Abstract
Bone is commonly affected in cancer. Cancer-induced bone disease results from the primary disease, or from therapies against the primary condition, causing bone fragility. Bone-modifying agents, such as bisphosphonates and denosumab, are efficacious in preventing and delaying cancer-related bone disease. With evidence-based care pathways, guidelines assist physicians in clinical decision-making. Of the 57 million deaths in 2008 worldwide, almost two thirds were due to non-communicable diseases, led by cardiovascular diseases and cancers. Bone is a commonly affected organ in cancer, and although the incidence of metastatic bone disease is not well defined, it is estimated that around half of patients who die from cancer in the USA each year have bone involvement. Furthermore, cancer-induced bone disease can result from the primary disease itself, either due to circulating bone resorbing substances or metastatic bone disease, such as commonly occurs with breast, lung and prostate cancer, or from therapies administered to treat the primary condition thus causing bone loss and fractures. Treatment-induced osteoporosis may occur in the setting of glucocorticoid therapy or oestrogen deprivation therapy, chemotherapy-induced ovarian failure and androgen deprivation therapy. Tumour skeletal-related events include pathologic fractures, spinal cord compression, surgery and radiotherapy to bone and may or may not include hypercalcaemia of malignancy while skeletal complication refers to pain and other symptoms. Some evidence demonstrates the efficacy of various interventions including bone-modifying agents, such as bisphosphonates and denosumab, in preventing or delaying cancer-related bone disease. The latter includes treatment of patients with metastatic skeletal lesions in general, adjuvant treatment of breast and prostate cancer in particular, and the prevention of cancer-associated bone disease. This has led to the development of guidelines by several societies and working groups to assist physicians in clinical decision making, providing them with evidence-based care pathways to prevent skeletal-related events and bone loss. The goal of this paper is to put forth an IOF position paper addressing bone diseases and cancer and summarizing the position papers of other organizations.
Keywords: Bone, Cancer, IOF, Skeletal-related events
Epidemiology of cancer-associated bone disease
Bone metastasis
Cancer affects nearly 12.7 million people and is associated with over 7 million deaths in 2008 [1]. Cancer is a rising global health burden and it is estimated that in 2030, cancer deaths will be tallied at over 13 million (World Health Organization (WHO) 2010 Global Status Report [2]). Addressing the morbidity and mortality of cancer is an important public health concern. On purpose, we are limiting our analysis to cancer bone involvement in adults and do not discuss cancer in children.
Metastases from cancer are associated with 90 % of cancer deaths [3]. It was estimated that one half of people who die from cancer in the USA have bone involvement [4–6]. Different tumour types may have preferential sites of metastases; however, the vast majority of tumours metastasize to bone, albeit at varying frequencies. The term metastatic bone disease reflects the spread of a tumour to the bone. This term may be applied to solid tumours, as well as to multiple myeloma, where the tumour is intrinsic to the bone marrow. In multiple myeloma, 70–95 % of patients have tumour bone disease [7]. In breast cancer, bone is often the first site of distant metastases [5] with approximately one half of patients experiencing bone metastases as the site of first relapse [8]. In advanced breast or prostate cancer, metastatic bone disease is present in the vast majority of patients. Bone metastases may also be seen in 15–30 % of cancers of the lung, gastrointestinal tract (colon and stomach) and the genitourinary (bladder, kidney and uterus) [9] (Table 1). In advanced thyroid cancer and melanoma, bone metastases are also frequently present [7]. Skeletal-related events (SREs) occur relatively commonly and include pathologic fractures (20.7 %), spinal cord compression (0.9 %), surgery (1.2 %) and radiotherapy (8.0 %) to bone and may or may not include hypercalcaemia of malignancy while skeletal complication also refers to pain and other symptoms, thus impairing quality of life, and also decreasing survival [7] and may encompass SREs as well. New anticancer and supportive care treatment modalities are alleviating symptoms, including bone-related ones, and maintaining or improving quality of life, at the expense in some instances of accelerated bone loss and fractures.
Table 1.
Incidence of metastatic bone disease [42]
| Incidence (%) | |
|---|---|
| Myeloma | 70–95 |
| Breast | 65–75 |
| Prostate | 65–75 (NCCN, Grawlow) |
| Renal | 20–25 |
| Melanoma | 14–45 |
| Thyroid | 60 |
| Lung | 30–40 |
| Bladder | 40 |
Cancer-related bone loss and fracture in patients who do not have bone metastases
Patients with cancer may be at increased risk of bone loss secondary to cancer disease treatment [10]. With improved survival rates in many types of cancer, early identification and treatment of osteoporosis among cancer patients could prevent unnecessary fractures, morbidity and reductions in quality of life. A recent prospective study in Germany of 1,041 cancer patients, mean age of 57 years, 78 % female, found elevated rates of osteoporosis compared to the general population [10]. The prevalence of osteoporosis in both men and women with cancer in complete remission was 16 % (95 % confidence interval (CI) 13.8–18.2) and osteopenia 44 % (defined using WHO criteria). Rates of osteoporosis were not statistically different among various cancer types, which included breast, gynaecological, prostate, colorectal and haematological cancers, although sample size was too small to detect potential differences in rates between subtypes [10].
Women who have been treated medically for breast cancer may be at increased risk for bone loss and fractures [11, 12]. In a case-controlled study of over 1,200 women with newly diagnosed breast cancer and no metastases, the annual incidence of vertebral fractures was 2.72 % compared to 0.53 % in the control arm, i.e. a fivefold increase, with rates adjusted for age, prevalent fractures and duration of follow-up [12]. Similarly, in the prospective observational arm of the Women’s Health Initiative, fracture rates in breast cancer survivors were increased by 68.6 fractures per 10,000 person-years, which compared to rates in women without breast cancer is a 15 % increase, after adjustment for age, ethnicity, weight and geographic location [11]. Kanis et al. [12] found a fivefold higher prevalence of vertebral fractures in women with breast cancer, but without bone metastases, than in women of the same age (odds ratio 4.7, 95 % CI 2.3–9.9).
Women with oestrogen receptor positive (ER+) breast cancer treated with aromatase inhibitors (AIs) as adjuvant endocrine therapy are at increased risk of rapid bone loss, and of fractures [13–15]. For example in the landmark Arimidex, Tamoxifen Alone or in Combination (ATAC) trial, average bone loss rates in women assigned to the AI anastrozole were 1–2 %/year, well above those recorded in women in the tamoxifen arm [16].
Men with prostate cancer are at particularly high risk of osteoporosis [17] and of fracture [18] in part due to treatment with androgen deprivation therapy (ADT). Rates of bone mineral density (BMD) decrease could be as high as 3.0 to 5.6 % within the first year of ADT, depending on the measured site, with annual decreases of 1.1 to 2.3 % thereafter [19].
Pathophysiology
Fractures in cancer-associated bone disease can result from the direct or systemic effect of the tumour itself or from therapies used to treat the primary disease. In the former case, they are related to local effects of metastatic deposit in bone and/or to generalized bone loss from tumour-produced systemically circulating bone resorbing hormones or cytokines. These comprise parathyroid hormone-related protein (PTHrP), like in lung and breast cancer, or tumour stimulated secretion by the osteoblast of local bone resorbing factors such as receptor activator of nuclear factor kappa-B ligand (RANKL), interleukin (IL)-6 or IL-3, like in multiple myeloma. Alternatively, bone loss may result from gonadal ablation by chemotherapy or endocrine ablative therapy to treat the primary disease. In some tumours, more than one mechanism may be operating [20].
Bone metastasis
The bone microenvironment represents a fertile soil capable of favouring the growth of malignant cells coming from a distant tumour (metastasis) or of haematological origin (myeloma or lymphoma). Relationships between bone remodelling and metastatic cells are summarized by the “seed and the soil” theory proposed in 1879 by Stephan Paget, who noticed that some cancer cells had an increased propensity to migrate and expand in bone (cited in [21]). The preferential targeting of the skeleton by some tumours is in part explained by tumour-specific factors, and by relevant modulators in the bone microenvironment, that enhance tumour growth. Indeed, some particular cancer cells (breast, prostate, malignant lymphocytes and plasma cells) possess characteristics that favour their anchorage in the bone marrow. The pathophysiology of bone metastasis has been reviewed extensively elsewhere [22–24]. Here, we will summarize the main aspects of the interactions between malignant cells, bone cells and bone remodelling.
The metastatic cascade
From a primary tumour, malignant cells can acquire the capacity to metastasize due to an increased motility and invasiveness and a special tropism to bone or bone marrow. They may produce or express various adhesive molecules for, e.g. integrins, which can bind to ligand molecules–receptors expressed by the stromal cells of the bone marrow or to non-collagenic proteins present in the bone matrix, such as osteopontin. When circulating within the blood stream, cancer cells can reach the sinusoid capillaries of the bone marrow, which also contain large pores [25]. Malignant cells can adhere to these endotheliums and extravase into the bone marrow environment.
Bone metastases are usually classified as osteolytic, osteoblastic/osteosclerotic, or mixed (osteolytic and osteoblastic), based on their appearance on X-ray images. Many patients may have both osteolytic and osteosclerotic metastases, and individual bone lesions can harbour both features. Predominantly osteolytic metastases are typical of multiple myeloma, renal cell cancer, thyroid cancer, non-small lung cancer, some gastro-intestinal tumours, and melanomas. Predominantly osteosclerotic lesions are most often observed in prostate cancer, carcinoid, gastrinoma and medulloblastoma. Mixed lesions occur most commonly in breast cancer, gastrointestinal tumours and most squamous cell cancers at their primary site. In osteolytic metastases, bone resorption is due to a stimulation of osteoclastogenesis (and not by a direct action of the tumour cells), and areas of metaplastic bone at the margin of the lytic lesions are observed [26] such as in non-small lung carcinoma, non-Hodgkin’s lymphoma and thyroid cancer. In osteosclerotic tumours, there is an increase in osteoclastogenesis but the stimulation of bone formation is more pronounced.
Development of osteolytic metastasis
Malignant cells release a number of molecules that favour osteoclastogenesis via the receptor activator of nuclear factor kappa-B (RANK)/RANKL/osteoprotegerin (OPG) system. PTHrP may activate this bone resorption pathway and is detected by immunohistochemistry in about 90 % of bone metastases from breast cancer, i.e. more often than in soft tissue metastases [27]. RANKL has also been shown to trigger the migration to bone of melanoma and of some epithelial cancer cells that express the RANK receptor, such as breast cancer cells. In a mouse model, by blocking RANKL, OPG resulted in a reduction in tumour burden in bone, but not in other organs [28]. Other bone resorbing factors such IL-1, IL-6, IL-8, IL-11 and TNF-α have also been identified. Osteoclastogenesis in both cortical and trabecular bones is increased in the vicinity of the tumour by a paracrine mechanism. In advanced metastatic bone disease, hypercalcaemia reflects the release of large amounts of calcium mobilized due to breakdown of the calcified matrix. During resorption, large amounts of deeply entrapped growth factors in bone are released and activated in the microenvironment. Transforming growth factor-beta (TGF-β), insulin-like growth factor (IGF)-I, and IGF-II promote the growth of the tumour cells locally through their receptors, e.g. breast cancer cells express receptors for TGF-β [24]. αCTX fragments of degraded collagen are also released from the eroded areas and represent a strong chemoattractant for recruiting locally new malignant cells [29].
Development of osteosclerotic metastasis
Prostatic adenocarcinoma is predominantly associated with osteosclerosis metastases. On histological bone sections, a large number of osteoblasts building new trabeculae are observed in the vicinity of the tumour cells. The neotrabeculae made of woven bone are anchored at the surface of pre-existing trabeculae and fill the marrow cavity. Prostate adenocarcinoma cells are able to release a number of cytokines that induce osteoclastogenesis including PTHrP. However, malignant cells also express a variety of proteases, e.g. prostate-specific antigen (PSA) which partially degrades PTHrP or IGF-binding proteins, and limit osteoclastogenesis. One characteristic of prostate cancer cells is their production of cytokines that favour osteoblastogenesis: ET-1 [30, 31], IGF-I and IGF-II, FGF-1 and FGF-2, VEGF. In turn, activated osteoblasts can release large amounts of IL-6, TGF-β and PDGF-BB which are potent growth factors for the tumour cells [32–34]. In both osteoblastic and osteoclastic metastases, a vicious circle is established since the malignant cells stimulate osteoblast or osteoclastic activity, which in turn stimulates tumour growth and progression.
Bone loss in myelomas and lymphomas
Lytic bone lesions are observed on X-ray in about 95 % of patients with advanced myeloma, in contrast to what is observed in lymphomas, although both entities are B cells and Band T cells malignancies, respectively. Bone involvement is related to an excessive bone resorption through increased osteoclast number encountered in the close vicinity of myeloma cells [35] together with a decreased osteoblast activity [36–38]. In myeloma, the cytokines produced by plasma cells and bone cell progenitors (IL-6, macrophage inflammatory protein-1α/CCL-3) induce the genesis of mature multinucleated osteoclasts. In lymphoma, when B cells invade the bone [39], a mixed population of multi- and mononuclear osteoclasts is observed, mononuclear TRAcP+ cells being only capable of microresorption [40]. Osteolytic lesions are usually rare in B cell lymphoma (8–10 % of cases) and occur only when the number of these cells is high [41]. In myelomas and lymphomas, a marked reduction in osteoblast activity has been identified by histomorphometric analysis [36]. Plasma cells can release several factors such as DKK1, sFRP 2, which act on the Wnt pathway and reduce the osteoblast number and activity [38]. The lesions observed in myeloma are predominantly osteolytic and can produce removal of whole trabeculae and perforation of cortical bone.
Cancer-related bone loss and fracture
A variety of mechanisms are responsible for bone loss in patients with cancer treatment-associated bone loss. The mechanisms may vary according to patient profile and chemotherapeutic regimen used. These include hypogonadism induced by chemotherapy, hormone ablative therapy, glucocorticoid, surgical castration, irradiation [42–44] or any combination of the above. Indeed, bone loss has been observed in lymphoma survivors who received therapy regimens including corticosteroids, alkylating agents and radiation therapy, all of which can cause hypogonadism [45, 46]. The highest rates for bone loss observed are in premenopausal women who experience cytotoxic or endocrine-induced acute ovarian ablation, reaching 8 % at the spine and 4 % at the hip, within the first year [47, 48], compared to half those rates in postmenopausal women receiving AIs [49–51].
Chemotherapy-induced hypogonadism
Predictors of premature ovarian failure in women with breast cancer receiving cytotoxic chemotherapy include patient’s age and type of regimen used. It was reported to occur in 60–85 % of women receiving adjuvant therapy with CMF, and in 50 % of women receiving the FAC regimens, with age-specific rates of 33 % in women aged 30–39 years, 96 % for women between 40 and 49 years and 100 % in women above age 50 [52] (Table 2). Permanent ovarian failure was observed following individual therapeutic doses of various chemotherapy agents including cyclophosphamide, chlorambucil and mitomycin-C.
Table 2.
Cancer treatment regimens directly and indirectly associated with bone loss [43]
| Direct effects | Indirect effects |
|---|---|
| Androgen deprivation therapy | Hypogonadism |
| Oestrogen suppression | Hyperparathyroidism |
| Glucocorticoids/corticosteroids | Vitamin D deficiency |
| Methotrexate | Gastrectomy |
| Megestrol acetate | Hyperprolactinemia |
| Platinium compounds | |
| Cyclophosphamide | |
| Doxorubicin | |
| Interpheron-alpha | |
| Valproic acid | |
| Cyclosporine | |
| Vitamin A | |
| NSAIDS | |
| Estramustine | |
| Ifosfamide | |
| Radiotherapy | |
| Combination chemotherapy regimens |
Cyclophosphamide appears to be the most common agent implicated in chemotherapy-associated amenorrhea. Premature menopause is dependent on its cumulative dose [52]. Chlorambucil and cyclophosphamide can cause prolonged azoospermia in male patients [53]. In addition, gonadal toxicity was evident in patients, especially those with testicular cancer, receiving a cumulative dose of cisplatinum greater than 400 mg/m2 [54]. Bone loss was also observed in patients made hypogonadic by cytotoxic drugs used in haematopoietic stem cell transplantations [55]. In premenopausal women, tamoxifen treatment is associated with bone loss, through its antiestrogen effects, whereas it is rather bone protective after the menopause being a partial oestrogen agonist [56, 57].
Hormone deprivation therapy
Osteoporosis stemming from hypogonadism is frequently seen in survivors of breast and prostate cancer, as therapeutic hypogonadism is an important strategy for controlling these hormone-dependent tumours [58].
Aromatase inhibitors
In women with ER+ breast cancers (about 70 % of tumours), AIs aim to reduce oestrogen levels by inhibiting the aromatization of androgens and their conversion to oestrogens in peripheral tissues [15]. Third generation non-steroidal (anastrozole and letrozole) and steroidal (exemestane which is similar to androstenedione) AI drugs inhibit the aromatase enzyme by 96–99 % [51], with substantial reduction in oestrogen concentrations (Table 2). AI-induced bone loss is generally more rapid and severe than bone loss in normal postmenopausal women [15], and should be taken into account especially when treated women with low BMD and/or fracture [59]. The skeletal effects observed are inversely correlated with baseline BMD and serum estradiol concentrations. Osteoporosis is more prevalent in women starting AI early after menopause, and there is only a partial recovery of BMD following the withdrawal of AI. Bone loss is accompanied by higher fracture risk [12, 15, 60–63]. This differs greatly from the effects of tamoxifen or raloxifene given in the prevention of breast cancer recurrence where increases in bone turnover may be partially averted and bone loss prevented. AIs are superior to tamoxifen as adjuvant therapy of ER+ breast cancer, with longer disease-free survival and without the risk of endometrial hyperplasia and cancer, cerebrovascular and venous thromboembolic events, but exhibit other toxicities such as arthralgias [15, 64]. Recently, AIs have been shown to further reduce the risk of recurrence after the diagnosis of ER+ breast cancer, either when given instead of tamoxifen or when administered sequentially after few years of tamoxifen therapy, and are thus now recommended in the adjuvant setting [65]. There is evidence that even the low levels of oestrogen in postmenopausal women are important for bone health. In the MORE clinical trial, comparing raloxifene to placebo, there was an inverse correlation between oestrogen levels and prospectively recorded fracture risk in those women assigned to the placebo group [66]. Letrozole, anastrozole and exemestane increase bone turnover [64] and decrease BMD, and letrozole and anastrozole increase the relative risk of vertebral and non-vertebral fractures by 40 %, when compared with tamoxifen. After a few years of AI use, women have a 20–35 % increased fracture risk [67]. For instance, fracture risk has been reported to increase by 55–115 % with anastrozole in the ATAC [68] and Austrian Breast & Colorectal Cancer Study Group (ABCSG) [69] trials, by 15–50 % with letrozole in the BIG-198 [70] and MA17 [71] trials, and by 41 % with exemestane in the IES trial [72], as compared to tamoxifen or placebo. However, recent data show that bone loss associated to treatment with aromatase inhibitors in breast cancer patients is influenced by CYP19 polymorphisms [73].
Sex hormone deprivation therapy with GnRH agonists and antagonists
Long-acting gonadotropin-releasing hormone (GnRH) agonists with increased receptor affinity or prolonged half-live lead to persistent activation of gonadotropin-releasing hormone receptors, causing an initial release of pituitary gonadotropins followed by a down-regulation of GnRH receptor and suppression of gonadotropin secretion. Consequently, ovarian sex steroids production is suppressed. GnRH agonists are effective in the management of endometriosis and of breast cancer in premenopausal women by suppressing oestrogen levels but they are inducing bone loss (Table 2). A BMD decrease of about 6 %/year is observed in patients on GnRH agonists with a recovery of bone mass after discontinuation. GnRH agonists may not increase the risk of fragility fractures in women with normal BMD [43].
In men with hormone-sensitive prostate cancer, castration or ADT can be induced surgically or medically with GnRH agonists or antagonists and a combination of GnRH agonist with androgen biosynthetic blockade. ADT is effective in reducing tumour extension, growth and improving survival [74]. ADT can be enhanced by the addition of androgen biosynthetic blockade such as cyproterone. The latter two strategies have a different effect on the skeleton. ADT by either bilateral orchiectomy or GnRH agonist increases the risk of fractures [18, 75–77]. In contrast, anti-androgens alone increase rather than decrease BMD in two randomized trials [78, 79]. Androgen deficiency-mediated decrease in lean body mass, increase in fat mass and impaired muscular strength may contribute to increased fracture risk [80–84] (Table 2). Abiraterone is an inhibitor of CYP17A1, an enzyme required in androgen synthesis. It reduces androgen and precursors steroids, and is associated with a better survival. Abiraterone is administered with prednisone 5 mg twice daily, or use of another steroid. At this time, there is insufficient bone data on this treatment regimen [85].
In men with prostate carcinoma, BMD of hip, ultradistal radius and lumbar spine decreases by 2–5 % after 12 months of ADT, and the risk of vertebral and hip fractures increases by 40–50 % [18, 77]. Fracture risk increases linearly with the number of GnRH injections. It has been estimated that fracture risk in men on ADT is as high as 20 % by 5 years of treatment [18, 86]. Older men and those with osteoporosis risk factors in addition to age are at the highest risk [87].
Radiation-induced hypogonadism and direct toxic effects on bone
Both female and male cancer survivors who received irradiation to the cranium, ovaries or testes can display hypogonadism [88]. The association between male hypogonadism and osteoporosis is further supported by reports of fractures occurring after external beam radiation therapy to the prostate bed for prostate cancer [89–92] (Table 2). In addition, pelvic or rib fracture can occur in relation with bone local irradiation [93, 94].
Glucocorticoids
The majority of therapeutic regimens for many haematopoietic malignancies involve high-dose glucocorticoids, usually administered over extended periods of time. Glucocorticoids, which are often used as a pain adjuvant, palliative agent, antiemetic or as part of the treatment, initially increase bone resorption, then later suppress osteoblast activity reducing thereby bone formation [88] (Table 2). Prolonged exposure to corticosteroids is the third leading cause of osteoporosis, after hypogonadism and advancing age [95, 96]. The risk of fracture increases by 50–100 % in recipients of oral corticosteroids [97].
Other indirect effects of cancer therapies
There are other indirect effects on bone health that result from cancer therapies [43]. These effects include hypovitaminosis D, cachexia (sarcopenia) and decreased mobility (Table 2).
Fracture risk assessment in patients with cancer and adjuvant therapies
Patients with cancer-associated osteoporosis are generally younger than patients with postmenopausal osteoporosis. As people with cancer survive longer, cancer-related skeletal event and their treatments are becoming increasingly recognized as important co-morbidities [98]. Early evaluation of risk factors for osteoporosis, including family history of fracture and assessment of peripheral neuropathy that may have occurred secondary to cancer therapy, medication review, physical examination, fall risk assessment, diet and exercise assessment and counselling, as well as changes in BMD over time, are of prime importance. Fracture risk assessment in this unique population can be challenging. It should include evaluation of BMD and clinical risk factors as detailed below.
Bone mineral density
BMD measurement by dual X-ray absorptiometry (DXA) is the most common clinical tool to directly measure bone mineral mass and indirectly evaluate bone strength [99]. BMD should be measured by DXA at spine and hip with measurements at the 1/3 radius considered if either one of these sites is not available. Malignancies in bone may either be lytic (e.g. myeloma) or blastic (e.g. breast or prostate), and if present in the region of interest, artifact is introduced. Infrequently, DXA images may give an indication of skeletal metastases requiring other imaging follow-up. Although radius BMD changes little with osteoporosis therapy, hypogonadism (e.g. from ADT) and hyperparathyroidism often lead to loss of forearm BMD [100]. Several guidelines recommend that postmenopausal women with breast cancer on AI, premenopausal women with ovarian failure secondary to cancer treatment and prostate cancer patients on ADT should have their BMD measured [42, 101]. For instance, the American Society of Clinical Oncology (ASCO) recommends DXA BMD testing in postmenopausal women taking AIs and premenopausal women who develop treatment-related premature menopause [102]. The Belgian Bone Club recommends measuring DXA BMD in all women starting AIs or medical castration therapy [103]. The UK Expert Group recommends measuring DXA BMD within 3 to 6 months of commencing AIs in all women, except for those ≥75 years of age, in whom treatment decisions are based upon age and clinical risk factor assessment, independently of BMD [101]. Similarly, an International Expert Panel recommends BMD measurement in patients with breast cancer initiating or receiving AI therapy [104] as does also the European Society for Clinical and Economical Aspects of Osteoporosis and Osteoarthritis (ESCEO) [15]. Patients with bone metastases receiving monthly bone resorption inhibitors do not require BMD assessment.
Clinical risk factors: 10-year absolute fracture risk
WHO fracture risk assessment tool (FRAX) provides an algorithm applicable for men older than 40 years and for postmenopausal women: 10-year fracture risk of the hip and of major osteoporotic fracture (wrist, proximal humerus, hip and clinical spine) with and without BMD. Including BMD improves the predictive performance of the score, that is, improves sensitivity without decreasing specificity [105, 106]. Although the FRAX calculator has provided a major advance in our assessment of fracture risk, it does not take into consideration some other risk factors, due to the nature of the clinical information available in the cohorts the model was developed from [107].
There is no way of estimating the impact of malignancy or its treatment from the FRAX algorithm [108], though cancer treatment-induced bone loss could be considered as secondary osteoporosis, the role of which in FRAX fracture assessment is entirely captured by BMD. Clinicians should thus use their clinical judgement to quantify their patient’s individual fracture risk.
There are two studies that used the FRAX calculation in men on ADT. In a cross-sectional study [109], FRAX identified more men at risk for fracture than BMD alone. Age was a very important risk factor. Adler reported [87] that FRAX derived estimates using femoral neck BMD or calculated without BMD defined different populations at risk.
Other risk factors
Fall risk, bone turnover markers and many other risk factors for fracture and bone loss are not included in FRAX. Avariety of measures of prospective fall risk have been shown to be useful in predicting fractures. They include questions or questionnaire-based tools, simple physical performance tests, measurements of muscle mass and devices that measure some aspect of strength, balance or integrated function [110, 111]. Bone turnover markers hold promise in fracture risk prediction and for monitoring treatment. However, there are still uncertainties about their use in clinical practice [112].
Medications
Numerous medications other than glucocorticoids, such as proton pump inhibitors, can predispose to bone loss (Table 2). They have not been included in FRAX modelling due to the infrequency of their use in the general population, their lack of evaluation in epidemiologic studies and their presumed lesser effects on bone metabolism.
Prevalent fracture
Prevalent fracture is a powerful predictor for further fractures, e.g. hip fracture risk is increased by more than twofold by a prevalent hip or spine fracture, independently of BMD, some of them being pathologic fractures. Indeed, a spine fracture increases the risk of hip fracture (relative risk (RR)=2.5), subsequent spine fracture (RR=4.4), forearm fracture (RR=1.7) or proximal humerus fracture (RR=1.9) [113, 114]. A greater number or higher severity of vertebral fracture increases the risk of vertebral and non-vertebral fracture. However, number, severity, location of fractures and glucocorticoid dose and duration are not captured in the current FRAX algorithm [107, 115]. Pathologic fracture versus osteoporotic fracture may be difficult to distinguish from plain radiographs and consideration should be given to other imaging such as computed tomography (CT), magnetic resonance imaging (MRI) or isotope bone scanning. Asymptomatic vertebral fractures are very common when appropriate imaging is applied [116]. Concerns for metastatic osseous lesions may be confirmed by biopsy.
Detection of metastatic cancer to bone
Among the many techniques used for imaging metastases, each has advantages and limitations [117]. The two “classical” methods, radiography and scintigraphy, both have limited sensitivity and specificity. CT and MRI are preferable because they provide more accurate information [118, 119]. Modern devices permit scans that cover a large part of the body with acceptable radiations exposure (for CT) and in reasonable time (for MRI). While MRI is more suited to detect early infiltration when the tumour is still restricted to bone marrow and offers better insight into soft tissue involvement, CT provides a better characterization of bone integrity. More sophisticated approaches like positron emission tomography (PET), today usually used in conjunction with CT (PET/CT), may also be considered, but the choice of the radiotracer is important. FDG PET is most commonly used, but the magnitude of the improvement over scintigraphy is controversial [120, 121]. 18F-Fluoride PET or 18F-choline PET [122] may be better choices but are more expensive and rarely available. The choice of bone imaging is impacted by the primary tumour type, the patient’s symptoms and location of area(s) in question.
Compromised bone integrity may endanger the spinal cord or other critical structures. Urgent surgical intervention to stabilize the skeleton and relieve pressure on nerves may be required. For long bones, lesions that involve 50 % of the diameter of the cortex or that are larger than 2.5–3 cm are considered at risk for pathological fracture. Clinical judgment is required to evaluate all skeletal complications of metastatic disease. Particularly at the spine, the assessment of mechanical stability is complex. New approaches for evaluation of stability based on CT may be more accurate, allowing both a better quantification of regional bone density and specific imaging of bone at high risk of pathologic fracture. In addition, finite element analysis methods developed successfully for evaluation of bone strength in osteoporosis [123] may be adapted in the future to metastatic bone [124, 125].
Prevention and treatment
Prevention of skeletal-related events
Bisphosphonates
Several studies have shown that bisphosphonates have anti-tumour potentials with direct and indirect effects that result not only in less bone loss but less tumour burden as well. They interrupt the vicious cycle in the bone microenvironment between tumour cells and osteoclasts described in section on pathophysiology above. Bisphosphonates, in particular zoledronic acid, also enhance γδ T cell differentiation (a T lymphocyte subpopulation that plays a main role against tumour cells) and have also potent anti-angiogenic activity in the adjuvant setting [23]. There is no clear impact of osteoclast inhibiting therapy on tumour burden. However, two recent phase III studies suggest that potent osteoclast inhibition may impact overall survival [126, 127].
Breast cancer in the adjuvant setting
The efficacy of bisphosphonates in reducing SREs in women with breast cancer and bone metastatic disease is unequivocal, as demonstrated in a recent Cochrane meta-analysis [128]. While they reduce the occurrence of SREs by 15 to 40 % [129], they do not impact SREs in subjects without bone metastases [130]. In the frame of adjuvant therapies, evidence from a secondary endpoint in the Zometa-Femara Adjuvant Therapy Synergy Trial (ZO-Fast) supports the hypothesis that bisphosphonates have an anti-metastatic role in breast cancer patients. In more than 1,000 recruited patients, those in the upfront group had lower disease recurrence or death than patients in the delayed group (1.1 vs 2.3 %). No local disease recurrences occurred in upfront patients while it occurred in 0.6 % of the delayed group. Efficacy of zoledronic acid was confirmed also in premenopausal women with a significant improvement in disease-free survival shown in the ABCSG-12 trial [131]. Both loco-regional and extraskeletal metastases risk were reduced, suggesting a systemic anti-tumour effect exerted by zoledronic acid. The results of these two adjuvant bisphosphonate studies must be viewed in conjunction with the outcomes of three other phase III adjuvant bisphosphonates trials. The Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) trial randomized 3,360 women with stage II or III early stage breast cancer to receive standard adjuvant systemic therapy with or without zoledronic acid. The bisphosphonate dosing was incrementally decelerated over the course of 5 years; however, total dose of zoledronic administered was much greater than either ZoFast or ABCSG-12. The primary endpoint in AZURE was disease-free survival. At a median follow-up of 59 months, AZURE demonstrated disease recurrence or death in 377 women in the zoledronic acid group and 375 in the control group [130]. In subset analysis, postmenopausal women appeared to have gained benefit from the addition of zoledronic acid. Note that to reach a neutral overall study outcome, premenopausal women had to have displayer a worse outcome with the addition of zoledronic acid. There is much speculation as to account for these findings. In patients with bone metastasis, a retrospective analysis based on 578 patients showed that zoledronic acid treatment normalized N-terminal telopeptide (NTX) levels in 81 % of the treated patients within the third month. Moreover normalization of NTX was associated with reduced risk of SREs and improved overall survival [132]. The National Surgical Adjuvant Breast and Bowel Project study B-34 [133] randomized 3,323 women to adjuvant clodronate or placebo with disease-free survival in women with stage I, II or III breast cancer as primary endpoint. At a median follow-up of 90.7 months, there were 286 events in the clodronate group and 312 events in the controls, which was a non-significant difference (95 % CI 0.78, 1.07; p =0.27). Similarly, in the study GAIN, 3,023 women with lymph node positive breast cancer were randomized to either oral ibandronate or placebo. There was equal disease free and overall survival in the two groups [134]. In another phase III study, patients were randomized to clodronate, ibandronate or zoledronic acid (without control or placebo arm). The results are due soon [135].
Multiple meta-analyses have been performed using published data and came to different conclusions, depending on the studies selected. It is anticipated that the Early Breast Cancer Trialist’s Collaborative Group will perform a meta-analysis based upon raw data and provide thus greater insight into the situation.
Prostate cancer
Earlier studies using pamidronate and clodronate failed to demonstrate a reduction in SREs in patients with prostate cancer and bone metastases. In a randomized controlled trial (RCT) in patients with castration-resistant prostate cancer with bone metastases, zoledronic acid (4 mg, 3-weekly) or placebo reduced SREs (p =0.009) and prolonged the median time to first SRE from 321 to 488 days (p =0.009) [136]. Bisphosphonates have not been shown to prevent bone metastases due to prostate cancer in any study. An ongoing study in men with metastatic prostate cancer compares the early use of zoledronic acid (within 3 months of initiation of ADT) to standard zoledronic acid (on diagnosis of castration resistance). The primary end point is the proportion of subjects experiencing SREs.
Denosumab
RANKL is a key mediator of metastatic bone resorption. Denosumab is a human monoclonal antibody that binds and neutralizes human RANKL. It prevents RANKL from activating RANK on osteoclasts, inhibiting osteoclast formation, function and survival, and hence reducing bone resorption. Therefore, RANKL inhibition through denosumab is a therapeutic target for preventing and treating bone metastases.
Breast cancer
In a study evaluating the efficacy of denosumab in 2,046 breast cancer patients with bone metastases in a double-blind double-dummy trial, denosumab (120 mg monthly) was superior to zoledronic acid (4 mg monthly with adaptation of the dose to the renal function) in suppressing bone turnover and delaying or preventing SREs. Denosumab increased the time to first on-study SRE by 18 % compared with zoledronic acid (hazard ration (HR), 0.82; p <0.001 for non-inferiority and p =0.01 for superiority). The median time to first on-study SRE was 26.4 months for the zoledronic acid group and had not been reached for the denosumab treatment group. Denosumab also delayed the time to first and subsequent (multiple) on-study SREs by 23 % compared with zoledronic acid (multiple event analysis; p =0.001). The mean skeletal morbidity rate (defined as the ratio of the number of SREs per patient divided by the patient’s time at risk) was also lower with denosumab than with zoledronic acid (0.45 vs 0.58 events per patient per year; p =0.004), which represents a reduction of 22 % with denosumab. Overall survival and disease progression were similar in the two treatment groups. Safety profile, including onset of osteonecrosis of the jaw, was similar between both groups [137].
Prostate cancer
In a study comparing denosumab (120 mg monthly) to zoledronic acid (4 mg monthly) for the prevention of SREs in 1,904 men with castration-resistant prostate cancer metastatic to bone, the primary end point was the time to first SRE. Denosumab proved to be non-inferior (p =0.0002) and superior (p =0.008) compared to zoledronic acid. Denosumab delayed the time to first on-study SRE by 18 % compared with zoledronic acid (HR, 0.82) and the median time to first on-study SRE was 3.6 months longer with denosumab compared with zoledronic acid. Denosumab also significantly delayed the time to first and subsequent on-study SREs by 18 % compared with zoledronic acid (p =0.008). Overall survival and disease progression evaluated by changes in PSA levels did not differ between both groups [138].
In the adjuvant setting, when denosumab 120 mg every 4 weeks was compared to placebo in preventing bone metastases in 1,432 men with castration-resistant prostate cancer and at high risk of developing bone metastases, bone metastasis-free survival was higher (p =0.028) and time to first bone metastasis increased (p =0.032) in the denosumab group. Denosumab improved bone metastasis-free survival by a median of 4.2 months compared with placebo (29.5 vs 25.2 months; HR, 0.85; p =0.028). Time to symptomatic bone metastasis was also prolonged. Overall survival was similar in both groups, but study medications were stopped when the first bone metastasis was diagnosed. Denosumab was associated with higher incidence of hypocalcaemia (1.7 vs 0.3 %) and osteonecrosis of the jaw (4.6 vs 0 % at the end of year 3) [139].
Other solid tumours and multiple myeloma
In a third study with an identical design, 1,776 patients with multiple myeloma or bone metastases and solid tumours (except those of prostate or breast) were randomized to receive denosumab or zoledronic acid. Approximately 40 % of patients in the study had non-small cell lung cancer and 10 % multiple myeloma. Denosumab was non-inferior to zoledronic acid in delaying the time to first on-study SRE (HR, 0.84; p =0.0007), but, after adjustment for multiple comparisons, the difference between the groups was not statistically significant for treatment superiority (p =0.06). The median time to first on-study SRE was 20.6 months for denosumab and 16.3 months for zoledronic acid. Overall survival and disease progression were similar in both treatment groups [140].
Integrated analysis of the three phase III trials in metastatic patients
The identical design of the three phase III studies conducted in patients with metastatic bone disease allowed for a pre-planned integrated analysis of the data involving a total of more than 5,700 patients. Denosumab increased the time to first on-study SRE by 17 % over zoledronic acid (HR, 0.83; 95 % CI, 0.76–0.90; p <0.001 for superiority). The median time to first SRE was 27.7 months with denosumab and 19.4 months with zoledronic acid. This integrated analysis showed that denosumab delayed the time to first and subsequent on-study SREs by 18 % compared with zoledronic acid (HR, 0.82; 95 % CI, 0.75–0.89; p <0.001 for superiority). Overall survival and disease progression were similar with both treatments [132]. The integrated analysis showed that the cumulative incidence of confirmed osteonecrosis of the jaw (ONJ) after 3 years was not negligible (n =89 from total of 5, 723 patients; 1.6 %) with no significant difference between denosumab and zoledronic acid treatments (1.8 vs 1.3 %; p = 0.13) [141]. The role of denosumab in the management of hypercalcaemia of malignancy has not yet been evaluated and clinical trials should be conducted to more clearly document this effect.
Conclusion for the prevention of skeletal-related events in breast cancer
In a recent meta-analysis of 34 RCTs conducted in women with various stages of breast cancer, Wong et al. have demonstrated the unequivocal efficacy of bone resorption inhibitors in reducing the incidence of SREs in women with bone metastatic disease [128]. Bisphosphonates reduced the risk of SREs by 15 % (RR=0.85, 95 % CI, 0.77–0.94), in nine studies of 2,806 women with bone metastases. This benefit was observed with intravenous (IV) zoledronic acid (4 mg/3–4 weeks) with RR 0.59 (95 % CI, 0.42–0.82), IV pamidronate (90 mg/3–4 weeks) (RR=0.77; 95 % CI, 0.69–0.87) and IV ibandronate (RR= 0.80; 95 % CI, 0.67–0.96), with one direct comparison in a large study confirming the equivalent efficacy of zoledronate and pamidronate. In three studies comprising 3,405 patients with skeletal metastases, denosumab was superior to a bisphosphonate in reducing the risk of SREs (RR=0.78; 95 % CI, 0.72–0.85) [128]. Such therapies did not influence the outcomes of women without bone metastases at study entry. The authors also concluded that there is insufficient evidence to make a conclusion about the role of adjuvant bisphosphonates in reducing visceral metastases, loco-regional recurrence and total recurrence or improving survival. Toxicity was generally mild kidney toxicity and ONJ were reported at similar rates for patients on denosumab compared to zoledronic acid.
Prevention of bone loss and fractures
As in idiopathic osteoporosis, preventive measures to maintain bone health include lifestyle changes, adequate calcium and vitamin D, regular physical exercise and pharmacotherapy such as bisphosphonates, denosumab or selective oestrogen receptor modulators (SERMs).
Lifestyle, calcium and vitamin D
Lifestyle interventions are crucial in order to improve quality of life and maximize any pharmacological treatment in cancer patients. The American Cancer Society, the World Cancer Research Fund/American Institute for Cancer Research and the American College of Sports Medicine have released guidelines for cancer survivors [142–144], recommending at least 30 min daily of physical activity for at least 5 days per week. Exercise interventions are also beneficial for muscle strength and bone mass, reducing also the risk of falls and, in turn, potentially of hip fractures.
Patients with malignancies are exposed to risk factors that may predispose to vitamin D deficiency. Among them, inadequate sunlight exposure, poor dietary intakes and treatment with medications that reduce vitamin D absorption are the most common [145, 146]. Indeed, 76–88 % of breast cancer survivors have low vitamin D levels, i.e. <20 ng/mL [147–149]. A recent study has shown that low vitamin D levels are highly prevalent among newly diagnosed breast cancer patients with nearly 44 % with vitamin D insufficiency [150]. As expected, African-Americans are more affected than Caucasians [150]. Clinical trials using vitamin D in cancer patients are lacking. In a recent double-blind placebo-controlled randomized phase II trial, breast cancer patients on AIs, treated with vitamin D2, had less aromatase inhibitor-induced musculoskeletal symptoms than those on placebo. Vitamin D-treated patients had also a stable BMD at the femoral neck (0.45 %±0.72), compared to a 1.39 % decrease seen in the placebo group [151]. The ASCO recommends supplementation with calcium and vitamin D in breast cancer patients, mostly if treated with AIs [102]. However, routine 25-hydroxyvitamin D measurement is not part of the current guidelines in breast cancer patients. Given the lack of clinical trials, no guidelines on specific levels of supplementation for vitamin D or calcium are available. Vitamin D supplementation should be given in order to bring serum 25-hydroxyvitamin D levels to 20 ng/mL (50 nmol/L) or higher [152]. According to data obtained in postmenopausal women, a daily intake of at least 800 IU should be advised together with a daily calcium intake (from food and supplements) of 1,000 mg. The intake of calcium from the diet should be favoured because it has been suggested that high doses of calcium supplements were associated with increased cardiovascular risks [153]. These recommendations have been extended from National Comprehensive Cancer Network (NCCN) Bone Health in Cancer Care Task Force to premenopausal women at risk of cancer treatment-associated bone loss.
As advised by the NCCN Prostate Cancer Guidelines (available at www.nccn.org), similar preventive measures, with calcium and vitamin D supplementation, should be applied to patients with prostate cancer, particularly if on ADT. A sufficient intake of calcium and vitamin D should be emphasized in those on intravenous bisphosphonates or denosumab to avoid the risk of hypocalcaemia.
Bisphosphonates
Bisphosphonates have been successfully used in patients with metastatic bone diseases to reduce SREs [154]. However, their use has been extended to prevent bone loss in patients with both breast or prostate cancer in the adjuvant setting, who are treated with chemotherapy or hormonal therapy [131]. This approach is in accordance with the wide use of bisphosphonates in idiopathic osteoporosis [155].
Breast cancer
In premenopausal women with breast cancer, adjuvant chemotherapy induces early menopause, a strong factor of accelerated bone loss. Few trials have evaluated the efficacy of bisphosphonates to attenuate chemotherapy-induced bone loss in young women [156–164]. The administration of oral clodronate at daily doses of 1,600 mg, oral risedronate at a dose of 30 mg/day, cyclical intravenous pamidronate at doses of 60 mg every 3 months and intravenous zoledronic acid at a dose of 4 mg every 3 months prevented chemotherapy-induced bone loss in these studies. Numerous other studies have confirmed such findings and are summarized in Table 3.
Table 3.
Guidelines for bone management in breast cancer
| Resources | Target population | Indication | Type of treatment, concentration, duration | Monitoring | Other recommendations |
|---|---|---|---|---|---|
| American Society of Clinical Oncology (ASCO) [102, 154] | Patients with metastatic breast cancer | Patients with metastatic breast cancer with evidence of bone metastases | sc denosumab 120 mg every 4 weeks IV pamidronate 90 mg over 2 h every 3–4 weeks IV zoledronic acid 4 mg over 15 min every 3–4 weeks |
Serum creatinine monitored before each dose of BP If serum creatinine clearance >60 mL/min, no change Annual DXA test |
To prevent ONJ, dental examination and appropriate treatment before BP Start the current standards of care for cancer bone pain management at the onset of pain, in concert with the initiation of the bone-modifying agent therapy Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
| Postmenopausal patients treated with AI | Prevention of bone loss | BP therapy with AI | |||
| International panel [189] | Patients with metastatic breast-cancer | Patients with metastatic breast-cancer Patients who cannot attend regular hospital care |
IV pamidronate over 2 h every 3–4 weeks IV ibandronate over 15 min every 3–4 weeks IV zoledronic acid over 15 min every 3–4 weeks Oral ibandronate or clodronate |
Serum creatinine monitored before each dose of BP Dose of BP adapted to renal function Subsequent monitoring for zoledronic acid and pamidronate Treatment for 2 years even in case of bone event. Beyond 2 years, individual risk assessment BMT test for people with risk factors: T-score < −1.5, age >65 years, corticosteroids for more than 6 months, family history of hip fracture, or personal history of fragility fracture after 50 |
To prevent ONJ, dental examination and appropriate treatment before BP Pain control, analgesic therapy and BP by itself is a major factor of quality of life To avoid renal toxicity with IV BP, hydration before treatment Calcium and vitamin D supplements In case of oral administration, instruction to avoid GI problems |
| Adjuvant setting | Patients receiving AI | Preventive BP, and especially IV zoledronic acid | BMD test | ||
| International Society of Geriatric Oncology (SIOG) [190] | Elderly patients with bone metastases | Prevention of SREs | IV pamidronate 60 mg/h at max IV ibandronate. for creatinine clearance <30 mL/min, 2 m of ibandronate every 3–4 weeks (1-h infusion) IV zoledronic acid 4 mg, with dose adjustments for patients with mild to severe renal impairment and not recommended in patients with severe renal impairment (<30 mL/min) Oral clodronate 800 mg: creatinine clearance between 10 and 30 mL/min Oral ibandronate 50 mg weekly: creatinine clearance< −30 mL/min |
Creatinine clearance instead of serum creatinine should be monitored before each dose of BP Less renally toxic agent should be used |
To prevent ONJ, dental examination and appropriate treatment before BP Zoledronic acid, ibandronate and pamidronate useful in the management of pain Optimization of the hydration status In case of oral administration, instruction to avoid GI problems |
| UK guidance document [101, 191] | Patients with advanced breast cancer | Patients newly diagnosed with bone metastases Patients with pain Patients with long bone involvement and significant cortical destruction Patients with or at risk of spinal cord compression |
BPs Single fraction radiotherapy (8 Gy) Assessment by an orthopaedic surgeon, surgery intervention to prevent fracture Assessment by a spinal specialized team, surgery, corticosteroids, radiotherapy |
Supportive care | |
| Post-menopausal patients treated with AI for breast cancer | Treatment depends on baseline BMD (T-score < −2.0) or occurrence of a vertebral fragility fracture or previous low trauma hip fracture or an annual bone loss ≥4 % or age >75 years and ≥1 clinical risk factors | Oral alendronate 70 mg weekly or risedronate 35 mg, oral ibandronate 150 mg monthly, IV ibandronate 3 mg 3-monthly or IV zoledronic acid 4 mg 6-monthly | BMD (DXA) test at spine/hip within 3 months of start of the treatment and then DXA after 1 year | Calcium and vitamin D supplements Lifestyle advice |
|
| Patients who experience premature menopause due to chemotherapy, or ovarian suppression, ablation or removal | Patients with DXA T-score < −1.0 or vertebral fragility fracture or previous low trauma hip fracture | Oral alendronate 70 mg weekly or risedronate 35 mg, oral ibandronate 150 mg monthly, IV ibandronate 3 mg 3-monthly or IV zoledronic acid 4 mg 6-monthly | BMD (DXA) test at spine/hip within 3 months of start of the treatment and then DXA after 1 year Monitoring thereafter depends on baseline BMD |
Calcium and vitamin D supplements Lifestyle advice |
|
| Belgian Bone Club [103] | Patients treated with AI for breast cancer | Patients with a T-score < −2.5 or history fragility fracture or a T-score between −1.0 and −2.5 with additional risk factors | IV zoledronic acid 4 mg every 6 months Other bisphosphonates may be considered |
At start of treatment, BMD test by DXA and assessment of risk factors for osteoporotic fracture BMD monitoring every 1–2 years of osteopenic, osteoporotic patients |
Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
| Practical guidance [104, 187] | Patients treated with AI for breast cancer | For patients with 2 risk factors (T-score < −1.5, age >65 years, low BMI, family history of hip fracture, corticosteroids, smoking) | IV zoledronic acid 4 mg every 6 months for at least 2 years and possibly as long as AI treatment Oral risedronate 35 mg/week (limited efficacy data) sc denosumab 60 mg every 6 months (limited efficacy data) |
Monitoring of BMD every 2 years For patients with a T-score ≥ −2.0 and no additional risk factors, BMD monitoring every 1–2 years |
Yo prevent ONJ, dental examination and appropriate treatment before BP Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
| ESCEO [15] | Women treated with AI for breast cancer | Osteoporotic women (T-score hip/spine < −2.5, or ≥1 prevalent fracture) Women aged ≥75 irrespective of BMD Patients with T-score < −1.5 + ≥1 clinical risk or T-score < −1.0 +≥2 clinical risk factors |
IV zoledronic acid 4 mg every 6 months sc denosumab Oral BPs For the entire period of AI: BP |
PTH, calcium, 25-hydroxyvitamin D to be tested before start of AI treatment Baseline risk of osteoporotic fracture by DXA and evaluation of clinical risk factors |
Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
AI aromatase inhibitor, BMD bone mineral density, BP bisphosphonate, DXA dual X-ray absorptiometry, ER+ oestrogen receptor positive, GI gastrointestinal, IV intravenous, ONJ osteonecrosis of the jaw, sc subcutaneous, SRE skeletal-related event
Bisphosphonates have also been shown to prevent AI-induced bone loss. The SABRE trial has tested in an open label approach the effect of risedronate in breast cancer patients on anastrozole. At 24 months follow-up, medium-risk patients on risedronate had a significant increase in lumbar spine and total hip BMD compared with anastrozole and placebo (2.2 vs −1.8 and 1.8 vs −1.1 %, respectively). In the high-risk group, lumbar spine and total hip BMD increased (3.0 and 2.0 %) while those on anastrozole had a 2.1 % decrease [165]. In the ARIBON study, early stage breast cancer patients receiving anastrozole were treated with ibandronate (high risk), ibandronate or placebo (medium risk), or anastrozole only (low risk). At 24 months, ibandronate-treated patients (150 mg orally every month), compared to placebo, had a higher BMD and suppressed markers of bone turnover [166]. Overall, these data confirm that oral bisphosphonates improve BMD and may normalize bone turnover in AI-treated patients.
Prevention of AI-induced bone loss has been tested also using intravenous infusions of zoledronic acid. The Z-Fast and ZO-Fast were designed to compare effects of zoledronic acid (4 mg intravenously) initiated either with letrozole and every 6 months thereafter (upfront group) or given only when bone loss became significant or a fragility fracture occurred (delayed group) [147]. In over 1,667 patients, the upfront regimen significantly improved lumbar spine BMD (+5.2 %) and total hip BMD (+3.5 %) versus the delayed group. N-telopeptide and ALP dropped by 21.3 and 12.8 % in the upfront group and increased by 21.7 and 24.9 % in the delayed group. No differences were found between the two groups in terms of fractures, although the study was not designed to test this endpoint. Disease recurrence was less frequent in the upfront group and no case of osteonecrosis of the jaw was reported. Encouraging data were also obtained in premenopausal women in the ABCSG where zoledronic acid prevented bone loss in a subgroup of premenopausal cancer patients [50]. These and other studies are presented in Table 3.
Prostate cancer
Alendronate, risedronate, zoledronic acid, denosumab and teriparatide are approved for the treatment/prevention of osteoporotic fractures in men with osteoporosis with some limitations according to regional policies. The use of teriparatide is not recommended in cancer-treated patients. Available RCTs in the setting of ADT and prostate cancer have shown efficacy in the prevention of bone loss, a significant increase in BMD and normalization of bone turnover in the dosages normally recommended for idiopathic osteoporosis [167, 168]. Pamidronate (60 mg IV 12-weekly) in a small prospective study in men with non-metastatic prostate cancer significantly increased BMD at the spine and hip [83]. Zoledronic acid (4 mg IV as single dose) in a RCT of ADT and non-metastatic prostate cancer significantly increased BMD at spine and hip, when compared to placebo [169]. There is presently no published trial of the effect of a bisphosphonate on fracture risk in the ADT/prostate cancer setting. These and other studies are presented in Table 5.
Table 5.
Guidelines for bone management in prostate cancer
| Resources | Target population | Indication | Type of treatments, concentration, duration | Monitoring | Other recommendations |
|---|---|---|---|---|---|
| International panel [189] | Patients with prostate cancer | Patients with hormone refractory prostate cancer metastatic to bone | IV zoledronic acid 4 mg every 3 weeks for 15 min | Serum creatinine before each dose Dose of BP adapted to renal function Subsequent monitoring for zoledronic acid Treatment for 2 years even in case of bone event; beyond 2 years, individual risk assessment |
To prevent ONJ, dental examination and appropriate treatment before BP Pain control, analgesic therapy and BP by itself is a major factor of quality of life To avoid renal toxicity with iv BP, hydration before treatment Calcium and vitamin D In case of oral administration, instruction to avoid GI problems |
| International Society of Geriatric Oncology (SIOG) [190] | Elderly patients with prostate cancer | Prevention of SREs | IV zoledronic acid 4 mg with dose adjustments for patients with mild to severe renal impairment and not recommended in patients with severe renal impairment (<30 mL/min) | Creatinine clearance instead of serum creatinine monitored in each patient before each dose Less renally toxic agent should be used |
To prevent ONJ, dental examination and appropriate treatment before BP BP useful in the management of pain Optimization of the hydration status |
| Belgian Bone Club [103] | Patients with prostate cancer | Patients treated with ADT and T-score < −2.5 or history fragility fracture or a T-score between −1.0 and −2.5 with also risk factors | IV zoledronic acid 4 mg once a year Oral alendronate, weekly |
At start of treatment, BMD test by DXA and assessment of risk factors for osteoporotic fracture BMD monitoring every 1–2 years of osteopenic, osteoporotic patients |
Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
| ESA, ANZBMS and USANZ [197] | Patients with non-metastatic prostate cancer | Patients receiving ADT | BPs (pamidronate, zoledronic acid, alendronate, risedronate) | DXA and thoracolumbar spine DXA in case of osteopenia Monitoring of metabolic health |
Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
| Expert panel [198] | Patients with prostate cancer | Patients beginning or receiving ADT with T-score < −2.5 | IV zoledronic acid 4 mg 15-min infusion every 3–4 weeks | DXA test at baseline and repeat every 12 months | Adequate lifestyle modifications (smoking, alcohol, exercise) Calcium and vitamin D supplements |
ADT androgen deprivation therapy, BMD bone mineral density, BP bisphosphonate, DXA dual X-ray absorptiometry, GI gastrointestinal, IV intravenous, ONJ osteonecrosis of the jaw, sc subcutaneous, SRE skeletal-related event
Denosumab
Breast cancer
The efficacy of denosumab was evaluated in 252 patients receiving adjuvant AI in the HALT-BC trial. Patients randomly received either placebo or denosumab (60 mg) every 6 months. In the denosumab group, BMD in all examined skeletal sites was increased already after 1 month of treatment, and at 12 and 24 months was significantly higher than placebo [170, 171]. At 24 months, the difference between both treatment groups was 7.6 % at the lumbar spine and 4.7 % at the total hip. At the lumbar spine, BMD increased by more than 3 % in 80 % of denosumab-treated patients compared with 10 % in the placebo group. An increase in BMD with denosumab was observed at lumbar spine after 1 year (+5.5 %) and at radius after 2 years (+6.1 %). No serious adverse events were attributed to denosumab in the trial. An ongoing clinical trial is investigating the anti-fracture efficacy of denosumab in women with breast cancer receiving AI in an adjuvant setting [172].
Prostate cancer
In the HALT Prostate trial, 1,468 men receiving ADT for prostate cancer and being at high risk for fracture (history of osteoporotic fracture, age ≥70 years or low BMD) were randomized to either denosumab (60 mg subcutaneously 6-monthly) or placebo for 3 years. At 2 years, BMD of the lumbar spine (the primary endpoint) increased by 5.6 % in the denosumab group as compared with a decrease of 1.0 % in the placebo group (p <0.001). Denosumab increased BMD at various skeletal sites (femoral neck, total hip, distal radius) and reduced the 3-year incidence of new vertebral fractures by 62 % (1.5 vs 3.9 % in the placebo group; HR, 0.38; 95 % CI, 0.19–0.78; p =0.006), fractures at any site by 72 % (p =0.10) and multiple fractures at any site by 72 % (p =0.006). In a further post hoc analysis, a trend was found toward a positive effect on non-vertebral fractures [173].
Selective oestrogen receptor modulators
In a RCT of 646 men with prostate cancer and ADT, subjects randomly received the SERM toremifene (80 mg oral daily) or placebo for 2 years. Toremifene significantly reduced the risk of new vertebral fractures by 50 % (p =0.05), increased BMD at the spine and hip, and decreased markers of bone turnover [174]. Raloxifene improved BMD at the hip and tended to improve BMD at the spine in another small open-label study, but has not been studied with fracture endpoints [175].
Official recommendations and comparison of guidelines
Metastatic bone disease
Tables 3 (breast), 4 (multiple myeloma) and 5 (prostate) summarize the various national and international recommendations for the management of bone disease in cancer patients and the suggested approaches to bone assessment and follow-up. The 2003 recommendations from the ASCO for the prevention and treatment SREs in patients with metastatic breast cancer [102] were updated in 2011 [154] (Table 3). The 2011 differ from the 2003 ones in the addition of denosumab to intravenous bisphosphonates. Bone-modifying agent therapy is only recommended for patients with breast cancer with evidence of bone metastases; denosumab 120 mg subcutaneously every 4 weeks, intravenous pamidronate 90 mg over no less than 2 h or zoledronic acid 4 mg over no less than 15 min every 3 to 4 weeks is recommended. ASCO also issued clinical practice guidelines to manage lytic bone disease or compression fractures in patients with multiple myeloma with intravenous bisphosphonates [176, 177]. IV zoledronic acid or pamidronate is recommended for preventing SREs in patients with multiple myeloma. Zoledronic acid is preferred over oral clodronate in newly diagnosed patients with multiple myeloma because of its potential anti-myeloma effects and survival benefits. Bisphosphonates should be administered every 3 to 4 weeks IV during initial therapy. Zoledronic acid or pamidronate should be continued in patients with active disease and should be resumed after disease relapse, if discontinued in patients achieving complete or very good partial response (Table 4).
Table 4.
Guidelines for bone management in multiple myeloma
| Resources | Target population | Indication | Type of treatments, concentration, duration | Monitoring | Other recommendations |
|---|---|---|---|---|---|
| American Society of Clinical Oncology (ASCO) [176, 177] | Multiple myeloma (MM) patients | For MM patients with lytic destruction of bone or spine compression fracture from osteopenia on plain radiographs or imaging and for MM patients with osteopenia, but no radiographic evidence of lytic bone disease | IV pamidronate 90 mg over 2 h every 3–4 weeks for 2 years IV zoledronic acid 4 mg over 15 min every 3–4 weeks for 2 years Oral clodronate |
Serum creatinine before each dose of pamidronate or Zoledronic acid Regular check of serum calcium, electrolytes, phosphate magnesium, haematocrit/haemoglobin Intermittent evaluation (every 3–6 months) of albuminuria patients who develop renal deterioration with no apparent cause during BP therapy should stop it |
For patients with pre-existing renal impairment, reduced dosage of zoledronic acid and initial pamidronate dose Zoledronic acid is not recommended in patients with severe renal impairment IV pamidronate or zoledronic acid for patients with pain caused by osteolytic disease as an adjuvant therapy To prevent ONJ, dental examination and appropriate treatment before BP |
| Haematology-oncology Task Force of the British Committee for Standards In Haematology and UK Myeloma Forum [192] | MM patients | For symptomatic MM patients with evident bone lesions or not For MM patients with fracture |
IV pamidronate IV zoledronic acid Oral clodronate Stabilization of long bone fractures and subsequent radiotherapy (8 Gy single fraction). Local radiation of 8 Gy single fraction for pain control |
Renal function should be carefully monitored and doses reduced according to manufacturers’ guidance | To prevent ONJ, dental examination and appropriate treatment before BP Oral calcium and vitamin D supplements with zoledronic acid |
| European Myeloma Network [193] | MM patients | For MM patients with lytic bone disease or severe osteoporosis | IV (or oral) pamidronate over 2–4 h for 2 years IV zoledronic acid Oral clodronate |
creatinine clearance | To prevent ONJ, dental examination and appropriate treatment before BP Patients with compromised renal function should have creatinine clearance rates, serum electrolytes, albuminuria BP useful in the management of pain |
| Mayo Clinic Consensus Statement [194] | MM patients | For MM patients with lytic disease evident on plain radiographs or osteopenia/osteoporosis | IV pamidronate monthly for 2 years IV zoledronic acid for monthly for 2 years |
Pamidronate is favoured over zoledronic acid | To prevent ONJ, dental examination and appropriate treatment before BP |
| National Comprehensive Cancer Network (NCCN) [195] | MM patients | For MM patients receiving primary myeloma therapy For MM patients with long-bone fractures or bony compression of spinal cord For MM patients with symptomatic vertebral compression fractures |
IV pamidronate IV zoledronic acid Radiotherapy (10–30 Gy) as a palliative treatment for uncontrolled pain Orthopaedic consultation Vertebroplasty or kyphoplasty |
Renal function | To prevent ONJ, dental examination and appropriate treatment before BP |
| ESMO [196] | MM patients | Patients with stage III or relapsed disease receiving conventional dose therapy | IV or oral long-term administration of bisphosphonates | ||
| International Society of Geriatric Oncology (SIOG) [190] | Elderly patients with MM | Prevention of SREs | IV pamidronate 60 mg/h at max. IV zoledronic acid 4 mg, with dose adjustments for patients with mild to severe renal impairment and not recommended in patients with severe renal impairment (<30 mL/min) IV ibandronate oral clodronate 800 mg: creatinine clearance between 10 and 30 mL/min |
Creatinine clearance instead of serum creatinine should be monitored in each patient before each dose Less renally toxic agent should be used |
To prevent ONJ, dental examination and appropriate treatment before BP BP useful in the management of pain Optimization of the hydration status In case of oral administration, instruction to avoid GI problems |
BMD bone mineral density, BP bisphosphonate, DXA dual X-ray absorptiometry, GI gastrointestinal, IV intravenous, MM multiple myeloma, ONJ osteonecrosis of the jaw, sc subcutaneous, SRE skeletal-related event
Although several studies suggest the potential usefulness of bone-modifying agents in preventing extension in other parenchymas, including skeletal metastases, in patients without metastatic bone disease at study entry, the evidence is not conclusive. The Cancer Care Ontario group has also issued recommendations to use zoledronic acid in adult patients with renal carcinoma and bone metastases [133].
Treatment-induced bone loss
The ASCO Guidelines Update committee on adjuvant endocrine therapy for women with ER+ breast cancer recommends that postmenopausal women with hormone receptor-positive breast cancer consider incorporating an aromatase inhibitor therapy at some point during adjuvant treatment, either as upfront therapy or as sequential treatment after tamoxifen. The optimal timing and duration of aromatase inhibition remain unresolved. The ASCO panel supports careful consideration of side effect profiles and patient preferences in deciding whether and when to incorporate AI therapy (Table 3). Risk stratification based on BMD T-score and clinical risk factors has been the recommended approach by several organizations to identify patients who most benefit from inhibitors of bone resorption. The issue of use of bone-modifying agents in the management of adjuvant-associated bone loss in patients with breast cancer is to be covered by ASCO in a separate guideline update that is eagerly awaited [154, 178].
General recommendations
Cancer without known skeletal metastases and not requiring therapy to lower sex steroid
Risk assessment should be applied as in non-cancer patients [179–182]. A detailed history and a focused physical examination are recommended to identify risk factors for low BMD, falls and fractures, as well as undiagnosed vertebral fractures. Hip and spine BMD should be measured with DXA according to the local guidelines for DXA use in non-cancer patients. FRAX should be calculated using femoral neck BMD and pharmacotherapy introduced according to guidelines for non-cancer patients.
For postmenopausal women or men over age 50 with densitometric osteoporosis (T-score ≤ −2.5 at the total hip, femoral neck or lumbar spine) or prevalent fragility fracture, laboratory investigations to rule out secondary causes of bone loss are recommended (blood cell count, calcium, phosphate, alkaline phosphatase, TSH, 25-hydroxyvitamin D, creatinine, serum protein electrophoresis) as in idiopathic osteoporosis. The use of bone turnover markers to improve fracture risk assessment or to monitor therapy remains controversial. However, in patients with skeletal metastatic disease, elevated markers of bone resorption appear to be associated with poorer prognosis and increased mortality [183].
Lateral thoracic and lumbar spine radiography, or VFA by DXA, if clinical evidence is suggestive of a vertebral fracture, should be performed. Vertebral fractures are defined as deformities of vertebrae with reduction greater than 20 % of vertebral dimension. Thirty percent of vertebral fractures are asymptomatic and therefore imaging in patients at risk should be encouraged. Such fractures are highly predictive of future fracture and have a marked influence on FRAX outcome.
Non-metastatic cancer treated with endocrine therapy
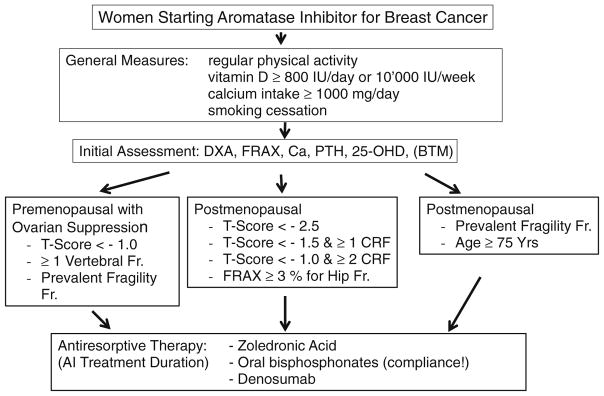
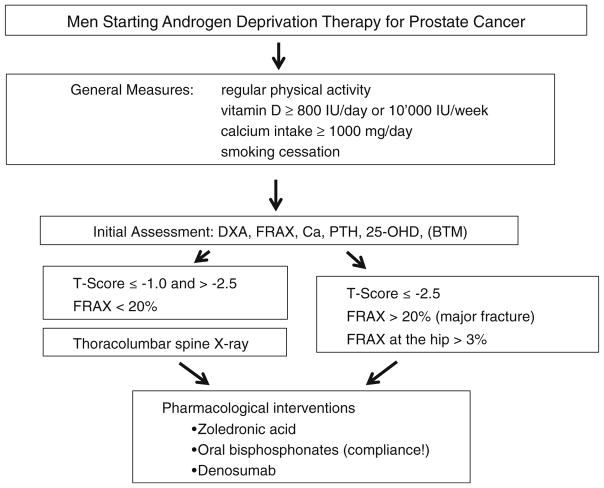
All of the above can be applied to this group of patients. Women who are taking AI and men who are undergoing ADT should be assessed for fracture risk, and osteoporosis therapy to prevent fractures should be considered (Figs. 1 and 2).
Fig. 1.
Management of patients with breast cancer treated with aromatase inhibitors (AIs). Adapted from [15]
Fig. 2.
Management of patients with non-metastatic prostate cancer on androgen deprivation therapy (ADT)
Follow-up
Baseline status, osteoporosis and bone fracture risk factors, and the type of cancer therapy used influence the frequency, profile and duration of examinations included in the follow-up of these patients [76, 101, 104].
Most of the cancer treatment-induced bone loss is explained by the hypogonadal state induced by the therapies. Thus, the follow-up currently focusses on the measurement of BMD and perhaps of bone turnover markers (BTMs) [101]. The goal of therapy is the maintenance (with some agents) or the increase (with other agents) in BMD. BTMs may respond with increases, with some therapies and decreases with other therapies. Measuring BMD or BTMs may increase adherence to therapy in individual patients.
A general approach and practical algorithm is presented in Figs. 1 and 2, which also emphasize the important role played by osteoporosis risk factors in the follow-up and management of these patients. Other organizations such as ESCEO, ASCO and the UK Expert Group have also issued other algorithms that are also anchored on BMD T-scores cut-offs and risk factors [15, 101, 102]. Indications and limitations can be region-specific according to the approval or insurance reimbursement policies.
Breast cancer
BTMs may be used to monitor response to antiresorptive therapy. The most commonly used specific markers are serum cross-linked terminal telopeptide (CTX) on a serum sample collected between 7 and 10 h in the morning in fasting state and urinary NTX expressed as a ratio to creatinine and measured on a second morning void urine sample. With the use of bisphosphonates as antiresorptive therapy, a 50–70 % reduction of CTX and NTX is expected in the first 3 months of treatment, a plateau is observed thereafter. It is generally accepted that the goal is to reduce bone resorption by more than the least significant change, keeping the bone resorption markers into the lower half of the reference range for healthy young women [101, 184]. Currently, a reduction in bone resorption greater than 50 % indicates that the least significant change has been achieved. It has been shown that concomitant diseases and recent fractures can influence BTM levels, thus caution needs to be taken when interpreting the results [185].
BMD value and the number of risk factors of each individual patient should be known before the initiation of any kind of bone-sparing therapy in all cancer treatment-associated bone loss (Figs. 1 and 2). Conversely, as the increases in BMD on treatment are small, BMD should be measured preferably at lumbar spine where the least significant change is around 3 %. BMD should be measured every 18–24 months [101, 186] in patients treated with bone resorption inhibitors.
Elderly women (>65 years) and patients with T-score < −2.0 and at least one more additional risk factor should receive bone protection with bisphosphonates irrespective of BMD if they are receiving AI therapy (Fig. 1).
Different management [101] is proposed in cancer therapy-induced premature menopause compared with biologically occurring menopause. In treatment-induced menopause or in programmed ovarian suppression before the age of 45 years, the evaluation of BMD should be indicated in two sites (spine and hip) and BMD should be repeated 12 months [187] after post-chemotherapy amenorrhea occurrence. The frequency of further monitoring would depend on the baseline BMD and the type of treatment. When AIs are concomitantly used, monitoring (every 18–24 months) and therapeutic intervention with calcium, vitamin D supplements and bisphosphonates are recommended [101].
Patients with a T-score between −1 and −2 should be considered “medium risk”. However, patients with medium risk with a decline of BMD >4 % per year (either site) should initiate bone protection therapy. Finally if BMD is ≥1, the 10-year risk for osteoporosis is very low and only lifestyle measures should be prescribed with re-evaluation in 24 months.
Prostate cancer
Bisphosphonates have been shown to attenuate bone loss, but optimal regimen (IV vs oral route of administration, every 6 months vs every 12 months) and the long-term effect on fracture risk remain to be established. Therefore, there is a need for bone monitoring measuring BMD with DXA. Denosumab has also been shown to increase bone mass in ADT patients who are at high risk of fracture and decrease BTMs. BMD and BTMs should be followed during treatment [84].
The frequency of monitoring will depend on the baseline DXA, the presence of osteoporosis risk factors and the use of bisphosphonates. Differently to breast cancer, high, medium and low-risk groups have been clearly established in prostate cancer, but a similar approach to breast cancer for monitoring is currently adopted [188].
If T-score is ≤ −2.5 patients should be monitored and managed as high-risk patients. If T-score is between −1 and −2.5, a more frequent monitoring (12 months) is advised. Patients with a T-score ≥ −1 can be considered at lower risk and BMD studies repeated every 18–24 months [101, 186]. If bisphosphonates are used in the frame of ADT, BMD should be measured every 18 to 24 months (Fig. 2).
Conclusions
Bone is often affected in the course of cancer either as bone metastases or as bone loss and fragility resulting from anticancer therapies. These various disease complications are associated with an important morbidity and with a largely compromised quality of life. To preserve bone and reduce this morbidity, efficacious therapies are available, with a highly favourable risk–benefits ratio. Expert reports from various societies are providing guidance for the identification of patients at risk of bone disease, their management and follow-up.
Acknowledgments
The authors thank Ms Aida Farha, Medical Information Specialist, Saab Medical Library, American University of Beirut, for her assistance in designing comprehensive and complex searches of the various medical literature resources.
Abbreviations
- ABCSG
Austrian Breast & Colorectal Cancer Study Group
- ADT
Androgen deprivation therapy
- AI
Aromatase inhibitor
- ALP
Alkaline phosphatase
- ASCO
American Society of Clinical Oncology
- ATAC
Arimidex, Tamoxifen Alone or in Combination
- AZURE
Adjuvant Zoledronic Acid to Reduce Recurrence
- BTM
Bone turnover marker
- BMD
Bone mineral density
- CI
Confidence interval
- CMF
Cyclophosphamide–methotrexate–5 fluorouracil
- CT
Computed tomography
- CTX
Cross-linked terminal telopeptide
- DKK1
Dickkopf-related protein
- DXA
Dual-energy X-ray absorptiometry
- ER
Estrogen receptor
- ET
Endothelin
- ESCEO
European Society for Clinical and Economical Aspects of Osteoporosis and Osteoarthritis
- FAC
5-Fluorouracil–doxorubicin–cyclophosphamide
- FDG
Fluorodeoxyglucose
- FGF
Fibroblast growth factor
- FRAX
WHO fracture risk assessment tool
- GnRH
Gonadotropin-releasing hormone
- HR
Hazard ratio
- IGF
Insulin-like growth factor
- IES
Intergroup Exemestane Study
- IL
Interleukin
- IV
Intravenous
- MRI
Magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- NTX
N-terminal telopeptide
- ONJ
Osteonecrosis of the jaw
- OPG
Osteoprotegerin
- PDGF-BB
Platelet-derived growth factor-BB
- PET
Positron emission tomography
- PSA
Prostate-specific antigen
- PTHrP
Parathyroid hormone-related protein
- RANK
Receptor activator of nuclear factor kappa-B
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- RCT
Randomized controlled trial
- RR
Relative risk
- SABRE
Study of Anastrozole with the Bisphosphonate Risedronate
- SERM
Selective oestrogen receptor modulator
- sFRP
Secreted frizzled-related protein
- SRE
Skeletal-related event
- TGF-β
Transforming growth factor-beta
- TNF-α
Tumour necrosis factor-alpha
- TRAcP
Tartrate-resistant acid phosphatase
- TSH
Thyroid-stimulating hormone
- VEGF
Vascular endothelial growth factor
- VFA
Vertebral fracture assessment
- WHO
World Health Organization
- Z-Fast and ZO-Fast
Zometa-Femara Adjuvant Synergy Trials
- αCTX
Alpha cross-linked telopeptides type
Footnotes
This position paper has been endorsed by the Committee of Scientific Advisors of IOF
Conflicts of interest René Rizzoli has received speaker or advisory board fees from Amgen, MSD, GSK, Servier, Danone and Takeda. Jean-Jacques Body has received speaker and consulting fees from Amgen and Novartis. Jorge B. Cannata-Andía has received research grants and speaker or advisory board fees from Amgen, Abbott, Servier and Shire. David Kendler has received honoraria for speaking, consulting and/or research grants from Amgen, Novartis, GSK, Eli Lily, Merck, Johnson&Johnson, Pfizer and Roche. Alexandra Papaioannou has been a consultant, or on a speaker’s bureau, or received unrestricted grants from Amgen, Eli Lilly, Merck Canada Inc., Novartis, Pfizer and Warner Chilcott. Tobias J de Villiers has received speaker or advisory board fees from Amgen, Merck, Adcock Ingram and Pfizer. Catherine Van Poznak has received research support from Amgen and Novartis. Ghada El-Hajj Fuleihan has received grants to support courses on osteoporosis from Novartis Pharmaceuticals and Les Laboratoires Servier. Abdellah El Maghraoui, Nicola Napoli, Dominique D Pierroz and Maria Rahme have no conflict of interest.
Contributor Information
R. Rizzoli, Division of Bone Diseases, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland
J.-J. Body, Department of Medicine, CHU Brugmann, Université Libre de Bruxelles, Brussels, Belgium
M.-L. Brandi, Department of Internal Medicine, University of Florence, Florence, Italy
J. Cannata-Andia, Bone and Mineral Research Unit, Instituto Reina Sofía, REDinREN, ISCIII, Hospital Universario Central de Asturias, Universidad de Oviedo, Oviedo, Spain
D. Chappard, GEROM-Research Group on Bone Remodeling and bioMaterials, LUNAM University, Angers, France
A. El Maghraoui, Rheumatology Department, Military Hospital Mohammed V, Rabat, Morocco
C.C. Glüer, Sektion Biomedizinische Bildgebung, Klinik für Diagnostische Radiologie, Universitätsklinikum Schleswig-Holstein, Kiel, Germany
D. Kendler, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada
N. Napoli, Division of Endocrinology, University Campus Bio-Medico, Rome, Italy
A. Papaioannou, Department of Medicine, McMaster University, Hamilton, Ontario, Canada
D.D. Pierroz, International Osteoporosis Foundation (IOF), Nyon, Switzerland
M. Rahme, Calcium Metabolism and Osteoporosis Program, WHO Collaborating Center for Osteoporosis and Metabolic Bone Disorders, American University of Beirut Medical Center, Beirut, Lebanon
C.H. Van Poznak, University of Michigan, Ann Arbor, MI, USA
T.J. de Villiers, Panorama MediClinic and Department of Gynaecology, Faculty of Health Sciences, Stellenbosch University, Cape Town, South Africa
G. El Hajj Fuleihan, Calcium Metabolism and Osteoporosis Program, WHO Collaborating Center for Osteoporosis and Metabolic Bone Disorders, American University of Beirut Medical Center, Beirut, Lebanon
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Description of the global burden of NCDs, their risk factors and determinants. WHO; Geneva: 2011. Global status report on noncommunicable diseases 2010. [Google Scholar]
- 3.Weigelt B, Peterse JL, van ’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 8.Cazzaniga M, Pronzato P, Leto di Priolo SL, De Matteis A, Di Costanzo F, Passalacqua R, Rosso R, Torri V. Patterns of relapse and modalities of treatment of breast cancer: the ‘IRIS’ Project, a multicenter observational study. Oncology. 2004;66:260–268. doi: 10.1159/000078325. [DOI] [PubMed] [Google Scholar]
- 9.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 10.Reuss-Borst M, Hartmann U, Scheede C, Weiss J. Prevalence of osteoporosis among cancer patients in Germany: prospective data from an oncological rehabilitation clinic. Osteoporos Int. 2012;23:1437–1444. doi: 10.1007/s00198-011-1724-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, Gass M, Leboff MS. Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:552–558. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179–1181. doi: 10.1038/sj.bjc.6690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastell R. Aromatase inhibitors and bone. J Steroid Biochem Mol Biol. 2007;106:157–161. doi: 10.1016/j.jsbmb.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 14.McCloskey E. Effects of third-generation aromatase inhibitors on bone. Eur J Cancer. 2006;42:1044–1051. doi: 10.1016/j.ejca.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Rizzoli R, Body JJ, De Censi A, Reginster JY, Piscitelli P, Brandi ML European Society for Clinical Economical Aspects of Osteoporosis and Osteoarthritis (ESCEO) Guidance for the prevention of bone loss and fractures in postmenopausal women treated with aromatase inhibitors for breast cancer: an ESCEO position paper. Osteoporos Int. 2012;23:2567–2576. doi: 10.1007/s00198-011-1870-0. [DOI] [PubMed] [Google Scholar]
- 16.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 17.Morote J, Morin JP, Orsola A, Abascal JM, Salvador C, Trilla E, Raventos CX, Cecchini L, Encabo G, Reventos J. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–504. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 19.Morote J, Orsola A, Abascal JM, Planas J, Trilla E, Raventos CX, Cecchini L, Encabo G, Reventos J. Bone mineral density changes in patients with prostate cancer during the first 2 years of androgen suppression. J Urol. 2006;175:1679–1683. doi: 10.1016/S0022-5347(05)00999-7. discussion 1683. [DOI] [PubMed] [Google Scholar]
- 20.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 21.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 22.Chappard D, Bouvard B, Basle MF, Legrand E, Audran M. Bone metastasis: histological changes and pathophysiological mechanisms in osteolytic or osteosclerotic localizations. A review. Morphologie. 2011;95:65–75. doi: 10.1016/j.morpho.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Clézardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007;24:599–608. doi: 10.1007/s10585-007-9112-8. [DOI] [PubMed] [Google Scholar]
- 24.Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer. 2000;88:2892–2898. doi: 10.1002/1097-0142(20000615)88:12+<2892::aid-cncr2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Pugsley MK, Tabrizchi R. The vascular system. An overview of structure and function. J Pharmacol Toxicol Methods. 2000;44:333–340. doi: 10.1016/s1056-8719(00)00125-8. [DOI] [PubMed] [Google Scholar]
- 26.Chappard D, Libouban H, Legrand E, Ifrah N, Masson C, Basle MF, Audran M. Computed microtomography of bone specimens for rapid analysis of bone changes associated with malignancy. Anat Rec (Hoboken) 2010;293:1125–1133. doi: 10.1002/ar.21150. [DOI] [PubMed] [Google Scholar]
- 27.Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059–3061. [PubMed] [Google Scholar]
- 28.Jones DH, Nakashima T, Sanchez OH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nat Geosci. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 29.Leeming DJ, Delling G, Koizumi M, Henriksen K, Karsdal MA, Li B, Qvist P, Tanko LB, Byrjalsen I. Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion. Cancer Epidemiol Biomarkers Prev. 2006;15:1392–1395. doi: 10.1158/1055-9965.EPI-05-0909. [DOI] [PubMed] [Google Scholar]
- 30.Granchi S, Brocchi S, Bonaccorsi L, Baldi E, Vinci MC, Forti G, Serio M, Maggi M. Endothelin-1 production by prostate cancer cell lines is up-regulated by factors involved in cancer progression and down-regulated by androgens. Prostate. 2001;49:267–277. doi: 10.1002/pros.10022. [DOI] [PubMed] [Google Scholar]
- 31.Takuwa Y, Masaki T, Yamashita K. The effects of the endothelin family peptides on cultured osteoblastic cells from rat calvariae. Biochem Biophys Res Commun. 1990;170:998–1005. doi: 10.1016/0006-291x(90)90491-5. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Zhang J, Dai J, Dehne LA, Mizokami A, Yao Z, Keller ET. Osteoblasts induce prostate cancer proliferation and PSA expression through interleukin-6-mediated activation of the androgen receptor. Clin Exp Metastasis. 2004;21:399–408. doi: 10.1007/s10585-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 34.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 35.Bataille R, Chappard D, Basle M. Excessive bone resorption in human plasmacytomas: direct induction by tumour cells in vivo. Br J Haematol. 1995;90:721–724. doi: 10.1111/j.1365-2141.1995.tb05609.x. [DOI] [PubMed] [Google Scholar]
- 36.Bataille R, Chappard D, Klein B. Mechanisms of bone lesions in multiple myeloma. Hematol Oncol Clin North Am. 1992;6:285–295. [PubMed] [Google Scholar]
- 37.Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O, Yaccoby S, Epstein J, Barlogie B, Shaughnessy JD., Jr The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109:4470–4477. doi: 10.1182/blood-2006-11-056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 39.Barretina J, Junca J, Llano A, Gutierrez A, Flores A, Blanco J, Clotet B, Este JA. CXCR4 and SDF-1 expression in B-cell chronic lymphocytic leukemia and stage of the disease. Ann Hematol. 2003;82:500–505. doi: 10.1007/s00277-003-0679-0. [DOI] [PubMed] [Google Scholar]
- 40.Josselin N, Libouban H, Dib M, Ifrah N, Legrand E, Basle MF, Audran M, Chappard D. Quantification of dendritic cells and osteoclasts in the bone marrow of patients with monoclonal gammopathy. Pathol Oncol Res. 2009;15:65–72. doi: 10.1007/s12253-008-9092-2. [DOI] [PubMed] [Google Scholar]
- 41.Marcelli C, Chappard D, Rossi JF, Jaubert J, Alexandre C, Dessauw P, Baldet P, Bataille R. Histologic evidence of an abnormal bone remodeling in B-cell malignancies other than multiple myeloma. Cancer. 1988;62:1163–1170. doi: 10.1002/1097-0142(19880915)62:6<1163::aid-cncr2820620620>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Coleman RE, Lipton A, Roodman GD, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stava CJ, Jimenez C, Hu MI, Vassilopoulou-Sellin R. Skeletal sequelae of cancer and cancer treatment. J Cancer Surviv. 2009;3:75–88. doi: 10.1007/s11764-009-0083-4. [DOI] [PubMed] [Google Scholar]
- 44.VanderWalde A, Hurria A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J Clin. 2011;61:139–156. doi: 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- 45.Michaud LB. Managing cancer treatment-induced bone loss and osteoporosis in patients with breast or prostate cancer. Am J Health Syst Pharm. 2010;67:S20–30. doi: 10.2146/ajhp100078. quiz S31-23. [DOI] [PubMed] [Google Scholar]
- 46.Wadhwa VK, Parr NJ. Peripheral or axial bone density measurements to identify osteoporosis in prostate cancer patients undergoing androgen deprivation therapy? Urology. 2009;73:1347–1351. doi: 10.1016/j.urology.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 47.Hadji P. Guidelines for osteoprotection in breast cancer patients on an aromatase inhibitor. Breast Care (Basel) 2010;5:290–296. doi: 10.1159/000321426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 49.Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230) J Bone Miner Res. 2006;21:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 50.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 51.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 53.Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer. 2009;53:261–266. doi: 10.1002/pbc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol. 1996;14:2923–2932. doi: 10.1200/JCO.1996.14.11.2923. [DOI] [PubMed] [Google Scholar]
- 55.Weilbaecher KN. Mechanisms of osteoporosis after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2000;6:165–174. doi: 10.1016/s1083-8791(00)70039-5. [DOI] [PubMed] [Google Scholar]
- 56.Love RR, Barden HS, Mazess RB, Epstein S, Chappell RJ. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med. 1994;154:2585–2588. [PubMed] [Google Scholar]
- 57.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 58.Hu MI, Gagel RF, Jimenez C. Bone loss in patients with breast or prostate cancer. Curr Osteoporos Rep. 2007;5:170–178. doi: 10.1007/s11914-007-0013-1. [DOI] [PubMed] [Google Scholar]
- 59.Bouvard B, Hoppe E, Soulie P, et al. High prevalence of vertebral fractures in women with breast cancer starting aromatase inhibitor therapy. Ann Oncol. 2012;23:1151–1156. doi: 10.1093/annonc/mdr356. [DOI] [PubMed] [Google Scholar]
- 60.Ghazi M, Roux C. Hormonal deprivation therapy-induced osteoporosis in postmenopausal women with breast cancer. Best Pract Res Clin Rheumatol. 2009;23:805–811. doi: 10.1016/j.berh.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Reid DM. Prevention of osteoporosis after breast cancer. Maturitas. 2009;64:4–8. doi: 10.1016/j.maturitas.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Rozenberg S, Carly B, Liebens F, Antoine C. Risks of osteoporosis associated with breast cancer treatment: the need to access to preventive treatment. Maturitas. 2009;64:1–3. doi: 10.1016/j.maturitas.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto DS, Viale PH. Update on identifying and managing osteoporosis in women with breast cancer. Clin J Oncol Nurs. 2009;13:E18–29. doi: 10.1188/09.CJON.E18-E29. [DOI] [PubMed] [Google Scholar]
- 64.McCloskey EV, Hannon RA, Lakner G, Fraser WD, Clack G, Miyamoto A, Finkelman RD, Eastell R. Effects of third generation aromatase inhibitors on bone health and other safety parameters: results of an open, randomised, multi-centre study of letrozole, exemestane and anastrozole in healthy postmenopausal women. Eur J Cancer. 2007;43:2523–2531. doi: 10.1016/j.ejca.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 65.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 66.Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA Multiple Outcomes of Raloxifene Evaluation T. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- 67.Gibson K, O’Bryant CL. Screening and management of osteoporosis in breast cancer patients on aromatase inhibitors. J Oncol Pharm Pract. 2008;14:139–145. doi: 10.1177/1078155208091866. [DOI] [PubMed] [Google Scholar]
- 68.Arimidex T, Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 69.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 70.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 71.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 72.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 73.Napoli N, Rastelli A, Ma C, et al. Genetic polymorphism at Val(80) (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER (+) breast cancer. Bone. 2013;55:309–314. doi: 10.1016/j.bone.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malcolm JB, Derweesh IH, Kincade MC, DiBlasio CJ, Lamar KD, Wake RW, Patterson AL. Osteoporosis and fractures after androgen deprivation initiation for prostate cancer. Can J Urol. 2007;14:3551–3559. [PubMed] [Google Scholar]
- 75.Alibhai SM, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 76.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 77.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 78.Sieber PR, Keiller DL, Kahnoski RJ, Gallo J, McFadden S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. J Urol. 2004;171:2272–2276. doi: 10.1097/01.ju.0000127738.94221.da. quiz 2435. [DOI] [PubMed] [Google Scholar]
- 79.Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22:2546–2553. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 80.Allain TJ. Prostate cancer, osteoporosis and fracture risk. Gerontology. 2006;52:107–110. doi: 10.1159/000090956. [DOI] [PubMed] [Google Scholar]
- 81.McLeod N, Huynh CC, Rashid P. Osteoporosis from androgen deprivation therapy in prostate cancer treatment. Aust Fam Physician. 2006;35:243–245. [PubMed] [Google Scholar]
- 82.Moyad MA. Osteoporosis and prostate cancer. Urol Oncol. 2003;21:374. doi: 10.1016/s1078-1439(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 83.Smith MR. Management of treatment-related osteoporosis in men with prostate cancer. Cancer Treat Rev. 2003;29:211–218. doi: 10.1016/s0305-7372(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 84.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ryan CJ, Molina A, Griffin T. Abiraterone in metastatic prostate cancer. N Engl J Med. 2013;368:1458–1459. doi: 10.1056/NEJMc1301594. [DOI] [PubMed] [Google Scholar]
- 86.Townsend MF, Sanders WH, Northway RO, Graham SD., Jr Bone fractures associated with luteinizing hormone-releasing hormone agonists used in the treatment of prostate carcinoma. Cancer. 1997;79:545–550. doi: 10.1002/(sici)1097-0142(19970201)79:3<545::aid-cncr17>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 87.Adler RA. Management of osteoporosis in men on androgen deprivation therapy. Maturitas. 2011;68:143–147. doi: 10.1016/j.maturitas.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Aksnes LH, Bruland OS. Some musculo-skeletal sequelae in cancer survivors. Acta Oncol. 2007;46:490–496. doi: 10.1080/02841860701218642. [DOI] [PubMed] [Google Scholar]
- 89.Chaganti RK, Parimi N, Lang T, Orwoll E, Stefanick ML, Nevitt M, Lane NE. Bone mineral density and prevalent osteoarthritis of the hip in older men for the Osteoporotic Fractures in Men (MrOS) Study Group. Osteoporos Int. 2010;21:1307–1316. doi: 10.1007/s00198-009-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol. 1997;157:439–444. [PubMed] [Google Scholar]
- 91.Daniell HW, Clark JC, Pereira SE, Niazi ZA, Ferguson DW, Dunn SR, Figueroa ML, Stratte PT. Hypogonadism following prostate-bed radiation therapy for prostate carcinoma. Cancer. 2001;91:1889–1895. doi: 10.1002/1097-0142(20010515)91:10<1889::aid-cncr1211>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 92.Grigsby PW, Perez CA. The effects of external beam radiotherapy on endocrine function in patients with carcinoma of the prostate. J Urol. 1986;135:726–727. doi: 10.1016/s0022-5347(17)45831-9. [DOI] [PubMed] [Google Scholar]
- 93.Igdem S, Alco G, Ercan T, Barlan M, Ganiyusufoglu K, Unalan B, Turkan S, Okkan S. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:818–823. doi: 10.1016/j.ijrobp.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 94.Overgaard M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose–response relationships on late bone damage. Acta Oncol. 1988;27:117–122. doi: 10.3109/02841868809090331. [DOI] [PubMed] [Google Scholar]
- 95.El Maghraoui A. Corticosteroid-induced osteoporosis. Presse Med. 2004;33:1213–1217. doi: 10.1016/s0755-4982(04)98891-4. [DOI] [PubMed] [Google Scholar]
- 96.Sambrook PN. How to prevent steroid induced osteoporosis. Ann Rheum Dis. 2005;64:176–178. doi: 10.1136/ard.2003.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sambrook PN, Diamond T, Ferris L, Fiatarone-Singh M, Flicker L, MacLennan A, Nowson C, O’Neill S, Greville H. Corticosteroid induced osteoporosis. Guidelines for treatment. Aust Fam Physician. 2001;30:793–796. [PubMed] [Google Scholar]
- 98.Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol. 2012;30:3665–3674. doi: 10.1200/JCO.2012.42.2097. [DOI] [PubMed] [Google Scholar]
- 99.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, Scientific A Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bruder JM, Ma JZ, Basler JW, Welch MD. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152–155. doi: 10.1016/j.urology.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 101.Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 102.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 103.Body JJ, Bergmann P, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, Reginster JY, Rozenberg S, Kaufman JM. Management of cancer treatment-induced bone loss in early breast and prostate cancer—a consensus paper of the Belgian Bone Club. Osteoporos Int. 2007;18:1439–1450. doi: 10.1007/s00198-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 104.Hadji P, Body JJ, Aapro MS, Brufsky A, Coleman RE, Guise T, Lipton A, Tubiana-Hulin M. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol. 2008;19:1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 105.van den Bergh JP, van Geel TA, Lems WF, Geusens PP. Assessment of individual fracture risk: FRAX and beyond. Curr Osteoporos Rep. 2010;8:131–137. doi: 10.1007/s11914-010-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watts NB. The Fracture Risk Assessment Tool (FRAX(R)): applications in clinical practice. J Womens Health (Larchmt) 2011;20:525–531. doi: 10.1089/jwh.2010.2294. [DOI] [PubMed] [Google Scholar]
- 107.Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395–2411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 108.Kanis JA, McCloskey EV, Johansson H, Oden A, Strom O, Borgstrom F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(Suppl 2):S407–413. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- 109.Saylor PJ, Kaufman DS, Michaelson MD, Lee RJ, Smith MR. Application of a fracture risk algorithm to men treated with androgen deprivation therapy for prostate cancer. J Urol. 2010;183:2200–2205. doi: 10.1016/j.juro.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2012;12:CD005465. doi: 10.1002/14651858.CD005465.pub3. [DOI] [PubMed] [Google Scholar]
- 111.Karlsson MK, Magnusson H, von Schewelov T, Rosengren BE. Prevention of falls in the elderly—a review. Osteoporos Int. 2013;24:747–762. doi: 10.1007/s00198-012-2256-7. [DOI] [PubMed] [Google Scholar]
- 112.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 113.Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–1037. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 114.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 115.McCloskey EV, Vasireddy S, Threlkeld J, Eastaugh J, Parry A, Bonnet N, Beneton M, Kanis JA, Charlesworth D. Vertebral fracture assessment (VFA) with a densitometer predicts future fractures in elderly women unselected for osteoporosis. J Bone Miner Res. 2008;23:1561–1568. doi: 10.1359/jbmr.080515. [DOI] [PubMed] [Google Scholar]
- 116.Diamond TH, Bucci J, Kersley JH, Aslan P, Lynch WB, Bryant C. Osteoporosis and spinal fractures in men with prostate cancer: risk factors and effects of androgen deprivation therapy. J Urol. 2004;172:529–532. doi: 10.1097/01.ju.0000130508.61020.66. [DOI] [PubMed] [Google Scholar]
- 117.Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–614. doi: 10.1016/S1470-2045(09)70088-9. [DOI] [PubMed] [Google Scholar]
- 118.Bristow RG. Biomarkers of clinical trials using molecular inhibitors and radiotherapy: state-of-the-art. Preface. Cancer Metastasis Rev. 2008;27:337–338. doi: 10.1007/s10555-008-9136-9. [DOI] [PubMed] [Google Scholar]
- 119.Steinborn MM, Heuck AF, Tiling R, Bruegel M, Gauger L, Reiser MF. Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J Comput Assist Tomogr. 1999;23:123–129. doi: 10.1097/00004728-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 120.Shie P, Cardarelli R, Brandon D, Erdman W, Abdulrahim N. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med. 2008;33:97–101. doi: 10.1097/RLU.0b013e31815f23b7. [DOI] [PubMed] [Google Scholar]
- 121.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing (1)(8)FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 122.Cook GJ. PET and PET/CT imaging of skeletal metastases. Cancer Imaging. 2010;10:1–8. doi: 10.1102/1470-7330.2010.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keaveny TM, Kopperdahl DL, Melton LJ, 3rd, Hoffmann PF, Amin S, Riggs BL, Khosla S. Age-dependence of femoral strength in white women and men. J Bone Miner Res. 2010;25:994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Keyak JH, Kaneko TS, Rossi SA, Pejcic MR, Tehranzadeh J, Skinner HB. Predicting the strength of femoral shafts with and without metastatic lesions. Clin Orthop Relat Res. 2005;439:161–170. doi: 10.1097/01.blo.0000174736.50964.3b. [DOI] [PubMed] [Google Scholar]
- 125.Tanck E, van Aken JB, van der Linden YM, Schreuder HW, Binkowski M, Huizenga H, Verdonschot N. Pathological fracture prediction in patients with metastatic lesions can be improved with quantitative computed tomography based computer models. Bone. 2009;45:777–783. doi: 10.1016/j.bone.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 126.Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474. doi: 10.1002/14651858.CD003474.pub3. [DOI] [PubMed] [Google Scholar]
- 129.Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y, Takashima S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 130.Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 131.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 132.Lipton A, Siena S, Rader M, Bilynsky B, Viniegra M, Richardson G, Beuzeboc P, Clemens MR, Ke C, Jun S. Comparison of denosumab versus zoledronioc acid (za) for treatment of bone diseases in advanced cancer patients: an integrated analysis of 3 pivotal trials. Annal Oncol. 2010;21(viii):379. [Google Scholar]
- 133.MacKenzie M, Quinton-Gladstone C, Haynes AE, Hotte S. Zoledronic acid for the treatment of bone metastases secondary to renal cell carcinoma. Toronto (ON): Cancer Care Ontario; 2010. program in Evidence-based Care CED-CCO Special Advice Report No 18. [Google Scholar]
- 134.Möbus V, Diel IJ, Harbeck N, et al. GAIN Study: a phase III trial to compare ETC. vs. EC-TX and Ibandronate vs. observation in patients with node-positive primary breast cancer- 1st interim efficacy analysis. 34th annual CTRC-AACR San Antonio Breast Cancer Symposium.2011. [Google Scholar]
- 135.S0307 Zoledronate, Clodronate, or Ibandronate in Treating Women Who Have Undergone Surgery for Stage I, Stage II, or Stage III Breast Cancer. [Accessed June 2013]; http://clinicaltrialsgov/ct2/show/NCT00127205?term=s0307&rank=2.
- 136.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 137.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 138.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith MR. Denosumab to prevent bone metastasis-free survival in men with castrate-resistant prostate cancer: results of a global Phase 3, randomised, double-blind trial. AUA meeting; Washington DC: Oral presentation; 2011. [Google Scholar]
- 140.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 141.Brown JE, Barrios CH, Diel IJ, et al. Incidence and outcomes of osteonecrosis of the jaw from an integrated analysis of three pivotal randomized double-blind, double-dummy phase 3 trials comparing denosumab and zoledronic acid for treatment of bone metastases in advanced cancer patients or myeloma. Bone Suppl. 2010;1:S18–S19. [Google Scholar]
- 142.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 143.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 144.WCRF/AICR. Food, nutrition and the prevention of cancer: a global perspective expert report. WCRF/AICR; London: 2007. [Google Scholar]
- 145.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 146.Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res. 2005;20:1327–1333. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- 147.Brufsky A, Bundred N, Coleman R, Lambert-Falls R, Mena R, Hadji P, Jin L, Schenk N, Ericson S, Perez EA. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 148.Geisler J, Lonning PE, Krag LE, et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomised, placebo-controlled study. Eur J Cancer. 2006;42:2968–2975. doi: 10.1016/j.ejca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 149.Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133–139. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Napoli N, Vattikuti S, Ma C, Rastelli A, Rayani A, Donepudi R, Asadfard M, Yarramaneni J, Ellis M, Armamento-Villareal R. High prevalence of low vitamin D and musculoskeletal complaints in women with breast cancer. Breast J. 2010;16:609–616. doi: 10.1111/j.1524-4741.2010.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat. 2011;129:107–116. doi: 10.1007/s10549-011-1644-6. [DOI] [PubMed] [Google Scholar]
- 152.Rizzoli R, Boonen S, Brandi ML, Bruyere O, Cooper C, Kanis JA, Kaufman JM, Ringe JD, Weryha G, Reginster JY. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Curr Med Res Opin. 2013;29(4):305–313. doi: 10.1185/03007995.2013.766162. [DOI] [PubMed] [Google Scholar]
- 153.Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk in the Women’s Health Initiative. Osteoporos Int. 2013;24(8):2371–2372. doi: 10.1007/s00198-013-2356-z. [DOI] [PubMed] [Google Scholar]
- 154.Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 155.Rizzoli R. Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM. 2011;104:281–300. doi: 10.1093/qjmed/hcq259. [DOI] [PubMed] [Google Scholar]
- 156.Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol. 1997;15:955–962. doi: 10.1200/JCO.1997.15.3.955. [DOI] [PubMed] [Google Scholar]
- 157.Fuleihan Gel H, Salamoun M, Mourad YA, Chehal A, Salem Z, Mahfoud Z, Shamseddine A. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:3209–3214. doi: 10.1210/jc.2004-1444. [DOI] [PubMed] [Google Scholar]
- 158.Hershman DL, McMahon DJ, Crew KD, Cremers S, Irani D, Cucchiara G, Brafman L, Shane E. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26:4739–4745. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hershman DL, McMahon DJ, Crew KD, Shao T, Cremers S, Brafman L, Awad D, Shane E. Prevention of bone loss by zoledronic acid in premenopausal women undergoing adjuvant chemotherapy persist up to one year following discontinuing treatment. J Clin Endocrinol Metab. 2010;95:559–566. doi: 10.1210/jc.2009-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim JE, Ahn JH, Jung KH, et al. Zoledronic acid prevents bone loss in premenopausal women with early breast cancer undergoing adjuvant chemotherapy: a phase III trial of the Korean Cancer Study Group (KCSG-BR06-01) Breast Cancer Res Treat. 2011;125:99–106. doi: 10.1007/s10549-010-1201-8. [DOI] [PubMed] [Google Scholar]
- 161.Powles TJ, McCloskey E, Paterson AH, Ashley S, Tidy VA, Nevantaus A, Rosenqvist K, Kanis J. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst. 1998;90:704–708. doi: 10.1093/jnci/90.9.704. [DOI] [PubMed] [Google Scholar]
- 162.Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol. 1997;15:1341–1347. doi: 10.1200/JCO.1997.15.4.1341. [DOI] [PubMed] [Google Scholar]
- 163.Shapiro CL, Halabi S, Hars V, et al. Zoledronic acid preserves bone mineral density in premenopausal women who develop ovarian failure due to adjuvant chemotherapy: final results from CALGB trial 79809. Eur J Cancer. 2011;47:683–689. doi: 10.1016/j.ejca.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37:2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 165.Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, Barlow D, Makris A, Eastell R. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28:967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 166.Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, Horsman JM, Brown JE, Hannon RA, Coleman RE. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14:6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 167.Greenspan SL, Resnick NM, Nelson JB. Once weekly oral alendronate prevents bone loss in men on androgen deprivation therapy for prostate cancer. J Urol. 2006;175(suppl 4):975. doi: 10.7326/0003-4819-146-6-200703200-00006. abstract. [DOI] [PubMed] [Google Scholar]
- 168.Taxel P, Dowsett R, Richter L, Fall P, Klepinger A, Albertsen P. Risedronate prevents early bone loss and increased bone turnover in the first 6 months of luteinizing hormone-releasing hormone-agonist therapy for prostate cancer. BJU Int. 2010;106:1473–1476. doi: 10.1111/j.1464-410X.2010.09329.x. [DOI] [PubMed] [Google Scholar]
- 169.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, Finkelstein JS, Smith MR. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Fan M, Kim D. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009;118:81–87. doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 171.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, Fan M, Jun S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 172. [Accessed June 2013];Study to determine treatment effects of denosumab in patients with breast cancer receiving aromatase inhibitor therapy. http://wwwclinicaltrialsgov/ct2/show/NCT00556374?term=denosumab+and+cancer&rank=10.
- 173.Saad F, Smith MR, Egerdie B, Tammela TL, Feldman RA, Heracek J, Szwedowski M, Ke C, Leder B, Goessl C. Denosumab for prevention of fractures in men receiving androgen deprivation therapy (ADT) for prostate cancer. J Clin Oncol. 2009;27:15s. abstract 5056. [Google Scholar]
- 174.Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D, Hancock ML, Steiner MS. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–1321. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 176.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 177.Terpos E, Morgan G, Dimopoulos MA, et al. International myeloma working group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31:2347–2357. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Van Poznak CH, Von Roenn JH, Temin S. American society of clinical oncology clinical practice guideline update: recommendations on the role of bone-modifying agents in metastatic breast cancer. J Oncol Pract. 2011;7:117–121. doi: 10.1200/JOP.2011.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lentle B, Cheung AM, Hanley DA, et al. Osteoporosis Canada 2010 Guidelines for the Assessment of Fracture Risk. Can Assoc Radiol J. 2011;62(4):243–250. doi: 10.1016/j.carj.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Leslie WD, Schousboe JT. A review of osteoporosis diagnosis and treatment options in new and recently updated guidelines on case finding around the world. Curr Osteoporos Rep. 2011;9:129–140. doi: 10.1007/s11914-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 181.Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/ep.16.s3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Coleman R, Costa L, Saad F, et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80:411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 184.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 185.Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int. 2000;11(Suppl 6):S30–44. doi: 10.1007/s001980070004. [DOI] [PubMed] [Google Scholar]
- 186.Watts NB, Lewiecki EM, Bonnick SL, et al. Clinical value of monitoring BMD in patients treated with bisphosphonates for osteoporosis. J Bone Miner Res. 2009;24:1643–1646. doi: 10.1359/jbmr.090818. [DOI] [PubMed] [Google Scholar]
- 187.Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, Gnant M, Guise T, Lipton A. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22:2546–2555. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 188.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 189.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 190.Body JJ, Coleman R, Clezardin P, Ripamonti C, Rizzoli R, Aapro M International Society of Geriatric Oncology. International Society of Geriatric Oncology (SIOG) clinical practice recommendations for the use of bisphosphonates in elderly patients. Eur J Cancer. 2007;43:852–858. doi: 10.1016/j.ejca.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 191.Coleman RE, Bertelli G, Beaumont T, Kunkler I, Miles D, Simmonds PD, Jones AL, Smith IE. UK guidance document: treatment of metastatic breast cancer. Clin Oncol (R Coll Radiol) 2012;24:169–176. doi: 10.1016/j.clon.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 192.Bird JM, Owen RG, D’Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 193.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 194.Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047–1053. doi: 10.4065/81.8.1047. [DOI] [PubMed] [Google Scholar]
- 195.National Comprehensive Cancer Network. NCCN Guidelines version 1.2012 Multiple Myeloma. 2012. [Google Scholar]
- 196.Harrouseau JL, Greil R, Kloke O, Force EGT. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of multiple myeloma. Ann Oncol. 2005;16(Suppl 1):i45–47. doi: 10.1093/annonc/mdi818. [DOI] [PubMed] [Google Scholar]
- 197.Grossmann M, Hamilton EJ, Gilfillan C, Bolton D, Joon DL, Zajac JD. Bone and metabolic health in patients with nonmetastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust. 2011;194:301–306. doi: 10.5694/j.1326-5377.2011.tb02979.x. [DOI] [PubMed] [Google Scholar]
- 198.Saad F, Higano CS, Sartor O, Colombel M, Murray R, Mason MD, Tubaro A, Schulman C. The role of bisphosphonates in the treatment of prostate cancer: recommendations from an expert panel. Clin Genitourin Cancer. 2006;4:257–262. doi: 10.3816/CGC.2006.n.004. [DOI] [PubMed] [Google Scholar]