [Google Scholar]
- 35.Murray J, Clarke B. 1966. The inheritance of polymorphic shell characters in Partula (Gastropoda). Genetics 54, 1261–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray J, Clarke B. 1976. Supergenes in polymorphic land snails. II. Partula suturalis. Heredity 37, 271–282. [DOI] [PubMed] [Google Scholar]
- 37.Degner E. 1952. Der Erbgang der Inversion bei Laciniaria biplicata MTG (Gastropoda, Pulmonata) nebst Bemerkungen zur Biologie dieser Art. Mitt. Hamburg Zool. Mus. Inst. 51, 3–61. [Google Scholar]
- 38.Utsuno H, Kasem S, Fyhjidam G, Asami T. 2010. Genetic basis of racemism and ease of interchiral mating in a clausiliid species of snails. Mollusc. Res. 30, 37–47. [Google Scholar]
- 39.Utsuno H, Asami T. 2009. Maternal inheritance of racemism in the terrestrial snail Bradybaena similaris. J. Hered. 101, 11–19. ( 10.1093/jhered/esp058) [DOI] [PubMed] [Google Scholar]
- 40.Schilthuizen M, Craze PG, Cabanban AS, Davison A, Stone J, Gittenberger E, Scott BJ. 2007. Sexual selection maintains whole-body chiral dimorphism in snails. J. Evol. Biol. 20, 1941–1949. ( 10.1111/j.1420-9101.2007.01370.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutcharit C, Asami T, Panha S. 2007. Evolution of whole-body enantiomorphy in the tree snail genus Amphidromus. J. Evol. Biol. 20, 661–672. ( 10.1111/j.1420-9101.2006.01246.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gittenberger E, Hamann TD, Asami T. 2012. Chiral speciation in terrestrial pulmonate snails. PLoS ONE 7, e34005 ( 10.1371/journal.pone.0034005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boycott AA, Diver C. 1923. On the inheritance of sinistrality in Limnaea peregra. Proc. R. Soc. Lond. B 95, 207–213. ( 10.1098/rspb.1923.0033) [DOI] [Google Scholar]
- 44.Boycott AE, Diver C, Garstang SL, Turner FM. 1931. The inheritance of sinistrality in Limnaea peregra (Mollusca, Pulmonata). Phil. Trans. R. Soc. Lond. B 219, 51–131. ( 10.1098/rstb.1931.0002) [DOI] [Google Scholar]
- 45.Abe M, Takahashi M, Kuroda R. 2014. Spiral cleavages determine the left-right body plan by regulating Nodal pathway in monomorphic gastropods, Physa acuta. Int. J. Dev. Biol. 58, 513–520. ( 10.1387/ijdb.140087rk) [DOI] [PubMed] [Google Scholar]
- 46.Policansky D. 1982. Flatfish and the inheritance of asymmetries. Behav. Brain Sci. 5, 262–265. ( 10.1017/S0140525X0001181X) [DOI] [Google Scholar]
- 47.Hubbs CL, Hubbs LC. 1945. Bilateral asymmetry and bilateral variation in fishes. Pap. Mich. Acad. Sci. Arts Lett. 30, 229–311. [Google Scholar]
- 48.Edelaar P, Postma E, Knops P, Phillips R. 2005. No support for a genetic basis of mandible crossing direction in crossbills (Loxia spp). Auk 122, 1123–1139. ( 10.1642/0004-8038(2005)122%5B1123:NSFAGB%5D2.0.CO;2) [DOI] [Google Scholar]
- 49.Benkman CW. 1988. A 3:1 ratio of mandible crossing direction in white-winged crossbills. Auk 105, 578–579. ( 10.2307/4087505) [DOI] [Google Scholar]
- 50.Jesson LK, Barrett SCH. 2002. The genetics of mirror-image flowers. Proc. R. Soc. Lond. B 269, 1835–1839. ( 10.1098/rspb.2002.2068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lilienfeld FA. 1959. Dextrality and sinistrality in plants. III. Medicago tuberculata Willd. and M. litoralis Rohde. Proc. Jpn. Acad. Sci. 35, 475–481. [Google Scholar]
- 52.Buschmann H, Hauptmann M, Niessing D, Lloyd CW, Schäffner AR. 2009. Helical growth of the Arabidopsis mutant tortifolia2 does not depend on cell division patterns but involves handed twisting of isolated cells. Plant Cell 21, 2090–2106. ( 10.1105/tpc.108.061242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govind CK. 1992. Claw asymmetry in lobsters: case study in developmental neuroethology. J. Neurobiol. 23, 1423–1445. ( 10.1002/neu.480231006) [DOI] [PubMed] [Google Scholar]
- 54.Govind CK, Pearce J. 1992. Mechanoreceptors and minimal reflex activity determining claw laterality in developing lobsters. J. Exp. Biol. 171, 149–162. [Google Scholar]
- 55.Govind CK, Kent KS. 1982. Transformation of fast fibers to slow prevented by lack of activity in developing lobster muscle. Nature 298, 755–757. ( 10.1038/298755a0) [DOI] [PubMed] [Google Scholar]
- 56.Govind CK, Pearce J. 1986. Differential reflex activity determines claw and closer muscle asymmetry in developing lobsters. Science 233, 354–356. ( 10.1126/science.233.4761.354) [DOI] [PubMed] [Google Scholar]
- 57.Govind CK, Pearce J. 1989. Critical period for determining claw asymmetry in developing lobsters. J. Exp. Zool. 249, 31–35. ( 10.1002/jez.1402490107) [DOI] [Google Scholar]
- 58.Govind CK, Pearce J. 1989. Delayed determination of claw laterality in lobsters following loss of target. Development 107, 547–551. [Google Scholar]
- 59.Gwynne DT. 1995. Phylogeny of the Ensifera (Orthoptera): a hypothesis supporting multiple origins of acoustical signalling, complex spermatophores and maternal care in crickets, katydids, and weta. J. Orthopt. Res. 4, 203–218. ( 10.2307/3503478) [DOI] [Google Scholar]
- 60.Masaki S, Kataoka M, Shirato K, Nakagahara M. 1987. Evolutionary differentiation of right and left tegmina in crickets. In Evolutionary biology of orthopteroid insects (ed. Baccetti B.), pp. 347–357. Chichester, UK: Horwood. [Google Scholar]
- 61.Graber V. 1872. Uber der Tonapparat der Locustiden, ein Beitrage zum Darwinismus. Z. Wiss Zool. 22, 100–119. [Google Scholar]
- 62.Kamimura Y. 2006. Right-handed penises of the earwig Labidura riparia (Insecta, Dermaptera, Labiduridae): Evolutionary relationships between structural and behavioral asymmetries. J. Morphol. 267, 1381–1389. ( 10.1002/jmor.10484) [DOI] [PubMed] [Google Scholar]
- 63.Palmer AR. 2006. Caught right-handed. Nature 444, 689–691. ( 10.1038/444689a) [DOI] [PubMed] [Google Scholar]
- 64.Kamimura Y, Lee C-Y. 2014. Mating and genital coupling in the primitive earwig species Echinosoma denticulatum (Pygidicranidae): implications for genital evolution in dermapteran phylogeny. Arthrop. Syst. Phylog. 72, 11–21. [Google Scholar]
- 65.Kamimura Y, Iwase R. 2010. Evolutionary genetics of genital size and lateral asymmetry in the earwig Euborellia plebeja (Dermaptera: Anisolabididae). Biol. J. Linn. Soc. 101, 103–112. ( 10.1111/j.1095-8312.2010.01491.x) [DOI] [Google Scholar]
- 66.Haigo SL, Bilder D. 2011. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331, 1071–1074. ( 10.1126/science.1199424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viktorinová I, Dahmann C. 2013. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Curr. Biol. 23, 1472–1477. ( 10.1016/j.cub.2013.06.014) [DOI] [PubMed] [Google Scholar]
- 68.Hejnol A. 2010. A twist in time: the evolution of spiral cleavage in the light of animal phylogeny. Integr. Comp. Biol. 50, 695–706. ( 10.1093/icb/icq103). [DOI] [PubMed] [Google Scholar]
- 69.Wilson EB. 1928. The cell in development and heredity, 3rd edn New York, NY: Macmillan. [Google Scholar]
- 70.Arenas-Mena C. 2007. Sinistral equal-size spiral cleavage of the indirectly developing polychaete Hydroides elegans. Dev. Dyn. 236, 1611–1622. ( 10.1002/dvdy.21164) [DOI] [PubMed] [Google Scholar]
- 71.Luetjens CM, Dorresteijn AWC. 1998. The site of fertilisation determines dorsoventral polarity but not chirality in the zebra mussel embryo. Zygote 6, 125–135. ( 10.1017/S0967199498000045) [DOI] [PubMed] [Google Scholar]
- 72.Crampton HE. 1894. Reversal of cleavage in a sinistral gastropod. Ann. NY Acad. Sci. 8, 167–170. ( 10.1111/j.1749-6632.1894.tb55419.x) [DOI] [Google Scholar]
- 73.van den Biggelaar JAM, Dictus W, van Loon AE. 1997. Cleavage patterns, cell-lineages and cell specification are clues to phyletic lineages in Spiralia. Sem. Cell Dev. Biol. 8, 367–378. ( 10.1006/scdb.1997.0161) [DOI] [PubMed] [Google Scholar]
- 74.Meshcheryakov VN, Beloussov LV. 1975. Asymmetrical rotations of blastomeres in early cleavage of Gastropoda. Roux’s Arch. Dev. Biol. 177, 193–203. ( 10.1007/BF00848080) [DOI] [PubMed] [Google Scholar]
- 75.Kuroda R, Endo B, Abe M, Shimizu M. 2009. Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature 462, 790–794. ( 10.1038/nature08597) [DOI] [PubMed] [Google Scholar]
- 76.Morgan TH, Tyler A. 1930. The point of entrance of the spermatozoon in relation to the orientation of the embryo in eggs with spiral cleavage. Biol. Bull. 58, 59–73. ( 10.2307/1537119) [DOI] [Google Scholar]
- 77.Guerrier P. 1970. Les caractères de la segmentation et la détermination de la polarité dorsoventrale dans le développement de quelques Spiralia. III. Pholas dactylus et Spisula subtruncata (Mollusques Lamellibranches). J. Embryol. Exp. Morph. 23, 667–692. [PubMed] [Google Scholar]
- 78.Dorresteijn AWC. 1990. Quantitative analysis of cellular differentiation during early embryogenesis of Platynereis dumerilii. Roux’s Arch. Dev. Biol. 199, 14–30. ( 10.1007/BF01681530) [DOI] [PubMed] [Google Scholar]
- 79.Scholtz G, Wolff C. 2002. Cleavage, gastrulation, and germ disc formation of the amphipod Orchestia cavimana (Crustacea, Malacostraca, Peracarida). Contrib. Zool. 71, 9–28. [Google Scholar]
- 80.Alwes F, Scholtz G. 2004. Cleavage and gastrulation of the euphausiacean Meganyctiphanes norvegica (Crustacea, Malacostraca). Zoomorphology 123, 125–137. ( 10.1007/s00435-004-0095-6) [DOI] [Google Scholar]
- 81.Gerberding M, Browne WE, Patel NH. 2002. Cell lineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates. Development 129, 5789–5801. ( 10.1242/dev.00155) [DOI] [PubMed] [Google Scholar]
- 82.Bregazzi PK. 1973. Embryological development in Tryphosella kergueleni (Miers) and Cheirimedon femoratus (Pfeffer) (Crustacea: Amphipoda). Br. Antarct. Survey Bull. 32, 63–74. [Google Scholar]
- 83.Hertzler PL. 2005. Cleavage and gastrulation in the shrimp Penaeus (Litopenaeus) vannamei (Malacostraca, Decapoda, Dendrobranchiata). Arthropod. Struct. Dev. 34, 455–469. ( 10.1016/j.asd.2005.01.009) [DOI] [PubMed] [Google Scholar]
- 84.Biffis C, Alwes F, Scholtz G. 2009. Cleavage and gastrulation of the dendrobranchiate shrimp Penaeus monodon (Crustacea, Malacostraca, Decapoda). Arthropod. Struct. Dev. 38, 527–540. ( 10.1016/j.asd.2009.06.003) [DOI] [PubMed] [Google Scholar]
- 85.Shibazaki Y, Shimizu M, Kuroda R. 2004. Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr. Biol. 14, 1462–1467. ( 10.1016/j.cub.2004.08.018) [DOI] [PubMed] [Google Scholar]
- 86.Nielsen C. 2010. Some aspects of spiralian development. Acta Zool. 91, 20–28. ( 10.1111/j.1463-6395.2009.00421.x). [DOI] [Google Scholar]
- 87.Palmer AR. 2012. Developmental origins of normal and anomalous random right-left asymmetry: lateral inhibition versus developmental error in a threshold trait. Contrib. Zool. 81, 111–124. [Google Scholar]
- 88.Socha R, Nedved O, Zrzavy J. 1993. Unstable forewing polymorphism in a strain of Pyrrhocoris apterus (Hemiptera: Pyrrhocoridae). Ann. Entomol. Soc. Am. 86, 484–489. ( 10.1093/aesa/86.4.484) [DOI] [Google Scholar]
- 89.Daugeron D, Plant A, Winkler I, Stark A, Baylac M. 2011. Extreme male leg polymorphic asymmetry in a new empidine dance fly (Diptera: Empididae). Biol. Lett. 7, 11–14. ( 10.1098/rsbl.2010.0726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrick FH. 1895. The American lobster: a study of its habits and development. Fish. Bull. US 15, 1–252. [Google Scholar]
- 91.Darby H. 1934. The mechanism of asymmetry in the Alpheidae. Carnegie Inst. Wash. Pap. Tortugas Lab. 28, 349–361. [Google Scholar]
- 92.Yamaguchi T. 1977. Studies on the handedness of the fiddler crab, Uca lactea. Biol. Bull. 152, 424–436. ( 10.2307/1540430) [DOI] [Google Scholar]
- 93.Ichikawa A, Takagaki N. 1942. The reversible asymmetry in the opercula of Hydroides ezoensis. I. Observations on the intact opercula. J. Fac. Sci. Hokkaido Univ. 8, 1–8. [Google Scholar]
- 94.Socha R. 1995. Selection for an unstable micropterism in Pyrrhocoris apterus (Heteroptera, Pyrrhocoridae). J. Zool. (Lond). 236, 407–415. ( 10.1111/j.1469-7998.1995.tb02721.x) [DOI] [Google Scholar]
- 95.Sollas IBJ. 1909. Inheritance of colour and of supernumerary mammae in guinea-pigs, with a note on the occurrence of a dwarf form. Rep. Evol. Committee R. Soc. (Lond.) 5, 51–79. [Google Scholar]
- 96.Radwan J, Unrug J, Tomkins JL. 2002. Status-dependence and morphological trade-offs in the expression of a sexually selected character in the mite, Sancassania berlesei. J. Evol. Biol. 15, 744–752. ( 10.1046/j.1420-9101.2002.00444.x) [DOI] [Google Scholar]
- 97.Walden MJ et al. . 2013. Cervical ribs: identification on MRI and clinical relevance. Clin. imaging 37, 938–941. ( 10.1016/j.clinimag.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 98.Whitten MJ. 1966. The quantitative analysis of threshold characters using asymmetry: a study of the witty character in Drosophila melanogaster. Genetics 54, 465–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritchie MG, Vahed K. 2011. Sexual selection: do flies lie with asymmetric legs? Curr. Biol. 21, R233–R234. ( 10.1016/j.cub.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 100.Delattre M, Félix M-A. 2001. Development and evolution of a variable left–right asymmetry in nematodes: the handedness of P11/P12 migration. Dev. Biol. 232, 362–371. ( 10.1006/dbio.2001.0175) [DOI] [PubMed] [Google Scholar]
- 101.Cetera M, Ramirez-San Juan GR, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, Horne-Badovinac S. 2014. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun. 5, 1–12. ( 10.1038/ncomms6511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duguid WDP. 2010. The enigma of reversed asymmetry in lithodid crabs: absence of evidence for heritability or induction of morphological handedness in Lopholithodes foraminatus. Evol. Dev. 12, 74–83. ( 10.1111/j.1525-142X.2009.00392.x) [DOI] [PubMed] [Google Scholar]
- 103.Crane J. 1975. Fiddler crabs of the world. Princeton, NJ: Princeton University Press. [Google Scholar]
- 104.McLaughlin PA. 2014. Ch. 2. Systematics of king crabs. In King crabs of the world (ed. Stevens BG.), pp. 31–46. Boca Raton, FL: CRC Press. [Google Scholar]
- 105.Keiler J, Richter S, Wirkner CS. 2015. The anatomy of the king crab Hapalogaster mertensii Brandt, 1850 (Anomura: Paguroidea: Hapalogastridae) – new insights into the evolutionary transformation of hermit crabs into king crabs. Contrib. Zool. 84, 149–165. [Google Scholar]
- 106.Campodonico GI. 1978. A case of inversion of the abdominal asymmetry of females of Paralomis granulosa (Jacquinot) (Decapoda, Anomura, Lithodida). Anal. Inst. Patagonia, Punta Arenas 9, 231–232. [Google Scholar]
- 107.Przibram H. 1901. Experimentelle Studien über Regeneration. Arch. Entwicklungsmech. Org. 11, 321 ( 10.1007/BF02278218) [DOI] [Google Scholar]
- 108.Read AT, Govind CK. 1997. Claw transformation and regeneration in adult snapping shrimp: test of the inhibition hypothesis for maintaining bilateral asymmetry. Biol. Bull. 193, 401–409. ( 10.2307/1542942) [DOI] [PubMed] [Google Scholar]
- 109.Emmel VE. 1908. The experimental control of asymmetry at different stages in the development of the lobster. J. Exp. Zool. 5, 474–484. ( 10.1002/jez.1400050403) [DOI] [Google Scholar]
- 110.Ahmed M. 1978. Development of asymmetry in the fiddler crab Uca cumulanta Crane, 1943 (Decapoda, Brachyura). Crustaceana 34, 294–300. ( 10.1163/156854078X00853) [DOI] [Google Scholar]
- 111.Zeleny C. 1905. Compensatory regulation. J. Exp. Zool. 2, 1–102. ( 10.1002/jez.1400020102) [DOI] [Google Scholar]
- 112.Vernberg FJ, Kostlow JD. 1966. Handedness in fiddler crabs (Genus Uca). Crustaceana 11, 61–64. ( 10.1163/156854066X00450) [DOI] [Google Scholar]
- 113.Hamilton PV, Nishimoto RT, Halusky JG. 1976. Cheliped laterality in Callinectes sapidus (Crustacea: Portunidae). Biol. Bull. 150, 393–401. ( 10.2307/1540680) [DOI] [PubMed] [Google Scholar]
- 114.Abby-Kalio NJ, Warner GF. 1989. Heterochely and handedness in the shore crab Carcinus maenas (L.) (Crustacea: Brachyura). Zool. J. Linn. Soc. 96, 19–26. ( 10.1111/j.1096-3642.1989.tb01819.x) [DOI] [Google Scholar]
- 115.Norman CP, Jones MB. 1991. Limb loss and its effect on handedness and growth in the velvet swimming crab Necora puber (Brachyura, Portunidae). J. Nat. Hist. 25, 639–645. ( 10.1080/00222939100770401) [DOI] [Google Scholar]
- 116.Przibram H. 1907. Claw reversal in decapod Crustacea (At the same time: experimental studies on regeneration. Fourth Communication) [in German]. Arch. Entwicklungsmech. Org. 25, 266–343. ( 10.1007/BF02292169) [DOI] [Google Scholar]
- 117.Masunari N, Hiro-oku M, Dan S, Takahiro N, Kondo M, Goto M, Takada Y, Saigusa M. 2015. Chela asymmetry in a durophagous crab: predominance of right-handedness and handedness reversal is linked to chela size and closing force. J. Exp. Biol. 218, 3658–3670. ( 10.1242/jeb.120196) [DOI] [PubMed] [Google Scholar]
- 118.Scalici M, Gherardi F. 2008. Heterochely and handedness in the river crab, Potamon potamios (Olivier, 1804) (Decapoda, Brachyura). Crustaceana 81, 507–511. ( 10.1163/156854008783797525) [DOI] [Google Scholar]
- 119.Simonson JL. 1985. Reversal of handedness, growth, and claw stridulatory patterns in the stone crab Menippe mercenaria (Say) (Crustacea: Xanthidae). J. Crust. Biol. 5, 281–293. ( 10.2307/1547875) [DOI] [Google Scholar]
- 120.Davis TA. 1978. Reversible and irreversible lateralities in some animals. Behav. Brain Sci. 2, 291–293. ( 10.1017/S0140525X0007463X) [DOI] [Google Scholar]
- 121.Bush SF. 1930. Asymmetry and relative growth of parts in the two sexes of the hermit crab, Pagurus prideauxi. Roux’s Arch. Dev. Biol. 123, 39–79. ( 10.1007/BF00644695) [DOI] [PubMed] [Google Scholar]
- 122.Backwell PRY, Christy JH, Telford SR, Jennions MD, Passmore NI. 2000. Dishonest signalling in a fiddler crab. Proc. R. Soc. Lond. B 267, 719–724. ( 10.1098/rspb.2000.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamaguchi T. 2001. Dimorphism of chelipeds in the fiddler crab, Uca arcuata. Crustaceana 74, 913–923. ( 10.1163/15685400152682665) [DOI] [Google Scholar]
- 124.Abelló P, Pertierra JP, Reid DG. 1990. Sexual size dimorphism, relative growth and handedness in Liocarcinus depurator and Macropipus tuberculatus (Brachyura: Portunidae). Scient. Mar. 54, 195–202. [Google Scholar]
- 125.Lewis JE. 1969. Reversal of asymmetry of chelae in Calappa Weber 1795 (Decapoda: Oxystomata). Proc. Biol. Soc. Wash. 82, 63–80. [Google Scholar]
- 126.Smith LD, Hines AH. 1991. Autotomy in blue crab (Callinectes sapidus Rathbun) populations: geographic, temporal, and ontogenetic variation. Biol. Bull. 180, 416–431. ( 10.2307/1542342) [DOI] [PubMed] [Google Scholar]
- 127.Yamaguchi T, Tokunaga S. 1995. Cheliped handedness in four species of Charybdis, Portunus and Thalamita (Brachyura: Portunidae). Crust. Res. 24, 33–38. [Google Scholar]
- 128.Brock RE, Smith LD. 1998. Recovery of claw size and function following autotomy in Cancer productus (Decapoda: Brachyura). Biol. Bull. 194, 53–62. ( 10.2307/1542513) [DOI] [PubMed] [Google Scholar]
- 129.Govind CK, Blundon JA. 1985. Form and function of the asymmetric chelae in blue crabs with normal and reversed handedness. Biol. Bull. 168, 321–331. ( 10.2307/1541244) [DOI] [Google Scholar]
- 130.Grunberg W, Havelec L. 1981. Morphometric studies on heterochely in the Californian crayfish Pacifastacus. 1. Leniusculus Dana 1852 (Decapoda, Astacidea). Wiener Tier. Monat. 68, 364–376. [Google Scholar]
- 131.Falck CL, Bowman TE. 1994. Commensal life, sexual dimorphism, and handedness in the canuellid harpacticoid Parasunaristes chelicerata (Por & Marcus, 1972). Hydrobiologia 292–293, 455–459. ( 10.1007/BF00229972) [DOI] [Google Scholar]
- 132.Agar WE. 1930. A statistical study of regeneration in two species of Crustacea. J. Exp. Biol. 7, 349–369. [Google Scholar]
- 133.Mellon dFJ, Stephens PT. 1978. Limb morphology and function are transformed by contralateral nerve section in snapping shrimp. Nature 272, 246–248. ( 10.1038/272246a0) [DOI] [PubMed] [Google Scholar]
- 134.Jesson LK, Barrett SCH. 2003. The comparative biology of mirror-image flowers. Int. J. Plant Sci. 164, S237–S249. ( 10.1086/378537) [DOI] [Google Scholar]