Abstract
Investigating the cumulative rate of deficits and the change of a frailty index (FI) chronologically is helpful in clinical and research settings in the elderly. However, limited evidence for the change of frailty before and after some nonfatal adverse health event such as a major osteoporotic fracture (MOF) is available. Data from the Global Longitudinal Study of Osteoporosis in Women 3-Year Hamilton cohort were used in this study. The changes of FI before and after onset of MOF were compared between the women with and without incident MOF. We also evaluated the relationship between risk of MOF, falls, and death and the change of FI and the absolute FI measures. There were 3985 women included in this study (mean age 69.4 years). The change of FI was significantly larger in the women with MOF than those without MOF at year 1 (0.085 versus 0.067, p = 0.036) and year 2 (0.080 versus 0.052, p = 0.042) post-baseline. The FI change was not significantly related with risk of MOF independently of age. However, the absolute FI measures were significantly associated with increased risk of MOF, falls, and death independently of age. In summary, the increase of the FI is significantly larger in the elderly women experiencing a MOF than their peer controls, indicating their worsening frailty and greater deficit accumulation after a MOF. Measures of the FI change may aid in the understanding of cumulative aging nature in the elderly and serve as an instrument for intervention planning and assessment.
Keywords: FRAILTY, FRAILTY INDEX, MAJOR OSTEOPOROTIC FRACTURE, AGING, GLOW
Introduction
Frailty is a clinical condition with increased vulnerability, resulting from aging-related decline across physical, psychological, and social functioning.(1–3) Frailty in the elderly is becoming a serious and increasing public health issue as the worldwide population aging accelerates.(2) The approach, frailty index (FI) of deficit accumulation, has been used widely to measure frailty in the elderly.(2,4) The concept of an FI relies on the fact that individuals with more health deficits are more likely to be frail; therefore, an FI of deficit accumulation could quantify the frailty status in the elderly.(5) An FI of deficit accumulation significantly increases with chronological age.(5) However, compared with others of the same age, an individual with a higher FI is always at greater risk of adverse health outcomes.(2,6) Currently, the concept of an FI has been increasingly accepted, with more and more studies using an FI to measure frailty and reporting significant associations with risk of fractures, falls, disability, and mortality.(7–11)
Investigating the cumulative rate of deficits and the change of an FI chronologically may assist in understanding the aging process, quantifying the increased frailty status, and evaluating the impact of interventions to reduce frailty.(5,6) Some studies assessed the long-term trajectories of the FI change with chronological age and reported that the increased FI could predict risk of death independently of chronological age.(12–14) Nevertheless, limited evidence for the change of frailty before and after some nonfatal adverse health events is available. For instance, little is known about the improvement or decline of the frailty status after an elderly individual experiences a major osteoporotic fracture (MOF). Moreover, whether and to what extent the FI change can predict the following adverse outcomes remain unexplored.
In this study, we used data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) 3-year Hamilton cohort to assess the change of FI in the elderly. Our primary objective was to investigate the change of FI in individuals experiencing a MOF (hip, upper arm or shoulder, spine, or wrist) during the follow-up. The secondary objective was to evaluate the relationship between the FI change and risk of MOF, falls, and mortality.
Materials and Methods
Participants and setting
Details about GLOW have been described elsewhere.(15) Briefly, GLOW is a multicenter longitudinal cohort study involving 17 sites in 10 countries (Belgium, Italy, Spain, France, Germany, Netherlands, UK, Australia, Canada, and US). GLOW was designed to explore health consequences and risk factors for fragility fractures in women aged ≥55 years who had consulted their physician practices in the prior 2 years. The women who had no language barriers or cognitive impairment and were not institutionalized or too ill to finish the study survey were included in GLOW. The study participants were stratified by age to ensure that two-thirds of women aged ≥65 years were included into the study.(15)
This study was a longitudinal analysis of the 3-year GLOW Hamilton, Canada cohort, with a sample of approximately 4000 women enrolled between May 2008 and March 2009. Data were collected by self-report at baseline and annual follow-up using mailed questionnaires, in which the questionnaires covered the domains of participant characteristics, medication use, perception about fracture risk and osteoporosis, comorbidities, physical activity, healthcare utilization, physical function, and quality of life.(15) Telephone interviews were performed if the woman needed assistance with completing the questionnaire or did not return the mailed survey.
The FI of deficit accumulation
The multidimensional FI of deficit accumulation at baseline and during follow-up covered 34 health deficits, including comorbidities (n = 15), healthcare utilization (n = 1), symptoms and signs (n = 6), and activity of daily living (n = 12). Details on the construction of the FI have been described elsewhere based on the GLOW Hamilton cohort.(16) The deficit variables and their coding are shown in Supplemental Table S1. Briefly, for the 34 deficit variables, each deficit was dichotomized or polychotomized to map the interval 0 to 1 so that the coding could reflect the frequency or severity of deficits. For example, for the question of “feels tired” in the symptoms and signs, the response of “all the time” was coded as 1, “most of time” as 0.75, “some time” as 0.25, “a little time” as 0.25, and “none of time” as 0 (Supplemental Table S1). To calculate an FI for an individual woman, all of her values of deficits were summed up and then divided by the total number of deficit variables (n = 34), with her FI ranging from 0 to 1. For instance, if a participant had 6 deficits scoring 1 point for each deficit, 1 deficit scoring 0.5 point, and the other 27 deficits with each score of 0 point, then her FI would therefore be 6.5 divided by 34, giving an FI = 0.19.
Outcomes
At baseline, the participants were asked whether they had a previous history of falls or fractures. During the follow-up, they documented their incident fractures and the dates in the questionnaires. For falls, the women recorded incident falls in the previous 12 months but were not required to report the dates for falls.(15) Regarding death, some family members and spouses informed the study site of the participant’s death when they received questionnaires from us or when we telephoned the homes of the nonresponders. If the household of the nonresponder could not be contacted, electronic databases of obituaries were searched for entries that matched the participant’s full name and date of birth.(16) All the outcomes were self-reported in the GLOW, and no information from medical records was available.(15)
The primary outcome in this study was survival time to incident MOF (hip, upper arm or shoulder, spine, or wrist fracture) during the follow-up. The secondary outcomes included death and incident falls.
Statistical analyses
We presented data as mean and standard deviation (SD) for continuous variables and frequency and percentages for categorical variables, respectively. The density distribution of the FIs at baseline, year 1, year 2, and year 3 post-baseline was smoothed and graphed using the Gaussian kernel function.(17) To calculate the population-averaged rate of deficit accumulation across ages, we used generalized estimating equations (GEE) and robust standard error estimators to account for the effect of within-group correlation on the FI which was repeatedly measured at baseline and during follow-up.(18) The changes of FI before and after onset of MOF at year 1 and year 2 post-baseline were compared between the women with and without incident MOF using the Student’s t test, in which the FI change for year 1 was calculated from year 2 FI minus baseline FI, and the FI change for year 2 was from year 3 FI minus year 1 FI, respectively. For simplicity, we denoted the change of FI before and after onset of MOF for year 1 as FI(year 2 – baseline), and the change of FI for year 2 as FI(year 3 – year 1), respectively.
To investigate the relationship between the FI change and risk of MOF, Cox proportional hazards regression models were conducted and hazard ratios (HRs) were used to quantify the associations. We used the change of FI to predict the risk of MOF in the following year. Specifically, we used the change of FI from baseline (ie, year 1 FI minus baseline FI, denoted as FI(year 1 – baseline)) to predict the risk of MOF at year 2 post-baseline, and the change from year 1 (ie, year 2 FI minus year 1 FI, denoted as FI(year 2 – year 1)) to evaluate the risk of MOF at year 3 post-baseline, respectively. Both a statistical test and a graphical examination using Schoenfeld residuals were performed to test the proportional hazards assumption in the Cox models.(19) For the risk of falls and death, binary logistic regression was conducted to evaluate their relationship with the FI change because no data on the dates for falls and death were available in the GLOW.
We also used the absolute values of the FI from the preceding year to predict the risk of events in the following year. For example, we used the absolute FI measures at year 1 to investigate their relationship with the risk of MOF at year 2 post-baseline. All the models were adjusted for age. Results were reported using the per-0.10 increment of the FI change and the FI absolute measures.(8,20)
For missing data, 10 multiple imputations were performed to impute missing data if the data were missing ≥10%, assuming they were randomly missing.(21,22) If the data of <10% on a variable were missing, we used the median or mean of the variable for imputation. All tests were two-sided with a significance level of 0.05. All analyses were conducted with the software package STATA, version 12 (Stata Corp., College Station, TX, USA) and SAS, version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Ethics
This study was reviewed and approved by the Western Institutional Review Board. Written informed consent was obtained from all the participants before the survey.
Results
There were 3985 women included in this study (Table 1). The mean age was 69.4 years at baseline (SD 8.89), and their mean body mass index (BMI) was 27.7 kg/m2 (SD 5.77). The percentages of participants who smoked, had a family history of factures, and had a previous fracture since age 45 years were 11.30%, 23.81%, and 22.31%, respectively. There were approximately 49% of the women drinking.
Table 1.
Baseline Characteristics of Participants
| Baseline characteristics | Participants (n = 3985)a |
|---|---|
| Age (years), mean (SD) | 69.4 (8.89) |
| BMI (kg/m2), mean (SD) | 27.7 (5.77) |
| Smoking, n (%) | 447 (11.30) |
| Drinking, n (%) | 1931 (48.79) |
| Family history of fractures, n (%) | 898 (23.81) |
| Prior fractures since age 45 years, n (%) | 862 (22.31) |
| FI at baseline, mean (SD) | 0.24 (0.13) |
SD = standard deviation; BMI = body mass index; FI = frailty index.
Mean follow-up = 3.01 years.
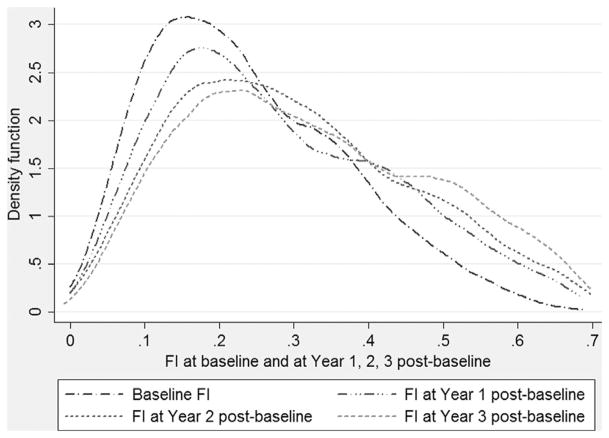
The baseline FI was 0.24 (SD 0.13), and the age-invariant 99% upper limit was 0.59. During follow-up, the FIs at year 1, year 2, and year 3 post-baseline had a mean of 0.29 (SD 0.16), 0.31 (SD 0.17), and 0.34 (SD 0.19), respectively. All the submaximal, age-invariant 99% upper limits of the FIs post-baseline were less than 0.70, in which the limit was 0.65 for year 1, 0.67 for year 2, and 0.67 for year 3, respectively. Fig. 1 displays the density distributions of the FIs at baseline and during follow-up, showing the right skewness for all the density distribution of the four FIs. The GEE model yielded a mean rate of deficit accumulation across ages of 0.020 (95% confidence interval [CI] 0.017–0.022, p <0.001) on a log scale, or 0.006 (95% CI 0.006–0.007, p <0.001) on a crude scale.
Fig. 1.
Distribution of frailty index at baseline, year 1, year 2, and year 3 post-baseline.
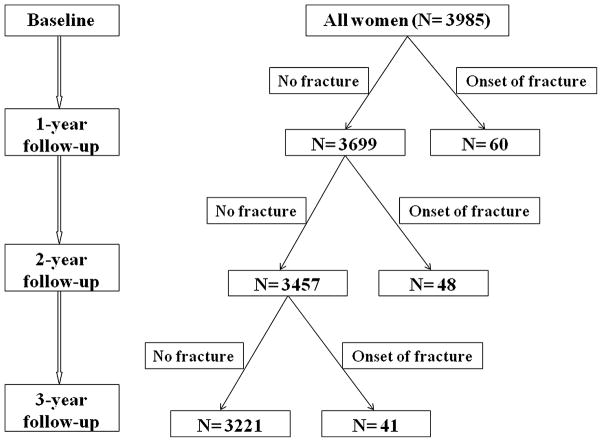
Fig. 2 shows the flow diagram of onset of incident MOF during the 3-year follow-up. There were 60, 48, and 41 new incident MOF reported during the years 1, 2, and 3 post-baseline, respectively. There were 187 (4.7%), 211 (5.6%), and 218 (6.2%) women who were alive but lost to follow-up at years 1, 2, and 3 post-baseline, respectively. However, there were <10% missing data on their FIs for the participants included for analyses. Table 2 shows the FI changes before and after onset of MOF at years 1 and 2 post-baseline. For year 1 post-baseline, both the FIs before and after MOF were significantly higher in the women with MOF compared with the participants without MOF (p <0.01). The change of FI for year 1 was also more substantial (p = 0.036) in the women with MOF (FI(year 2 – baseline) = 0.085) than those without MOF (FI(year 2 – baseline) = 0.067). Similarly, the FIs before and after MOF at year 2 post-baseline in the participants with fractures differed significantly from those controls (Table 2). The increase of FI was significantly larger in the women with MOF (FI(year 3 – year 1) = 0.080) compared with those without MOF (FI(year 3 – year 1) = 0.052), with a p value of 0.042. However, the increase of ages was not significantly different in the women with and without MOF at year 1 (2.06 versus 1.99 years, p = 0.71) and year 2 (2.03 versus 2.00 years, p = 0.76) post-baseline.
Fig. 2.
Flow diagram of onset of incident major osteoporotic fracture during 3-year follow-up.
Table 2.
Age and FI Before and After Onset of MOF at Year 1 and Year 2 Post-baseline
| Factors | Year 1 post-baseline
|
Year 2 post-baseline
|
||||
|---|---|---|---|---|---|---|
| With MOF (n = 60) | Without MOF (n = 3699) | p Value | With MOF (n = 48) | Without MOF (n = 3457) | p Value | |
| Before MOFa | ||||||
| Age (years), mean (SD) | 71.9 (9.53) | 69.2 (8.74) | 0.019 | 70.3 (8.80) | 69.8 (8.59) | 0.65 |
| FI, mean (SD) | 0.28 (0.15) | 0.24 (0.14) | 0.008 | 0.34 (0.18) | 0.28 (0.16) | 0.003 |
| After MOFb | ||||||
| Age (years), mean (SD) | 74.0 (9.50) | 70.9 (8.84) | 0.017 | 72.4 (8.78) | 71.8 (8.58) | 0.63 |
| FI, mean (SD) | 0.37 (0.19) | 0.30 (0.17) | 0.004 | 0.42 (0.21) | 0.33 (0.18) | <0.001 |
| Change before and after the onset of MOFc | ||||||
| FI, mean (SD) | 0.085 (0.086) | 0.067 (0.077) | 0.036 | 0.080 (0.11) | 0.052 (0.097) | 0.042 |
FI = frailty index; MOF = major osteoporotic fracture; SD = standard deviation.
For year 1, information before MOF was collected from baseline; for year 2, information before MOF was collected from year 1.
For year 1, information after MOF was collected from year 2; for year 2, information after MOF was collected from year 3.
The change of FI for year 1 is denoted as FI(year 2 – baseline); the change of FI for year 2 is denoted as FI(year 3 – year 1).
Table 3 shows the relationship between the per-0.10 increment of the FI change and risk of incident MOF at years 2 and 3 post-baseline. The FI(year 1 – baseline) (ie, change of FI from baseline) had a mean of 0.046 (SD 0.077), whereas the mean of the FI(year 2 – year 1) (ie, change of FI from year 1) was 0.022 (SD 0.089). The FI change was not significantly related with risk of MOF independently of age: HR = 1.38 (95% CI 0.96–1.94, p = 0.08) for year 2, HR = 1.32 (95% CI 0.94–1.85, p = 0.10) for year 3, respectively. Nevertheless, the absolute FI measures from the previous year significantly predicted risk of MOF in the following year: HR = 1.33 (95% CI 1.12–1.58, p = 0.001) for year 2; HR = 1.25 (95% CI 1.05–1.50, p = 0.017) for year 3, respectively.
Table 3.
Relationship Between per-0.10 Increment of the FI and Risk of Onset of Incident MOF
| Factors | Year 2 post-baseline (n= 48)
|
Year 3 post-baseline (n= 41)
|
||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Changed valuesa | ||||
| Age (years) | 1.29 (0.49–3.42) | 0.60 | 1.23 (0.81–1.86) | 0.33 |
| FI | 1.38 (0.96–1.94) | 0.08 | 1.32 (0.94–1.85) | 0.10 |
| Absolute measures from previous yearb | ||||
| Age (years) | 0.99 (0.96–1.03) | 0.75 | 1.04 (1.01–1.08) | 0.026 |
| FI | 1.33 (1.12–1.58) | 0.001 | 1.25 (1.05–1.50) | 0.017 |
FI = frailty index; MOF = major osteoporotic fracture; HR = hazard ratio; CI = confidence interval.
For year 2, changed values were from year 1 minus baseline, denoted as FI(Year1-basline); for year 3, changed values were from year 2 minus year 1, denoted as FI(Year2-Year1).
For year 2, information was from year 1; for year 3, information was from year 2.
There were 483 (21.33%) women with incident falls and 43 (1.09%) deaths during year 2, and 288 (17.04%) falls and 25 (0.64%) deaths documented during year 3 post-baseline, respectively. Similarly, as shown in Table 4, the change of FIs was not significantly associated with risk of falls and death at years 2 and 3 (p >0.05), except for risk of death at year 2 (OR = 1.84, 95% CI 1.34–2.53, p <0.001). Using the absolute FI measures, it was found that the FI was consistently and significantly related with increased risk of falls and death independently of age: OR = 1.18 (95% CI 1.10–1.27) at year 2 and OR = 1.12 (95% CI 1.02–1.22) at year 3 for falls; OR = 1.58 (95% CI 1.33–1.89) at year 2 and OR = 1.24 (95% CI 1.07–1.46) at year 3 for death, respectively (Table 4).
Table 4.
Relationship Between per-0.10 Increment of the FI and Risk of Onset of Incident Falls and Deatha
| Outcomes | Year 2 post-baseline
|
Year 3 post-baseline
|
||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Changed valuesb | ||||
| Falls | ||||
| Age (years) | 1.05 (0.75–1.46) | 0.79 | 1.02 (0.87–1.19) | 0.82 |
| FI | 1.10 (0.97–1.25) | 0.15 | 1.72 (0.96–3.06) | 0.34 |
| Death | ||||
| Age (years) | 1.23 (0.98–1.56) | 0.081 | 0.74 (0.33–1.64) | 0.45 |
| FI | 1.84 (1.34–2.53) | <0.001 | 1.33 (0.87–2.03) | 0.20 |
| Absolute measures from previous yearc | ||||
| Falls | ||||
| Age (years) | 1.00 (0.99–1.02) | 0.85 | 1.01 (1.00–1.03) | 0.13 |
| FI | 1.18 (1.10–1.27) | <0.001 | 1.12 (1.02–1.22) | 0.018 |
| Death | ||||
| Age (years) | 1.06 (1.03–1.10) | <0.001 | 1.05 (1.02–1.09) | 0.004 |
| FI | 1.58 (1.33–1.89) | <0.001 | 1.24 (1.07–1.46) | 0.006 |
FI = frailty index; OR = odds ratio; CI= confidence interval.
Incident falls: n = 483 (21.33%) for year 2 post-baseline; n = 288 (17.04%) for year 3 post-baseline. Incident death: n = 43 (1.09%) for year 2 post-baseline; n = 25 (0.64%) for year 3 post-baseline.
For year 2, changed values were from year 1 minus baseline, denoted as FI(year 1– baseline); for year 3, changed values were from year 2 minus year 1, denoted as FI(year 2 – year 1).
For year 2, information was from year 1; for year 3, information was from year 2.
Because the risk of mortality increased after a fracture, we also performed a post hoc analyses by adjusting prior fractures in the model to assess the relationship between the FI and risk of death. All the results remained consistent, yielding an OR of 1.80 (95% CI 1.27–2.55) for death at year 2 and 1.29 (95% CI 0.79–2.11) for death at year 3 using the change of FIs, and with an OR of 1.58 (95% CI 1.32–1.88) for death at year 2 and 1.25 (95% CI 1.00–1.58) for death at year 3 using the absolute FI measures, respectively.
Discussion
In this study using the data from GLOW Hamilton 3-year cohort, we assessed the change of FI in the elderly women experiencing a MOF. The increase of FI was significantly larger in the participants with MOF compared with those without MOF, indicating increasing frailty and faster deficit accumulation for the elderly women after a MOF. Nevertheless, the FI change was not significantly related with the following MOF, falls, or death. By contrast, the absolute FI measures in the previous year could predict MOF, falls, and death in the following year.
The FI increased significantly with chronological age, with a mean rate of deficit accumulation of 0.020 across ages on a log scale. The average increase of deficit accumulation, as well as the right skewness of the FI distribution, was consistent with other longitudinal studies in different countries for the community-dwelling elderly.(5,12,23–25) The age-invariant 99% upper limit of the FIs increased from 0.59 at baseline to 0.67 at years 2 and 3 post-baseline, which was also in line with other findings that the submaximal upper limit of an FI was less than 0.70.(12,25–27) This implied that once the elderly accumulated health deficits to a highly frail state and they could not tolerate any additional stressors, then death would be inevitable.(6,12,24,25)
In this study, we found that for the women experiencing MOF, their FI increase was significantly higher than those without MOF (Table 2). The trajectories of the frailty status could vary in the elderly substantially, which reflected a different aging rate in individuals.(6) The women developing MOF may accelerate deficit accumulation and transfer to a higher state of frailty, which therefore resulted in a larger increase of the FI compared with their controls. As recommended as an estimate of “biological age,”(6,28,29) the FI would be better to assess the frailty transition in the elderly than chronological age. In our study, even though the change of chronological ages was similar, significantly higher increase of the FI was observed in the women experiencing MOF (Table 2). Therefore, measuring the “biological age” may aid in our understanding of the cumulative aging process and the transition of frailty or fitness status in the elderly, especially for those who developed nonfatal adverse health events.
The change in FIs was not significantly associated with risk of outcomes in the following year (Tables 3 and 4). Part of the interpretation may rely on the short-term intervals that were used to document the outcomes annually during the follow-up. For instance, if the FI change from baseline to year 1 were assessed for the relationship with outcomes at year 3 post-baseline, it would produce a hazard ratio of 1.41 for MOF, an OR of 1.21 for falls, and an OR of 1.36 for death (all p values < 0.05), respectively. Moreover, the short-term FI assessment may also possibly yield an improvement on individual frailty trajectories because of health interventions or changes in lifestyles, even though deficits accumulated over time on average for the elderly.(6,29) Therefore, in our study, the annual FI change measures may reflect more individual variation of their frailty transition in the yearly follow-up than quantifying the increased risk of short-term adverse health outcomes in their peer comparison. Nevertheless, the absolute FI measures from the previous year were significantly related with increased risk of MOF, falls, and death in the following year (Tables 3 and 4). Compared with the significant associations between the baseline FI and risk of adverse health outcomes during the 3-year follow-up, which had been published elsewhere,(16,20,30) this study also found that the FI in the previous year was positively related with risk of fractures, falls, and death in the following 12 months. These findings reinforced the concept of the FI that compared with her peers, an elderly individual with more deficits was always more likely to experience adverse health outcomes independently of age,(5,6,31) even though the outcomes were evaluated using annual short-term intervals.
There were several studies using an FI to measure the degree of frailty and assess the change of FI over time in different countries.(12,14,27,32,33) As the population aged, the mean FI increased and, accordingly, higher risk of death was observed. For instance, the Honolulu-Asia Aging Study included 3801 aged Japanese-American males and followed them to the cumulative death rate of >90% with seven waves.(12) They reported that the FI changed from 0.14 at baseline to 0.22 in the final assessment, and the median survival time decreased from 9.12 years at wave 1 to 1.81 years at wave 7.(12) Nevertheless, none of these studies looked at the frailty change before and after nonfatal events.(12,14,27,32,33) Therefore, little could be known about the intrinsic aging process (ie, deterioration or recovery) for the elderly who developed a nonfatal event. By contrast, our study may provide some insight into the short-term cumulative aging nature in the aged, especially for those experiencing MOF. Furthermore, the change of FI may be used to serve as an indicator for care or intervention planning and assessment.(34) For example, in a fracture intervention study with an FI instrument as outcome measures, the change of FI could assist in the identification of the minimally important differences, taking the frailty transition nature into account.
There are some limitations of our study. The data used in this study were from participants’ self-report and could not be validated by medical records,(15) although evidence had shown reasonable credibility of the self-reported data in different settings and populations.(35–37) The data from year 1 post-baseline did not include several deficit variables of chronic comorbidities to calculate the FI, such as rheumatoid arthritis, chronic bronchitis, celiac disease, cancer, etc. (Supplemental Table S1). For the purpose of calculation of the FI at year 1, we used the same comorbidity data from baseline for imputation, assuming no change of the chronic diseases for the women. This, however, may underestimate the FI measures at year 1 because we could not capture the new deficits for the participants without baseline chronic comorbidities, even though it was reasonable that the patients with baseline comorbidities were not expected to recover within 1 year. Furthermore, we could not analyze the individual components of MOF, including hip, upper arm or shoulder, spine, and wrist fracture, because of insufficient sample size for the specific fracture types. For example, there were only 11 spine fractures documented within year 1, and the numbers were 10 and 7 for years 2 and 3, respectively. Likewise, the numbers of hip fracture were only 7, 6, and 6 for years 1, 2, and 3 post-baseline, respectively. Besides, no analyses could be performed for the relationship between the time-dependent FI and risk of onset of MOF. Because the data on FIs were entirely from the annual questionnaires, only four FIs for each participant were collected in this study (at baseline, year 1, year 2, and year 3 post-baseline, respectively). Therefore, for the relationship between the change of FI and risk of onset of MOF in the following year, we could only treat the FI as a fixed (time-independent) covariate in the Cox model, rather than a time-dependent covariate.(38) Lastly, the GLOW only included female participants,(15) thus findings from this study may not be generalizable to elderly males.
Our study investigated the change of frailty status before and after nonfatal events for the elderly, which may provide some value to the existing evidence on the short-term cumulative aging nature. Furthermore, measures of the relationship with risk of adverse health outcomes using both the FI changes and absolute FI values suggested the predictive validity of the FI instrument in this study.(34) Other strengths of this study included the large sample size and the representative sample because of the unique sampling method in the GLOW.(15) The participants were recruited according to the lists provided by the physician practices with few exclusion criteria, which thus would lead to the women being nonselected and representative of the practices in real world.(15,39,40)
Based on the data from GLOW Hamilton 3-year cohort, the increase of the FI is significantly larger in the elderly women experiencing a MOF than their peer controls, implying their worsening frailty and greater deficit accumulation after a MOF. Measures of the FI change may aid in the understanding of cumulative aging nature in the elderly and serve as an instrument for intervention planning and assessment.
Supplementary Material
Acknowledgments
The authors acknowledge the whole GLOW research group investigators herein.
Financial support for the GLOW was provided by Warner Chilcott Company, LLC, and Sanofi-Aventis to the Center for Outcomes Research, University of Massachusetts Medical School. GL received a Father Sean O’Sullivan Research Award, the Research Institute of St. Joe’s Hamilton, and a doctoral award from the CSC. The sponsors did not get involved in the study design or conduct; data collection, analyses, or interpretation; or writing, review, or approval of this manuscript.
Authors’ roles: All of the authors contributed to the study conception and design. GL and JC were responsible for data analyses. GL and JDA contributed to the drafting of the manuscript. AP, LT, and JC provided professional and statistical support and made critical revision to the manuscript. All authors approved the final version to be published and agreed to act as guarantors of the work.
Footnotes
Disclosures
AP: Advisory board member: Amgen, Eli Lilly, Merck Frosst, Novartis, and Procter & Gamble; consultant: Amgen, Aventis Pharma, Eli Lilly, Lundbeck Canada Inc., Merck Frosst, Novartis, Procter & Gamble, Servier, Warner Chillcott, and Wyeth Ayerst; clinical trials: Novartis and Pfizer. JDA: consultant/speaker: Amgen, Astra Zeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, Sanofi-Aventis, Servier, Wyeth, and Bristol-Myers Squibb; clinical trials: Amgen, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi-Aventis, Wyeth, and Bristol-Myers Squibb. All other authors state that they have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nursing Outlook. 2010;58(2):76–86. doi: 10.1016/j.outlook.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Fang X, Shi J, Song X, et al. Frailty in relation to the risk of falls, fractures, and mortality in older Chinese adults: results from the Beijing Longitudinal Study of Aging. J Nutr Health Aging. 2012;16(10):903–7. doi: 10.1007/s12603-012-0368-6. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy C, Ioannidis G, Rockwood K, et al. A Frailty Index predicts 10-year fracture risk in adults age 25 years and older: results from the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2014;25(12):2825–32. doi: 10.1007/s00198-014-2828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan M, Beck S, Havelock W, Eeles E, Hubbard RE, Johansen A. Predicting outcome after hip fracture: using a frailty index to integrate comprehensive geriatric assessment results. Age Ageing. 2014;43(1):122–6. doi: 10.1093/ageing/aft084. [DOI] [PubMed] [Google Scholar]
- 10.Tom SE, Adachi JD, Anderson FA, Jr, et al. Frailty and fracture, disability, and falls: a multiple country study from the global longitudinal study of osteoporosis in women. J Am Geriatr Soc. 2013;61(3):327–34. doi: 10.1111/jgs.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabue-Teguo M, Kelaiditi E, Demougeot L, Dartigues JF, Vellas B, Cesari M. Frailty index and mortality in nursing home residents in France: results from the INCUR study. J Am Med Dir Assoc. 2015;16(7):603–6. doi: 10.1016/j.jamda.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K. Frailty in the Honolulu-Asia Aging Study: deficit accumulation in a male cohort followed to 90% mortality. J Gerontol A Biol Sci Med Sci. 2015;70(1):125–31. doi: 10.1093/gerona/glu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–94. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitnitski A, Song X, Rockwood K. Trajectories of changes over twelve years in the health status of Canadians from late middle age. Exp Gerontol. 2012;47(12):893–9. doi: 10.1016/j.exger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Hooven FH, Adachi JD, Adami S, et al. The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int. 2009;20(7):1107–16. doi: 10.1007/s00198-009-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Ioannidis G, Pickard L, et al. Frailty index of deficit accumulation and falls: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Hamilton cohort. BMC Musculoskelet Disord. 2014;15(1):185. doi: 10.1186/1471-2474-15-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsay JO. Kernel smoothing approaches to nonparametric item characteristic curve estimation. Psychometrika. 1991;56(4):611–30. [Google Scholar]
- 18.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 20.Li G, Thabane L, Papaioannou A, Adachi JD. Comparison between frailty index of deficit accumulation and fracture risk assessment tool (FRAX) in prediction of risk of fractures. Bone. 2015;77:107–14. doi: 10.1016/j.bone.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham JW. Missing data analysis: making it work in the real world. Ann Rev Psychol. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 22.Horton NJ, Kleinman KP. Much ado about nothing. Am Stat. 2007;61(1):79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53(12):2184–9. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 24.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127(5):494–6. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Yang Z, Song X, et al. Sex differences in the limit to deficit accumulation in late middle-aged and older Chinese people: results from the Beijing Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2014;69(6):702–9. doi: 10.1093/gerona/glt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing. 2013;42(3):372–7. doi: 10.1093/ageing/afs180. [DOI] [PubMed] [Google Scholar]
- 28.Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60(8):1046–51. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 29.Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14(6):709–17. doi: 10.1007/s10522-013-9446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Thabane L, Ioannidis G, Kennedy C, Papaioannou A, Adachi JD. Comparison between frailty index of deficit accumulation and phenotypic model to predict risk of falls: data from the global longitudinal study of osteoporosis in women (GLOW) Hamilton cohort. PloS One. 2015;10(3):e0120144. doi: 10.1371/journal.pone.0120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockwood K, Mitnitski A. How might deficit accumulation give rise to frailty. J Frailty Aging. 2012;1(1):7–10. doi: 10.14283/jfa.2012.2. [DOI] [PubMed] [Google Scholar]
- 32.Fallah N, Mitnitski A, Searle SD, Gahbauer EA, Gill TM, Rockwood K. Transitions in frailty status in older adults in relation to mobility: a multistate modeling approach employing a deficit count. J Am Geriatr Society. 2011;59(3):524–9. doi: 10.1111/j.1532-5415.2011.03300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitnitski A, Bao L, Rockwood K. Going from bad to worse: a stochastic model of transitions in deficit accumulation, in relation to mortality. Mech Ageing Dev. 2006;127(5):490–3. doi: 10.1016/j.mad.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age Ageing. 2015;44(4):545–7. doi: 10.1093/ageing/afv043. [DOI] [PubMed] [Google Scholar]
- 35.Hundrup YA, Hoidrup S, Obel EB, Rasmussen NK. The validity of self-reported fractures among Danish female nurses: comparison with fractures registered in the Danish National Hospital Register. Scand J Public Health. 2004;32(2):136–43. doi: 10.1080/14034940310017490. [DOI] [PubMed] [Google Scholar]
- 36.Rahman A, Gibney L, Person SD, et al. Validity of self-reports of reasons for hospitalization by young adults and risk factors for discordance with medical records: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2005;162(5):491–8. doi: 10.1093/aje/kwi215. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause (NY) 2004;11(3):264–74. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 38.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Ann Rev Public Health. 1999;20(1):145–57. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 39.Diez-Perez A, Adachi JD, Adami S, et al. Risk factors for treatment failure with antiosteoporosis medication: the Global Longitudinal Study of Osteoporosis in Women (GLOW) J Bone Miner Res. 2014;29(1):260–7. doi: 10.1002/jbmr.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guggina P, Flahive J, Hooven FH, et al. Characteristics associated with anti-osteoporosis medication use: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) USA cohort. Bone. 2012;51(6):975–80. doi: 10.1016/j.bone.2012.08.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.