Abstract
Summary
A procedure for creating a simplified version of fracture risk assessment tool (FRAX®) is described. Calibration, fracture prediction, and concordance were compared with the full FRAX tool using two large, complementary Canadian datasets.
Introduction
The Canadian Association of Radiologists and Osteoporosis Canada (CAROC) system for fracture risk assessment is based upon sex, age, bone mineral density (BMD), prior fragility fracture, and glucocorticoid use. CAROC does not require computer or web access, and categorizes 10-year major osteoporotic fracture risk as low (<10%), moderate (10–20%), or high (>20%).
Methods
Basal CAROC fracture risk tables (by age, sex, and femoral neck BMD) were constructed from Canadian FRAX probabilities for major osteoporotic fractures (adjusted for prevalent clinical risk factors). We assessed categorization and fracture prediction with the updated CAROC system in the CaMos and Manitoba BMD cohorts.
Results
The new CAROC system demonstrated high concordance with the Canadian FRAX tool for risk category in both the CaMos and Manitoba cohorts (89% and 88%). Ten-year fracture outcomes in CaMos and Manitoba BMD cohorts showed good discrimination and calibration for both CAROC (6.1–6.5% in low-risk, 13.5–14.6% in moderate-risk, and 22.3–29.1% in high-risk individuals) and FRAX (6.1–6.6% in low-risk, 14.4–16.1% in moderate-risk, and 23.4–31.0% in high-risk individuals). Reclassification from the CAROC risk category to a different risk category under FRAX occurred in <5% for low-risk, 20–24% for moderate-risk, and 27–30% for high-risk individuals. Reclassified individuals had 10-year fracture outcomes that were still within or close to the original nominal-risk range..
Conclusion
The new CAROC system is well calibrated to the Canadian population and shows a high degree of concordance with the Canadian FRAX tool. The CAROC system provides s a simple alternative when it is not feasible to use the full Canadian FRAX tool.
Keywords: Bone mineral density, Canada, CAROC, Fracture risk prediction, FRAX, Osteoporosis
Introduction
In the absence of a defining fracture, the diagnosis of osteoporosis is based on the measurement of bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA). The World Health Organization has provided an operational definition of osteoporosis as a BMD that lies 2.5 standard deviations or more below the average mean value for young healthy women (T-score≤−2.5 standard deviations (SD)) based upon a standardized reference site (the femoral neck) and reference population (the NHANES III data for women aged 20–29 years) [1–3].
Although reduced bone mass is an important and easily quantifiable measurement, studies have shown that most fractures occur in individuals with a BMD T-score above the operational threshold [4–6]. Recognizing the limitations of BMD alone, a simple semi-quantitative approach for BMD reporting based upon 10-year major osteoporotic fracture risk prediction was developed in 2005 by the Canadian Association of Radiologists and Osteoporosis Canada (CAROC) [7]. The CAROC system integrates the independent effects of sex, age, BMD (originally based on minimum T-score) and two clinical risk factors (prior fragility fracture and prolonged glucocorticoid use). The original CAROC system used available 10-year fracture probability data from Sweden [8]. These two clinical risk factors (CRF) were supported by evidence that they were strong and consistent predictors of fracture risk; subsequent meta-analyses have confirmed their importance as BMD-independent risk factors [9–11]. The CAROC system has been very popular in Canada with primary care physicians and radiologists since it is intuitive and easily summarized on a pocket card.
The original CAROC system was found to overestimate fracture risk in Canadians [12, 13]. This was attributable to several factors: Sweden has among the highest fracture rates in the world; substitution of minimum T-score for femoral neck T-score gives systematically higher risk estimates; the published Swedish fracture probabilities were for an average individual and included some with the CAROC clinical risk factors which results in “double-counting”; and the published Swedish fracture probabilities were from almost 20 years ago and do not reflect secular decreases in hip fracture rates that have been observed in Canada [14].
Released in 2008, the WHO fracture risk assessment tool (FRAX®) allows for quantitative estimation of individual 10-year major osteoporotic and hip fracture probability [15]. In addition to age, sex, and (optionally) BMD, the following risk factors are considered: prior fragility fracture, body mass index (BMI), prior use of glucocorticoids, secondary osteoporosis, rheumatoid arthritis, a parental history of hip fracture, current cigarette smoking, and alcohol intake of 3 or more units/day. Analyses have confirmed that there is an improvement in fracture prediction in the use of BMD and CRFs together when compared with BMD alone or CRFs alone [16].
A Canadian FRAX tool has recently been developed and calibrated with Canadian hip fracture epidemiology (FRAX web version 3.1) [17]. Although FRAX provides a more complete description of clinical risk factors not included in the CAROC system, there is uncertainty over the incremental benefit of including additional weaker clinical risk factors [18]. Furthermore, using FRAX requires specialized software and/or access to the website. The FRAX software is still not widely available on BMD machines in Canada. The objective of the current study was to update the CAROC risk tables based upon the Canadian FRAX tool, thereby providing a simple alternative in primary care for individuals for do not have access to computer-based FRAX calculations. Classification and fracture prediction were evaluated in two complementary populations, namely the Canadian Multicentre Osteoporosis Study (CaMos) and the Manitoba Bone Density Program cohort. CaMos is a population-based study and therefore provides information on potential intervention rates for the general population. The Manitoba Bone Density Program cohort is a large clinical referral population and therefore provides information on implications for routine clinical practice.
Methods
CaMos cohort
CaMos is an ongoing, prospective cohort study of 9,423 community-dwelling, randomly selected women (6,539) and men (2,884), aged 25 years and older at baseline, living within 50 km of nine Canadian cities (St John’s, Halifax, Quebec City, Kingston, Toronto, Hamilton, Saskatoon, Calgary, and Vancouver) that began cohort recruitment in 1997–1998. CaMos objectives, methodology and sampling framework are described in detail elsewhere [19]. Briefly, data collection at baseline included an extensive interviewer administered questionnaire and a clinical assessment. The questionnaire included socio-demographic information, medical and fracture history, family history, dietary intake, physical activity, and tobacco smoking. Clinical assessments included height, weight, and BMD by DXA. Yearly fracture information are collected by phone interviews or interviews when the participant is due for an extensive follow-up (years 3 (40–60 years old), 5 and 10). All study participants gave written informed consent in accordance with the Helsinki declaration. Ethics approval was granted through McGill University and the appropriate ethics review boards for each participating center.
The present study included women and men aged 50 years and older at baseline with complete data on FRAX risk factors [20]. Weight and height were recorded at the time of the DXA examination. Prior history of osteoporotic fracture after age 50 was assessed from questionnaire, and excluded fractures of the head, hands, ankle, or feet and those due to high trauma. Current smoking (cigarettes, cigars, or pipe) and number of daily alcohol servings were obtained from the CaMos questionnaire. Glucocorticoid use was defined as current and regular use of oral or intravenous glucocorticoids identified by drug codes. Since self-reported rheumatoid arthritis and osteoarthritis are often confused, we required that patients reporting rheumatoid arthritis also indicate treatment with one of the following drug codes: glucocorticoid, methotrexate, hydroxychloroquine, leflunomide, etanercept, or infliximab. All FRAX risk factors were based on baseline measures with the exception of parental hip fracture which was a composite; history of parental hip fracture was used for everyone with year 5 data, whereas history of any parental osteoporotic fracture was used from the baseline questionnaire for those without year 5 data.
Femoral neck BMD was measured by DXA using Hologic or Lunar DPX densitometers, depending on the CaMos regional center. A detailed description of BMD quality control is available elsewhere [21]. Briefly, longitudinal stability was monitored using a spine phantom, local to each center. Lunar data were converted into equivalent Hologic values by standard methods [22]. All densitometers were calibrated at the start of the study and once each year thereafter using a single European Spine Phantom to ensure site-to-site comparability. All measurements were re-analyzed centrally by the same three technicians.
Manitoba cohort
The study population for this historical cohort study consisted of all women and men in the Province of Manitoba, Canada, age 50 years or older at the time of baseline femoral neck DXA. Subjects were required to have medical coverage from Manitoba Health during the observation period ending March 2008 without other exclusions. For those with more than one eligible set of measurements, only the first record was included. The study was approved by the Research Ethics Board for the University of Manitoba and the Health Information Privacy Committee of Manitoba.
Fractures can be assessed in Manitoba through a combination of hospital discharge abstracts (diagnoses and procedures coded using the ICD-9-CM and ICD-10-CA systems) and physician billing claims (in-patient, out-patient, and office-based) [23]. Use of systemic glucocorticoids was obtained by linkage to the provincial Drug Program Information Network database with drugs classified according to the Anatomical Therapeutic Chemical system of the WHO [24]. The pharmacy database is accurate both for capture of drug dispensations as well as the prescription details [25]. Prolonged glucocorticoid use was defined as over 90 days dispensed in the year prior to DXA testing at a mean prednisone-equivalent dose of 7.5 mg per day or greater. Longitudinal health service records were assessed for the presence of hip, clinical vertebral, forearm, and humerus fracture codes (collectively designated as “osteoporotic”) before and after BMD testing that were not associated with trauma codes [26]. We required that hip fractures and forearm fractures be accompanied by a site-specific fracture reduction, fixation, or casting code which enhances the diagnostic and temporal specificity for an acute fracture. Prior fragility fracture was taken be a fracture prior to BMD testing based upon the previous definition. A diagnosis of rheumatoid arthritis testing was taken from physician office visits and/or hospitalizations with a compatible ICD-9-CM/ICD-10-CA code in a 3-year period prior to BMD testing. Parental hip fracture information was only collected in the last 2 years (2005 and onwards) and was therefore missing for earlier cases. Proxies were used for smoking (chronic obstructive pulmonary disease (COPD) diagnosis) and high alcohol intake (alcohol or substance abuse diagnosis). Weight and height were recorded at the time of the DXA examination (prior to 2000, this was by self-report and starting in 2000 height was assessed with a wall-mounted stadiometer and weight was assessed without shoes using a standard floor scale).
Bone density testing with DXA has been available in Manitoba since 1990 and managed as an integrated program since 1997 using targeted case-finding and standard criteria as previously published [27, 28]. The program maintains a database of all DXA results which can be linked with other population-based computerized health databases through an anonymous personal identifier [29]. The DXA database has been previously described with completeness and accuracy in excess of 99%. DXA scans were performed and analyzed in accordance with manufacturer recommendations. Prior to 2000, DXA measurements were performed with a pencil-beam instrument (Lunar DPX, GE Lunar, Madison WI) and after this date a fan-beam instrument was used (Lunar Prodigy, GE Lunar, Madison WI). Instruments were cross-calibrated using anthropomorphic phantoms and 59 volunteers. No clinically significant differences were identified (femoral neck T-score differences <0.2). Therefore all analyses are based upon the unadjusted numerical results provided by the instrument. Densitometers showed stable long-term performance and satisfactory in vivo precision [30].
CAROC system
Under the original CAROC system [7], each subject was assigned a basal fracture risk category (low risk <10%, moderate risk 10–20%, and high risk >20%) based upon BMD, sex and age using 10-year fracture probability risk tables from Sweden [8]. The presence of prior fragility fractures and/or prolonged systemic glucocorticoid substantially elevates fracture risk. This was operationalized under the CAROC system by increasing the risk categorization to the next level: from low risk to moderate risk, or from moderate risk to high risk. When both factors were present (i.e., prior fragility fractures and prolonged systemic glucocorticoid use), the patient was considered to be at high fracture risk regardless of the BMD result.
To update the CAROC risk tables, we used the sex-specific major osteoporotic fracture probability tables for the Canadian FRAX tool calibrated by the WHO Coordinating Centre from 2005 national hip fracture epidemiology [17]. These risk tables give 10 year fracture probabilities according to BMD (femoral neck T-score from −4.0 to +1.0 in 0.5 SD increments) and age (from 50 to 90 in 5 year increments). The FRAX basal risk tables (i.e., without additional clinical risk factors) would slightly underestimate fracture risk under the CAROC system since there are additional FRAX risk factors (rheumatoid arthritis, parental history of hip fracture, current cigarette smoking, and alcohol intake of 3 or more units/day) that contribute to higher fracture risk. To adjust for the effect of these additional FRAX clinical risk factors which are not part of the CAROC formulation, we derived sex-and age-specific factors from the Manitoba cohort that reflect their average effects. Firstly, the basal FRAX risk tables were bilinearly interpolated to derive intermediate ages (nearest year) and T-scores (0.1 SD steps), and a risk probability (FRAXbasal) was determined for every person in the Manitoba cohort. Secondly, that person’s FRAX probability (FRAXCAROC) was computed without prior fragility fracture or prolonged glucocorticoid use (i.e., both responses set to “no”) but including all other FRAX risk factors as originally defined. The average ratio FRAXCAROC/FRAXbasal was computed for 5-year age groupings in men (range, 1.03 to 1.12) and women (range, 1.03 to 1.10), and reflects the additional effect of the non-CAROC risk factors on fracture probability. Finally, values in the FRAX basal risk tables (i.e., without additional clinical risk factors) were multiplied by the corresponding ratio. Sex-and age-specific cutoffs corresponding to fracture probabilities of 10% and 20% (the cutoffs that categorize individuals into low, moderate or high fracture risk under CAROC) were identified and defined the updated CAROC basal risk values (Table 1). Additional details are available on request from the authors.
Table 1.
CAROC basal risk tables for women and men based upon femoral neck T-scores (female white NHANES III). Missing values indicate no suitable cutoff
| Age (years) | Women
|
Men
|
||
|---|---|---|---|---|
| 10% cutoffa | 20% cutoffa | 10% cutoffa | 20% cutoffa | |
| 50 | −3.4 | – | −3.2 | – |
| 55 | −2.9 | – | −2.9 | −3.9 |
| 60 | −2.3 | −3.7 | −2.5 | −3.7 |
| 65 | −1.9 | −3.5 | −2.4 | −3.7 |
| 70 | −1.7 | −3.2 | −2.3 | −3.7 |
| 75 | −1.2 | −2.9 | −2.3 | −3.8 |
| 80 | −0.5 | −2.6 | −2.1 | −3.8 |
| 85 | 0.1 | −2.2 | −2.0 | – |
| 90 | −0.1 | −2.5 | −2.9 | – |
A value above the 10% cutoff indicates low risk; a value below the 20% cutoff indicates high risk; and intermediate values indicate moderate risk
For each individual in the CaMos and Manitoba cohorts, the CAROC risk category was then calculated based upon the category from the basal CAROC risk table with a risk category increase for each of the two CAROC clinical risk factors (prior fragility fracture and prolonged glucocorticoid use). For comparison purposes, we also categorized major osteoporotic fracture probability from the full Canadian FRAX tool for all individuals in the CaMos and Manitoba cohorts using the same criteria (low <10%, moderate risk 10–20%, and high risk >20%).
Statistics
Descriptive statistics for demographic and baseline characteristics are presented as mean ± SD for continuous variables or count (percent) for categorical variables. The CAROC risk category was compared with the FRAX risk category computed for each individual from the Canadian FRAX model. Concordance in risk category designation overall and stratified by the number of CAROC clinical risk factors was computed by summing the diagonal elements and dividing by the number of subjects. Observed 10-year major osteoporotic fractures (hip, forearm, humerus, clinical spine) rates and risk reclassification were then studied in relation to the three CAROC categories according to the method of Janes et al. [31]. Ten-year estimates of osteoporotic fracture were derived using the Kaplan–Meier method with observations censored at end of follow-up or 10 years (whichever came sooner) and death treated as a competing hazard. Separate analyses were performed for both cohorts. Statistical analyses were performed with Statistica (version 7.0, StatSoft Inc, Tulsa, OK).
Results
The CaMos cohort included 6,697 subjects (4,778 women and 1,919 men) age 50 years old or greater. The Manitoba cohort included 39,603 subjects (36,730 women and 2,873 men). Baseline characteristics are summarized in Table 2. Differences between the two cohorts are consistent with expected clinical referral bias in the Manitoba cohort, particularly among men. Men included in the Manitoba cohort were on average 3 years older than CaMos men and had lower femoral neck T-scores, while women had similar age and femoral neck T-score. CaMos women and men had a lower prevalence of prior fracture, parental hip fracture, rheumatoid arthritis and glucocorticoid use than the Manitoba cohort. Differences in the prevalence of smoking and high alcohol intake were relatively small and potentially related to the use of proxy measures (COPD and substance abuse diagnosis) in the Manitoba cohort.
Table 2.
Baseline characteristics of the CaMos and Manitoba cohorts
| CaMos
|
Manitoba
|
|||
|---|---|---|---|---|
| Women (N=4,778) | Men (N=1,919) | Women (N=36,730) | Men (N=2,873) | |
| Age | 65.8±8.8 | 65.3±9.1 | 65.7±9.8 | 68.2±10.1 |
| BMI | 27.1±4.9 | 27.3±3.8 | 26.8±5.2 | 27.1±4.4 |
| Prior fragility fracture | 11.3% | 4.9% | 13.6% | 15.0% |
| Parental hip fracture | 8.3% | 5.8% | 13.2%a | 10.6%a |
| Rheumatoid arthritis | 0.9% | 0.3% | 3.6% | 7.6% |
| Current/recent glucocorticoid use | 1.4% | 1.4% | 4.2% | 22.1% |
| Current smoking (or proxy) | 13.3% | 17.8% | 8.0% | 18.1% |
| Alcohol use, ≥3 units (or proxy) | 0.9% | 6.8% | 2.4% | 4.2% |
| Femoral neck T-score (white female) | −1.5±1.1 | −0.5±1.2 | −1.5±1.0 | −1.2±1.1 |
Date are mean ± SD or percent
For 2005–2007 (N=8439 women, 814 men).
The CAROC system demonstrated a high level of concordance with Canadian FRAX risk category in both cohorts with overall concordance of 89% in CaMos (Table 3) and 88% in the Manitoba cohort (Table 4). Concordance was highest in individuals without prior fragility fractures and without prolonged glucocorticoid use (92% and 93%), followed by those with both clinical risk factors (100% and 76%), and was lowest for those with a single clinical risk factor (61% and 74%). Reclassification of the CAROC low-risk category to a different risk category under the Canadian FRAX system (i.e., the proportion of the low-risk category in CAROC reclassified to moderate or high risk under FRAX) was less than 5% in both CaMos (Table 5) and the Manitoba cohort (Table 6). Reclassification of the CAROC moderate-risk category under FRAX (to low or high) was seen in 24.4% of the participants in CaMos and 19.5% in the Manitoba cohort. For the CAROC high-risk category, reclassification (to low or moderate) was seen in 29.9% and 27.0%, respectively. However, virtually all of the reclassification was to an adjacent risk category (only a single individual changed two categories, from high to low).
Table 3.
CaMos concordance in risk stratification for Canadian FRAX versus CAROC for 10 year major osteoporotic fracture
| CAROC | Canadian FRAX
|
|||
|---|---|---|---|---|
| Low (<10%) | Moderate (10–20%) | High (>20%) | Total | |
| All subjects | ||||
| Low (<10%) | 4,295 (64) | 191 (3) | 0 (0) | 4,486 (67) |
| Moderate (10–20%) | 312 (5) | 1,169 (17) | 65 (1) | 1,546 (23) |
| High (>20%) | 0 (0) | 199 (3) | 466 (7) | 665 (10) |
| Total | 4,607 (69) | 1,559 (23) | 531 (8) | 6,697 (100) |
| Concordance | 5,930 (89) | |||
| No-risk factors | ||||
| Low (<10%) | 4,295 (72) | 191 (3) | 0 (0) | 4,486 (75) |
| Moderate (10–20%) | 205 (3) | 981 (16) | 47 (1) | 1,233 (21) |
| High (>20%) | 0 (0) | 55 (1) | 213 (4) | 268 (4) |
| Total | 4,500 (75) | 1,227 (20) | 260 (4) | 5,987 (100) |
| Concordance | 5,489 (92) | |||
| One-risk factors | ||||
| Low (<10%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderate (10–20%) | 107 (16) | 188 (27) | 18 (3) | 313 (45) |
| High (>20%) | 0 (0) | 143 (21) | 234 (34) | 377 (55) |
| Total | 107 (16) | 331 (48) | 252 (37) | 690 (100) |
| Concordance | 422 (61) | |||
| Two-risk factors | ||||
| Low (<10%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderate (10–20%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| High (>20%) | 0 (0) | 0 (0) | 20 (100) | 20 (100) |
| Total | 0 (0) | 0 (0) | 20 (100) | 20 (100) |
| Concordance | 20 (100) | |||
Results are stratified by the number of additional clinical risk factors (prior fragility fracture and prolonged glucocorticoid use)
Table 4.
Manitoba concordance in risk stratification for Canadian FRAX versus CAROC for 10-year major osteoporotic fracture
| CAROC | Canadian FRAX
|
|||
|---|---|---|---|---|
| Low (<10%) | Moderate (10–20%) | High (>20%) | Total | |
| All subjects | ||||
| Low (<10%) | 20,857 (53) | 889 (2) | 0 (0) | 21,746 (55) |
| Moderate (10–20%) | 2,028 (30) | 9,894 (25) | 364 (1) | 12,286 (31) |
| High (>20%) | 5 (0) | 1,497 (4) | 4,069 (10) | 5,571 (14) |
| Total | 22,890 (342) | 12,280 (31) | 4,433 (11) | 39,603 (100) |
| Concordance | 34,820 (88) | |||
| No-risk factors | ||||
| Low (<10%) | 20,857 (65) | 889 (3) | 0 (0) | 21,746 (67) |
| Moderate (10–20%) | 704 (12) | 7,684 (24) | 306 (1) | 8,694 (27) |
| High (>20%) | 0 (0) | 350 (1) | 1,464 (5) | 1,814 (6) |
| Total | 21,561 (360) | 8,923 (28) | 1,770 (5) | 32,254 (100) |
| Concordance | 30,005 (93) | |||
| One-risk factors | ||||
| Low (<10%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderate (10–20%) | 1,325 (19) | 2,927 (41) | 170 (2) | 4,422 (62) |
| High (>20%) | 0 (0) | 375 (5) | 2,310 (33) | 2,685 (38) |
| Total | 1,325 (19) | 3,302 (46) | 2,480 (35) | 7,107 (100) |
| Concordance | 5,237 (74) | |||
| Two-risk factors | ||||
| Low (<10%) | 0 (0) | 0 (0) | 4 (2) | 4 (2) |
| Moderate (10–20%) | 0 (0) | 0 (0) | 55 (23) | 55 (23) |
| High (>20%) | 0 (0) | 0 (0) | 183 (76) | 183 (76) |
| Total | 0 (0) | 0 (0) | 242 (100) | 242 (100) |
| Concordance | 183 (76) | |||
Results are stratified by the number of additional clinical risk factors (prior fragility fracture and prolonged glucocorticoid use)
Table 5.
CaMos risk reclassification for Canadian FRAX versus CAROC for 10-year major osteoporotic fracture
| CAROC | Canadian FRAX
|
|||
|---|---|---|---|---|
| Total | Low (<10%) | Moderate (10–20%) | High (>20%) | |
| Low (<10%) | ||||
| N fracture | 253 | 233 | 20 | 0 |
| N total | 4,486 | 4,295 | 191 | 0 |
| Percent fracture | 5.6% | 5.4% | 10.5% | N/A |
| Percent 10-year fracture outcomesa | 6.1% | 5.9% | 10.9% | N/A |
| Total reclassified | 4.3% | – | 4.3% | 0.0% |
| Moderate (10–20%) | ||||
| N fracture | 192 | 26 | 154 | 12 |
| N total | 1,546 | 312 | 1,169 | 65 |
| Percent fracture | 12.4% | 8.3% | 13.2% | 18.5% |
| Percent 10-year fracture outcomesa | 13.5% | 9.2% | 14.3% | 19.9% |
| Total reclassified | 24.4% | 20.2% | – | 4.2% |
| High (>20%) | ||||
| N fracture | 136 | 0 | 34 | 102 |
| N total | 665 | 0 | 199 | 466 |
| Percent fracture | 20.5% | N/A | 17.1% | 21.9% |
| Percent 10-year fracture outcomesa | 22.3% | N/A | 18.4% | 24.0% |
| Total reclassified | 29.9% | 0.0% | 29.9% | – |
| Total | ||||
| N fracture | 581 | 259 | 208 | 114 |
| N total | 6,697 | 4,607 | 1,559 | 531 |
| Percent fracture | 8.7% | 5.6% | 13.3% | 21.5% |
| Percent 10-year fracture outcomesa | 9.3% | 6.1% | 14.4% | 23.4% |
Kaplan–Meier estimate
Table 6.
Manitoba risk reclassification for Canadian FRAX versus CAROC for 10-year major osteoporotic fracture
| CAROC | Canadian FRAX
|
|||
|---|---|---|---|---|
| Total | Low (<10%) | Moderate (10–20%) | High (>20%) | |
| Low (<10%) | ||||
| N fracture | 21,746 | 20,857 | 889 | 0 |
| N total | 731 | 690 | 41 | 0 |
| Percent 10-year fracture outcomesa | 6.5% | 6.3% | 14.3% | N/A |
| Total reclassified | 4.1% | – | 4.1% | 0.0% |
| Moderate (10–20%) | ||||
| N fracture | 12,286 | 2,028 | 9,894 | 364 |
| N total | 937 | 106 | 807 | 24 |
| Percent 10-year fracture outcomesa | 14.6% | 9.0% | 15.5% | 14.3% |
| Total reclassified | 19.5% | 16.5% | – | 3.0% |
| High (>20%) | ||||
| N fracture | 5,571 | 5 | 1,497 | 4,069 |
| N total | 875 | 1 | 157 | 717 |
| Percent 10-year fracture outcomesa | 29.1% | N/A | 21.4% | 31.9% |
| Total reclassified | 27.0% | 0.1% | 26.9% | – |
| Total | ||||
| N fracture | 39,603 | 22,890 | 12,280 | 4,433 |
| N total | 2,543 | 797 | 1,005 | 741 |
| Percent 10-year fracture outcomesa | 12.0% | 6.6% | 16.1% | 31.0% |
Kaplan–Meier estimate
In a well-calibrated model, 10-year fracture risk would be expected to be below 10% for low-risk individuals, close to 15% for moderate-risk individuals, and above 20% for high-risk individuals. In Tables 5 and 6, 10-year fracture outcomes (Kaplan–Meier estimates) in both study cohorts showed good calibration for both CAROC (6.1–6.5% in low-risk, 13.5–14.6% in moderate-risk, and 22.3–29.1% in high-risk individuals) and Canadian FRAX (6.1–6.6% in low-risk, 14.4–16.1% in moderate-risk, and 23.4–31.0% in high-risk individuals). Both systems generated fracture rates that were well within the nominal-risk ranges for both cohorts. Furthermore, reclassified individuals had fracture outcomes that were still within or close to the original nominal-risk ranges. For example, when the CAROC moderate-risk category was reclassified under Canadian FRAX, the fracture outcomes at 10 years were 9.0–9.2% in those reclassified to low risk and 14.3–19.9% in those reclassified to high risk. Conversely, when the moderate-risk category under Canadian FRAX was reclassified under CAROC, the 10 year fracture outcomes were 10.9–14.3% in those reclassified to low risk and 18.4–21.4% in those reclassified to high risk.
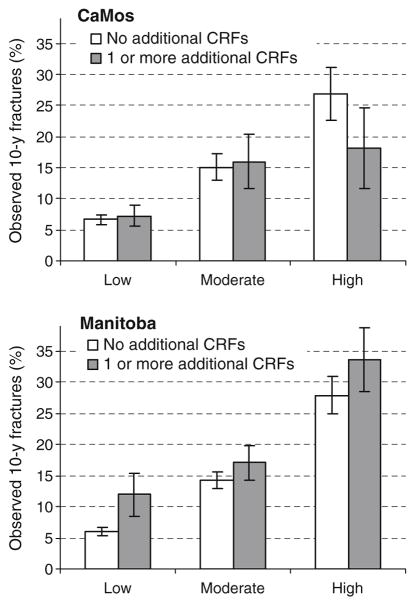
The study populations were further stratified according to the presence or absence of FRAX clinical risk factors that do not contribute to CAROC (i.e., parental history of hip fracture, smoking (or COPD), excess alcohol intake [or substance abuse diagnosis], rheumatoid arthritis). One or more additional FRAX risk factors were identified in 23% of the CaMos cohort and 17% of the Manitoba cohort. When there were no additional FRAX risk factors, 90% concordance was seen between the CAROC and Canadian FRAX risk categories in both the CaMos and Manitoba cohorts. Slightly lower concordance was seen when there were additional FRAX risk factors: 81% in the CaMos and 77% in the Manitoba cohorts. Observed 10 year fracture outcomes showed a stepwise increase according to the CAROC risk category (Fig. 1). In the Manitoba cohort, the subgroup categorized as low risk under CAROC but with additional FRAX risk factors was at higher risk than those without additional FRAX risk factors but no difference was seen for the corresponding CaMos subgroups. For subgroups categorized as moderate or high risk under CAROC, the observed 10-year fracture outcomes stratified according to presence or absence of additional FRAX risk factors were similar.
Fig. 1.
Observed 10-year major osteoporotic fracture outcomes by CAROC risk category according to the absence (white bar) or presence (gray bar) of one or more additional FRAX clinical risk factors (CRFs). 95% CI bars are shown
Discussion
The updated CAROC risk system shows good calibration and discrimination in two independent Canadian cohorts, one a population-based cohort and the other representative of patients seen in clinical practice. Moreover, these risk categories agreed closely with risk categories derived from the Canadian FRAX tool. No major benefit in using the full Canadian FRAX system over CAROC was evident, and reclassified individuals had 10-year fracture outcomes that were still within or close to the original nominal-risk range. Therefore, CAROC provides a simple tool for assessing fracture risk in primary care. FRAX is based upon a more complete set of clinical risk factors, and offers greater versatility since it allows for risk assessment in the absence of a BMD measurement and is more quantitatively accurate for those patients with one of more of the risk factors that contribute to FRAX but not to CAROC (i.e., parental history of hip fracture, smoking, excess alcohol intake, rheumatoid arthritis). The two-risk assessment tools are therefore seen as complementary versions of the same system, and the choice of using Canadian FRAX or CAROC is largely a matter of personal preference and convenience.
In the absence of a Canadian FRAX tool, Swedish fracture data have previously been used for estimating fracture risk in Canada [32]. This is not optimal because of possible miscalibration due to known international variation in fracture risk and the fact that Sweden has among the highest fracture rates in the world [33, 34]. We confirmed that the derivation of the original CAROC risk tables from Swedish data resulted in thresholds which overestimated fracture risk among Canadians [7]. The risk cutoffs for men and women from the updated CAROC system are substantially lower than the original CAROC cutoffs for many sex and age combinations (notably women 50–75 years and men 70–85 years). For example, at age 65 a women without other risk factors would need a femoral neck T-score of −1.9 or lower to be at moderate risk and −3.5 or lower to be at high risk. This compares with the original CAROC cutoffs −1.0 and −2.7. The change is attributable to the use of 2005 Canadian fracture data for construction of the updated CAROC risk tables, whereas the original CAROC tables used published Swedish fracture probabilities [8]. The published Swedish fracture probabilities were from almost 20 years ago and do not reflect secular decreases in hip fracture rates that have been observed in Canada [14].
FRAX was created as a quantitative fracture risk assessment tool and is available free of charge on the internet at www.shef.ac.uk/FRAX and more recently as an iPhone application [20]. It was derived from nine prospective cohorts (which included 5-year data from CaMos) and was validated in 11 different population-based prospective cohorts from Europe, the USA, Australia, and Japan [16]. Recent work has validated calibration and discrimination of the Canadian FRAX tool using 10-year CaMos data and the Manitoba Bone Density Program [35, 36]. Our study does not indicate that the additional clinical risk factors used in FRAX are unimportant, as the derivation meta-analyses and a recent report work in the Manitoba cohort show independent contributions to fracture risk prediction in individuals with those risk factors [15, 16, 36]. However, the additional benefit is relatively small and therefore not readily apparent when averaged across groups of individuals.
Strengths of this investigation include the use of two independent Canadian cohorts. Similar findings in both population-based and clinical referral populations increase confidence in the robustness of the findings. These two cohorts are complementary and used different data sources, slightly different risk factor definitions and methods for fracture ascertainment. Hence, limitations of one population may not apply to the other population. In the Manitoba cohort, proxies were used for two of the risk factors utilized to generate the FRAX probabilities: COPD diagnosis instead of smoking, alcohol or substance abuse diagnosis for high alcohol intake (more than 2 units of alcohol per day). As a result, patients with these risk factors in the Manitoba cohort are likely to have greater exposure than the CaMos participants with these risk factors. In the CaMos cohort, a definition was used for rheumatoid arthritis that would only include those on disease-modifying treatment. The parental hip fracture variable was a composite construction in the CaMos cohort and partially missing in the Manitoba cohort. Although the current analysis was particular to the Canadian FRAX tool and population, the procedure outlined for creating a simplified risk assessment system should be generalizable to other FRAX tools.
In conclusion, the updated CAROC system is well calibrated in terms of osteoporotic fracture outcomes and shows a high overall degree of concordance with the full FRAX system. The full Canadian FRAX system provided little additional fracture risk stratification compared with the simplified CAROC system. However, FRAX is based upon a more complete assessment of risk factors (including some associated with secondary osteoporosis) and is therefore preferred to the CAROC system for fracture risk assessment. The simplified CAROC system is an acceptable alternative in Canada where it is not possible to adopt the full Canadian FRAX system.
Acknowledgments
We would like to thank Ms. Helena Johansson and Dr. John Kanis for their generating the Canadian FRAX results for both the CaMos and Manitoba cohorts. We thank all those participants in CaMos whose careful responses and attendance made this analysis possible. The authors are indebted to Manitoba Health for the provision of data (HIPC File No. 2007/2008–49). The results and conclusions are those of the authors, and no official edndorsement by Manitoba Health is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee. The analyses and conclusions in this report reflect the opinions of individual experts and not thier affiliated organizations.
Source of funding: The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research (CIHR), Merck Frosst Canada Ltd., Eli Lilly Canada Inc., Novartis Pharmaceuticals Inc., The Alliance for Better Bone Health: Sanofi-Aventis, Procter & Gamble Pharmaceuticals Canada Inc., Amgen, The Dairy Farmers of Canada and The Arthritis Society.
Footnotes
CaMos Research Group: David Goltzman (co-principal investigator, McGill University, Montreal), Nancy Kreiger (co-principal investigator, University of Toronto, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto), CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Claudie Berger (study statistician). Memorial University, St. John’s New-foundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator). Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator). Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator). Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator). University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator) McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator). University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator). University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator). University of British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Millan Patel (co-director), Brian Lentle (radiologist), Yvette Vigna (coordinator).
Conflicts of interest: William Leslie is part of a speaker bureau for Merck Frosst and Amgen. He has also received unrestricted educational and/or research grants from Amgen; Merck Frosst; sanofi-Aventis; Procter & Gamble; Genzyme and is a member of the following advisory boards: Genzyme; Novartis; and Amgen. Lisa Lix received an unrestricted research grant from Amgen. In the past 3 years, Eugene McCloskey has received speaker fees and/or unrestricted research grants from Novartis, Amgen, AstraZeneca, Pfizer, Bayer, Procter & Gamble, Lilly, Roche, Servier, and Hologic.
Contributor Information
W. D. Leslie, Department of Medicine, University of Manitoba, Winnipeg, MB, Canada. Department of Medicine (C5121), St. Boniface General Hospital, 409 Tache Avenue, Winnipeg, MB, CanadaR2H 2A6
C. Berger, CaMos National Coordinating Centre, McGill University, Montreal, QC, Canada
L. Langsetmo, CaMos National Coordinating Centre, McGill University, Montreal, QC, Canada
L. M. Lix, School of Public Health, University of Saskatchewan, Saskatoon, SK, Canada
J. D. Adachi, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada
D. A. Hanley, Departments of Medicine and Community Health Sciences, University of Calgary, Calgary, AB, Canada
G. Ioannidis, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada
R. G. Josse, Department of Medicine, University of Toronto, Toronto, ON, Canada
C. S. Kovacs, Discipline of Medicine, Memorial University, St. John’s, Newfoundland, Canada
T. Towheed, Department of Medicine, Queen’s University, Kingston, ON, Canada
S. Kaiser, Department of Medicine, Dalhousie University, Halifax, NS, Canada
W. P. Olszynski, Department of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
J. C. Prior, Department of Medicine and Endocrinology, University of British Columbia, Vancouver, BC, Canada
S. Jamal, Department of Medicine, University of Toronto, Toronto, ON, Canada
N. Kreiger, School of Public Health, University of Saskatchewan, Saskatoon, SK, Canada. Department of Public Health Sciences, University of Toronto, Toronto, ON, Canada
D. Goltzman, School of Public Health, University of Saskatchewan, Saskatoon, SK, Canada. Department of Medicine, McGill University, Montreal, QC, Canada
References
- 1.Kanis JA, Melton LJ, III, Christiansen C, et al. The diagnosis of osteoporosis [see comments] J Bone Miner Res. 1994;9:1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42:467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–12. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 5.Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575–80. doi: 10.1503/cmaj.070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langsetmo L, Goltzman D, Kovacs CS, et al. Repeat low-trauma fractures occur frequently among men and women who have osteopenic BMD. J Bone Miner Res. 2009;24:1515–22. doi: 10.1359/jbmr.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada: a shift to absolute fracture risk assessment. J Clin Densitom. 2007;10:120–3. doi: 10.1016/j.jocd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Johnell O, Oden A, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 11.Adachi JD, Olszynski WP, Hanley DA, et al. Management of corticosteroid-induced osteoporosis. Semin Arthritis Rheum. 2000;29:228–51. doi: 10.1016/s0049-0172(00)80011-6. [DOI] [PubMed] [Google Scholar]
- 12.Leslie WD, Tsang JF, Lix LM. Simplified system for absolute fracture risk assessment: clinical validation in Canadian women. J Bone Miner Res. 2009;24:353–60. doi: 10.1359/jbmr.081012. [DOI] [PubMed] [Google Scholar]
- 13.Leslie WD, Lix LM. Simplified 10-year absolute fracture risk assessment: a comparison of men and women. J Clin Densitom. 2010;13:141–6. doi: 10.1016/j.jocd.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Leslie WD, O’Donnell S, Jean S, et al. Trends in hip fracture rates in Canada. JAMA. 2009;302:883–9. doi: 10.1001/jama.2009.1231. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Oden A, Johansson H, et al. FRAX and its applications to clinical practice. Bone. 2009;44:734–43. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 17.Leslie WD, Lix D, Langsetmo L, et al. Construction of a FRAX model for the assessment of fracture probability in Canada and implications for treatment. Osteoporis Int. 2010 doi: 10.1007/s00198-010-1464-2. in press. [DOI] [PubMed]
- 18.Chen P, Krege JH, Adachi JD, et al. Vertebral fracture status and the World Health Organization risk factors for predicting osteoporotic fracture risk. J Bone Miner Res. 2009;24:495–502. doi: 10.1359/jbmr.081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiger N, Tenenhouse A, Joseph L, et al. Research notes: the Canadian Multicentre Osteoporosis Study (CaMos) - background, rationale, methods. Can J Aging. 1999;18:376–87. [Google Scholar]
- 20.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger C, Langsetmo L, Joseph L, et al. Association between change in BMD and fragility fracture in women and men. J Bone Miner Res. 2009;24:361–70. doi: 10.1359/jbmr.081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genant HK, Grampp S, Gluer CC, et al. Universal standardization for dual x-ray absorptiometry: Patient and phantom cross-calibration results. J Bone Miner Res. 1994;9:1503–14. doi: 10.1002/jbmr.5650091002. [DOI] [PubMed] [Google Scholar]
- 23.Roos NP, Shapiro E. Revisiting the manitoba centre for health policy and evaluation and its population-based health information system. Med Care. 1999;37:JS10–JS14. doi: 10.1097/00005650-199906001-00005. [DOI] [PubMed] [Google Scholar]
- 24.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment. Norwegian Institute for Public Health; Oslo: 2005. [Google Scholar]
- 25.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998;32:1152–7. doi: 10.1345/aph.18117. [DOI] [PubMed] [Google Scholar]
- 26.Leslie WD, Tsang JF, Caetano PA, et al. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab. 2007;92:77–81. doi: 10.1210/jc.2006-1415. [DOI] [PubMed] [Google Scholar]
- 27.Leslie WD, Metge C. Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom. 2003;6:275–82. doi: 10.1385/jcd:6:3:275. [DOI] [PubMed] [Google Scholar]
- 28.Leslie WD, MacWilliam L, Lix L, et al. A population-based study of osteoporosis testing and treatment following introduction of a new bone densitometry service. Osteoporos Int. 2005;16:773–82. doi: 10.1007/s00198-004-1756-5. [DOI] [PubMed] [Google Scholar]
- 29.Leslie WD, Caetano PA, MacWilliam LR, et al. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8:25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 30.Leslie WD. The importance of spectrum bias on bone density monitoring in clinical practice. Bone. 2006;39:361–8. doi: 10.1016/j.bone.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008;149:751–60. doi: 10.7326/0003-4819-149-10-200811180-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J. 2005;56:178–88. [PubMed] [Google Scholar]
- 33.Leslie WD, O’Donnell S, Lagace C, et al. Population-based Canadian hip fracture rates with international comparisons. Osteoporos Int. 2009;21:1317–1322. doi: 10.1007/s00198-009-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanis JA, Johnell O, De Laet C, et al. International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res. 2002;17:1237–44. doi: 10.1359/jbmr.2002.17.7.1237. [DOI] [PubMed] [Google Scholar]
- 35.Fraser L, Langsetmo L, Berger C, et al. Fracture prediction and calibration of a Canadian FRAX tool: a population-based report from CaMos. Osteoporis Int. 2010 doi: 10.1007/s00198-010-1465-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie WD, Lix LM, Johansson H, et al. Independent clinical validation of a Canadian FRAX((R)) tool: Fracture prediction and model calibration. J Bone Miner Res. 2010 doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]