Abstract
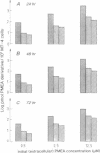
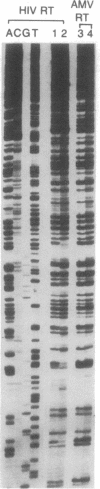
9-(2-Phosphonylmethoxyethyl)adenine (PMEA) is a potent and selective inhibitor of retrovirus (i.e., human immunodeficiency virus) replication in vitro and in vivo. Uptake of PMEA by human MT-4 cells and subsequent conversion to the mono- and diphosphorylated metabolites (PMEAp and PMEApp) are dose-dependent and occur proportionally with the initial extracellular PMEA concentrations. Adenylate kinase is unable to phosphorylate PMEA. However, 5-phosphoribosyl-1-pyrophosphate synthetase directly converts PMEA to PMEApp with a Km of 1.47 mM and a Vmax that is 150-fold lower than the Vmax for AMP. ATPase, 5'-phosphodiesterase, and nucleoside diphosphate kinase are able to dephosphorylate PMEApp to PMEAp, albeit to a much lower extent than the dephosphorylation of ATP. PMEApp has a relatively long intracellular half-life (16-18 hr) and has a much higher affinity for the human immunodeficiency virus-specified reverse transcriptase than for the cellular DNA polymerase alpha (Ki/Km: 0.01 and 0.60, respectively). PMEApp is at least as potent an inhibitor of human immunodeficiency virus reverse transcriptase as 2',3'-dideoxyadenosine 5'-triphosphate. Being an alternative substrate to dATP, PMEApp acts as a potent DNA chain terminator, and this may explain its anti-retrovirus activity.
Full text
PDF

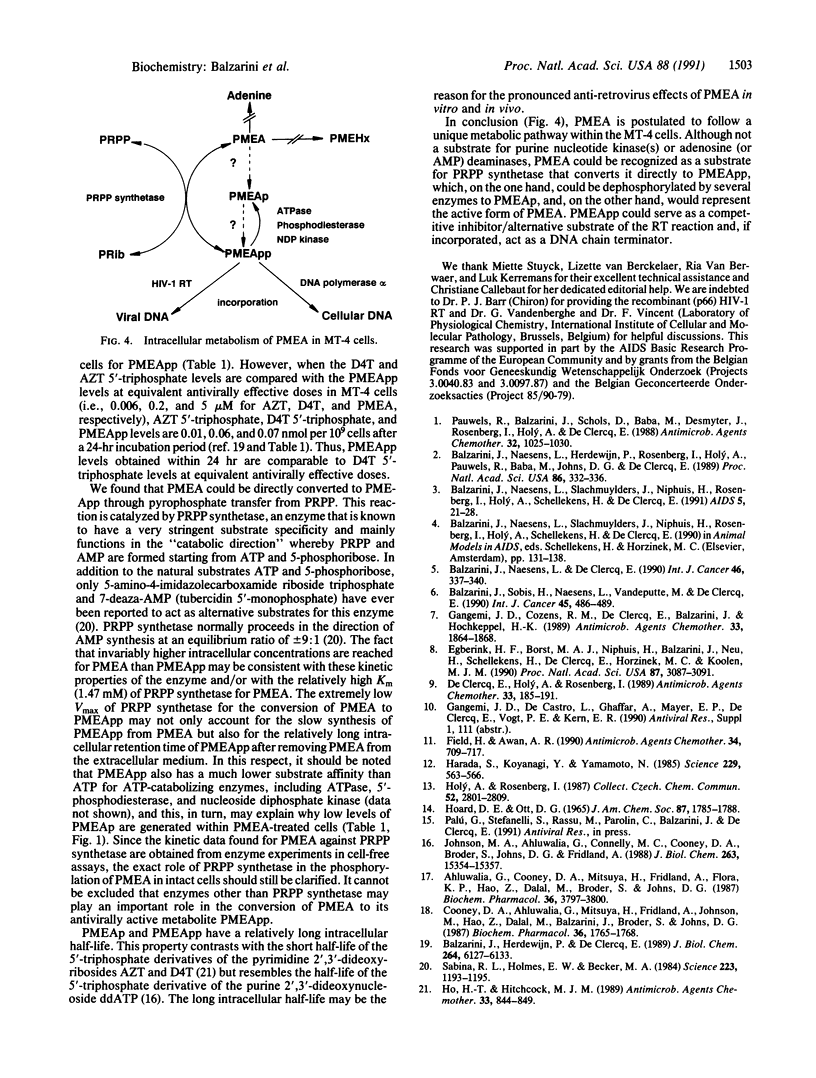
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aduma P. J., Gupta S. V., De Clercq E. Antiherpes virus activity and effect on deoxyribonucleoside triphosphate pools of (E)-5-(2-bromovinyl)-2'-deoxycytidine in combination with deaminase inhibitors. Antiviral Res. 1990 Mar;13(3):111–125. doi: 10.1016/0166-3542(90)90027-5. [DOI] [PubMed] [Google Scholar]
- Ahluwalia G., Cooney D. A., Mitsuya H., Fridland A., Flora K. P., Hao Z., Dalal M., Broder S., Johns D. G. Initial studies on the cellular pharmacology of 2',3'-dideoxyinosine, an inhibitor of HIV infectivity. Biochem Pharmacol. 1987 Nov 15;36(22):3797–3800. doi: 10.1016/0006-2952(87)90440-0. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Herdewijn P., De Clercq E. Differential patterns of intracellular metabolism of 2',3'-didehydro-2',3'-dideoxythymidine and 3'-azido-2',3'-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J Biol Chem. 1989 Apr 15;264(11):6127–6133. [PubMed] [Google Scholar]
- Balzarini J., Naesens L., De Clercq E. Anti-retrovirus activity of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in vivo increases when it is less frequently administered. Int J Cancer. 1990 Aug 15;46(2):337–340. doi: 10.1002/ijc.2910460233. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Naesens L., Herdewijn P., Rosenberg I., Holy A., Pauwels R., Baba M., Johns D. G., De Clercq E. Marked in vivo antiretrovirus activity of 9-(2-phosphonylmethoxyethyl)adenine, a selective anti-human immunodeficiency virus agent. Proc Natl Acad Sci U S A. 1989 Jan;86(1):332–336. doi: 10.1073/pnas.86.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J., Naesens L., Slachmuylders J., Niphuis H., Rosenberg I., Holý A., Schellekens H., De Clercq E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS. 1991 Jan;5(1):21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Sobis H., Naesens L., Vandeputte M., De Clercq E. Inhibitory effects of 9-(2-phosphonylmethoxyethyl)adenine and 3'-azido-2',3'-dideoxythymidine on tumor development in mice inoculated intracerebrally with Moloney murine sarcoma virus. Int J Cancer. 1990 Mar 15;45(3):486–489. doi: 10.1002/ijc.2910450319. [DOI] [PubMed] [Google Scholar]
- Cooney D. A., Ahluwalia G., Mitsuya H., Fridland A., Johnson M., Hao Z., Dalal M., Balzarini J., Broder S., Johns D. G. Initial studies on the cellular pharmacology of 2',3'-dideoxyadenosine, an inhibitor of HTLV-III infectivity. Biochem Pharmacol. 1987 Jun 1;36(11):1765–1768. doi: 10.1016/0006-2952(87)90235-8. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Holý A., Rosenberg I. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob Agents Chemother. 1989 Feb;33(2):185–191. doi: 10.1128/aac.33.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberink H., Borst M., Niphuis H., Balzarini J., Neu H., Schellekens H., De Clercq E., Horzinek M., Koolen M. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3087–3091. doi: 10.1073/pnas.87.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Awan A. R. Effective chemotherapy of equine herpesvirus 1 by phosphonylmethoxyalkyl derivatives of adenine demonstrated in a novel murine model for the disease. Antimicrob Agents Chemother. 1990 May;34(5):709–717. doi: 10.1128/aac.34.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi J. D., Cozens R. M., De Clercq E., Balzarini J., Hochkeppel H. K. 9-(2-Phosphonylmethoxyethyl)adenine in the treatment of murine acquired immunodeficiency disease and opportunistic herpes simplex virus infections. Antimicrob Agents Chemother. 1989 Nov;33(11):1864–1868. doi: 10.1128/aac.33.11.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Ho H. T., Hitchcock M. J. Cellular pharmacology of 2',3'-dideoxy-2',3'-didehydrothymidine, a nucleoside analog active against human immunodeficiency virus. Antimicrob Agents Chemother. 1989 Jun;33(6):844–849. doi: 10.1128/aac.33.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. A., Ahluwalia G., Connelly M. C., Cooney D. A., Broder S., Johns D. G., Fridland A. Metabolic pathways for the activation of the antiretroviral agent 2',3'-dideoxyadenosine in human lymphoid cells. J Biol Chem. 1988 Oct 25;263(30):15354–15357. [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Schols D., Baba M., Desmyter J., Rosenberg I., Holy A., De Clercq E. Phosphonylmethoxyethyl purine derivatives, a new class of anti-human immunodeficiency virus agents. Antimicrob Agents Chemother. 1988 Jul;32(7):1025–1030. doi: 10.1128/aac.32.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina R. L., Holmes E. W., Becker M. A. The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science. 1984 Mar 16;223(4641):1193–1195. doi: 10.1126/science.6199843. [DOI] [PubMed] [Google Scholar]