Abstract
P-glycoprotein (Pgp, encoded by ABCB1, commonly known as MDR1), an ATP-dependent transporter with a broad range of hydrophobic drug substrates, has been associated with the in vitro intracellular transport of cholesterol; however, these findings have not been confirmed in vivo. In this manuscript we tested the contributions of Pgp to in vivo cholesterol homeostasis by comparing the cholesterol phenotype of wild type mice with mice lacking both murine isoforms of Pgp (Abcb1a−/−/1b−/−) by measuring cholesterol absorption, circulating cholesterol, and lipoprotein cholesterol profiles. The mice were fed diets containing normal or high levels of dietary fat (25% vs. 45% kcal from fat) and cholesterol (0.02% vs. 0.20% w/w) for 8 weeks to challenge their capacity to maintain homeostasis.
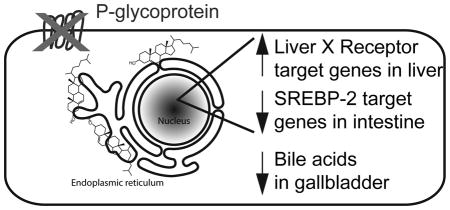
There were no significant differences in cholesterol absorption, circulating cholesterol levels, and lipoprotein profiles between Pgp knockout and wild type mice fed matching diets. Compensatory shifts were observed in the activation of two key transcription factors involved in maintaining cholesterol balance, the Liver X Receptor and SREBP-2, which may have maintained the wild type phenotype in the knockout mice. Deletion of Pgp affected the molar composition of gallbladder bile when the mice were fed diets containing high levels of dietary fat, cholesterol, or both. The mole fraction of bile salts was reduced in the gallbladder bile of Pgp knockout mice while the mole fraction of cholesterol was increased.
In this paper, we provide evidence that Pgp knockout mice maintain cholesterol homeostasis, even when challenged with high cholesterol diets. We suggest that the specific shifts in cholesterol regulatory networks identified in the jejunum and liver of the knockout mice may have compensated for the lack of Pgp. Our finding that Pgp knockout mice were unable to maintain gallbladder bile composition when challenged with high dietary fat and/or cholesterol compliments recent reports that Pgp may be a secondary bile salt export pump.
Keywords: P-glycoprotein, MDR1, cholesterol, bile, bile acids, ATP-binding cassette transporters
Graphical abstract
INTRODUCTION
In vivo cholesterol homeostasis is maintained through the coordinated interplay of trafficking, biosynthesis, absorption, and efflux of cholesterol. These processes contribute to a cholesterol phenotype that can be characterized by measuring fractional cholesterol absorption from the jejunum, serum cholesterol levels, the distribution of cholesterol into specific lipoproteins, and the elimination of cholesterol from the liver into bile. Excess cholesterol is removed from the body by the liver primarily through catabolism into bile acids but also by direct secretion into bile via ATP-binding cassette (ABC) transporters1,2. The dimer formed by the half transporters ABCG5 and ABCG8 is the primary efflux mechanism for unmodified cholesterol from cells of the small intestine and the liver. Deletion of ABCG5 and ABCG8 does not completely ablate cholesterol efflux suggesting that a secondary mechanism for the removal of cholesterol may exist in mice3,4, with confirmatory findings in humans5.
There are several lines of evidence suggesting that the ABC transporter encoded by the ABCB1 gene, P-glycoprotein (Pgp), may be involved in the cellular disposition of cholesterol. Pgp is a 170kDa full ABC transporter with extensive N-linked glycosylation that effluxes a wide range of hydrophobic drugs6,7. Pgp function is intricately linked with membrane cholesterol content, though the details of the association are controversial8,9. Mechanistic in vitro studies demonstrated that Pgp binds cholesterol10 and that the association between cholesterol and Pgp impacted its drug efflux activity8,11. Furthermore, Pgp was proposed to be an ATP-dependent “flippase” of cholesterol, maintaining cholesterol on the extracellular leaflet of the membrane bilayer12. Non-specific Pgp inhibitors disrupt trafficking of cholesterol from the plasma membrane to the endoplasmic reticulum, reducing synthesis and esterification of cholesterol without direct inhibition of ACAT, the enzyme responsible for cholesterol esterification13–17. Similar findings were reported when new inhibitors with improved specificity for Pgp were used18. Two groups have used specific overexpression of ABCB1 as part of their experimental design with contradictory results19,20.
Only two preliminary studies have been published investigating the role of Pgp in cholesterol homeostasis in vivo. Luker et al. showed that Abcb1a−/−/1b−/− knockout mice had unchanged cholesterol absorption but slight alterations to liver accumulation of cholesterol. The authors speculated that diets enriched in fat or cholesterol would be required to elucidate the role of Pgp but these studies were never published21. Our group published a pilot study demonstrating increased Liver X Receptor (LXR) levels in the liver of Abcb1a−/−/1b−/− mice22. In the current manuscript we evaluated the cholesterol phenotype of wild type and Abcb1a−/−/1b−/− mice after chronic feeding of diets enriched in fat and/or cholesterol to determine if Pgp contributes to in vivo cholesterol homeostasis. Although we challenged the mice with the dietary conditions that were predicted to be required to elucidate a role for Pgp in cholesterol balance we did not observe differences in the cholesterol phenotype between wild type and knockout mice, as characterized by fractional cholesterol absorption, serum cholesterol levels, and lipoprotein cholesterol profiles. This was perhaps explained by altered activation of two key cholesterol regulatory pathways. We noted that the Pgp knockout mice were unable to maintain the gallbladder bile composition of wild type mice when fed the high-fat and high-cholesterol diets suggesting that future studies should focus on the impact of diet and Pgp on bile homeostasis.
EXPERIMENTAL METHODS
Ethics Statement
All animal studies were conducted in strict accordance with the guidelines of the Canadian Council on Animal Care in Science. The protocol was reviewed and approved by the University of British Columbia Animal Care Committee (certificate A05-0032) prior to beginning the studies.
In vivo studies
All experiments compared male wild type FVB mice (WT) with male mice lacking both isoforms of P-glycoprotein23 backcrossed for 12 generations on the FVB background (KO: Abcb1a−/−/1b−/−) purchased from Taconic Farms (Hudson, NY). The test diets were developed with the assistance of Research Diets (New Brunswick, NJ). The fat content in the diets was based on the well-established “high” fat diet in which 45% of the kcal are from fat24. Lard was selected to increase the fat content, based on the “Western” pattern diet25.
When the mice were five-weeks old, each mouse was placed on one of the four test diets, (n=10) (NFNC: 25% kcal from fat and 0.02% w/w cholesterol, HFNC: 45% kcal from fat and 0.02% w/w cholesterol, NFHC: 25% kcal from fat and 0.20% w/w cholesterol, or a “Western” diet HFHC: 45% kcal from fat and 0.20% w/w cholesterol). After eight weeks on the test diets26, each mouse received a single oral bolus of either 75μL water or 75μL of ezetimibe solution (10mg/kg dose as a positive control for reduced cholesterol absorption). After 60min each mouse received a second oral bolus of 75μL of soybean oil containing 1μCi of [4-14C]-cholesterol and 2μCi of [5,6-3H]-β-sitostanol. The mice were placed in metabolic-style cages containing shelter and enrichment items for 96h. Total fecal output was collected at 24h, 48h, 72h, and 96h post administration. The mice were then returned to conventional-style housing and recovered for 72–120h. Under anesthetic (ketamine/xylazine) the common bile duct was ligated and the gallbladders filled for 60min prior to sacrifice. Body temperature was maintained with heating pads. The mice were euthanized by cardiac puncture exsanguination and cervical dislocation. Serum was obtained from whole blood. The gallbladder and liver were removed, weighed and washed in PBS and frozen in liquid nitrogen. The small intestine was removed and split into three segments approximating the duodenum, jejunum, and ileum. The medial portion of each segment was cleaned in PBS and frozen in liquid nitrogen.
Chemicals
All solvents were purchased from Fisher Scientific (Waltham, MA) and were of HPLC grade unless otherwise noted. Dry chemicals were purchased from Sigma (St. Louis, MO) and were of SigmaUltra quality unless otherwise noted. The soybean oil was USP grade from Xenex Labs (Coquitlam, BC, Canada), [4-14C]-cholesterol was purchased from GE Healthcare (Waukesha, WI) and [5,6-3H]-β-sitostanol was purchased from American Radiolabeled Chemicals (St. Louis, MO). Scintillation cocktail was sourced from MP Biomedicals (Solon, OH).
Bile analysis
Each gallbladder was thawed and punctured with a 25-gauge needle and syringe to remove aliquots of bile for analysis. Bile phospholipid content was quantified using phospholipase D and choline oxidase from Wako Diagnostics (Richmond, VA). Cholesterol content was measured using the Amplex Red Cholesterol Assay by Life Technologies (Carlsbad, CA). Bile samples from five mice per group were randomly selected and sent to the Harvard Digestive Diseases Center, where the bile acids were extracted and quantified by HPLC27.
Lipid analyses
Total serum cholesterol and unesterified cholesterol was measured with cholesterol oxidase-based colorimetric assays from Wako Diagnostics (Richmond, VA). Tissue lipid concentrations were determined after extracting the total lipid content from 50mg of tissue following the Folch method28. The lipid films were reconstituted in a bile acid buffer solution (100mM potassium phosphate, 5mM cholic acid, 0.1% Triton X-100, pH 7.4). The cholesterol content was determined using the Amplex Red Cholesterol Assay. Serum samples from each dietary group were pooled and sent to Dr. D.E. Cohen’s laboratory at the Harvard Digestive Diseases Center for separation by fast protein liquid chromatography (FPLC) and subsequent enzymatic analysis for cholesterol29.
Fecal analysis
Cholesterol was isolated from the fecal matter using a method based on work by Miettinen, Ahrens and Grundy30 incorporating the modifications published by Turley et al.31 Fecal samples collected from individual mice over a 24h period were dried overnight in a 60°C oven and crushed using a pestle and mortar. The total lipid content was extracted from fecal matter with 19 volumes of 2:1 (v/v) chloroform:methanol at 60°C for 3min. The insoluble material was pelleted by centrifugation (1000g for 5min at 4°C) and the supernatant retained for analysis. The chloroform:methanol extraction was repeated on the insoluble material and the supernatant pooled with the first extraction. Solvent was removed by drying under N2 gas. The lipid films were saponified with 3mL of 1:1 (v/v) methanol:2N NaOH(aq) for 60min in a 60°C water bath. The neutral sterols were isolated with sequential petroleum ether extractions: 3mL of petroleum ether was added to each tube, the tubes were vigorously mixed and the phases separated by centrifugation (1000g for 5min at 4°C). The upper, organic, phase was transferred to a scintillation vial. The petroleum ether extraction was repeated twice more on the aqueous phase. A 300μL aliquot of the final aqueous phase was transferred to a scintillation vial for determination of aqueous radiolabel. The contents of the scintillation vials were dried under N2 prior to addition of scintillation cocktail. The samples were incubated overnight and the radioactivity was quantified by liquid scintillation counting. [14C]-cholesterol in the feces was compared to the dose given as an indirect measurement of cholesterol absorption. The dosing mixture contained a dosing control of [3H]-sitostanol, a derivative of cholesterol that is not absorbed. Cholesterol absorption was calculated using the following equation:
Gene expression studies
The details of the RT-qPCR studies were provided to reviewers according to the MIQE guidelines32 (Supplementary Table 5). Total RNA was extracted from ~50mg of frozen jejunum or liver using TRIzol according to the manufacturer’s instructions. RNA was quantified using RiboGreen by Life Technologies and all samples were diluted to 0.2mg/mL in Tris-EDTA buffer prepared in DEPC-treated water. The reverse transcription reaction was completed using 1.0μg of RNA and a combination of OligodT20 and random hexamers in the SuperScript III First-Strand SuperMix from Life Technologies. The cDNA products were characterized using the OliGreen Assay by Life Technologies and all samples diluted to 2ng/μL. The real-time PCR reactions (3min at 95°C followed by 40 cycles of 95°C for 15sec and 60°C for 1min) were performed on an Applied Biosystems 7900HT real-time thermocycler in standard mode. TaqMan gene expression assays from Life Technologies (Supplementary Table 4) were used with iTaq SuperMix by Bio-Rad (Hercules, CA) with 10ng of cDNA as the template. The semi-quantitative gene expression was calculated using the ΔΔCt method using Actb as the reference gene. There were no changes to Actb Ct values between any treatment groups. LXR activity was determined by measuring the expression of Abcg5, Abcg8 and Abca1 in the jejunum. In the liver, murine Abca1 is not a target of LXR33; therefore, Cyp7a1 was used instead34. SREBP-2 activity was determined by measuring the expression of Ldlr, Hmgcr, and Npc1l1. Hepatic farnesoid X receptor (FXR) activity was probed by measuring the expression of Abcb4, Abcb11, Shp, Abcc2, and Slc27a.
Data analysis
All data sets were analyzed for statistical significance by parametric methods using SigmaStat (version 3.5). An alpha value of 0.05 was selected a priori as the threshold at which the null hypothesis can be rejected for all tests. If the data set did not meet the assumptions required of parametric methods they were Ln-transformed. The data determined the selection of statistical method used. When comparisons were made between two groups, unpaired two-tailed t-tests were used. If there were several groups to compare, a one-way ANOVA was selected. If the data were influenced by two independent factors, for example diet and genotype, then a two-way ANOVA was run. If the ANOVA revealed that the difference in means of the main groups was greater than could be expected by chance (p-value < 0.05), then Tukey post hoc tests were used to determine which groups were statistically different from the others.
RESULTS
Effect of diet on Pgp gene expression
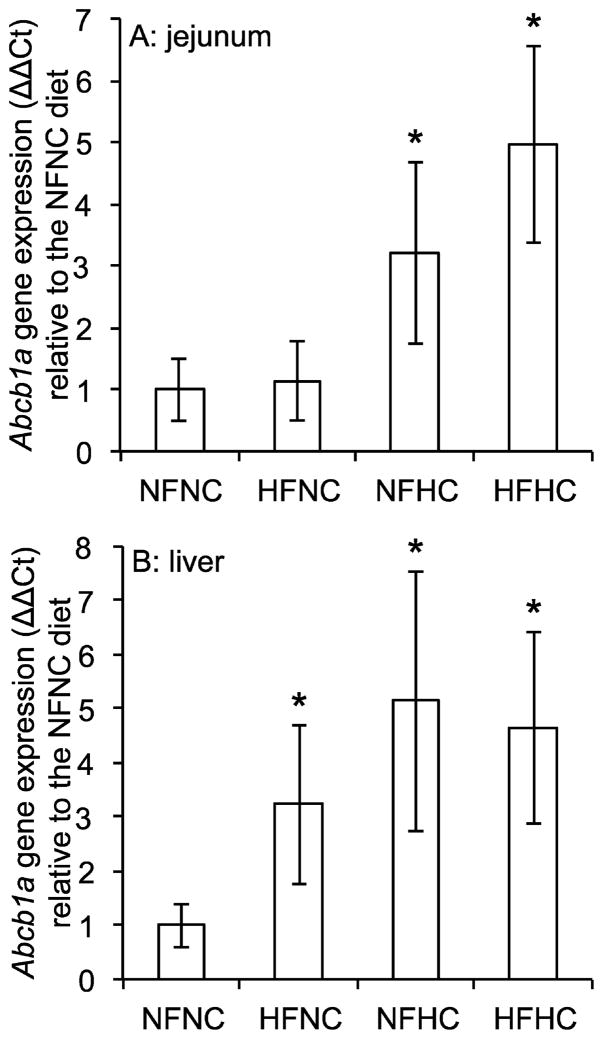
Wild type mice fed diets enriched in fat and cholesterol had increased expression of Abcb1a, the isoform of the gene encoding Pgp expressed by intestinal cells and hepatocytes, relative to mice fed the standard NFNC diet. Dietary cholesterol but not fat increased Abcb1a mRNA in the jejunum of wild type mice (Figure 1A). Addition of cholesterol alone to the diet induced the expression of Abcb1a by 3.2-fold over the NFNC diet. Abcb1a was further induced when the mice were fed the HFHC diet (p<0.001 vs. NFNC by one-way ANOVA). Liver Abcb1a expression was induced by both dietary fat and cholesterol (Figure 1B). Dietary fat alone induced hepatic Abcb1a expression by 3.2-fold over the NFNC diet and Abcb1a was further increased by dietary cholesterol (p<0.001 vs. NFNC by one-way ANOVA).
Figure 1.
Effect of diet on Pgp gene expression in wild type mice. The expression of Abcb1a, the isoform of Pgp expressed in liver and intestinal cells, was measured in (A) jejunum samples and (B) liver samples isolated from mice on each of the four diets by RT-qPCR. NFNC diet (25% kcal fat + 0.02% w/w cholesterol), HFNC diet (45% kcal fat + 0.02% w/w cholesterol), NFHC diet (25%kcal fat + 0.20% w/w cholesterol), HFHC diet (45% kcal fat + 0.20% w/w cholesterol). The mean relative expression is shown ± SD, n=9–10. *p<0.05 vs. NFNC by one-way ANOVA and Tukey post-hoc tests
Cholesterol phenotype
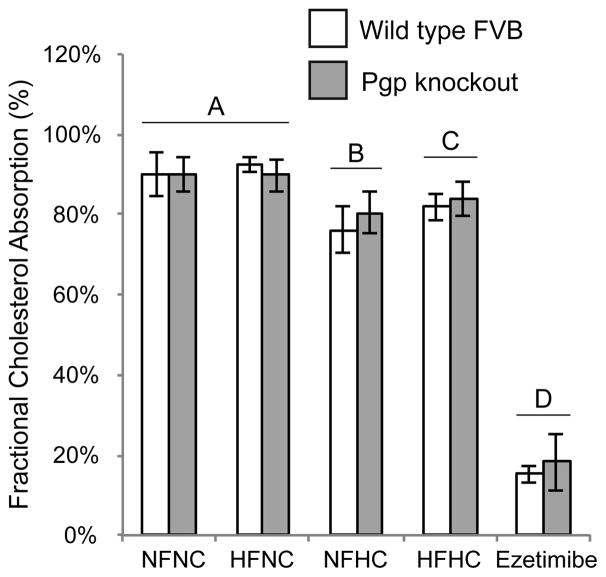
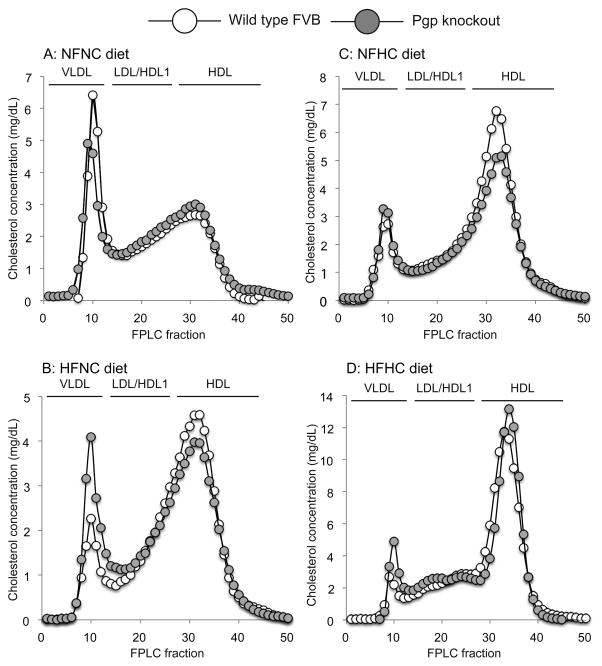
Previous studies predicted that the role of Pgp in cholesterol absorption would only be observed by feeding the mice diets enriched in fat and/or cholesterol21. In our study fractional cholesterol absorption was not affected by deletion of Pgp regardless of the diet the mice were fed (Figure 2: p>0.05 by two-way ANOVA). Fractional cholesterol absorption was decreased in all mice fed the high cholesterol diets (NFHC and HFHC) relative to the NFNC diet (Figure 2: p<0.001 by two-way ANOVA). Similarly, diet but not deletion of Pgp affected serum cholesterol concentrations (Table 1). Qualitative FPLC analysis of lipoprotein cholesterol revealed that Pgp knockout mice challenged with either high fat or high cholesterol had slightly increased cholesterol in VLDL compared to the WT mice. In the knockout mice, this change was accompanied by decreased HDL cholesterol (Figure 3). Prior studies with Pgp knockout mice showed that the deletion of Pgp affected cholesterol accumulation in the liver when mice were fed a standard chow diet21. This observation was confirmed in our study: both diet and genotype affected cholesterol accumulation in the liver (p<0.001 for diet, p=0.009 for genotype – Supplementary Table 1). The two-way ANOVA revealed a statistically significant interaction between diet and genotype. The interaction indicates that the effect of diet on cholesterol accumulation depends on genotype and vice versa and, consequently, the contributions of each factor cannot be distinguished. Conversely, Pgp deletion had no effect on liver phospholipid or triacylglycerol content (Supplementary Table 2).
Figure 2.
Effects of diet and deletion of Pgp on the fractional absorption of cholesterol. The results of the fecal dual isotope cholesterol assay are displayed for the wild type FVB mice (WT: hollow bars) and Pgp knockout mice (Abcb1a−/−/1b−/− KO: grey bars) after eight weeks on the test diets. Mice were administered a single dose of cholesterol mixed with β-sitostanol (non-absorbed control) and feces were collected for 96h. The ezetimibe group was given a single 10mg/kg dose 60min prior to the administration of cholesterol as a positive control for reduced cholesterol absorption. NFNC diet (25% kcal fat + 0.02% w/w cholesterol), HFNC diet (45% kcal fat + 0.02% w/w cholesterol), NFHC diet (25%kcal fat + 0.20% w/w cholesterol), HFHC diet (45% kcal fat + 0.20% w/w cholesterol). The mean cholesterol absorption values are shown ± SD, n=8–10. There are no statistically significant differences between WT and KO mice within any dietary group. Dietary groups designated by different letters have statistically significant differences. p<0.05 by two-way ANOVA with Tukey post-hoc tests.
Table 1.
Effect of diet and deletion of P-glycoprotein on serum cholesterol parameters after nine weeks on the test diets.
| Genotype | Diet | n | Serum cholesterol content (mg/dL)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Total cholesterol | Unesterified cholesterol | |||||||
| mean | ± | SD | mean | ± | SD | |||
| Wild Type | NFNC | 8 | 164.0 | ± | 48.0 | 44.2 | ± | 44.2 |
| HFNC | 8 | 150.1 | ± | 30.6 | 59.0 | ± | 59.0 | |
| NFHC | 7 | 188.4 | ± | 28.0 | 53.9 | ± | 53.9 | |
| HFHC | 7 | #216.3 | ± | 34.8 | #79.5 | ± | 79.5 | |
|
| ||||||||
| Pgp knockout | NFNC | 8 | 144.8 | ± | 33.9 | 48.8 | ± | 21.8 |
| HFNC | 8 | 165.5 | ± | 33.0 | 50.4 | ± | 22.2 | |
| NFHC | 8 | 177.4 | ± | 42.9 | 52.8 | ± | 9.1 | |
| HFHC | 8 | #209.3 | ± | 38.5 | #79.9 | ± | 21.9 | |
Serum was collected and analyzed for total and unesterified cholesterol content using cholesterol oxidase-based assays.
p<0.05 relative to the NFNC diet by two-way ANOVA with Tukey post-hoc tests; n=7–8.
Figure 3.
Effect of P-glycoprotein deletion on lipoprotein cholesterol. Equal volumes of pooled serum were separated by fast protein liquid chromatography. Each panel contains the total cholesterol concentration in the fractions obtained from wild-type FVB mice (WT: hollow markers) or Pgp knockout mice (KO: grey markers) administered the following test diets: (A) NFNC (25% kcal fat + 0.02% w/w cholesterol), (B) HFNC (45% kcal fat + 0.02% w/w cholesterol), (C) NFHC (25%kcal fat + 0.20% w/w cholesterol), (D) HFHC (45% kcal fat + 0.20% w/w cholesterol). Pools consisted of equal volume aliquots from 6–10 mice. The cholesterol was distributed into VLDL, LDL/sub fraction 1 of HDL (HDL1), and HDL.
Effect of diet on cholesterol regulatory networks
Cellular cholesterol homeostasis is maintained primarily through the SREBP-2 and LXR transcription factors in the liver and in the jejunum. To test the activation of these pathways we measured the expression of genes regulated by each transcription factor in the jejunum and in the liver. Dietary cholesterol induced LXR activation in the jejunum of both wild type and Pgp knockout mice (NFHC and HFHC diets) as measured by expression of Abcg5, Abcg8 and Abca1 (Table 2); p<0.05 vs. NFNC by one way ANOVA). Conversely, neither increased dietary fat nor cholesterol had a consistent effect on the expression of SREBP-2 target genes, Ldlr, Hmgcr, and Npc1l1 (Table 3). Similarly, the expression of LXR-target genes Abcg5 Abcg8, and Cyp7a1 was induced in the liver by dietary cholesterol for both wild type and knockout mice (Table 4); p<0.05 vs. NFNC by one-way ANOVA). The relationship between dietary conditions and the expression genes regulated by the bile acid nuclear receptor, FXR, was not consistent. Future investigations into the role of Pgp in bile acid homeostasis should include thorough assessment of FXR-related transcriptional activity in the liver and ileum.
Table 2.
Effects of diet on the expression of LXR-target genes in the jejunum of wild type and Pgp knockout mice.
| Genotype | Gene | 0.02% (w/w) cholesterol | 0.20% (w/w) cholesterol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 25% kcal fat | 45% kcal fat | 25% kcal fat | 45% kcal fat | ||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| Wild type | Abca1 | 1.00 | ± | 0.33 | 0.98 | ± | 0.48 | *2.39 | ± | 0.54 | *2.94 | ± | 0.69 |
| Abcg5 | 1.00 | ± | 0.30 | *0.59 | ± | 0.10 | *1.78 | ± | 0.40 | *2.54 | ± | 1.05 | |
| Abcg8 | 1.00 | ± | 0.37 | *0.46 | ± | 0.14 | *2.29 | ± | 0.58 | *2.74 | ± | 0.95 | |
|
| |||||||||||||
| Pgp knockout | Abca1 | 0.69 | ± | 0.25 | 1.15 | ± | 0.46 | *3.32 | ± | 1.53 | *2.65 | ± | 1.38 |
| Abcg5 | 0.78 | ± | 0.35 | 0.91 | ± | 0.35 | *2.09 | ± | 0.63 | *2.84 | ± | 1.60 | |
| Abcg8 | 0.86 | ± | 0.43 | 1.02 | ± | 0.38 | *2.28 | ± | 0.81 | *2.48 | ± | 1.43 | |
Gene expression was determined by semi-quantitative RT-qPCR from RNA isolated from frozen jejunum samples. The mean ΔCt value of the WT-NFNC dietary group was set as the reference to which all samples were compared using the ΔΔCt methodology. The mean relative expression and SD is shown to indicate the variability of the response to dietary stimulus.
p<0.05 vs. NFNC diet by one-way ANOVA with Tukey post-hoc tests, n=9–10.
Table 3.
Effects of diet on the expression of SREBP-2-target genes in the jejunum of wild type and Pgp knockout mice.
| Genotype | Gene | 0.02% (w/w) cholesterol | 0.20% (w/w) cholesterol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% kcal fat | 45% kcal fat | 25% kcal fat | 45% kcal fat | ||||||||||
|
| |||||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| Wild type | Ldlr | 1.00 | ± | 0.39 | 1.31 | ± | 0.35 | 0.85 | ± | 0.28 | *1.38 | ± | 0.23 |
| Npc1l1 | 1.00 | ± | 0.55 | 0.73 | ± | 0.37 | 1.06 | ± | 0.30 | 1.12 | ± | 0.30 | |
| Hmgcr | 1.00 | ± | 0.46 | 1.09 | ± | 0.30 | 0.78 | ± | 0.22 | *2.18 | ± | 0.57 | |
|
| |||||||||||||
| Pgp knockout | Ldlr | 0.54 | ± | 0.15 | *1.45 | ± | 0.42 | *0.71 | ± | 0.26 | *1.06 | ± | 0.17 |
| Npc1l1 | 0.34 | ± | 0.19 | *1.00 | ± | 0.52 | *1.27 | ± | 0.98 | *1.19 | ± | 0.75 | |
| Hmgcr | 0.55 | ± | 0.31 | *1.53 | ± | 0.45 | 0.91 | ± | 0.46 | *1.60 | ± | 0.61 | |
Gene expression was determined by semi-quantitative RT-qPCR from RNA isolated from frozen jejunum samples. The mean ΔCt value of the WT-NFNC dietary group was set as the reference to which all samples were compared using the ΔΔCt methodology. The mean relative expression and SD is shown to indicate the variability of the response to dietary stimulus.
p<0.05 vs. NFNC diet by one-way ANOVA with Tukey post-hoc tests, n=9–10.
Table 4.
Effects of diet on the expression of LXR-target genes in the liver of wild type and Pgp knockout mice.
| Genotype | Gene | 0.02% (w/w) cholesterol | 0.20% (w/w) cholesterol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 25% kcal fat | 45% kcal fat | 25% kcal fat | 45% kcal fat | ||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| Wild type | Abcg5 | 1.00 | ± | 0.40 | 1.10 | ± | 0.29 | *3.51 | ± | 0.89 | *2.54 | ± | 0.65 |
| Abcg8 | 1.00 | ± | 0.34 | *1.47 | ± | 0.40 | *3.04 | ± | 0.68 | *3.14 | ± | 0.76 | |
| Cyp7a1 | 1.00 | ± | 0.66 | *6.83 | ± | 4.47 | *8.52 | ± | 3.24 | *7.44 | ± | 3.82 | |
|
| |||||||||||||
| Pgp knockout | Abcg5 | 1.48 | ± | 0.60 | 0.98 | ± | 0.21 | *3.34 | ± | 0.75 | *2.54 | ± | 0.50 |
| Abcg8 | 1.31 | ± | 0.51 | 1.33 | ± | 0.35 | *3.07 | ± | 0.68 | *3.04 | ± | 0.59 | |
| Cyp7a1 | 2.38 | ± | 1.06 | 5.14 | ± | 2.51 | *6.44 | ± | 2.66 | *9.10 | ± | 6.02 | |
Gene expression was determined by semi-quantitative RT-qPCR from RNA isolated from frozen liver samples. The mean ΔCt value of the WT-NFNC dietary group was set as the reference to which all samples were compared using the ΔΔCt methodology. The mean relative expression and SD is shown to indicate the variability of the response to dietary stimulus.
p<0.05 vs. NFNC diet by one-way ANOVA with Tukey post-hoc tests, n=9–10.
Effect of Pgp deletion on cholesterol regulatory networks
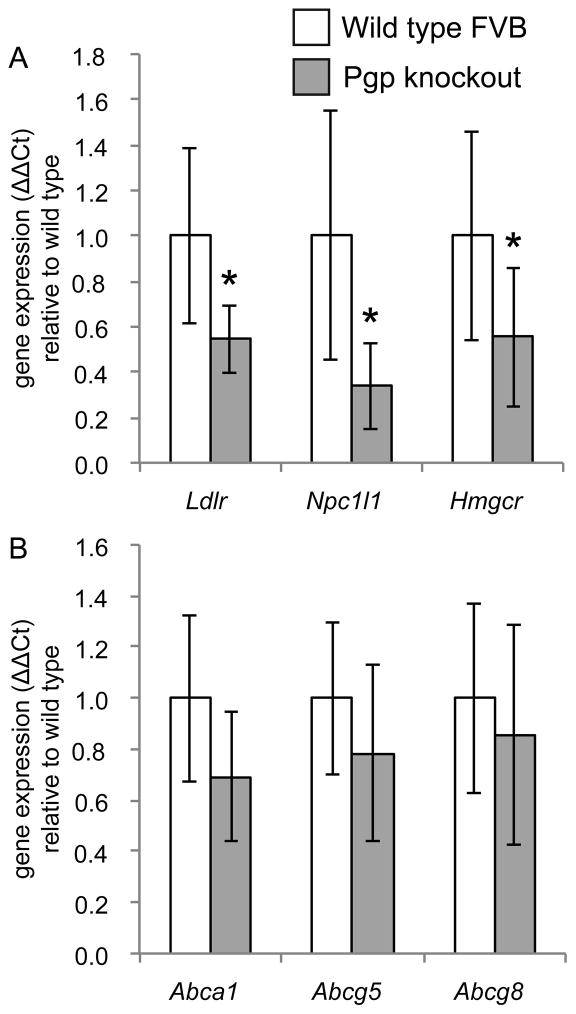
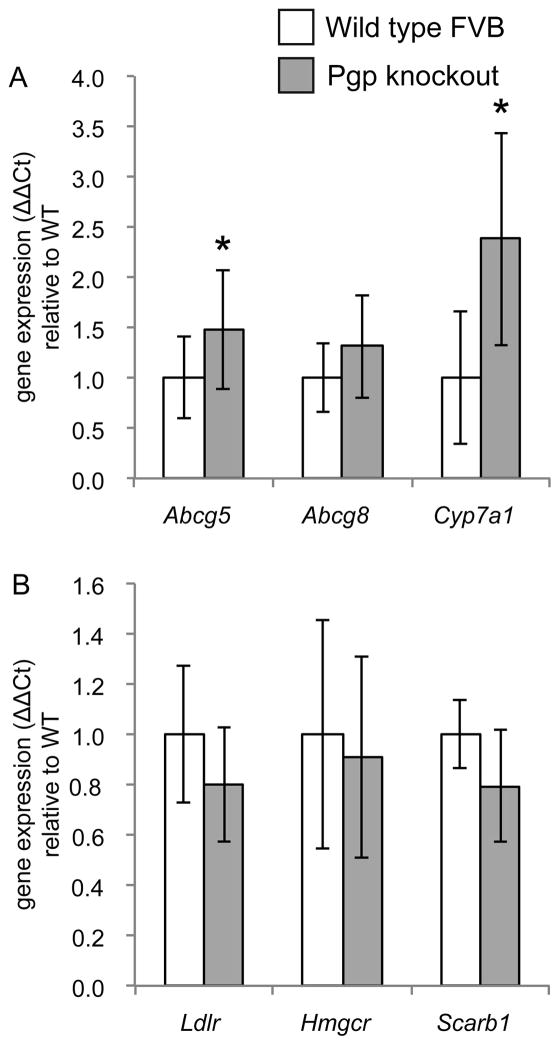
After determining the effect of diet on SREBP-2 and LXR activation we examined the effect that Pgp deletion had on each pathway. The expression of SREBP-2 target genes was reduced in the jejunum of Pgp knockout mice administered the NFNC diet relative to the wild type mice (Figure 4A; p<0.05 vs. WT by t-test). This was not accompanied by changes to the expression of LXR target genes (Figure 4B) nor was total accumulation of cholesterol was affected by Pgp deletion (Supplementary Table 3). Hepatic expression of the LXR-target genes Abcg5 and Cyp7a1 was increased in Pgp knockout mice fed the NFNC diet relative to wild type littermates but there were no changes to SREBP-2 target genes Hmgcr, Ldlr, or Scarb1 (Figure 5; p<0.05 vs. WT by t-test). There were no consistent changes to LXR, SREBP-2, or FXR target genes attributable to deletion of Abcb1a/Abcb1b in the other dietary groups (Tables 2–5).
Figure 4.
Effect of Pgp deletion on SREBP-2 and LXR activation in the jejunum. (A) Relative to wild type mice, Pgp knockout mice have reduced expression of genes under the transcriptional control of SREBP-2 after administration of the NFNC diet. (B) Wild type and Pgp knockout mice do not show alterations to the expression of genes under transcriptional control of LXR. Mean values shown ± SD, n=9–10. *p<0.05 vs. WT by unpaired t-test
Figure 5.
Effect of Pgp deletion on SREBP-2 and LXR activation in the liver. (A) Pgp knockout mice have increased expression of LXR target genes after chronic administration of the NFNC diet; similar results were seen with the HFHC diet. (B) Wild type and Pgp knockout mice have unchanged hepatic expression of SREBP-2 target genes. Mean values shown ± SD, n=9–10. *p<0.05 vs. WT by unpaired t-test
Table 5.
Effects of diet on the expression of FXR-target genes in the liver of wild type and Pgp knockout mice.
| Genotype | Gene | 0.02% (w/w) cholesterol | 0.20% (w/w) cholesterol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 25% kcal fat | 45% kcal fat | 25% kcal fat | 45% kcal fat | ||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| Wild type | Abcb4 | 1.00 | ± | 0.23 | *1.68 | ± | 0.35 | *1.39 | ± | 0.24 | *1.65 | ± | 0.28 |
| Abcb11 | 1.00 | ± | 0.29 | 1.06 | ± | 0.36 | 0.95 | ± | 0.21 | 1.00 | ± | 0.22 | |
| Mrp2 | 1.00 | ± | 0.79 | 1.50 | ± | 1.09 | 1.13 | ± | 1.37 | 1.98 | ± | 2.29 | |
| Shp | 1.00 | ± | 1.22 | 2.31 | ± | 2.1 | 1.24 | ± | 1.25 | 4.34 | ± | 4.67 | |
| Slc27a | 1.00 | ± | 0.82 | 1.24 | ± | 1.25 | 1.91 | ± | 1.45 | 1.79 | ± | 0.66 | |
|
| |||||||||||||
| Pgp knockout | Abcb4 | 0.90 | ± | 0.27 | *1.88 | ± | 0.44 | *1.58 | ± | 0.32 | 1.12 | ± | 0.24 |
| Abcb11 | 0.76 | ± | 0.23 | *1.41 | ± | 0.49 | 0.65 | ± | 0.17 | *1.14 | ± | 0.35 | |
| Mrp2 | 0.76 | ± | 0.99 | *0.97 | ± | 1.52 | 0.69 | ± | 0.54 | 4.65 | ± | 5.24 | |
| Shp | 0.67 | ± | 0.66 | 0.44 | ± | 0.59 | 1.26 | ± | 1.09 | *3.22 | ± | 3.58 | |
| Slc27a | 0.63 | ± | 1.05 | 0.66 | ± | 1.09 | 0.53 | ± | 0.55 | *1.69 | ± | 1.14 | |
Gene expression was determined by semi-quantitative RT-qPCR from RNA isolated from frozen liver samples. The mean ΔCt value of the WT-NFNC dietary group was set as the reference to which all samples were compared using the ΔΔCt methodology. The mean relative expression and SD is shown to indicate the variability of the response to dietary stimulus.
p<0.05 vs. NFNC diet by one-way ANOVA with Tukey post-hoc tests, n=8–10.
Effect of Pgp deletion on gallbladder bile composition
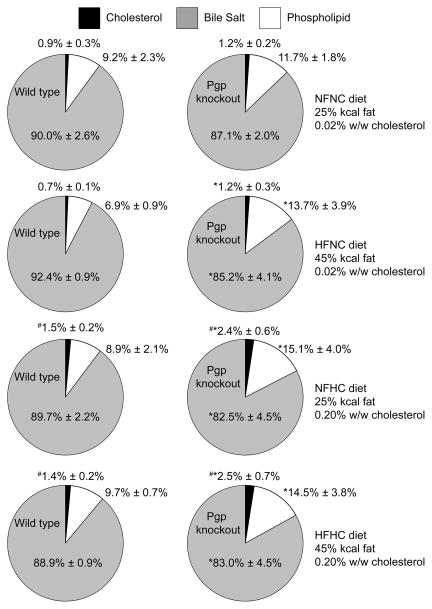
Our group has published a preliminary study in which we found increased cholesterol in the feces of Pgp knockout mice22. In the current study we determined that the fractional cholesterol absorption was unaffected by Pgp deletion; consequently, we evaluated the gallbladder bile composition to determine if there were perturbations to biliary cholesterol in the knockout mice. The mole fraction of gallbladder cholesterol, phospholipid, and bile salts was calculated for each mouse and the mean values presented in Figure 6. Consistent with previous studies, the gallbladder bile in this study was unaffected by Pgp deletion when the mice were fed a normal-fat, normal-cholesterol diet 23,35,36. Pgp deletion affected gallbladder bile composition when mice were fed diets containing elevated fat (45%kcal vs. 25%kcal) or elevated cholesterol (0.20% vs. 0.02% w/w). Knockout mice had decreased molar ratio of bile acids, while the molar ratio of cholesterol and phospholipids increased relative to diet-matched wild type mice (n=5 mice per group except KO-HFNC where n=4 due to the inability to collect a complete data set from one mouse). Neither gallbladder size nor phospholipid content in the gallbladder was unaffected by Pgp deletion (Supplementary Figures 1 and 3) indicating that the changes in gallbladder mole fractions are due to a reduction in bile salt in the knockout mice. The knockout mice have unchanged mole fraction of each type of bile acid measured in the gallbladder (Tauromuricholate, Tauroursodeoxycholate, Glycoursodeoxycholate, Taurocholate, Taurochenodeoxycholate, and Taurodeoxycholate) indicating that the defect in transport is not limited to a particular class of bile salt (Supplementary Figure 2).
Figure 6.
Effects of diet and Pgp deletion on the molar composition of gallbladder bile. The mean molar percentage of cholesterol, phospholipid, and bile salts in gallbladder bile is shown ± SD for each of the four diets tested. NFNC: 25%kcal fat and 0.02% (w/w) cholesterol. HFNC: 45%kcal fat and 0.02% (w/w) cholesterol. NFHC: 25%kcal fat and 0.20% (w/w) cholesterol. HFHC: 45%kcal fat and 0.20% (w/w) cholesterol for wild type mice (WT) and Pgp knockout mice (KO). * p<0.05 vs. WT on the same diet by two-way ANOVA with Tukey post-hoc tests; #p<0.05 vs. NFNC by two-way ANOVA with Tukey post hoc tests, n=5 for all groups except KO-HFNC where n=4.
DISCUSSION
In this study we tested whether or not P-glycoprotein contributes to cholesterol homeostasis in vivo. We hypothesized that by challenging the mice with increased dietary fat and cholesterol, dietary conditions lacking from earlier studies, we would stress the ability of the mice to maintain homeostasis and clearly demonstrate differences between the wild type and Pgp knockout mice. Both dietary fat and cholesterol induced Pgp expression (Figure 1) suggesting that Pgp may have a physiological purpose under these dietary conditions. We found that mice lacking Pgp were able to maintain cholesterol homeostasis when fed any of the test diets but were unable to maintain gallbladder bile composition relative to the wild type mice when fed high fat and/or cholesterol. An examination of the activation state of the SREBP-2 and LXR transcription factors revealed alterations to cholesterol regulatory pathways in the knockout mice that may have contributed to the maintenance of cholesterol homeostasis.
Cell culture studies clearly showed that inhibition of Pgp disrupted intracellular cholesterol trafficking14–18 but the in vivo contributions of Pgp to cholesterol homeostasis have not been described. Our hypothesis was that Abcb1a/1b deletion would affect the cholesterol phenotype of mice challenged with cholesterol-rich diets but our results do not support the hypothesis. Pgp knockout mice and wild type mice maintained similar fractional cholesterol absorption, circulating cholesterol levels, and lipoprotein profiles on each diet (Figure 2, Figure 3, and Table 1). With knockout mice, it is important to examine the regulatory pathways that maintain the phenotype under investigation as compensatory shifts can maintain homeostasis and obscure the role of the protein. We chose to examine the jejunum and the liver as these are the sites of cholesterol absorption37 and elimination2, respectively. To test whether we were able to detect changes in cholesterol homeostasis, we probed the impact of dietary cholesterol on two regulatory pathways: LXR and SREBP-2. The data in Table 2 and Table 4 clearly show that LXR was activated in response to the consumption of high cholesterol diets, as measured by the increased expression of LXR target genes. As expected, elevated dietary fat and cholesterol did not affect the expression of SREBP-2 target genes in the jejunum (Table 3). Prior studies demonstrated that SREBP-2 is activated by low levels of cholesterol 38–40. The responses of LXR and SREBP-2 in to dietary stimulus in our study are consistent with previous reports confirming that we were successfully detecting shifts in cholesterol homeostasis.
When comparing the wild type and knockout mice fed the NFNC diet we determined that SREBP-2 activity was reduced in the jejunum of Pgp knockout mice (Figure 4) while LXR activity was increased in the liver (Figure 5) relative to wild type mice. Small increases to intracellular cholesterol deactivate SREBP-239,41 while LXR is activated by increased oxysterols, oxidized metabolites of cholesterol42,43. The combined deactivation of SREBP-2 and activation of LXR is consistent with increased intracellular cholesterol and its metabolites in the Pgp knockout mice. This indirect evidence supports the idea that the Pgp knockout mice had a reduced capacity to efflux cholesterol in the jejunum and liver. These shifts in the activation of LXR and SREBP-2 were not observed in knockout mice fed the diets enriched with fat and/or cholesterol. We speculate that the activation of LXR linked with a ten-fold increase in dietary may have obscured any effects that Pgp deletion may have had. The stimulus of a ten-fold increase in dietary cholesterol was expected to deactivate SREBP-2 regardless of any knockout effect.
Our finding that Pgp knockout mice do not maintain gallbladder bile composition (Figure 6) compliments the recent report by Ling et al. who used triple knockout mice to demonstrate that Pgp acts as a compensatory bile salt efflux transporter in the absence of the Bile Salt Export Pump (BSEP)36,44. Our gallbladder bile data suggest that Pgp may also be required for bile salt homeostasis under physiologically relevant dietary conditions, even in the presence of BSEP. Several groups have investigated the bile salt export capacity of Pgp knockout mice and all have found that the mice are phenotypically similar to wild type mice23 with slight increases36 or decreases35 to bile salt efflux relative to total lipid efflux, similar to our observations for the NFNC diet (Figure 6). In each of these studies the authors examined the bile salt efflux of mice after administration of a normal chow diet or a cholestatic diet containing 0.5% (w/w) cholic acid; our study is the first to measure bile composition in Pgp knockout mice fed high-fat, high-cholesterol diets. BSEP knockout mice have an accumulation of hydrophobic bile acids, indicating that BSEP preferentially recognizes certain bile salts45. When we examined the bile salt composition we found no differences in the molar ratio of the six most prevalent bile salts between knockout and wild type mice, indicating that Pgp recognized all bile salt species non-specifically (Supplementary Figure 2)
There are several limitations to this set of studies. Mice are commonly used as a first in vivo model to evaluate the physiological relevance of in vitro data; however, they are poor models for human cholesterol metabolism46. Their lipoprotein pool is predominantly HDL, while humans have higher levels of LDL. Mice lack cholesteryl ester transfer protein47, they require genetic manipulation in order to develop human-like atherosclerosis48,49, and produce a different bile acid pool than humans45. Despite these limitations, a diverse set of genetic tools exist for working with mice which make them a good first in vivo model, even for atherosclerosis studies50. In this manuscript we provided preliminary evidence suggesting that murine Pgp may be required for to maintain gallbladder bile homeostasis in mice that consumed elevated dietary fat and/or cholesterol. These data support conducting future studies designed to elucidate the role of Pgp in bile salt efflux. These studies should test the impact of dietary lipids on bile salt pool size and directly measure bile salt efflux rates in Pgp knockout mice by bile duct cannulation. By determining the expression of SREBP-2 target genes, we only have an indirect measurement of the activity of the transcription factor. We attempted to confirm our findings by measuring the activation of SREBP-2 directly by examining cleavage to its active form39–41 but there was insufficient jejunum remaining after the lipid extractions and RT-qPCR analyses to conduct the experiment as described51. Similarly, we postulate that the increased LXR activity was linked to increased cholesterol metabolites but we were not able to quantify hepatic oxysterol content.
The pattern of perturbations to SREBP-2 and LXR provide indirect evidence that a cholesterol efflux mechanism is lacking from the Pgp knockout mice. Future mechanistic ex vivo studies are warranted to test the cholesterol efflux capacity of Pgp.
CONCLUSIONS
We conducted this study in order to test the hypothesis that deletion of P-glycoprotein would affect the ability of mice to maintain cholesterol homeostasis after chronic consumption of a range of high-fat, high-cholesterol diets. Contrary to our hypothesis, the Pgp knockout mice maintained similar cholesterol phenotypes to wild type mice fed the matching diet. We identified compensatory perturbations to the LXR and SREBP-2 cholesterol regulatory pathways in the knockout mice that may explain the unchanged cholesterol phenotype in the knockout mice. When examining gallbladder bile composition we determined that deletion of Pgp affected the ability of the mice to maintain bile homeostasis after eating high-fat and high-cholesterol diets. This novel discovery compliments a recent publication by Victor Ling et al. who reported the capacity of Pgp to act as a secondary bile salt export pump in the absence of BSEP36. These data support further investigation of the contribution of Pgp to bile salt efflux after chronic consumption of physiologically relevant high-fat, high-cholesterol diets.
Acknowledgments
Funding Sources
Funding for this project was provided in the form of an operating grant to KMW and SJT from the Canadian Institutes of Health Research. SDL was supported by a “Frederick Banting and Charles Best Canada Graduate Scholarship” from the Canadian Institutes of Health Research and by a “Senior Graduate Studentship” from the Michael Smith Foundation for Health Research.
We would like to thank Dr. David Cohen’s laboratory at the Harvard Digestive Diseases Center for their contributions to the FPLC and HPLC experiments described in this paper. We would also like to thank Jackie Fleischer and Ian Wong for their assistance with several of the hepatic analyses included in this manuscript. We are grateful to The Center for Drug Research and Development for providing training and access to their real time thermocycler for our gene expression studies.
ABBREVIATIONS
- ABC
ATP-binding cassette
- Pgp
P-glycoprotein
- LXR
Liver X Receptor
- SREBP-2
Sterol Response Element Binding Protein 2
- WT
wild type FVB mice
- KO
Pgp knockout mice
- NFNC
normal fat and normal cholesterol diet
- HFNC
high fat and normal cholesterol diet
- NFHC
normal fat and high cholesterol diet
- HFHC
high fat and high cholesterol diet
- FPLC
fast protein liquid chromatography
- FXR
Farnesoid X Receptor
- BSEP
bile salt export pump; gene names were obtained from the HUGO Human Gene Name Nomenclature Committee52 or the mouse genome database53, as appropriate
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supporting Information. Three additional figures (gallbladder phospholipids, gallbladder bile acid composition, and gallbladder size) and five additional data tables (hepatic cholesterol content, hepatic phospholipid and triglyceride content, jejunum cholesterol content, TaqMan assay information, and a summary of the RT-qPCR controls) are available as supplementary information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Claudel T, Zollner G, Wagner M, Trauner M. Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease. Biochim Biophys Acta. 2011;1812:867–878. doi: 10.1016/j.bbadis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 3.Plösch T, van der Veen JN, Havinga R, Huijkman NCA, Bloks VW, Kuipers F. Abcg5/Abcg8-independent pathways contribute to hepatobiliary cholesterol secretion in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G414–23. doi: 10.1152/ajpgi.00557.2005. [DOI] [PubMed] [Google Scholar]
- 4.Wang HH, Patel SB, Carey MC, Wang DQH. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(−/−) mice. Hepatology. 2007;45:998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geuken E, Visser DS, Leuvenink HGD, de Jong KP, Peeters PMJG, Slooff MJH, Kuipers F, Porte RJ. Hepatic expression of ABC transporters G5 and G8 does not correlate with biliary cholesterol secretion in liver transplant patients. Hepatology. 2005;42:1166–1174. doi: 10.1002/hep.20886. [DOI] [PubMed] [Google Scholar]
- 6.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penzotti JE, Lamb ML, Evensen E, Grootenhuis PDJ. A computational ensemble pharmacophore model for identifying substrates of P-glycoprotein. J Med Chem. 2002;45:1737–1740. doi: 10.1021/jm0255062. [DOI] [PubMed] [Google Scholar]
- 8.Eckford PDW, Sharom FJ. Interaction of the P-glycoprotein multidrug efflux pump with cholesterol: effects on ATPase activity, drug binding and transport. Biochemistry. 2008;47:13686–13698. doi: 10.1021/bi801409r. [DOI] [PubMed] [Google Scholar]
- 9.Orlowski S, Martin S, Escargueil A. P-glycoprotein and “lipid rafts”: some ambiguous mutual relationships (floating on them, building them or meeting them by chance?) Cell Mol Life Sci. 2006;63:1038–1059. doi: 10.1007/s00018-005-5554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura Y, Kioka N, Kato H, Matsuo M, Ueda K. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem J. 2007;401:597–605. doi: 10.1042/BJ20060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radeva G, Perabo J, Sharom FJ. P-Glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains. FEBS J. 2005;272:4924–4937. doi: 10.1111/j.1742-4658.2005.04905.x. [DOI] [PubMed] [Google Scholar]
- 12.Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc Natl Acad Sci US A. 2002;99:10347–10352. doi: 10.1073/pnas.162366399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metherall JE, Waugh K, Li H. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J Biol Chem. 1996;271:2627–2633. doi: 10.1074/jbc.271.5.2627. [DOI] [PubMed] [Google Scholar]
- 14.Metherall JE, Li H, Waugh K. Role of multidrug resistance P-glycoproteins in cholesterol biosynthesis. J Biol Chem. 1996;271:2634–2640. doi: 10.1074/jbc.271.5.2634. [DOI] [PubMed] [Google Scholar]
- 15.Debry P, Nash EA, Neklason DW, Metherall JE. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J Biol Chem. 1997;272:1026–1031. doi: 10.1074/jbc.272.2.1026. [DOI] [PubMed] [Google Scholar]
- 16.Field FJ, Born E, Chen H, Murthy S, Mathur SN. Esterification of plasma membrane cholesterol and triacylglycerol-rich lipoprotein secretion in CaCo-2 cells: possible role of p-glycoprotein. J Lipid Res. 1995;36:1533–1543. [PubMed] [Google Scholar]
- 17.Field FJ, Born E, Murthy S, Mathur SN. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane is constitutive in CaCo-2 cells and differs from the transport of plasma membrane cholesterol to the endoplasmic reticulum. J Lipid Res. 1998;39:333–343. [PubMed] [Google Scholar]
- 18.Luker GD, Nilsson KR, Covey DF, Piwnica-Worms D. Multidrug resistance (MDR1) P-glycoprotein enhances esterification of plasma membrane cholesterol. J Biol Chem. 1999;274:6979–6991. doi: 10.1074/jbc.274.11.6979. [DOI] [PubMed] [Google Scholar]
- 19.Tessner TG, Stenson WF. Overexpression of MDR1 in an intestinal cell line results in increased cholesterol uptake from micelles. Biochem Biophys Res Commun. 2000;267:565–571. doi: 10.1006/bbrc.1999.1996. [DOI] [PubMed] [Google Scholar]
- 20.Le Goff W, Settle M, Greene DJ, Morton RE, Smith JD. Reevaluation of the role of the multidrug-resistant P-glycoprotein in cellular cholesterol homeostasis. J Lipid Res. 2006;47:51–58. doi: 10.1194/jlr.M500255-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Luker GD, Dahlheimer JL, Ostlund RE, Piwnica-Worms D. Decreased hepatic accumulation and enhanced esterification of cholesterol in mice deficient in mdr1a and mdr1b P-glycoproteins. J Lipid Res. 2001;42:1389–1394. [PubMed] [Google Scholar]
- 22.Thornton SJ, Wong E, Lee SD, Wasan KM. Effect of dietary fat on hepatic liver X receptor expression in P-glycoprotein deficient mice: implications for cholesterol metabolism. Lipids Health Dis. 2008;7:21. doi: 10.1186/1476-511X-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schinkel AH, Mayer U, Wagenaar E, Mol CAAM, van Deemter L, Smit JJM, van der Valk M, Voordouw AC, Spits H, van Tellingen O, Zijlmans JMJM, Fibbe WE, Borst P. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci US A. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 26.de Vogel-van den Bosch HM, de Wit NJW, Hooiveld GJEJ, Vermeulen H, van der Veen JN, Houten SM, Kuipers F, Müller M, van der Meer R. A cholesterol-free, high-fat diet suppresses gene expression of cholesterol transporters in murine small intestine. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1171–80. doi: 10.1152/ajpgi.00360.2007. [DOI] [PubMed] [Google Scholar]
- 27.VanPatten S, Ranginani N, Shefer S, Nguyen LB, Rossetti L, Cohen DE. Impaired biliary lipid secretion in obese Zucker rats: leptin promotes hepatic cholesterol clearance. Am J Physiol Gastrointest Liver Physiol. 2001;281:G393–404. doi: 10.1152/ajpgi.2001.281.2.G393. [DOI] [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 29.Wu MK, Cohen DE. Phosphatidylcholine transfer protein regulates size and hepatic uptake of high-density lipoproteins. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1067–74. doi: 10.1152/ajpgi.00194.2005. [DOI] [PubMed] [Google Scholar]
- 30.Miettinen TA, Ahrens EH, Grundy SM. Quantitative Isolation and Gas-Liquid Chromatographic Analysis of Total Dietary and Fecal Neutral Steroids. J Lipid Res. 1965;6:411–424. [PubMed] [Google Scholar]
- 31.Turley SD, Daggy BP, Dietschy JM. Psyllium augments the cholesterol-lowering action of cholestyramine in hamsters by enhancing sterol loss from the liver. Gastroenterology. 1994;107:444–452. doi: 10.1016/0016-5085(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 32.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 33.Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- 34.Agellon LB, Drover VAB, Cheema SK, Gbaguidi GF, Walsh A. Dietary cholesterol fails to stimulate the human cholesterol 7alpha-hydroxylase gene (CYP7A1) in transgenic mice. J Biol Chem. 2002;277:20131–20134. doi: 10.1074/jbc.C200105200. [DOI] [PubMed] [Google Scholar]
- 35.Plösch T, Bloks VW, Baller JFW, Havinga R, Verkade HJ, Jansen PLM, Kuipers F. Mdr P-glycoproteins are not essential for biliary excretion of the hydrophobic heme precursor protoporphyrin in a griseofulvin-induced mouse model of erythropoietic protoporphyria. Hepatology. 2002;35:299–306. doi: 10.1053/jhep.2002.30900. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V. Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology. 2009;50:948–956. doi: 10.1002/hep.23089. [DOI] [PubMed] [Google Scholar]
- 37.Wang DQH. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 38.Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol Cell. 2002;10:237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci US A. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 44.Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry. 2005;44:12598–12605. doi: 10.1021/bi050943e. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci US A. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 47.Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, Breslow JL, Tall AR. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991;266:10796–10801. [PubMed] [Google Scholar]
- 48.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 49.Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 51.Sheng Z, Otani H, Brown MS, Goldstein JL. Independent regulation of sterol regulatory element-binding proteins 1 and 2 in hamster liver. Proc Natl Acad Sci US A. 1995;92:935–938. doi: 10.1073/pnas.92.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seal RL, Gordon SM, Lush MJ, Wright MW, Bruford EA. genenames.org: the HGNC resources in 2011. Nucleic Acids Res. 2011;39:D514–9. doi: 10.1093/nar/gkq892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE Mouse Genome Database Group. The Mouse Genome Database (MGD): comprehensive resource for genetics and genomics of the laboratory mouse. Nucleic Acids Res. 2012;40:D881–6. doi: 10.1093/nar/gkr974. [DOI] [PMC free article] [PubMed] [Google Scholar]