Abstract
This study described prescribing trends before and after implementing a provincial strategy aimed at improving osteoporosis and fracture prevention in Ontario long-term care (LTC) homes. Data were obtained from a pharmacy provider for 10 LTC homes in 2007 and 166 homes in 2012. We used weighted, multiple linear regression analyses to examine facility-level changes in vitamin D, calcium, and osteoporosis medication prescribing rates between 2007 and 2012. After five years, the estimated increase in vitamin D, calcium, and osteoporosis medication prescribing rates, respectively, was 38.2 per cent (95% confidence interval [CI]: 29.0, 47.3; p < .001), 4.0 per cent (95% CI: −3.9, 12.0; p = .318), and 0.2 per cent (95% CI: −3.3, 3.7; p = .91). Although the study could not assess causality, findings suggest that wide-scale knowledge translation activities successfully improved vitamin D prescribing rates, although ongoing efforts are needed to target homes with low uptake.
Keywords: aging, calcium, long-term care, nursing home, osteoporosis, prescribing, vitamin D
An estimated 60 to 80 per cent of long-term care (LTC) residents have osteoporosis (Gloth & Simonson, 2008; Zimmerman et al., 1999) and in Canada, it is estimated that the fracture rate for LTC residents is approximately two to four times that of similarly aged community-dwelling residents (Crilly, Tanner, Kloseck, & Chesworth, 2010). Combined with age-related losses in bone quantity and quality (Chen, Zhou, Fujita, Onozuka, & Kubo, 2013), the high prevalence of sarcopenia (Landi et al., 2012), frailty (Kanwar et al., 2013) and falls (Norris, Walton, Patterson, Feightner, & the Canadian Task Force on Preventive Health Care, 2003) in LTC residents may synergistically increase susceptibility for fractures (Ensrud et al., 2007; Ensrud et al., 2009; Gielen et al., 2012; Frisoli, Chaves, Ingham, & Fried, 2011). Furthermore, many LTC residents have sub-optimal vitamin D levels (Flicker et al., 2003; Ioannidis, Kennedy, Dykeman, Dudziak, & Papaioannou, 2012a), which is associated with lower bone mineral density (Bischoff-Ferrari, Dietrich, Orav, & Dawson-Hughes, 2004a; Bischoff-Ferrari et al., 2009; Hanley, Cranney, Jones, Whiting, & Leslie, 2010), decreased lower extremity function (Bischoff-Ferrari et al., 2004b; Dawson-Hughes, 2008), falls (Dawson-Hughes, 2008; Flicker et al., 2003), and fractures (Bischoff-Ferrari, Giovannucci, Willett, Dietrich, & Dawson-Hughes, 2006; Cauley et al., 2011; Holvik et al., 2013; Looker & Mussolino, 2008). In a Canadian LTC study (Ioannidis, et al., 2012a), 54 per cent of all residents and 69 per cent of residents taking ≤ 400 IU/day had sub-optimal levels [25-hydroxyvitamin D (25(OH)D) < 75 nmol/L] for bone health (Hanley et al., 2010).
In 2005, the Ontario Ministry of Health and LTC launched the Ontario Osteoporosis Strategy (Jaglal et al., 2010; Osteoporosis Action Plan Committee, 2003). This ongoing, population-based, strategic action plan is targeted at improving osteoporosis prevention and care across all residents in Ontario, with the overall goal of reducing morbidity, mortality, and costs from osteoporosis-related fractures. Its five main objectives are (a) health promotion; (b) access and appropriate utilization for bone mineral density testing; (c) targeted post-fracture care including improved assessment and treatment for osteoporosis; (d) professional education; and (e) research and evaluation (Jaglal et al., 2010; Osteoporosis Action Plan Committee, 2003). To accomplish and implement these objectives, several initiatives are targeted at distinct populations. In 2007, an LTC-focused component of this provincial strategy was added (the remainder of the article focuses on that component, the Ontario Osteoporosis Strategy for LTC).
The Ontario Osteoporosis Strategy for LTC has undertaken a province-wide program of outreach activities to increase awareness about fracture prevention specifically in LTC, with a focus on the importance of appropriate vitamin D and calcium intake, and on falls prevention. To date, knowledge translation activities have included (a) environmental scans, (b) systematic reviews, (c) barrier analysis, (d) creating and disseminating a 10-minute educational video and Fracture Prevention Toolkits, (e) launching a website (www.osteoporosislongtermcare.ca), and (f) educational outreach (Ioannidis et al., 2012a; Ioannidis et al., 2012b; Kennedy et al., 2011b; Kennedy et al., 2012a; 2012b; Lau et al., 2010; Sawka, Ismaila, Cranney, et al., 2010; Sawka, Ismaila, Raina, et al., 2010).
Osteoporosis and fracture prevention in LTC is multi-faceted and includes falls prevention activities, risk assessments, ensuring adequate intake of calcium and vitamin D, and balance and strengthening exercises (Demontiero, Hermann, & Duque, 2011; Papaioannou et al., 2010b). For residents at highest risk of fractures, hip protectors and osteoporosis medications are options that should be considered (Sawka, Ismaila, Cranney, et al., 2010; Papaioannou et al., 2010b). Pharmacologic therapy is indicated for patients at (1) high absolute fracture risk (> 20% probability over 10 years) based on risk tools (Leslie et al., 2010; Leslie et al., 2011a) and (2) individuals over age 50 with a hip or vertebral fracture or more than one fragility fracture (Papaioannou et al., 2010b). Several studies in Ontario (Giangregorio et al., 2009; Ioannidis et al., 2012a) and other regions (Colon-Emeric et al., 2007b; Jachna, Shireman, Whittle, Ellerbeck, & Rigler, 2005; Kamel, 2007; Parikh, Avorn, & Solomon, 2009; Parikh, Mogun, Avorn, & Solomon, 2008; Wright, 2007) have demonstrated that the management of osteoporosis and fractures is sub-optimal in LTC residents.
In 2007, at the outset of the Ontario Osteoporosis Strategy for LTC, we conducted an environmental scan to examine the prescribing of vitamin D, calcium, and osteoporosis medications in a convenience sample of 10 Ontario LTC homes. In 2012, we had access to prescribing records and facility characteristics for a large, unselected cohort of Ontario LTC homes (n = 166). Thus, the primary purpose of this analysis was to describe and compare vitamin D, calcium, and osteoporosis medication prescribing rates before and after implementing the Ontario Osteoporosis Strategy for LTC. A secondary objective was to examine the association between resident/facility characteristics and prescribing rates. Although we cannot infer any causal associations in this descriptive study, our analysis of prescribing trends and correlates will highlight the impact of wide-scale outreach activities and provide guidance regarding the direction of future knowledge translation efforts.
Methods
Setting
In Ontario, all LTC homes are licensed or approved by the provincial health ministry. Termed “nursing homes” or “aged care homes” in other jurisdictions, these facilities provide assistance with activities of daily living and access to 24-hour nursing care (Ontario Ministry of Health and Long-Term Care, 2013).
Study Cohorts
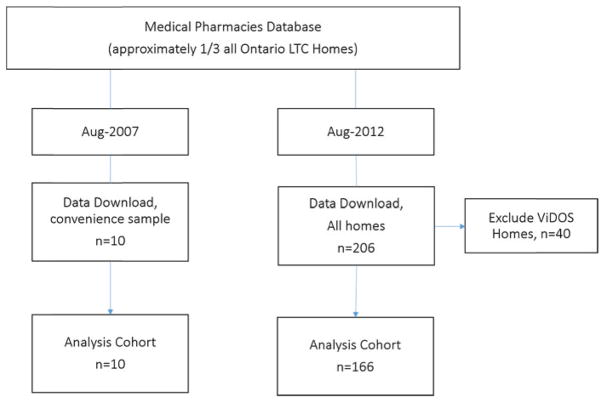
Data for both cohort years (2007 and 2012) were obtained from Medical Pharmacies, a large pharmacy provider that provides medications, clinical support, and consulting services to approximately one-third of all LTC homes in Ontario (> 40,000 residents). As outlined in Figure 1, in August 2007, de-identified medication and demographic data were downloaded from a sample of 10 LTC homes from across Ontario. The homes were quasi-randomly selected by the pharmacy database manager (i.e., no formal randomization technique was employed, but the manager selected a convenience sample of homes to ensure geographical coverage across the province). In August 2012, data were downloaded for all Ontario LTC homes serviced by Medical Pharmacies (n = 206), excluding 40 homes that participated in the Vitamin D and Osteoporosis Study (ViDOS). Briefly, ViDOS was a pilot, cluster randomized trial examining the feasibility and effectiveness of a more intensive, multifaceted, knowledge translation intervention targeting fracture prevention in LTC (Kennedy et al., 2012a). Professional Advisory Committees (physicians, nurses, pharmacists, and other staff) at intervention homes participated in three small-group, interactive educational meetings over 12 months. Content at the sessions, which were facilitated by an expert opinion leader, included a standardized presentation, question-and-answer session, action planning for quality improvement, and audit and feedback review. Control homes received the same knowledge translation as all other LTC homes in Ontario.
Figure 1.
Flowchart of the study population
Knowledge Translation Activities
Targeting LTC health care professionals (including medical, nursing, pharmacy, rehabilitation, and dietary), the Ontario Osteoporosis Strategy for LTC has implemented several key knowledge translation activities including educational meetings, educational outreach, and development and dissemination of educational materials.
Physicians and nurse consultants with expertise in osteoporosis and geriatrics provide ongoing continuous medical education including presentations at annual conferences [Ontario Long Term Care Physicians, Registered Nurses Association of Ontario (RNAO)] and LTC forums; materials and practice tools are distributed at exhibitor booths.
Partnerships with these professional organizations and other stakeholder groups – Ontario College of Family Physicians, Residents and Family Councils, Ontario Long Term Care Association, Ontario Association of Non-Profit Homes & Services for Seniors, corporate multi-facility chains, provincial falls prevention strategy – have resulted in opportunities to engage LTC professionals, corporate leaders, and policy makers. For example, representatives from these organizations serve on the Ontario Osteoporosis Strategy for LTC’s advisory council and have facilitated surveys regarding awareness and information needs among their members.
The development and dissemination of educational materials has been another key component of the Ontario Osteoporosis Strategy for LTC. Fracture Prevention Toolkits were developed and delivered to all LTC homes in the province. The toolkits provide practical, evidence-based materials tailored specifically to the LTC setting including posters, best-practices checklists, pocket cards, and point-of-care tools. In addition, a 10-minute DVD (Meeting the Challenge of Osteoporosis and Fracture Prevention) was developed as a resource for staff training and education. Optional training webinars were developed to introduce LTC homes to the concepts and materials contained in the toolkits. In 2011, the Ontario Osteoporosis Strategy for LTC website was launched and promoted in all LTC homes across Ontario. In addition to providing information and resources (e.g., PowerPoint modules), registered users receive e-newsletters and have access to an online community of practice that encourages the sharing of ideas and best practices.
Targeted educational outreach is also delivered by 13 Osteoporosis Canada area managers who are responsible for implementing and integrating all Ontario Osteoporosis Strategy (including non-LTC) projects, building relationships, and disseminating information in the community and institutions (Jaglal et al., 2010). Within LTC, these area managers deliver in-services to front-line staff and families and encourage the implementation of toolkits and best practices for fracture prevention.
Data Sources and Outcomes
Data were downloaded from a central pharmacy database that contained all residents' medication/supplement orders. In 2007, we included the bisphosphonates etidronate, alendronate, and risedronate as osteoporosis medications. In 2012, we added more recently approved medications [i.e., zoledronic acid (November 2007), teriparidide (February 2010), and denosumab (August 2010)]. We calculated the total quantity of vitamin D (IU) and calcium (mg) consumed daily, which included multivitamins/minerals, and medications containing calcium and vitamin D. Daily values were derived from weekly and monthly formulations. In 2012, validation checks were performed, comparing our program with a method that included manual identification of medications by pharmacists; discrepancies were reviewed until matching results were obtained.
A binary outcome was created for prescription of any osteoporosis medication. Based on daily intakes, we created binary outcomes for vitamin D ≥ 800 IU/day and calcium ≥ 500 mg/day. These were chosen to be consistent with 2010 Osteoporosis Canada clinical practice guidelines (Papaioannou et al., 2010b), which recommend vitamin D supplementation ≥ 800 IU/day for adults over age 50, and 1200 mg/day of elemental calcium from both diet and supplementation. Typically, supplementation with 500 mg/day of calcium would be required to meet daily targets, as dietary intake of calcium among Canadian LTC residents has been estimated to be far below the recommended amount. In one small Canadian study (Lengyel, Whiting, & Zello, 2008), mean dietary intake was 600 mg/day (SD = 261) for women and 780 mg/day (SD = 268) for men in LTC. Facility characteristics such as number of beds, profit status, geographical location, and chain affiliation were collected from publicly available information on the Health Ministry website.
Analyses
Descriptive statistics [means, standard deviations (SD), counts (%), ranges (min, max) and quartiles (Q1, Q3; i.e., middle 50%)] were tabulated as appropriate. Only facility-level demographic characteristics (mean age, percentage female, and number of resident beds) were available for the 2007 cohort; additional characteristics including profit status, chain affiliation, and mean number of doctors per facility are reported for 2012. Differences in demographic characteristics between cohort years were examined using the general linear model (GLM) procedure.
Facility-level prescribing rates were calculated as point prevalence estimates: the numerator was all residents with the relevant medication/supplement order on the day of the data download, and the denominator was all current residents on that day. Box-plots were constructed to describe the distribution of prescribing rates across LTC homes. The average change in facility-level prescribing rates between cohort years (2012 compared to 2007) was determined using weighted multiple linear regression analyses, adjusted for age, sex, and home size. This technique accounts for differences in precision, which is a function of the sample size and the estimate itself. Each facility-level prescribing rate was assigned a weight equal to the reciprocal of its variance.
Correlates of prescribing were examined only for the 2012 cohort, as additional resident and facility-level data were available. The generalized estimating equations technique (Hardin & Hilbe, 2003), assuming an exchangeable correlation structure, was used to examine the relationship between resident/facility characteristics and prescribing rates. Facility-level variables included (a) home size (small: < 100; medium: 100–199: large ≥ 200 beds), (b) profit status (for-profit, municipal/government, non-profit), (c) chain affiliation (chain/non-chain), (d) number of prescribing physicians per home, and (e) the population size of the community in which the home was located. Resident variables included age and sex. The LTC home was the clustered variable in all analyses. The results are reported as odds ratios [OR’s] and 95 per cent CI’s.
All analyses were conducted separately for vitamin D, calcium, and osteoporosis medications using SAS Institute’s SAS version 9.1 and IBM SPSS Statistics version 20. The criterion for statistical significance was alpha = 0.05. Ethics approval was received from the Hamilton Health Sciences/McMaster University Faculty of Health Sciences Research Ethics Board.
Results
The 2007 cohort was n = 2,098 residents living in 10 LTC homes, and the 2012 cohort was n = 21,699 residents living in 166 LTC homes (see Figure 1). Facility characteristics are displayed in Table 1. The mean facility size (i.e., number of beds) was greater for the 2007 versus 2012 cohort (p < .05). The percentage of all residents taking vitamin D (≥ 800 IU/day), calcium (≥ 500 mg/day), and osteoporosis medication, respectively, was 31.3 per cent (34.9% of women; 25.8% of men), 26.2 per cent (31.1% of women; 16.8% of men), and 17.2 per cent (21.6% of women; 8.8% of men) in 2007, and 59.4 per cent (63.2% of women; 50.4% of men), 33.0 per cent (37.5% of women; 22.3% of men), and 18.1 per cent (22.2% of women; 8.3% of men) in 2012.
Table 1.
Comparison of LTC home baseline characteristics for 2007 and 2012 cohorts
| Characteristic | 2007
|
2012
|
|---|---|---|
| n = 10 | n = 166 | |
| Resident age, mean (SD) | 82.9 (1.73) | 83.7 (2.66) |
| Proportion female, mean (SD) | 0.66 (0.06) | 0.70 (0.07) |
| Home size (number of beds) | ||
| mean (SD) | 209.8 (45.0) | 130.7 (77.6)a |
| min, max | 118, 286 | 16, 459 |
| Prescribers per home, mean (SD) | NA | 5.83 (3.82) |
| For-profit, % | NA | 56.6% |
| Corporate chain affiliation, % | NA | 44.6% |
| Community size (location of home), median | NA | 53,203 |
| (Q1, Q3) | (7638, 507 096) | |
p < .05
NA = data not available
SD = standard deviation
Change in Facility Prescribing Rates: 2007 to 2012
Table 2 presents the weighted, mean facility-level prescribing rates for 2007 and 2012, and the estimated change between cohort years. Between 2007 and 2012, prescribing rates increased by 38.2 per cent (95% CI: 29.0, 47.3; p < .001) for vitamin D and by 4.0 per cent (95% CI: = −3.9, 12.0; p = .318) for calcium, but the latter was not significant. There was no significant difference in osteoporosis medication prescribing rates between cohort years (0.2%, 95% CI: −3.3, 3.7; p = .91).
Table 2.
Change in facility-level prescribing rates from 2007 to 2012, per cent (95% CI)
| Prescribing Practice | Mean, Weighted Facility Prescribing Rates* | Prescribing Changea | |
|---|---|---|---|
| 2007 | 2012 | 2012–2007 (95% CI) | |
| Vitamin D | 25.4 (16.7, 34.1) | 63.6 (60.8, 66.3) | 38.2 (29.0, 47.3) |
| Calcium | 23.5 (0.16, 0.31 | 27.6 (25.3, 29.8 | 4.0 (−3.9, 12.0) |
| OP medication | 15.4 (12.1, 18.7) | 15.6 (14.6, 16.6) | 0.2 (−3.3, 3.7) |
Weighted by the reciprocal of the error variance of facility prescribing rates and adjusted for age, sex, and facility size.
Distribution of Prescribing across LTC Homes
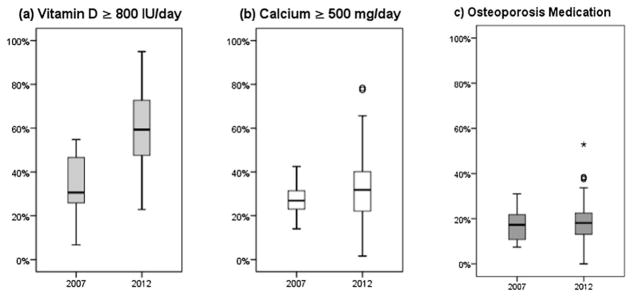
Figure 2a illustrates the spread in vitamin D (≥ 800 IU/day) prescribing rates across LTC homes, ranging from 7 to 55 per cent in 2007 and 23 to 95 per cent in 2012. The prescribing rates of vitamin D for the middle 50 per cent of homes (i.e., Q1, Q3) were 24 to 47 per cent in 2007 and 48 to 73 per cent in 2012.
Figure 2.
Distribution of facility-level prescribing rates across Ontario LTC homes for (a) vitamin D ≥ 800 IU/day, (b) calcium ≥ 500 mg/day, and (c) osteoporosis medication
Figure 2b shows the spread in calcium (≥500 mg/day) prescribing rates across LTC homes, ranging from 14 to 43 per cent in 2007 and 2 to 78 per cent in 2012. The calcium prescribing rates for the middle 50 per cent of homes were 22 to 32 per cent in 2007 and 22 to 40 per cent in 2012.
As displayed in Figure 2c, compared with the supplements, there appeared to be less dispersion in prescribing rates for osteoporosis medications across LTC homes, and the distributions were similar for both cohort years. Osteoporosis medication prescribing across LTC homes ranged from 7–31 per cent in 2007 and 0–53 per cent in 2012. Prescribing rates for the middle 50 per cent of homes were 10 to 23 per cent in 2007 and 13 to 23 per cent in 2012.
In 2012, we also examined the various types of osteoporosis medications prescribed. The percentage of all residents who received an osteoporosis medication, by sub-type, was 17.2 per cent bisphosphonate and 0.9 per cent denosumab.
Correlates of Prescribing (2012)
We examined several facility-level (home size, profit status, chain affiliation, number of prescribing physicians, community population size) and resident-level variables (age and sex) in relation to prescribing rates. As displayed in Table 3, increasing age, number of physicians, and community size were positively associated with prescribing. Males were less likely to be prescribed osteoporosis supplements/medications. There were no significant associations between prescribing and chain status, profit status, or LTC home size.
Discussion
This study examined prescribing patterns before and after the initiation of a provincial knowledge translation strategy focused on improving fracture prevention within Ontario LTC homes. Although we could not assess causality, our results suggest some improvement in evidence-based prescribing practices during the study period. There was increased uptake of the recommendation to prescribe appropriate amounts of vitamin D (i.e., ≥ 800 IU/day). Between 2007 and 2012, the estimated increase in vitamin D prescribing was nearly 40 per cent, and by 2012 vitamin D (≥ 800 IU/day) prescribing rates in the upper quartile of LTC homes were between 73 and 95 per cent (Figure 2a). Despite the substantial increase in overall vitamin D prescribing, the considerable spread in prescribing between homes suggests ongoing knowledge translation efforts are needed to target homes with low rates and that home-specific barriers should be addressed.
We observed a four per cent non-significant increase in calcium prescribing between 2007 and 2012; there appeared to be greater dispersion in facility prescribing rates for 2012 (see Figure 2b). We hypothesize that this spread may reflect some of the uncertainty about the risks and benefits of calcium in light of publications reporting an increased risk of cardiovascular events associated with calcium supplementation (Bolland et al., 2010). Furthermore, in the 2010 Osteoporosis Canada clinical practice guidelines (Papaioannou et al., 2010b), there is greater emphasis on obtaining calcium thru dietary means rather than through supplements. Few studies have reported on dietary calcium intake in LTC residents; however, one small Canadian study suggests that a 500 mg supplementation would be required for most LTC residents to meet the daily calcium target (Lengyel et al., 2008).
Despite the availability of newly approved medications, prescribing of osteoporosis medications did not appear to increase between 2007 and 2012. In both cohort years, three-quarters of LTC homes had prescribing rates below 23 per cent and with the exception of outliers, all homes had prescribing rates below 34 per cent (see Figure 2c). We are not able to comment on the appropriateness of the prescribing rates since we did not have access to information regarding the risk status of residents (i.e., documented osteoporosis or fractures). We do know from our recent surveys that many LTC physicians recognize the value of osteoporosis medications for high-risk residents (Sawka, Ismaila, Raina, et al., 2010; Wall et al., 2013), but there is still a great deal of uncertainty regarding (1) the assessment of fracture risk (e.g., bone mineral density testing is difficult in LTC residents; Kennedy et al., 2011b; Kennedy et al., 2012a; Wall et al., 2013) and application of existent tools (Leslie, Berger, et al., 2011; Leslie, Lix, et al., 2011) may be impractical; (2) treatment benefits for LTC residents; and (3) knowing whom to treat, particularly residents at moderate fracture risk (Wall et al., 2013). To address these practice-level barriers for managing osteoporosis and fractures in LTC, in early 2013 the Ontario Osteoporosis Strategy for LTC held a Canadian Institutes of Health Research (CIHR) consensus conference to adapt the 2010 Osteoporosis Canada clinical practice guidelines for frail elderly and LTC residents. Future knowledge translation efforts will be aimed at disseminating these guidelines to LTC practitioners.
Comparison with Other Studies
The 2007 prescribing rates in our study were similar or higher than other studies conducted prior to this time. In these studies only 6 to 25 per cent of residents, many of whom were selected based on high-risk status, received an osteoporosis medication (Colon-Emeric et al., 2007; Jachna et al., 2005; Parikh et al., 2011; Parikh et al., 2008). Several studies did not examine vitamin D and calcium use, as they utilized reimbursement databases, and supplements are not adequately captured. In a Canadian study based on 2005/2006 Resident Assessment Instrument –Minimum Data Set 2.0 data (RAI-MDS 2.0; n = 17 LTC homes in Ontario and Manitoba), approximately 27 per cent of high-risk residents (i.e., documented osteoporosis or fracture) were prescribed any calcium and vitamin D, with 6.5 per cent and 3.6 per cent prescribed calcium or vitamin D, respectively, and 19 per cent prescribed a multivitamin (Giangregorio et al., 2009). In American studies, Kamel (2007) reported that fewer than 12 per cent of all residents received any calcium or vitamin D supplementation, and Gupta and Aronow (2003) reported that 57 per cent of female residents received calcium and 32 per cent vitamin D, but this included low-dose supplementation (e.g., vitamin D 200 IU/day).
There were limited studies with which to compare our 2012 results. Of the few available, our prescribing rates were similar for calcium and substantially higher for vitamin D. In Canadian studies (2009–2010 data), 25 per cent to 45 per cent were taking calcium supplementation and less than 35 per cent were taking vitamin D ≥ 800 IU/day (Ioannidis et al., 2012a; Viveky et al., 2012). Similarly, in a recent American study based in an academic-affiliated LTC centre, 35 per cent of residents received vitamin D ≥ 800 IU/day prior to a quality improvement intervention (Yanamadala, Heflin, White, & Buhr, 2012).
Prescribing Correlates and Variation between Homes (2012)
In multivariable analyses, both female gender and increasing age were associated with prescription of vitamin D, calcium, and osteoporosis medications. The association with gender is similar to that in other studies (Colon-Emeric et al., 2007b; Parikh et al., 2011), but the association with age is in contrast to findings of another study, which found a reverse association with age in LTC residents with fractures (Parikh et al., 2011). When we examined facility-level variables, greater number of treating physicians per home was associated with greater prescribing of vitamin D and calcium. Although we are not entirely sure why this relationship existed, we know that some LTC homes in Ontario adopt standardized policies such as admission orders for vitamin D and calcium (Kennedy et al., 2011b). Although we were unable to examine the use of standard policies in this study, it is possible that homes with several physicians may have a greater need to employ standardized care policies such as standard orders for vitamin D and calcium. It is also possible that homes with a higher number of physicians have a greater chance of having at least one advocate for implementing osteoporosis best practices. There was only a small association between increasing community size and calcium and osteoporosis prescribing (e.g., approximate six per cent increase in odds for a community of 1,000,000 versus 100,000). Chain status, profit status, and LTC home size were not significantly related to prescribing rates, which was similar to results in other studies examining osteoporosis management (Colon-Emeric et al., 2007b; Parikh et al., 2011).
We observed considerable spread in prescribing rates between homes, particularly for vitamin D and calcium. We were not able to control for differences in resident case-mix among facilities, and it is possible that varied case-mix was in part responsible for observed differences in facility-level prescribing. However, given there are few contra-indications for calcium and vitamin D, it seems unlikely that this was a major contributing factor. Our results, and those of others (Colon-Emeric et al., 2007b; Parikh et al., 2011), indicate that most facility characteristics are not associated with osteoporosis-related prescribing. Similarly, studies examining variation in anti-psychotic prescribing have reported that substantial inter-home variation remained after adjusting for a range of facility and resident characteristics (Huybrechts et al., 2012; Rochon, 2007). In contrast, studies examining non-prescribing quality measures – including restraint use, pressure ulcer prevalence, staffing levels, complaints, and government regulatory measures – report that non-profit versus for-profit homes demonstrate higher quality of care (Comondore et al., 2009; McGregor et al., 2005; McGregor et al., 2011). If resident and facility characteristics cannot adequately account for differences in prescribing, further attention is needed to consider other potential factors including facility culture, operational policies, staffing levels, and prescriber characteristics. Interestingly, research by Curtis et al. (2009) suggested that for osteoporosis medications, prescribing sub-cultures within individual LTC homes were not as influential as individual physician preferences. In a three-level model, the physician clustering effect was not significant, and the authors emphasized the importance of targeting knowledge translation efforts at individual physicians. Similarly, a recent study in Ontario LTC homes indicated that prescriber characteristics were more influential than resident characteristics in influencing antibiotic treatment courses (Daneman et al., 2013).
Implications for Knowledge Translation
We are encouraged that adequate vitamin D and calcium prescribing improved after implementing the Ontario Osteoporosis Strategy for LTC. It is possible that other factors contributed to the observed uptake, particularly these two: (1) increased media, societal, and academic attention regarding the benefits of vitamin D; and (2) the publication of the updated Osteoporosis Canada clinical practice guidelines in 2010 (Papaiannou et al., 2010). While these were likely contributing factors, passive approaches to disseminating research evidence (e.g., publication of clinical guidelines, academic conferences) are not sufficient to produce large practice changes (Grol & Grimshaw, 2003). For example, despite the publication and dissemination of Canadian osteoporosis guidelines in 2002 (Brown & Josse, 2002), a considerable osteoporosis care gap remained in both community-dwelling and institutionalized cohorts (Leslie et al., 2012; Giangregorio, 2006; Papaioannou et al., 2004; 2008). Similarly, the benefits of calcium and vitamin D for LTC residents have been well known since Chapuy et al.’s (1992) widely cited publication, yet little uptake in their use occurred in the decade after the study was published (Kamel, 2007; Parikh et al., 2008; Rojas-Fernandez, Lapane, MacKnight, & Howard, 2002).
Considerable knowledge translation efforts are required to make substantial improvements in health care practices. As Grol (2010) suggested:
For guidelines to have an impact on actual care, they need to be integrated with other quality improvement initiatives, such as performance measurement and quality improvement programmes. This requires intensive collaboration between the organisations responsible for these tasks, which is lacking in most countries.
We believe initiatives such as the Ontario Osteoporosis Strategy (Jaglal et al., 2010) are taking important and necessary steps in translating research evidence into practice and policy via an integrated approach that involves multiple sectors including government, health care organizations, community agencies, patient associations, researchers, and front-line professionals.
In addition to practice-level barriers to osteoporosis and fracture care [i.e., impracticality of bone mineral density testing, difficulty in applying fracture risk assessment tools, uncertainty regarding benefits for LTC residents, and confusion regarding whom to treat] (Colon-Emeric et al., 2004; Kennedy et al., 2011b; Kennedy et al., 2012a; Lau et al., 2010; McKercher, Crilly, & Klosek, 2000; Sawka et al., 2010b; Wall et al., 2013), several organizational barriers also exist. These include (a) not including osteoporosis and fracture risk assessment as part of standardized processes (e.g., admission, quarterly reviews); (b) not capturing risk variables electronically; and (c) failure to incorporate preventative osteoporosis and fracture strategies into formal care-plans (Colon-Emeric et al., 2004; Colon-Emeric et al., 2007a; Kennedy et al., 2011b; Kennedy et al., 2012a; Kennedy et al., 2013). Given the interdisciplinary, team-based approach to care in LTC facilities, it is imperative that knowledge translation efforts target both practice-level and organizational changes.
Strengths and Limitations
The strengths of this study include the use of a pharmacy database that has a well-developed system for capturing vitamin D, calcium, and osteoporosis medication variables. Our sample of LTC homes, particularly in the smaller 2007 cohort, were subject to both sampling bias (i.e., non-representative sample of homes) and sampling error (i.e., even with random sampling, potential differences between the sample and population values). For both cohort years we lacked a complete range of resident variables, particularly patient case-mix. We were able to examine some facility and resident characteristics in our 2012 cohort, but were unable to consider other factors such as staffing ratios and prescriber characteristics. The benefits of using a single pharmacy database were the completeness and uniformity of its medication information; however, it is possible the LTC homes serviced by the provider are not representative of other LTC homes in the province. Due to our continued partnership that we have had with the provider, including initiatives to improve other types of prescribing (Kennedy et al., 2011a; Papaioannou et al., 2010a), it is possible that some homes would represent the best-case scenario. If this were the case, it is possible that overall our rates could be over-estimated; however, since we used the same pharmacy provider for both cohort years, it would not impact our estimates of five-year change.
Summary
For the past several years, the Ontario Osteoporosis Strategy for LTC has implemented wide-scale knowledge translation activities in LTC homes across the province. Although we cannot assess causality in our study, our findings suggest that wide-scale knowledge translation activities were successful in improving vitamin D prescribing within Ontario LTC, although ongoing efforts are needed to target homes with low uptake.
The consensus meeting we held in 2013, to adapt the Osteoporosis Canada clinical practice guidelines for the frail elderly and LTC residents, will provide more explicit guidance regarding the management of osteoporosis and fractures in LTC. Ongoing knowledge translation activities should be aimed at disseminating these adapted guidelines and decreasing variation in management practices between LTC homes. Wide-scale changes to knowledge management systems are also necessary for improving fracture risk assessment and better integrating practice guidelines. Even though this impact evaluation only examined prescribing outcomes, we are encouraged by the substantial uptake observed for vitamin D prescribing five years after initiating the Ontario Osteoporosis Strategy for LTC. Future evaluations should also consider process changes and examine falls and fractures outcomes.
Table 3.
Associations between prescribing rates and resident/facility characteristics in 2012
| Characteristic | Vitamin D | Calcium | Osteoporosis Medications |
|---|---|---|---|
|
| |||
| OR (95% CI) | |||
| Resident-level | |||
| Age, per 10 years | 1.08 (1.05, 1.11) | 1.05 (1.02, 1.09 | 1.06 (1.02, 1.11) |
| Male sex | 0.61 (0.57, 0.65) | 0.48 (0.45, 0.52) | 0.33 (0.30, 0.37) |
| Facility-level | |||
| Community population, per 100,000 persons | 1.00 (0.99, 1.00) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.01) |
| Number of prescribing physicians | 1.05 (1.02, 1.09) | 1.03 (1.01, 1.05) | 1.01 (0.99, 1.04) |
| Corporate chain affiliationa | 0.96 (0.69, 1.33) | 0.95 (0.67, 1.34) | 1.03 (0.81, 1.30) |
| LTC home size | |||
| Medium (100–199 beds)b | 0.97 (0.74, 1.27) | 1.04 (0.84, 1.28) | 1.08 (0.91, 1.27) |
| Large (≥ 200 beds)b | 0.78 (0.53, 1.13) | 0.90 (0.67, 1.19) | 0.85 (0.63, 1.14) |
| Profit status | |||
| Municipalc | 1.10 (0.76, 1.59) | 1.03 (0.71, 1.48) | 0.96 (0.73, 1.28) |
| Non-profitc | 1.06 (0.71, 1.58) | 1.35 (0.91, 2.02) | 1.20 (0.92, 1.58) |
Bolded estimates indicate significance at alpha < 0.05.
Reference category is no chain affiliation.
Reference category is small (< 100 beds).
Reference category is for-profit.
Footnotes
We thank the Ministry of Health and Long-Term Care in Ontario for their support of the Ontario Osteoporosis Strategy.
References
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. American Journal of Medicine. 2004a;116(9):634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. American Journal of Clinical Nutrition. 2004b;80(3):752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. American Journal of Clinical Nutrition. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. The Official Journal of the American Society of Bone and Mineral Restoration. 2009;24(5):935–942. doi: 10.1359/JBMR.081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. British Medical Journal. 2010;341(c3691):1–9. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. Canadian Medical Association Journal. 2002;167(10 Suppl):S1–S34. [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multi-ethnic cohort of women: The women's health initiative (WHI) The Official Journal of the American Society of Bone and Mineral Research. 2011;26(10):2378–2388. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. New England Journal of Medicine. 1992;327(23):1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY. Age-related changes in trabecular and cortical bone microstructure. International Journal of Endocrinology. 2013;2013(2013):1–9. doi: 10.1155/2013/213234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Emeric CS, Casebeer L, Saag K, Allison J, Levine D, Suh TT, et al. Barriers to providing osteoporosis care in skilled nursing facilities: Perceptions of medical directors and directors of nursing. Journal of the American Medical Directors Association. 2004;5(6):361–366. doi: 10.1097/01.JAM.0000141950.34986.EE. [DOI] [PubMed] [Google Scholar]
- Colon-Emeric CS, Lekan D, Utley-Smith Q, Ammarell N, Bailey D, Corazzini K, et al. Barriers to and facilitators of clinical practice guideline use in nursing homes. Journal of the American Geriatrics Society. 2007a;55(9):1404–1409. doi: 10.1111/j.1532-5415.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Emeric CS, Lyles KW, Levine DA, House P, Schenck A, Gorospe J, et al. Prevalence and predictors of osteoporosis treatment in nursing home residents with known osteoporosis or recent fracture. Osteoporosis International. 2007b;18(4):553–559. doi: 10.1007/s00198-006-0260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comondore VR, Devereaux PJ, Zhou Q, Stone SB, Busse JW, Ravindran NC, et al. Quality of care in for-profit and not-for-profit nursing homes: Systematic review and meta-analysis. BMJ. 2009;339(b2732):1–15. doi: 10.1136/bmj.b2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crilly RG, Tanner DA, Kloseck M, Chesworth BM. Hip fractures in long-term care: Is the excess explained by the age and gender distribution of the residents? Journal of Aging Research. 2010;2010(291258):1–5. doi: 10.4061/2010/291258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JR, Arora T, Xi J, Silver A, Allison JJ, Chen L, et al. Do physicians within the same practice setting manage osteoporosis patients similarly? implications for implementation research. Osteoporosis International. 2009;20(11):1921–1927. doi: 10.1007/s00198-009-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman N, Gruneir A, Bronskill SE, Newman A, Fischer HD, Rochon PA, et al. Prolonged antibiotic treatment in long-term care: Role of the prescriber. JAMA International Medicine. 2013;173(8):673–682. doi: 10.1001/jamainternmed.2013.3029. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. American Journal of Clinical Nutrition. 2008;88(2):537S–540S. doi: 10.1093/ajcn/88.2.537S. [DOI] [PubMed] [Google Scholar]
- Demontiero O, Herrmann M, Duque G. Supplementation with vitamin D and calcium in long-term care residents. Journal of the American Medical Directors Association. 2011;12(3):190–194. doi: 10.1016/j.jamda.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Flicker L, Mead K, MacInnis RJ, Nowson C, Scherer S, Stein MS, et al. Serum vitamin D and falls in older women in residential care in Australia. Journal of the American Geriatrics Society. 2003;51(11):1533–1538. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. Journal of Gerontology A: Biological Science Medical Science. 2007;62(7):744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatric Society. 2009;57(3):492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoli A, Jr, Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: Results from the women’s health and aging study (WHAS) II. Bone. 2011;48(4):952–957. doi: 10.1016/j.bone.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Jantzi M, Papaioannou A, Hirdes J, Maxwell CJ, Poss JW. Osteoporosis management among residents living in long-term care. Osteoporosis International. 2009;20(9):1471–1478. doi: 10.1007/s00198-009-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangregorio LM, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: An international phenomenon. Seminars in Arthritis and Rheumatism. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Gielen E, Verschueren S, O’Neill TW, Pye SR, O’Connell MD, Lee DM, et al. Musculoskeletal frailty: A geriatric syndrome at the core of fracture occurrence in older age. Calcified Tissue International. 2012;91(3):161–177. doi: 10.1007/s00223-012-9622-5. [DOI] [PubMed] [Google Scholar]
- Gloth FM, Simonson W. Osteoporosis is under-diagnosed in skilled nursing facilities: A large-scale heel BMD screening study. Journal of the American Medical Directors Association. 2008;9(3):190–193. doi: 10.1016/j.jamda.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Grol R, Grimshaw J. From best evidence to best practice: Effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- Grol R. Has guideline development gone astray? Yes. BMJ. 2010;340:c306. doi: 10.1136/bmj.c306. [DOI] [PubMed] [Google Scholar]
- Gupta G, Aronow WS. Underuse of procedures for diagnosing osteoporosis and of therapies for osteoporosis in older nursing home residents. Journal of the American Medical Directors Association. 2003;4(4):200–202. doi: 10.1097/01.JAM.0000073963.49915.FC. [DOI] [PubMed] [Google Scholar]
- Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD. Guidelines Committee of the Scientific Advisory Council of Osteoporosis Canada. Vitamin D in adult health and disease: A review and guideline statement from osteoporosis Canada (summary) Canadian Medical Association Journal. 2010;182(12):1315–1319. doi: 10.1503/cmaj.091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized estimating equations. 1. New York, NY: Chapman & Hall/CRC; 2003. [Google Scholar]
- Holvik K, Ahmed LA, Forsmo S, Gjesdal CG, Grimnes G, Samuelsen SO, et al. Low serum levels of 25-hydroxyvitamin D predict hip fracture in the elderly. A NOREPOS study. The Journal of Clinical Endocrinology and Metabolism. 2013;98(9):3341–3350. doi: 10.1210/jc.2013-1468. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Rothman KJ, Brookhart MA, Silliman RA, Crystal S, Gerhard T, et al. Variation in antipsychotic treatment choice across US nursing homes. Journal of Clinical Psychopharmacology. 2012;32(1):11–17. doi: 10.1097/JCP.0b013e31823f6f46. [DOI] [PubMed] [Google Scholar]
- Ioannidis G, Kennedy CC, Dykeman J, Dudziak S, Papaioannou A. Association between vitamin D3 supplementation and serum 25-hydroxyvitamin D levels in older individuals residing in long-term care in Ontario, Canada. Journal of the American Geriatrics Society. 2012a;60(5):985–987. doi: 10.1111/j.1532-5415.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis G, Papaioannou A, Kennedy CC, Giangregorio LM, Thabane L, Eappen J. What organizational factors influence vitamin D use in nursing homes? Baseline data from the ViDOS cluster randomized controlled trial. Journal of Bone and Mineral Research. 2012b;27(Suppl 1) Retrieved 17 February 2015 from http://www.asbmr.org/education/2012-abstracts. [Google Scholar]
- Jachna CM, Shireman TI, Whittle J, Ellerbeck EF, Rigler SK. Differing patterns of antiresorptive pharmacotherapy in nursing facility residents and community dwellers. Journal of the American Geriatrics Society. 2005;53(8):1275–1281. doi: 10.1111/j.1532-5415.2005.53401.x. [DOI] [PubMed] [Google Scholar]
- Jaglal SB, Hawker G, Cameron C, Canavan J, Beaton D, Bogoch E, et al. The ontario osteoporosis strategy: Implementation of a population-based osteoporosis action plan in Canada. Osteoporosis International. 2010;21(6):903–908. doi: 10.1007/s00198-010-1206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel HK. Update on osteoporosis management in long-term care: Focus on bisphosphonates. Journal of the American Medical Directors Association. 2007;8(7):434–440. doi: 10.1016/j.jamda.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Kanwar A, Singh M, Lennon R, Ghanta K, McNallan SM, Roger VL. Frailty and health-related quality of life among residents of long-term care facilities. Journal of Aging and Health. 2013;25(5):792–802. doi: 10.1177/0898264313493003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CC, Campbell G, Garg AX, Dolovich L, Stroud JB, McCallum RE, et al. Piloting a renal drug alert system for prescribing to residents in long-term care. Journal of the American Geriatrics Society. 2011a;59(9):1757–1759. doi: 10.1111/j.1532-5415.2011.03565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CC, Papaioannou A, Ioannidis G, Giangregorio LM, Marr S, Morin SN, et al. How can we improve bone health in the long-term care setting? lessons from the ViDOS study. Journal of Bone and Mineral Research. 2011b;26(Suppl 1) Retrieved 17 February 2015 from http://www.asbmr.org/education/2011-abstracts. [Google Scholar]
- Kennedy CC, Ioannidis G, Giangregorio LM, Adachi JD, Thabane L, Morin SN, et al. An interdisciplinary knowledge translation intervention in long-term care: Study protocol for the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Implementation Science. 2012a;7(1):1–12. doi: 10.1186/1748-5908-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CC, Lohfeld L, Adachi JD, Morin SM, Marr S, Crilly RG. Is a multifaceted knowledge translation intervention feasible and acceptable in long-term care? A qualitative study with ViDOS participants. Canadian Geriatrics Journal. 2013;16(2):67. [Google Scholar]
- Kennedy CC, Papaioannou A, Ioannidis G, Giangregorio LM, Thabane L, Soleas I. What predicts osteoporosis treatment in nursing home residents: Baseline data from the ViDOS cluster randomized controlled trial. Journal of Bone and Mineral Research. 2012b;27(Suppl 1) Retrieved 17 February 2015 from http://www.asbmr.org/education/2012-abstracts. [Google Scholar]
- Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. Journal of Gerontology: Series A: Biological Sciences and Medical Sciences. 2012;67(1):48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- Lau AN, Ioannidis G, Potts Y, Giangregorio LM, Van der Horst ML, Adachi JD, et al. What are the beliefs, attitudes and practices of front-line staff in long-term care (LTC) facilities related to osteoporosis awareness, management and fracture prevention? BMC Geriatrics. 2010;10(73):1–7. doi: 10.1186/1471-2318-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel CO, Whiting SJ, Zello GA. Nutrient inadequacies among elderly residents of long-term care facilities. Canadian Journal of Dietary Practice Research. 2008;69(2):82–88. doi: 10.3148/69.2.2008.82. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Berger C, Langsetmo L, Lix LM, Adachi JD, Hanley DA, et al. Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: Results from the CaMos and Manitoba cohorts. Osteoporosis International. 2011a;22(6):1873–1883. doi: 10.1007/s00198-010-1445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie WD, Giangregorio LM, Yogendran M, Azimaee M, Morin S, Metge C, et al. A population-based analysis of the post-fracture care gap 1996–2008: The situation is not improving. Osteoporosis International. 2012;23(5):1623–1629. doi: 10.1007/s00198-011-1630-1. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Independent clinical validation of a Canadian FRAX tool: Fracture prediction and model calibration. Journal of Bone Mineral Research. 2010;25(11):2350–2358. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Lix LM, Langsetmo L, Berger C, Goltzman D, Hanley DA, et al. Construction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatment. Osteoporosis International. 2011b;22(3):817–827. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- Looker AC, Mussolino ME. Serum 25-hydroxyvi-tamin D and hip fracture risk in older U.S. white adults. Journal of Bone and Mineral Research. 2008;23(1):143–150. doi: 10.1359/jbmr.071003. [DOI] [PubMed] [Google Scholar]
- McGregor MJ, Cohen M, McGrail K, Broemeling AM, Adler RN, Schulzer M, et al. Staffing levels in not-for-profit and for-profit long-term care facilities: Does type of ownership matter? Canadian Medical Association Journal. 2005;172(5):645–649. doi: 10.1503/cmaj.1040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor MJ, Cohen M, Stocks-Rankin CR, Cox MB, Salomons K, McGrail KM, et al. Complaints in for-profit, non-profit and public nursing homes in two Canadian provinces. Open Medicine. 2011;5(4):e183–e192. [PMC free article] [PubMed] [Google Scholar]
- McKercher HG, Crilly RG, Kloseck M. Osteoporosis management in long-term care. survey of Ontario physicians. Canadian Family Physician. 2000;46:2228–2235. [PMC free article] [PubMed] [Google Scholar]
- Norris MA, Walton RE, Patterson CJS, Feightner JW the Canadian Task Force on Preventive Health Care. Prevention of falls in long-term care facilities: Systematic review and recommendations. London, ON: Canadian Task Force; 2003. Report No.: CTFPHC Technical Report. [Google Scholar]
- Ontario Ministry of Health and Long-Term Care. Home, community and residential care services for seniors: Seniors’ care: Long-term care homes. Ontario, Canada: Queen’s Printer for Ontario; 2013. Retrieved 19 January 2014, from http://www.health.gov.on.ca/en/public/programs/ltc/15_facilities.aspx. [Google Scholar]
- Osteoporosis Action Plan Committee. Osteoporosis action plan: An osteoporosis strategy for Ontario. 2003. [Google Scholar]
- Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD. The osteoporosis care gap in Canada. BMC Musculoskeletal Disorder. 2004;5(11):1–6. doi: 10.1186/1471-2474-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Kennedy CC, Campbell G, Stroud JB, Wang L, Dolovich L, et al. A team-based approach to warfarin management in long term care: A feasibility study of the MEDeINR electronic decision support system. BMC Geriatrics. 2010a;10(38):1–10. doi: 10.1186/1471-2318-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Kennedy CC, Ioannidis G, Gao Y, Sawka AM, Goltzman D, et al. The osteoporosis care gap in men with fragility fractures: The Canadian multicentre osteoporosis study. Osteoporosis International. 2008;19(4):581–587. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ. 2010b;182(17):1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Avorn J, Solomon DH. Pharmacological management of osteoporosis in nursing home populations: A systematic review. Journal of the American Geriatrics Society. 2009;57(2):327–334. doi: 10.1111/j.1532-5415.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- Parikh S, Brookhart MA, Stedman M, Avorn J, Mogun H, Solomon DH. Correlations of nursing home characteristics with prescription of osteoporosis medications. Journal of Bone and Mineral Research. 2011;48(5):1164–1168. doi: 10.1016/j.bone.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Mogun H, Avorn J, Solomon DH. Osteoporosis medication use in nursing home patients with fractures in 1 US state. Archives of Internal Medicine. 2008;168(10):1111–1115. doi: 10.1001/archinte.168.10.1111. [DOI] [PubMed] [Google Scholar]
- Rochon PA. Exploring the variation in Ontario nursing home prescribing rates for antipsychotics. Healthcare Quarterly. 2007;10(4):20–22. doi: 10.12927/hcq.2013.19309. [DOI] [PubMed] [Google Scholar]
- Rojas-Fernandez CH, Lapane KL, MacKnight C, Howard KA. Undertreatment of osteoporosis in residents of nursing homes: Population-based study with use of the systematic assessment of geriatric drug use via epidemiology (SAGE) database. Endocrine Practice. 2002;8(5):335–342. doi: 10.4158/EP.8.5.335. [DOI] [PubMed] [Google Scholar]
- Sawka AM, Ismaila N, Cranney A, Thabane L, Kastner M, Gafni A, et al. A scoping review of strategies for the prevention of hip fracture in elderly nursing home residents. PLoS One. 2010a;5(3):1–10. doi: 10.1371/journal.pone.0009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka AM, Ismaila N, Raina P, Thabane L, Straus S, Adachi JD, et al. Hip fracture prevention strategies in long-term care: A survey of Canadian physicians’ opinions. Canadian Family Physician. 2010b;56(11):e392–e397. [PMC free article] [PubMed] [Google Scholar]
- Viveky N, Toffelmire L, Thorpe L, Billinsky J, Alcorn J, Hadjistavropoulos T, et al. Use of vitamin and mineral supplements in long-term care home residents. Applied Physiology Nutrition and Metabolism. 2012;37(1):100–105. doi: 10.1139/h11-141. [DOI] [PubMed] [Google Scholar]
- Wall M, Lohfeld L, Giangregorio L, Ioannidis G, Kennedy CC, Moser A, et al. Fracture risk assessment in long-term care: A survey of long-term care physicians. BMC Geriatrics. 2013;13(109):1–7. doi: 10.1186/1471-2318-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RM. Use of osteoporosis medications in older nursing facility residents. Journal of the American Medical Directors Association. 2007;8(7):453–457. doi: 10.1016/j.jamda.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamadala M, Heflin MT, White HK, Buhr GT. Ensuring vitamin D supplementation in nursing home patients – A quality improvement project. Journal of Nutrition in Gerontology and Geriatrics. 2012;31(2):158–171. doi: 10.1080/21551197.2012.678240. [DOI] [PubMed] [Google Scholar]
- Zimmerman SI, Girman CJ, Buie VC, Chandler J, Hawkes W, Martin A, et al. The prevalence of osteoporosis in nursing home residents. Osteoporosis International. 1999;9(2):151–157. doi: 10.1007/s001980050129. [DOI] [PubMed] [Google Scholar]