Abstract
Despite concerted international efforts, mortality from neonatal infections remains unacceptably high in some areas of the world, particularly for premature infants. Recent developments in flow cytometry and next-generation sequencing technologies have led to major discoveries over the past few years, providing a more integrated understanding of the developing human immune system in the context of its microbial environment. We review these recent findings, focusing on how in human newborns incomplete maturation of the immune system before a full term of gestation impacts on their vulnerability to infection. We also discuss some of the clinical implications of this research in guiding the design of more-accurate age-adapted diagnostic and preventive strategies for neonatal sepsis.
Keywords: Neonate, Immunology, Systems Biology, Ontogeny, Fetal development
INTRODUCTION
In 2015, one million neonates died of infection globally [1]. Despite broad, concerted international efforts, neonatal infections continue to be a main cause of mortality under 28 days of age. Improving global access to medical care, together with the continued implementation of sanitary measures, could substantially reduce neonatal mortality. Nevertheless, despite optimal medical care, neonatal infections remain prevalent even in developed countries, and especially in premature infants of low birth weight [2]. Sepsis can be difficult to diagnose in newborns, and is often fatal if not promptly treated. In clinical practice, treatment is also complicated by the lack of sensitivity of bacterial cultures and the lack of accurate diagnostic markers that go beyond measuring blood counts or other laboratory tests such C-reactive protein (CRP, see Glossary). Antibiotic treatments are also increasingly complicated by the emergence of bacterial resistance, which has become a real challenge in several nurseries across North America [3]. The complex nature of these problems mandates multidisciplinary efforts from the scientific community to improve our understanding of the immunological causes of newborn sepsis at the mechanistic level to improve diagnostic tools and therapeutic interventions. For example, many cases of sepsis could be preventable through immunization, either provided directly to the newborn baby or through the mother during gestation. However, current vaccines have been designed based upon assumptions that were drawn from our knowledge of the mature, adult immune system, which differs in many ways from the immune system of newborns and young infants. Recent technological advances, such as the development of multi-parameter flow cytometry, systems immunology, and high-throughput next-generation sequencing, now offer exciting new opportunities to dissect basic biological processes directly in humans both at the population level and across development. We review here the most recent discoveries that have been made in this area, include new insights gained into the development of mucosal immune defenses of human newborns, how interactions with the microbiome influence neonatal health outcomes, how changes in basic cell energy metabolism can impact immune cell activation, and how animal models have been used to gain a better knowledge on the specific vulnerabilities of the neonatal immune response to infection. Together, these discoveries provide a more integrated view of the developing human immune system during fetal life and the early neonatal period, opening new opportunities for improving the diagnosis and treatment of neonatal sepsis.
Immune development during fetal ontogeny
Important progress has been made over the past few years in our understanding of hematopoiesis in humans. In comparison with adults or even infants born at term, premature infants are at a much higher risk of infection (Box 1). This knowledge helps to situate these premature newborns in terms of their immune development (Figure 1). About a quarter of all infants born below 32 weeks of gestation will develop a serious infection during the neonatal period [2]. This early period of prematurity also coincides with major immunological vulnerability. Indeed, infants at this stage of development are considerably immunocompromised because of the incomplete maturation of both innate and adaptive immune defense mechanisms (Box 2).
Box 1. Prematurity in Humans.
The normal, full-term gestation in humans is 40 weeks (9 months). Prematurity is defined as when an infant is born below 37 weeks of gestation. The limit of viability in humans is about 22 weeks; unfortunately, infants born below this gestational age cannot survive even with optimal medical care. Premature infants face several short- and long-term health issues that require immediate medical attention at birth. Because of incompletely developed organs such as the lungs and bowels, preterm infants require intensive care interventions to survive after birth; these include supplemental oxygen, invasive mechanical ventilation, gavage feeds, and intravenous nutrition. Although life-saving, these interventions expose infants to a considerable risk of developing bloodstream infections from microbial pathogens present in the natural environment. When born below 32 weeks, newborns are considered to be immature both physiologically and immunologically, especially those under 29 weeks. Infections in premature infants usually occur within the first month of age and involve common organisms such as members of the Candida genus, Staphylococcus epidermidis, and Escherichia coli, that are not usually threatening to adults.
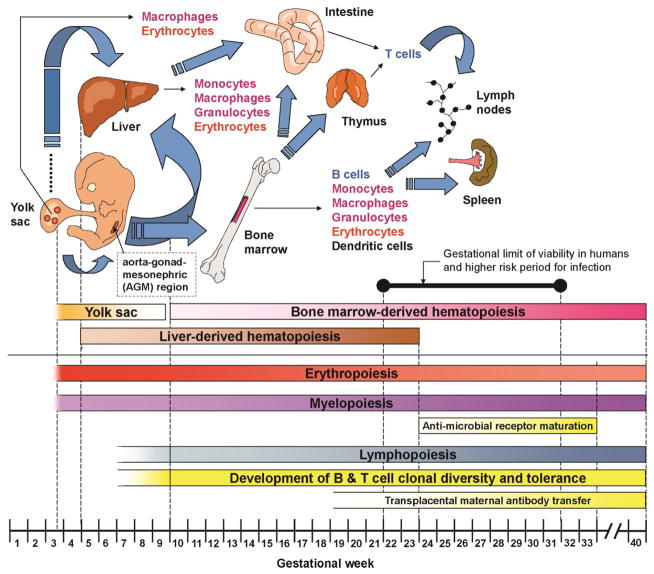
Figure 1. Immune Development during Gestation.
The development of hematopoiesis begins early in embryonic life in the yolk sac, followed by the liver, bone marrow, and other secondary hematopoietic organs. Lymphopoiesis begins after erythropoiesis and myelopoiesis. The blue arrows illustrate hematopoietic cells and their progenitors (and precursors) migrating between hematopoietic organs at different stages of embryonic and fetal life. The diagram also represents the aorta–gonad–mesonephric (AGM) region which is an important structure supporting the early colonization of hema-topoietic organs by hematopoietic cells and their progenitors. Infants born very prematurely, between 22 (defining the absolute gestational limit of viability in humans) and 32 weeks, are at high risk of infection. This period also corresponds to a critical period where substantial maturation of antimicrobial PRRs occurs, beginning with endosomal/cytoplasmic, followed by extracellular PRRs. This period also likely overlaps with the beginning of the development of mature, adult-like fetal T cells from an earlier wave of fetal T cells. Concomitant to this is the passive immunization of fetuses through trans-placental maternal antibody transfer. All these events contribute to the immaturity of the neonatal immune system in the context of a preterm birth.
Box 2. Relative Importance of the Innate and Adaptive Immune Systems in Neonates.
The innate immune system is considered to be a rapid, first-line of defense. Cells of the innate system comprise primarily myeloid cells such as macrophages, neutrophils, basophils, eosinophils, monocytes, and innate lymphoid cells. The adaptive immune system has the ability to acquire ‘immunological memory’ to a wide variety of antigens, thereby resulting in improved immune responses over subsequent pathogen encounters. Cells of the adaptive system are primarily composed of B and T lymphocytes. The development of immunological memory by the adaptive immune system involves the process of affinity maturation, which occurs through hypermutation of nucleotide sequences in the hypervariable regions of genes encoding T cell and B cell receptors. However, most recent data indicate that the innate immune system can also undergo a form of immunological memory termed ‘training’. Similar to adaptive immune responses, this training alters functional responses to subsequent pathogen exposures, and evidence suggests that this training is mediated through epigenetic changes [110]. During ontogeny, myeloid cells develop earlier in gestation than lymphoid cells. Adaptive immunity provides considerably improved immunological protection from infections, although this takes years of exposure to multiple variants of pathogens, in order to develop in humans. Therefore, in comparison with adults, the immunologically naïve newborn has to rely more heavily on innate immune defenses.
As illustrated in Figure 1, myelopoiesis begins early in the yolk sac around the 3rd week of gestation, followed by the liver after the 5th week. Until about the 20th to 22nd weeks the liver assumes most hematopoietic cell production, after which production of blood cells mainly takes place in the bone marrow [4]. The composition of myeloid cells has been well studied throughout gestation [5]. Neutrophils are very important in protecting newborns against infections. However, they form a very low proportion of blood cells until about 32 weeks [5]. Consequently, these cells have been the most difficult to study during this period. In preterm newborns, neutrophils lack the ability to form neutrophil extracellular traps, which are lattices of extracellular DNA, chromatin, and antibacterial proteins that mediate extracellular killing of microorganisms via the generation of potent reactive oxygen species [6].
In comparison to other myeloid cells, monocytes have been better studied, partly for historical reasons and partly because of their relatively high abundance in cord blood. Like other immune cell types, monocytes recognize pathogens through PRRs designed to recognize conserved microbial structures. These comprise endosomal, cytoplasmic, and extracellular antimicrobial detectors such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-containing protein (NOD) and NOD-like receptors (NLRs), C-type lectin receptors (CLRs), and the retinoic acid-inducible gene I (RIG-I) and RIG-I-like receptors (RLRs) [7]. Three main monocyte subsets have been defined in adult blood, according to their expression of the CD14 and CD16 cell surface markers [8]. Before 29 weeks of gestation, a majority of fetal monocytes display an immature phenotype characterized by low CD14 expression [8]. These monocytes are likely to play a more predominant role in tissue remodeling than in aggressive immune responses [4]. Anti-microbial immune recognition and antigen presentation are also significantly impaired at this gestation even in “classical” high CD14-expressing monocytes, because of reduced receptor expression and intracellular signaling [9, 10]. In fact, below 20–24 weeks of gestation, our data suggest little activity in extracellular PRR although this gestational age group was not included in studies [11–15]. By comparison, PRR activity in mononuclear cells increases until about 33 weeks of gestation, with the earliest activity detected in endosomal (TLR7, 8 and 9) and intracellular (e.g. NLRs), followed by extracellular PRRs such as the TLR1, 2, 4 and 5 and the dectin-1 receptor [9, 16].
The significance of this “inside-out” hierarchical development of PRR function during fetal ontogeny is unclear [14]. However, it may important clinical implications. It corroborates well with some of the microbial vulnerabilities clinically observable in early premature infants. For example, activity in TLR2, which plays a predominant role in the recognition of the coagulase-negative staphylococci (CoNS), develops late in gestation [13]. Correspondingly, infections with this pathogen are most common below 30 weeks of gestation [17]. Knowledge of the maturation in activity of PRRs across stages of development is also important because of the adjuvant role activation of these receptors play during immunizations. Accordingly, premature infants do tend to display significantly reduced antibody responses to routine vaccines [18], a pattern that is also mirrored in the elderly [19]. Further, the complement system is also functionally impaired in neonates born prematurely, as evidenced in a decrease in complement proteins C3a, factor H, factor I [20], also limiting pathogen recognition and clearance.
Lymphopoiesis begins after the initiation of myelopoiesis during ontogeny. Production of B and T cells commences between 8 and 10 weeks of gestation in the bone marrow and thymus. Recently, the use of next-generation sequencing has allowed an unprecedented view of the structural diversity of the antigen receptors of lymphocytes within human individuals at the single-cell resolution level. The occurrence of somatic hypermutation, which is a sign of the finely-tuned cognate encounter of a lymphocyte with its antigen, indicates that these interactions do occur during the fetal period [21]. A recent and more in-depth phenotypic characterization of neonatal CD4+ T cells obtained from newborn cord blood also showed that up to 5–10% of cells are differentiated into either memory or effector cells, confirming their activation in utero [22]. The antigenic source of this T and B cell activation remains unclear, although this may not be entirely surprising given that several studies in humans indicate that microorganisms are naturally present well before birth within the intra-amniotic environment [23, 24].
During gestation, the placenta assumes a major dual role protecting the fetus against infection while also preventing immune activation directed towards maternal antigens [25]. While microbial organisms may be the source of T and B cell activation, it is also possible that some of these cells may have been triggered by maternal antigens [26]. In this context, one vital requirement for the fetus is to maintain tolerance not only to self antigens but also to the allogeneic maternal antigens, while retaining the ability to become activated upon encountering infections. Recent data have provided important insights into how this may be achieved. According to current models, the production of B and T cells is not linear during ontogeny, but instead occur in waves which likely originate from distinct hematopoietic stem cell progenitors [27, 28]. In mice, two functionally distinct populations of B cells (B1 and B2) have been described. Recent data in humans identified similar innate-like B1 cells that predominate in cord blood, spontaneously produce IgM antibodies, and express immunoglobulins targeting a narrower repertoire of antigens such as phosphorylcholine and DNA [29]. Like B cells, early fetal T cells also appear functionally distinct from the late-gestation, more resembling “adult-like” T cells in that the former are prone to tolerance [26]. During the second trimester of pregnancy, maternal cells can be detected in fetal lymph nodes [26]. Instead of provoking a classic allogeneic response from fetal T cells, these maternal cells induce the development of suppressive fetal regulatory T cells, as shown by evidence of tolerance induction against non-inherited maternal allo-antigens (NIMAs), and this tolerance can persist at least until early adulthood [26]. Using transgenic mice, researchers have been able to show a systemic accumulation of NIMA-specific regulatory T cells in female offspring. Furthermore, micro-chimerism resulting from the persistence of maternal cells in the fetus not only increased fetal tolerance but also improved reproductive fitness in pregnancies sired by males with shared NIMA specificity [30]. These data potentially highlight a mechanism ensuring a successful pregnancy, although it is unclear how faulty tolerance mechanisms might play a role in the initiation of preterm labor in humans.
The immune system of a full-term infant is thought to be well-adapted to its developmental period [19, 31]. Most recently, studies have demonstrated other unique functional features of the newborn immune system, potentially allowing age-specific interactions between innate and adaptive immune cells during the course of an infection. For example, neonatal immune cells can produce developmentally unique cytokine isoforms, and two examples have been reported in humans with IL-4 and CXCL8 (also known as interleukin-8 (IL-8) [32, 33]. In addition, a large proportion of human naïve neonatal T cells are able to produce the CXCL8 chemokine upon stimulation [34]. The production of CXCL8 by neonatal T cells is unique in that it occurs in absence of effector differentiation, specifically in CD31 T cells that have recently emerged from the thymus [35]. Consistent with an innate-like phenotype of these, production of CXCL8 is enhanced by co-activation of extracellular PRRs. CXCL8 is a powerful neutrophil chemoattractant and is a chemokine that has no direct counterpart in mice [36]. Given the important role of neutrophils in neonatal sepsis, production of this chemokine may play a crucial role during an early response to infection as suggested by data showing an accumulation of CXCL8-producing CD4+ T cells during sepsis or necrotizing enterocolitis (NEC) [34]. In mice, innate γδ T cells have been shown to activate neutrophils directly during infections through a production of large amounts of IL-17, an important cytokine which facilitates the development of mucosal immunity [37]. However, human neutrophils generally respond poorly to IL-17 [34]. Presumably, CXCL8-producing naïve T cells could provide immediate help by sanctioning the activation of neutrophils in humans, in a PRR-dependent manner during an infection. Production of CXCL8 by naïve T cells may be particularly important in newborns who lack a rapidly responding immunological memory to most pathogens at birth. Interestingly, CXCL8 may also have broader trophic mucosal effects in the human fetus as suggested by the high constitutive expression of this cytokine by intestinal epithelial cells (IEC) resulting in protective effect against TNF-α-mediated apoptotic cell death [38].
Development of Mucosal Immune Defenses
Colonization of the fetus marks the beginning of an extraordinarily complex lifelong relationship with microbes (Box 3). Mouse models have been extremely useful in understanding how micro-organisms at mucosal interfaces are essential in shaping immune functions during the post-natal period. However, the existence of major functional differences across species has warranted human studies to confirm the relevance of findings from mouse models. Detailed reviews on the development of mucosal immune defenses during ontogeny have been published elsewhere [39–41]. However, additional relevant studies performed in the context of neonatal sepsis have been undertaken and are discussed here.
Box 3. Natural Microbial Colonization of the Newborn Infant.
Improving our understanding of human–microbe interactions brings a greater appreciation of the role of the human microbiome in long-term health outcomes. The intra-uterine environment is no longer considered sterile, and there is a greater appreciation of ‘normal’ placental and amniotic microbiota in utero. However, the abundance and diversity of this microbial flora probably increase considerably within days of birth as the mucosal and epidermal surfaces of the infant become colonized with nosocomial microbes. In the case of infants born at full term, a variety of factors have been evolutionarily selected to enhance colonization by beneficial microorganisms. Healthy term infants born vaginally are colonized by the maternal vaginal, intestinal, and skin microbiota shortly after birth [111]. Several factors will influence the diversity of the early microbiome. These include, for example, the way the infant is delivered, nutritional factors, antibiotic exposure, and, very likely, geographical and ethnic factors. In the case of preterm infants, nearly half are born via Caesarean section in North America, which likely affects colonization by normal human commensals. Premature infants also often have nutritional deficits, and are at risk of prolonged antibiotic use which may further disrupt their normal colonization.
The role of the intestine as a functionally distinct immunological organ is increasingly recognized [42]. Traditionally, our knowledge of the developing human intestinal immune system has been greatly complicated by tenuous immune phenotyping protocols coupled to a lack of accessibility to fetal samples. In the last couple of years, advances in the use of multi-parameter flow cytometry reagents and equipment, allowing more extensive, high-throughput characterization of cell populations using minuscule amount of biological samples obtainable from a human neonate has greatly facilitated discoveries in this area. For instance, invariant natural Killer (iNKT) cells are a relatively rare innate-like T cell type particularly important in protecting the host against colonizing micro-organisms at mucosal interfaces. These cells can be detected using fluorescence-labeled antigen-loaded tetramerized major histocompatibility complex molecules [43]. Recent data has shown that intestinal neonatal iNKT cells share unique functional properties in contrast to conventional T cells, intestinal neonatal iNKT cells produce robust Th1 and Th17 responses [44]. In humans, iNKT cells are abundant early on in fetal blood [45], although their tissue of origin had remained unclear until recent data showed that these cells accumulate in the small intestine during the second trimester of gestation [44].
Studies in mice have also revealed the crucial role of the small intestine in regulating both local and systemic Th17 responses [46]. Particularly, IL-17-mediated Th and γδ T cell responses appear very important to prevent invasion of micro-organisms at mucosal interfaces [47]. Earlier studies showed that neonatal CD4+ T cells are intrinsically biased towards a “default” Th2 response with an abundant, an epigenetically regulated production of IL-4 and IL-10 (reviewed in [48]). Recently, human neonatal CD4+ T cells were also shown to intrinsically differentiate poorly into IL-17-producing cells upon anti-CD3/CD28 activation in vitro [49, 50]. There are six homologs of IL-17 (IL-17A to F); IL-17A is the best characterized and predominant member of this family, whereas IL-17F also shares partly overlapping functions with IL-17A [51]. IL-17A/F-deficient mice exhibit increased susceptibility to a broad range of bacterial and fungal mucosal infections [51, 52]. In humans, the clinical impact of IL-17 deficiency is more limited, highlighting another important difference across species [47]. Loss of Th17 cells in humans with chronic HIV infection results in a loss of gut-barrier function and subsequent microbial translocation [53]. Notably, adult humans lacking production of IL-17 appear specifically vulnerable to neonatal pathogens such as Candida and Staphylococcus aureus [54]. To help promote systemic Th17 responses in the full-term neonate, neonatal antigen-presenting cells produce high levels of IL-1β, IL-6, but also high amounts of IL-23 [55]. By contrast, monocytes and dendritic cells from preterm neonates below 29 weeks of gestation produce low amounts of IL-1β and IL-23 [9, 15]. This lack of Th17-polarizing cytokine production by neonatal antigen presenting cells of infants born very prematurely may partly explain their increased vulnerability to mucosal infections caused by Staphylococcal and Candida species.
Increasing evidence suggest an important influence of the neonatal microbiome on health outcomes. In healthy term newborns, a diverse intestinal microbial flora promotes the development of mucosal barriers and prevents the outgrowth of pathogenic micro-organisms [56, 57]. Transient intestinal dysbiosis in the early post-natal period has recently been associated with an increased risk of asthma during early childhood [58]. Anomalies in the placental microbiome have also been associated with premature birth (reviewed in [59]). Moreover, accumulating evidence suggests that the composition of the microbiome plays an important role in protecting the newborn against infections. For example, disruption of the normal flora with broad spectrum antibiotics increases the risk of neonatal sepsis [60–62]. In preterm neonates, distinct microbial colonization of the gut linked to increased levels of CoNS and decreased colonization by protective commensals such as Bacteroides, Bifidobacterium, and Lactobacillus has also been associated to increased risks of neonatal sepsis [63, 64] and NEC [65, 66]. Data suggest that abnormal PRR signaling at mucosal surfaces in preterm infants may limit microbial diversity, thereby favoring the colonization of pathogenic micro-organisms [62, 64, 67]. These findings have direct clinical relevance. Indeed, administration of probiotics therapeutically reduces the risk of neonatal sepsis and NEC in premature infants [68]. Large clinical trials are also ongoing in developing countries; results so far indicate that administration of probiotics reduces neonatal mortality due to sepsis [69].
Most recently, other important observations with regards to the cellular composition [70] and presence of anti-microbial peptides [71]in breastmilk have been recently published. These data shed light on how the immune properties of human milk vary across periods of lactation and on the protective impact of breastfeeding on immune maturation [70, 71]. A main clinical relevance of these findings is that breastmilk can be used to protect against neonatal sepsis. For example, in the neonatal intensive care unit administration of human milk (as opposed to cow protein-based milk formula) has been repeatedly shown to protect against NEC in premature infants.
Mechanisms of Immune Cell Maturation and Function during Ontogeny
Despite major progress in the characterization of the neonatal immune system at various developmental stages, the fundamental mechanisms regulating the maturation of immune functions in humans during ontogeny remain completely unknown. Recent data has revealed an important immune regulatory role for erythrocytes. These authors suggested that CD71+ nucleated red blood cells (nRBCs) actively suppress both innate and adaptive immune responses through the expression of arginase, which inhibits nitric oxide synthesis [72]. However, this conclusion was disputed by others whose data suggested that the heightened intestinal immune activation in mice treated with anti-CD71 to deplete the nRBCs was unaffected by replenishment of these cells [73]. Of note, significant cross-contamination can occur between nRBCs and other white blood cells types during cell purification for ex vivo immune studies [74]. This type of technical problem therefore raises concern that previous conclusions from functional and genomic analyses of cord blood immune cells may have been biased because of the heterotopic cellular interactions with DNA/high rRNA-containing an potentially suppressing nRBCs that go undetected unless formally excluded by FACS [74].
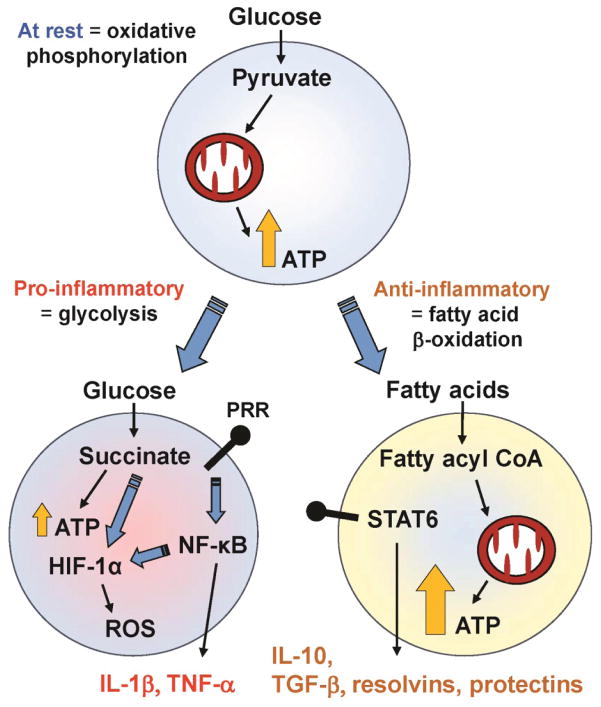
Another recent contribution to our understanding of the regulation of immune responses has been the demonstration that a major metabolic switch is required in immune cells, in order to accommodate the massive induction of genes following an activation of these cells [75–78]. The essence of these changes is depicted in Figure 2. At rest, immune cells largely utilize oxidative phosphorylation to generate energy. Upon encountering a pathogen, cells turn off oxidative phosphorylation to rely almost exclusively on glycolysis for energy production; these metabolic changes are followed by activation of inflammatory immune pathways. Mitochondria play a central role in operating this metabolic switch through controlling the metabolic fate of glucose, as well as the utilization of fatty acids. By contrast, upon encountering an anti-inflammatory stimulus, enhanced lipid metabolism results in increase production of anti-inflammatory cytokines (IL-10, TGF-β), as well as pro-resolving lipid mediators (e.g. resolvins and protectins) [79].
Figure 2. Metabolic Adaptation Occurring during Immune Activation.
Resting immune cells, such as monocytes or T cells primarily produce energy (ATP) from breaking down glucose into pyruvate, and through mitochondrial oxidative phosphorylation. The activation of pattern recognition receptors (PRRs) by microbes leads to the activation of the master transcription regulator NF-κB, which in turn results in inflammatory cytokine gene expression and production (e.g. IL-1β, TNF-α). Concurrently, cells also undergo a metabolic shift to aerobic glycolysis (a phenomenon also known as the Warburg effect), where energy production occurring through glycolysis breaks down glucose into succinate. Although less energy is produced for each glucose molecule, this pathway does provide large quantities of anabolic substrates necessary to feed into the high gene transcriptional activity occurring during immune activation. Succinate and NF-κB also stabilize the transcription factor HIF-1α, resulting in further upregulation of inflammatory cytokine gene expression. The shift away from oxidative phosphorylation also allows these cells to produce reactive oxygen species (ROS) more efficiently. By contrast, anti-inflammatory signaling (via STAT6) causes an increase in fatty acid uptake, mitochondrial biogenesis, and increased mitochondrial function. This metabolic shift towards fatty acid oxidation leads to upregulation of anti-inflammatory cytokines (e.g., IL-10, TGF-β) and pro-resolving lipid mediators such as resolvins and protectins.
Metabolic control over the pro or anti-inflammatory nature of an immune response appears to be a general requirement of immune cells, as suggested by similar changes observed during T cell [80], monocyte/macrophage [81] and dendritic cells activation [82]. Corresponding metabolic shifts to glycolysis in pro-inflammatory vs fatty acid oxidation in anti-inflammatory responses have been observed in both human and mouse cells [76, 77, 83]. As another example, activation of effector CD4+ T cells requires in a shift from oxidative phosphorylation to glycolysis while activation of regulatory T cells results in a shift in cell energy metabolism toward fatty acid oxidation [80]. The broad and conserved extent of these changes and the unique metabolic state of the fetus suggests that a lack of similar metabolic changes may contribute to immune impairments in preterm neonates. This hypothesis is supported by several primary metabolic differences that have been described between neonates born prematurely, at term, and adults. For instance, during fetal life, mitochondria have a fragmented appearance [84], reduced mass [85], and display a more limited capacity for energy production [86]. In addition, preterm neonates exhibit increased lipid metabolism relative to their glycolytic activity [87]; this is linked to an altered metabolic response during sepsis [88]. Also, oxidative phosphorylation pathways are down-regulated in preterm neonates [86, 89, 90], which compensate by increasing fatty acid oxidation [91, 92]. Hypoxia-Inducible Factor 1-alpha (HIF-1α), and mechanistic Target of Rapamycin (mTOR) have been shown to play an important role in regulating this metabolic switch [75]. HIF-1α is a transcription factor largely responsible for the switch from oxidative phosphorylation to glycolytic pathways; in addition, HIF-1α activity can be induced by inflammatory stimuli through via mTOR signaling [75]. Whereas the role of HIF-1α and mTOR has been relatively well defined in mature organisms, little is known about the regulation of these molecules during ontogeny. The fetus naturally evolves in a relatively hypoxic environment that is due to its unique blood circulation. Regulation through HIF-1α is influenced by a variety of stimuli, including inflammatory signaling [93] and oxygen levels [94], thereby providing a potential link between low oxygen concentration in utero and decreased immune responsiveness [78].
Another important question is whether observations of attenuated immune responses made in preterm neonates at birth can be extrapolated to explain the high risk of infection observed during the neonatal period. Indeed, our knowledge of the developing newborn immune system has been largely derived from cord blood. Nonetheless, recent studies have examined the maturation of immune cells during the neonatal period, at the time infants are most affected by neonatal pathogens (i.e. around 1–3 weeks of post-natal age). Indeed, using both whole blood and single-cell flow cytometry analyses, Marchant et al. have confirmed that PRR responses (e.g. TLR4, TLR7/8), remain attenuated in post-natal blood obtained from neonates born below 30 weeks of gestation [95]. These results provide important support to the premise that reduced immune activity is responsible for the high vulnerability to infections.
Premature infants are often born in pathological conditions such as following chorioamnionitis, which may potentially impact on functional measurements of immune responses in cord blood (Box 4). A vast majority of these infants are also exposed to corticosteroids (an immunosuppressive drug), which are often administered to pregnant mothers in order to promote fetal lung maturation. However, multivariable statistical analyses of immune responses in these infants following rigorous clinical definitions of antenatal exposures suggest that the functional immunological differences measured from cord blood are probably developmentally related, rather than due to clinical exogenous perinatal factors linked to their premature birth [14].
Box 4. Chorioamnionitis and the Developing Neonate.
Chorioamnionitis has been strongly associated with preterm labor. For example, there is evidence of chorioamnionitis in about 30–50% of mothers delivering an infant prematurely below 29 weeks of gestation. Chorioamnionitis is also a major risk factor for poor neonatal health outcomes. Infections of the fetal membranes expose the fetus to an inflammatory environment, which can be damaging to the developing brain and may also progress to neonatal sepsis. Based on animal models, chorioamnionitis can also modulate fetal immune responses, an effect that has been termed ‘immune-paralysis’ [112, 113].
Lessons from Neonatal Mouse Models
Studying the role of immune components in sepsis experimentally in newborn infants is virtually impossible for obvious ethical reasons. The miniaturization of immunological methods has greatly helped the development of neonatal mouse models of sepsis. These models have confirmed the limited myelopoietic capacity of the newborn and its impact on survival of newborn mice during sepsis [96]. Using a poly-microbial infection model, Wynn and Moldawer demonstrated that rodent neonates rely heavily on innate immune defense during sepsis [97]. Moreover, the brain of a developing newborn is also particularly vulnerable to inflammation, and neonatal infections carry an important risk of major long-term neurological disability in premature infants [98]. To study the mechanisms of brain injury during neonatal infections, Levy and Mallard developed an interesting mouse model [99] in which they demonstrated that inflammation due to Staphylococcus epidermidis caused brain injury even in the absence of bacterial entry into the cerebrospinal fluid space. These results suggest that low-grade inflammation produced during transient bacteremia could be sufficient to cause damage to a developing neonatal brain. The findings have important clinical implications, since earlier studies have indicated that transient bacteremia commonly occurs in infants with sepsis [100, 101]. Another study has demonstrated persistent sub-clinical inflammation in the absence of overt clinical signs of infection in these infants throughout the neonatal period, possibly due to an ongoing exposure to intensive care therapies [102]. In light of these findings, better strategies will be necessary to both limit the sustained exposure of premature infants to any potential triggers of inflammation or sources of transient bacteremia, and to prevent infections [103].
The coupling of neonatal mouse models to genome-wide transcriptome studies has also pointed to important functional differences between neonatal and adult responses [104]. Indeed, the nature of innate immune PRR pathways involved during sepsis differs between neonates and adults, highlighting the importance of age-appropriate models [96]. However, major differences in the nature of the transcriptome responses depend on the model used [105], and well-recognized differences between humans and mice may limit the application of findings across species. This justifies direct observations in humans [106]. Most recently, two important genome-wide gene expression studies have provided insights into the molecular pathways activated in newborns with severe infections [88, 107]. For instance, on the one hand marked transcriptional alterations in metabolic pathways associated with glucose transport, glycolysis, and cholesterol homeostasis, transport, and metabolism have been detected in newborns during active sepsis [88]. On the other hand, transcriptional pathways related to antigen presentation processes via MHC II were down-regulated in septic infants [88]. The specific up-regulation of immune suppressor signaling pathways, such as TNFAIP3, CD71, and SOCS1/3 in infants with sepsis and which have been reported might help to explain why some infants fail to clear infections, although it is possible that the lower gestational age of infants from the septic group in this study may have confounded some of these results [88]. The main clinical importance of these results is that they provide proof-of-concept validation that measuring genome-wide host immune responses can greatly help discriminate infants with or without bacterial infection more accurately than traditional clinical laboratory markers. These data should drive the development of a new generation of diagnostic tests that will inform clinical decision-making regarding antibiotic treatment in neonatal sepsis in a timely and pathogen-oriented manner, thereby reducing missed diagnoses and unnecessary interventions.
Concluding remarks and future research directions
Important progress has been made in our understanding of the maturation of immune functions in newborns, especially in the most vulnerable group of premature infants born at the lower end of gestation. However, translation of these findings into clinical practice requires answers to important remaining questions (see Outstanding Questions). First and foremost, we lack a clear understanding of the causal relationship between developmental changes in immune function in the neonate and the acquisition/risk of infection(s). This in turn limits a clear knowledge of how (and which) specific interventions might have the greatest effect on health. Indeed, despite being repeatedly expressed in the literature, the statement that immaturity in the neonatal immune system is responsible for the increased vulnerability of the newborn to neonatal sepsis remains largely assumptive. Prospective studies linking immunological phenotypes to detailed clinical outcomes are needed to address this question. Recent advances in transcriptomics, and in flow cytometry combined with mass spectrometry can allow the measurement of much more extensive cellular immune phenotypes in minuscule amounts of biological samples [108]. These technologies will greatly facilitate the study of immune diversity at the population level, across development, and in a high-throughput manner. Second, an understanding of the development of immune functions at the mucosal interfaces is important, given that a majority of cases of neonatal sepsis worldwide are due to respiratory or intestinal infections [109]. In addition, studying how changes in the microbiome in early life shape immunological responses and alter the risk of autoimmune or inflammatory diseases during adulthood may offer additional opportunities for clinical translation. Third, more detailed knowledge of the specific cell types and subtypes harboring important functions in neonatal sepsis are warranted. Indeed, the role of neutrophils, B cells, and other important immune cell types in neonatal sepsis has been largely neglected, partly owing to major practical limitations in working with small blood volumes or inaccessible tissue samples from fetuses and premature infants. Together, these studies will allow us to develop a better grasp of the potential impact of immunological issues amenable to clinical interventions. Fourth, next-generation sequencing technologies offer unique opportunities to revisit vaccine responses in neonates and preterm infants at the systems level. These approaches should also help to achieve a better understanding of the basic mechanisms regulating the maturation of immune responses during ontogeny. Advanced knowledge in this area is interesting and important from a fundamental perspective, but it also has the potential to lead to the innovation and development of novel and more age-specific immunization strategies, from the premature to the young infant. Finally, large prospective studies will be necessary to adapt and confirm the clinical utility of using transcriptomic signatures of the host’s immune response to improve the diagnosis of neonatal sepsis. Increasing the accuracy of current laboratory tests for neonatal sepsis could save billions of dollars in newborn hospitalization costs and antibiotic use in North America alone.
OUTSTANDING QUESTIONS.
How do functional variations of the immune system across developmental stages affect health outcomes in newborns, at the population level?
How do developmental changes in specific immune cell types (such as neutrophils and B cells) contribute to the increased vulnerability of newborns to infections?
How do mucosal immune defenses mature across development? How do early-life changes in the microbiota, influenced by specific factors (ethnicity, geography, nutrition prematurity, exposure to antibiotics), alter the risk of disease development later on (e.g., asthma)?
Can genome-wide transcriptomic host–response signatures measured from the blood of newborns with clinically suspected infection help diagnose sepsis? Could such timely information help reduce antibiotic treatment duration, thereby preventing unnecessary interventions and prolonged hospitalization?
What are the basic mechanisms regulating the maturation of immune responses during ontogeny (including a role for epigenetics)?
How can data on newborn susceptibility to infection and on the ontogeny of the immune system be used to better understand impaired immunity at the other end of the spectrum of life (e.g., the elderly)?
Given the major immunological differences between mice and humans during ontogeny, can other non-murine animal models (e.g., non-human primates) be used to improve our understanding of the vulnerability of newborns to sepsis?
In summary, although the distinct immunological characteristics of the newborn and the unique immunological immaturity of an infant in the context of premature birth present a challenging and complex medical picture, recent scientific advances in immunology and host–microbe interactions have significantly enhanced our understanding of immune development in humans. This creates unique research translation opportunities and the potential to develop novel therapeutic approaches to treat a variety of neonatal diseases.
TRENDS.
The study of immune development at the systems level using next-generation sequencing and advanced multi-parameter flow cytometry is allowing a better understanding of phenotypic and functional differences in innate and adaptive immunity. This facilitates a better appreciation of immunity in humans at the population level, and across developmental stages during ontogeny.
Immune responses in fetuses, newborns, and infants are not simply hypofunctional or underdeveloped, but are rather functionally distinct than that of adults.
The intestine plays a major role in immune development particularly during fetal ontogeny and in the early postnatal period.
Major developmental maturation occurs in antimicrobial pattern recognition receptors (PRRs) in humans, until about 33 weeks of gestation. Infants born prematurely during this period have immature PRR responses that are likely to play an important role increasing their infection risk.
Alterations in the infant’s microbial flora either due to prematurity, alternate mode of delivery, prolonged antibiotic exposure in early life or changes in diet, has been linked to dysbiosis, and in some cases to an increased risk of neonatal sepsis.
Acknowledgments
We are grateful to our colleague Dr Tobias Kollmann for providing insightful comments on this manuscript. H.R.R. is supported by a Mitacs Accelerate Scholarship, in partnership with STEMCELL Technologies Inc. and the British Columbia Children’s Hospital Foundation. P.M.L. is supported by a Career Investigator Award from the Michael Smith Foundation for Health Research, and a Clinician-Scientist Award from the Child and Family Research Institute. We thank Kelsey Lee for help in revising this manuscript. Part of the research presented in this article has been funded by the Hospital for Sick Children, the Canadian Institutes of Health Research (operating grants MOP-110938; MOP-123478), and the US March of Dimes Foundation.
GLOSSARY
- Aorta–Gonad–Mesonephros (AGM)
a region of the embryonic mesoderm that develops in the para-aortic splanchnopleuric area during embryogenic life. Definitive hematopoietic stem cell progenitors arise from the AGM to migrate to dedicated hematopoietic organs such as the liver, bone marrow, and spleen. In humans, this region forms around the 5th week of gestation.
- C-reactive protein (CRP)
an acute-phase protein produced by the liver during an infection. Its detection in blood is used in clinical neonatology to help to discriminate infections in newborns presenting clinical signs. Although an elevation in CRP has been shown to help to predict neonatal infections, other non-infectious causes can lead to elevation of this marker, thereby reducing the accuracy of this test. Its relatively late increase (>12 h) in the course of an infection limits its use as a marker to help clinicians to decide whether to start antibiotic treatment in sick newborns.
- Chorioamnionitis
inflammation of the fetal chorion and amnion membranes in the placenta, usually due to bacteria, that is associated with increased risk of preterm labor and/or neonatal sepsis.
- Coagulase-negative staphylococcus (CoNS)
a bacterium that is present ubiquitously in humans. Although rarely a threat to adults with a mature immune system, it is one of the most common causes of bacteremia related to indwelling catheter devices in premature newborns.
- CXCL8 (IL-8)
a chemokine produced in humans, but not in mice. It is a potent neutrophil chemotactic protein produced by a large proportion of naive neonatal T cells.
- Dectin-1 receptor
encoded by the gene CLEC7A, a PRR that recognizes b-glucans, which are carbohydrate components of fungal outer cell walls.
- Hematopoiesis
the process by which blood cells originate from hematopoietic progenitor stem cells. In the fetus, hematopoiesis progressively moves from the yolk sac to the AGM, to the fetal liver, and to the bone marrow, as these respective organs mature. In newborns and adults, hematopoiesis largely occurs in the bone marrow.
- Intestinal dysbiosis
disruption of the microbial flora within the intestinal lumen, which can be caused by factors such as multiple and/or prolonged antibiotic exposure.
- Invariant natural killer T (iNKT) and γδ T cells
are considered innate-like T cells in part because they can rapidly produce effector cytokine or cytotoxic responses upon activation. These cells also have structurally unique T cell receptors that generally recognize non-protein antigens.
- Lymphopoiesis
a process by which T and B cells, natural killer cells, some subset of dendritic cells, as well as the most recently described innate lymphoid cells are produced. T and B cells form the main cellular components of adaptive immunity.
- Myelopoiesis
a process by which monocytes, macrophages, granulocytes (i.e., neutrophils, basophils, and eosinophils), and some subsets of dendritic cells are produced.
- Necrotizing enterocolitis (NEC)
a medical complication most frequently encountered in premature infants, and resulting in vascular necrosis of the bowel wall, loss of bowel integrity, and sepsis. NEC is thought to be related in part to an abnormal host-response to colonizing microorganisms and is a major cause or mortality in infants born prematurely.
- Nosocomial infection
infection acquired in the hospital setting.
- Pro-resolving lipid mediators
these include resolvins and protectins, which are synthesized from polyunsaturated ω3 fatty acids, primarily eicosapentaenoic acid and docosahexaenoic acid but also docosapentaenoic acid. These metabolites have been shown to be potent anti -inflammatory mediators that regulate the active resolution of inflammation and promotion of wound healing following tissue injury.
References
- 1.Lawn JE, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantey JB, Milstone AM. Bloodstream infections: epidemiology and resistance. Clin Perinatol. 2015;42:1–16. doi: 10.1016/j.clp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie PM, Levy O. The mononuclear phagocyte system. In: Polin R, et al., editors. In Fetal and Neonatal Physiology. 5. Chapter 125. Elsevier; 2016. pp. 1208–1216. [Google Scholar]
- 5.Davies NP, et al. Fetal leucocyte count in rhesus disease. Arch Dis Child. 1992;67:404–406. doi: 10.1136/adc.67.4_spec_no.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yost CC, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker SW, et al. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol. 2014;289:135–139. doi: 10.1016/j.cellimm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Sharma AA, et al. Impaired NLRP3 inflammasome activity during fetal development regulates IL-1b production in human monocytes. Eur J Immunol. 2015;45:238–249. doi: 10.1002/eji.201444707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krow-Lucal ER, et al. Distinct functional programming of human fetal and adult monocytes. Blood. 2014;123:1897–1904. doi: 10.1182/blood-2013-11-536094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Förster-Waldl E, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 12.Sadeghi K, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 13.Strunk T, et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr Res. 2012;72:10–18. doi: 10.1038/pr.2012.48. [DOI] [PubMed] [Google Scholar]
- 14.Sharma AA, et al. Hierarchical maturation of innate immune defences in very preterm neonates. Neonatology. 2014;106:1–9. doi: 10.1159/000358550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavoie PM, et al. Profound lack of interleukin (IL)-12/IL- 23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J Infect Dis. 2010;202:1754–1763. doi: 10.1086/657143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philbin VJ, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchant EA, et al. Neonatal sepsis due to coagulase-negative staphylococci. Clin Dev Immunol. 2013;2013:586076. doi: 10.1155/2013/586076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baxter D. Vaccine responsiveness in premature infants. Hum Vaccin. 2010;6:506–511. doi: 10.4161/hv.6.6.12083. [DOI] [PubMed] [Google Scholar]
- 19.Kollmann TR, et al. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grumach AS, et al. Complement profile in neonates of different gestational ages. Scand J Immunol. 2014;79:276–281. doi: 10.1111/sji.12154. [DOI] [PubMed] [Google Scholar]
- 21.Rechavi E, et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med. 2015;7:276ra25. doi: 10.1126/scitranslmed.aaa0072. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med. 2014;6:238ra72. doi: 10.1126/scitranslmed.3008748. [DOI] [PubMed] [Google Scholar]
- 23.Aagaard K, et al. The placenta harbors a unique micro-biome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao B, Mysorekar IU. Intracellular bacteria in pla-cental basal plate localize to extravillous trophoblasts. Placenta. 2014;35:139–142. doi: 10.1016/j.placenta.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 25.PrabhuDas M, et al. Immune mechanisms at the mater-nal–fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mold JE, et al. Maternal alloantigens promote the devel-opment of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin DO, et al. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70- J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinder JMM, et al. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell. 2015;162:505–515. doi: 10.1016/j.cell.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma AA, et al. The developing human preterm neo-natal immune system: a case for more research in this area. Clin Immunol. 2012;145:61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebel K, et al. CD4+ T cells from human neonates and infants are poised spontaneously to run a non-classical IL-4 program. J Immunol. 2014;192:5160–5170. doi: 10.4049/jimmunol.1302539. [DOI] [PubMed] [Google Scholar]
- 33.Maheshwari A, et al. Developmental changes in circulating IL-8/CXCL8 isoforms in neonates. Cytokine. 2009;46:12–16. doi: 10.1016/j.cyto.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbons D, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 35.van den Broek T, et al. Neonatal thymectomy reveals differentiation and plasticity within human naive T cells. J Clin Invest. 2016;126:1126–1136. doi: 10.1172/JCI84997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remick DG. Interleukin-8. Crit Care Med. 2005;33:S466–S467. doi: 10.1097/01.ccm.0000186783.34908.18. [DOI] [PubMed] [Google Scholar]
- 37.Haas JD, et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari A, et al. Interleukin-8/CXCL8 forms an auto-crine loop in fetal intestinal mucosa. Pediatr Res. 2004;56:240–249. doi: 10.1203/01.PDR.0000133196.25949.98. [DOI] [PubMed] [Google Scholar]
- 39.Jain N, Walker WA. Diet and host–microbial cross-talk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14–25. doi: 10.1038/nrgastro.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulde M, Hornef MW. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol Rev. 2014;260:21–34. doi: 10.1111/imr.12190. [DOI] [PubMed] [Google Scholar]
- 41.Battersby AJ, Gibbons DL. The gut mucosal immune system in the neonatal period. Pediatr Allergy Immunol. 2013;24:414–421. doi: 10.1111/pai.12079. [DOI] [PubMed] [Google Scholar]
- 42.Maynard CL, et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma AA, et al. Ex vivo purification and characterization of human invariant natural killer T cells. J Immunol Methods. 2011;373:1–7. doi: 10.1016/j.jim.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loh L, et al. Invariant natural killer T cells developing in the human fetus accumulate and mature in the small intestine. Mucosal Immunol. 2014;7:1–11. doi: 10.1038/mi.2014.13. [DOI] [PubMed] [Google Scholar]
- 45.Ladd M, et al. Natural killer T cells constitutively expressing the interleukin-2 receptor / chain early in life are primed to respond to lower antigenic stimulation. Immunology. 2010;131:289–299. doi: 10.1111/j.1365-2567.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esplugues E, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald DR. TH17 deficiency in human disease. J Allergy Clin Immunol. 2012;129:1429–1435. doi: 10.1016/j.jaci.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson CB, et al. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 49.de Roock S, et al. Defective TH17 development in human neonatal T cells involves reduced RORC2 mRNA content. J Allergy Clin Immunol. 2013;132:754–756. doi: 10.1016/j.jaci.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Dijkstra KK, et al. TH17 differentiation capacity develops within the first 3 months of life. J Allergy Clin Immunol. 2014;133:891–894. doi: 10.1016/j.jaci.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Korn T, et al. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 52.Sheridan BS, et al. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuetz A, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10:e1004543. doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cypowyj S, et al. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol. 2012;42:2246–2254. doi: 10.1002/eji.201242605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanden Eijnden S, et al. Preferential production of the IL- 12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36:21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 56.Mirpuri J, et al. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J Immunol. 2010;184:7186–7195. doi: 10.4049/jimmunol.0903116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda S, et al. Bifidobacteria can protect from entero-pathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 58.Arrieta MC, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 59.Cao B, et al. Placental microbiome and its role in preterm birth. Neoreviews. 2014;15:537–545. doi: 10.1542/neo.15-12-e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuppala VS, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deshmukh HS, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arboleya S, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166:538–544. doi: 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Berrington JE, et al. The neonatal bowel microbiome in health and infection. Curr Opin Infect Dis. 2014;27:236–243. doi: 10.1097/QCO.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 64.Drell T, et al. The development of gut microbiota in critically ill extremely low birth weight infants assessed with 16S rRNA gene based sequencing. Gut Microbes. 2014;5:304–312. doi: 10.4161/gmic.28849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mai V, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrow AL, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in pre-term infants. Microbiome. 2013;1:13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Claud EC, et al. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin- induced inflammation. Proc Natl Acad Sci USA. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.AlFaleh K, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011;2011:CD005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Strunk T, et al. Probiotics to prevent early-life infection. Lancet Infect Dis. 2015;15:378–379. doi: 10.1016/S1473-3099(15)70088-5. [DOI] [PubMed] [Google Scholar]
- 70.Trend S, et al. Leukocyte populations in human preterm and term breast milk identified by multicolour flow cytometry. PLoS ONE. 2015;10:e0135580. doi: 10.1371/journal.pone.0135580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trend S, et al. Antimicrobial protein and peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS. 2015 doi: 10.1371/journal.pone.0117038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elahi S, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wynn JL, et al. Neonatal CD71+ erythroid cells do not modify murine sepsis mortality. J Immunol. 2015;195:1064–1070. doi: 10.4049/jimmunol.1500771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Goede OM, et al. Nucleated red blood cells impact DNA methylation and expression analyses of cord blood hemato-poietic cells. Clin Epigenetics. 2015;7:95. doi: 10.1186/s13148-015-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng SC, et al. mTOR- and HIF-1/-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Everts B, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKe supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGettrick AF, O’Neill LAJ. How metabolism generates signals during innate immunity and inflammation. J Biol Chem. 2013;288:22893–22898. doi: 10.1074/jbc.R113.486464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rius J, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF- 1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:24–35. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buck MD, et al. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galván-Peña S, O’Neill LAJ. Metabolic reprogram-ing in macrophage polarization. Front Immunol. 2014;5:1–6. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang SCC, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:1–12. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mintz HA, et al. Morphological and biochemical studies of isolated mitochondria from fetal, neonatal, and adult liver and from neoplastic tissues. J Cell Biol. 1967;34:513–523. doi: 10.1083/jcb.34.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mészáros G, et al. Altered mitochondrial response to activation of T-cells in neonate. Acta Physiol Hung. 2015;102:216–227. doi: 10.1556/036.102.2015.2.12. [DOI] [PubMed] [Google Scholar]
- 86.Valcarce C, et al. Mammalian adaptation to extrauterine environment: mitochondrial functional impairment caused by prematurity. Biochem J. 1994;303:855–862. doi: 10.1042/bj3030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsubahara M, et al. Early human development glucose metabolism soon after birth in very premature infants with small-and appropriate-for-gestational-age birth weights. Early Hum Dev. 2012;88:735–738. doi: 10.1016/j.earlhumdev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Smith CL, et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat Commun. 2014;5:1–15. doi: 10.1038/ncomms5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brauner P, et al. Induction of uncoupling protein 3 gene expression in skeletal muscle of preterm newborns. Pediatr Res. 2003;53:691–697. doi: 10.1203/01.PDR.0000054687.07095.0B. [DOI] [PubMed] [Google Scholar]
- 90.Wenchich L, et al. Mitochondrial energy metabolism in very premature neonates. Biol Neonate. 2002;81:229–235. doi: 10.1159/000056753. [DOI] [PubMed] [Google Scholar]
- 91.Oey NA, et al. Fatty acid oxidation in the human fetus: implications for fetal and adult disease. J Inherit Metab Dis. 2006;29:71–75. doi: 10.1007/s10545-006-0199-x. [DOI] [PubMed] [Google Scholar]
- 92.Alexandre-Gouabau MC, et al. Maternal and cord blood LC-HRMS metabolomics reveal alterations in energy and poly-amine metabolism, and oxidative stress in very-low birth weight infants. J Proteome Res. 2013;12:2764–2778. doi: 10.1021/pr400122v. [DOI] [PubMed] [Google Scholar]
- 93.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1b through HIF-1/ Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wen H, Ting JPY. Agitation by suffocation: how hypoxia activates innate immunity via the warburg effect. Cell Metab. 2013;17:814–815. doi: 10.1016/j.cmet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Marchant EA, et al. Attenuated innate immune defenses in very premature neonates during the neonatal period. Pediatr Res. 2015;78:492–497. doi: 10.1038/pr.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cuenca AG, et al. TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. J Immunol. 2015;194:1169–1177. doi: 10.4049/jimmunol.1302676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wynn JL, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strunk T, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis. 2014;14:751–762. doi: 10.1016/S1473-3099(14)70710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kronforst KD, et al. A neonatal model of intravenous Staphylococcus epidermidis infection in mice <24 h old enables characterization of early innate immune responses. PLoS ONE. 2012;7:e43897. doi: 10.1371/journal.pone.0043897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Storm W. Transient bacteremia following endotracheal suctioning in ventilated newborns. Pediatrics. 1980;65:487–490. [PubMed] [Google Scholar]
- 101.LeFrock JL, et al. Transient bacteremia associated with nasotracheal suctioning. JAMA. 1976;236:1610–1611. [PubMed] [Google Scholar]
- 102.Chang BA, et al. Early inflammation in the absence of overt infection in preterm neonates exposed to intensive care. Cytokine. 2011;56:621–626. doi: 10.1016/j.cyto.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chau V, et al. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41:83–103. doi: 10.1016/j.clp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 104.Gentile LF, et al. Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol. 2014;192:3156–3165. doi: 10.4049/jimmunol.1301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gentile LF, et al. Host responses to sepsis vary in different low-lethality murine models. PLoS ONE. 2014;9:e94404. doi: 10.1371/journal.pone.0094404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Efron PA. The future of murine sepsis and trauma research models. J Leukoc Biol. 2015;98:945–952. doi: 10.1189/jlb.5MR0315-127R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cernada M, et al. Genome-wide expression profiles in very low birth weight infants with neonatal sepsis. Pediatrics. 2014;133:e1203–e1211. doi: 10.1542/peds.2013-2552. [DOI] [PubMed] [Google Scholar]
- 108.O’Gorman WE, et al. Single-cell systems-level analysis of human Toll-like receptor activation defines a chemokine signature in patients with systemic lupus erythematosus. J Allergy Clin Immunol. 2015;136:1326–1336. doi: 10.1016/j.jaci.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu L, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 110.Saeed S, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jimenez-Truque N, et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics. 2012;129:e1252–e1259. doi: 10.1542/peds.2011-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kallapur SG, et al. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J Immunol. 2011;187:2688–2695. doi: 10.4049/jimmunol.1100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kramer BW, et al. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. 302. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]