Abstract
The current study examines anxiety and age associations with attention allocation and physiological response to threats and rewards. Twenty-two healthy-adults, 20 anxious-adults, 26 healthy-youth, and 19 anxious-youth completed two eye-tracking tasks. In the Visual Scene Task (VST), participants’ fixations were recorded while they viewed a central neutral image flanked by two threatening or two rewarding stimuli. In the Negative Words Task (NWT), physiological response was measured by means of pupil diameter change while negative and neutral words were presented. For both tasks, no interaction was found between anxiety and age-group. In the VST, anxious participants avoided the threatening images when groups were collapsed across age. Similarly, adults but not adolescents avoided the threatening images when collapsed across anxiety. No differences were found for rewarding images. In NWT, all subjects demonstrated increase in pupil dilation after word presentation. Only main effect of age emerged with stronger pupil dilation in adults than children. Finally, maximum pupil change was correlated with threat avoidance bias in the scene task. Gaze patterns and pupil dilation show that anxiety and age are associated with attention allocation to threats. The relations between attention and autonomic arousal point to a complex interaction between bottom-up and top-down processes as they relate to attention allocation.
Keywords: Anxiety, development, attention allocation, eye tracking, pupil dilation, social and non-social stimuli
Stimuli that elicit emotional responses capture attention, which in turn facilitates adaptive behavioral and physiological responses in normative populations. However, in patients with anxiety disorders, dysregulated emotional sensitivity is associated with aberrant patterns of attention capture, and this may in turn contribute to pathological responding (Bar-Haim et al., 2007; Mogg and Bradley, 1998). Although anxiety disorders typically first emerge during childhood and adolescence, research examining the effect of attention allocation on anxiety has primarily been conducted in adults, using stimuli developed for adults. The current study was designed to address potential developmental differences in attention allocation among anxious youth and adults using two novel eye-tracking tasks.
Although threat-related attention biases have consistently been found in anxious adults and children (Shechner et al., 2012), the direction and strength of biases are more variable in pediatric than adult patients (Bar-Haim, 2010). This is not surprising given the prominent age related changes in executive functioning, in brain structures that support attention control, in emotional regulation strategies and in individual learning histories (Luna et al., 2010; Paus, 2005). This developmental difference is particularly important when examining the effects of longer exposure of threatening stimuli on attention, a process that involves both involuntary immediate responses as well as attention processes which are under greater voluntary control that emerge with longer stimulus durations (LeDoux, 1996). Whereas the vigilance response is believed to be reflexive the avoidance response is generally thought to be under greater voluntary control. Avoidance may also be partially learned response which becomes more established with experience. Therefore, while we expected similar initial orienting response across development, we expected the avoidance response to be more strongly and consistently expressed in adults. In this regard, a common theoretical explanation described by the vigilance-avoidance model, postulates that anxious individuals initially orient their attention towards, but then rapidly shift their attention away from threats (Mogg et al., 1997; Waters et al., 2010). Hence, developmental differences in attention allocation are expected to affect patterns of vigilance-avoidance, which may explain the variability in threat related attention biases observed in anxious youth.
Further, current models suggest that both bottom up and top down factors influence attention allocation (Knudsen, 2007). Bottom up effects, such as threat detection, are likely related to responses in several early maturing brain regions such as amgydala, anterior cingulate and orbitofrontal cortex. Top down factors, such as working memory, response selection and effortful shifts of attention, are likely mediated by brain regions such as lateral prefrontal and posterior parietal cortex (Browning, Holmes, Murphy, Goodwin, & Harmer, 2010; Corbetta & Shulman, 2002; Knudsen, 2007), which are relatively late to reach functional maturity (Crone, 2009; Gogtay et al., 2004; Paus, 2005). Consequently, in mature adults with anxiety this may result in an organized goal of avoiding aversive experiences, whereas in children with anxiety this function may not be mature enough to influence attentional control (Amso and Scerif, 2015). This issue may be particularly relevant in the presence of stimuli that are highly salient (i.e. bottom up) during a specific developmental stage, as social stimuli appear to be for adolescents (Blakemore et al., 2007; Blakemore and Mills, 2014; Nelson et al., 2005).
Eye tracking is one promising method for investigating threat-related attentional biases. Unlike the typical dot-probe task, where attention patterns are inferred based on differences in behavioral reaction times (Macleod et al., 1986), recording eye movements allows for a direct, in-vivo, measure of attention allocation. In addition, continuous recording of eye movements, as opposed to series of single dot-probe trials, could be very useful in studying the temporal changes in attention allocation over time. And yet, only four studies to date have used these methods to study attention-anxiety relations in youth (Gamble and Rapee, 2009; In-Albon et al., 2010; Seefeldt et al., 2014; Shechner et al., 2013). While these studies varied in the exact definition of vigilance, three found differences in early but not later phases of attention. A fourth study found no bias in initial attention orientation towards negative or positive scenes, but some between-group differences emerged in later phases of stimulus display (In-Albon et al., 2010). These equivocal results may be the product of various methodological differences such as stimuli type (faces vs. scenes), presentation duration and age range.
In the present studies we used eye tracking to assess the degree to which attentional capture varies as a function of anxiety diagnosis and age. In a first study, participants passively viewed naturalistic scenes, which varied by valence (threatening vs. rewarding) and in whether they were embedded in a social context, which is highly salient during adolescence (Steinberg and Morris, 2001). As with previous eye tracking studies in anxious youth (Gamble and Rapee, 2009; In-Albon et al., 2010; Seefeldt et al., 2014; Shechner et al., 2013), visual attention was measured by patterns of eye fixation. In a second study, - negative social and negative non-socially relevant words were presented in both visual and auditory domains to the same participants. Here, pupil dilation was used as an index of autonomic response to stimuli with differential emotional valence (Siegle et al., 2003; Silk et al., 2007). Inclusion of both measures allowed us to independently track attention allocation and autonomic responses in the same participants.
We made the following hypotheses: 1) Anxiety will be associated with avoidance of threat related images and enhanced pupil diameter change to threatening words; 2) Age will be positively associated with attention allocation, as indexed by avoidance of threatening images, and negatively associated with pupil diameter change (because of emotional valence) across all groups; 3) Anxiety and age will interact such that greater differences in threat avoidance will be observed between anxious adults and anxious youth compare to the corresponding difference in the non-anxious groups; 4) Measures of attention (eye movement) and arousal (pupil diameter change) will be correlated in individuals across tasks.
Method
Participants
Eighty-seven participants completed the study: Healthy Adults (HA, n = 22); Anxious Adults (AA, n = 20); Healthy Youth (HY, n = 26); and Anxious Youth (AY, n = 19). Written informed consent was obtained from adult participants and parents; written assent was obtained from youth. The National Institute of Mental Health Institutional Review Board approved all procedures.
Individuals were recruited from the community using advertisements, flyers and word of mouth. Exclusion criteria were IQ < 70, current use of medication or any medical condition that could interfere with testing. These were conditions that were judged by the examining physician to have effects on the central nervous system, and included conditions such as seizures, neoplasms, or any medications with known neurological effects. During a screening visit, participants received a comprehensive psychiatric assessment with the Structured Clinical Interview for the DSM-IV-TR Axis I Disorders (SCID) for adults, and the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) was for youth. All clinicians were trained to an adequate level of reliability (kappa > 0.70) for all disorders, and diagnoses were confirmed by a clinical interview with a senior psychiatrist. Healthy participants were free of all psychiatric disorders. Anxious youths and adults met DSM-IV-TR criteria for a current anxiety disorder: Generalized anxiety disorder (GAD), Social phobia (SoPh), or Separation anxiety (SAD); secondary comorbid diagnoses of Panic disorder (PD) or Specific phobia (SpecPh) were not exclusionary. Patients were free of current or past diagnoses of obsessive-compulsive disorder (OCD) or post-traumatic disorder (PTSD), history of psychosis, bipolar disorder, or ongoing major depressive disorder. All participants were medication free and physically healthy.
Clinical and demographic profiles of each group are presented in Table 1. No significant differences emerged between the anxious and healthy youth and between anxious and healthy adults on age, SES or IQ, all ps > .176. Of note, the anxious adult group had a higher proportion of female participants (85%) than the three other groups – this was controlled for in all analyses. Finally, as expected, anxiety measures were higher for anxious, compared with healthy, participants (all ps < .001).
Table 1.
Sample demographics
| Whole Sample (n = 87) | Anxious | Healthy | ||
|---|---|---|---|---|
| Adult (n = 20) | Youth (n = 19) | Adult (n = 22) | Youth (n = 26) | |
| GAD | 4 | |||
| SoPh | 3 | 3 | ||
| SAD | 3 | |||
| SoPh & GAD | 10 | 4 | ||
| SoPh & Other | 1 | |||
| GAD & PD | 3 | |||
| SAD & GAD | 1 | |||
| SoPh & SAD | 1 | |||
| GAD & SpecPh | 1 | |||
| SoPh & GAD & SpecPh | 2 | |||
| SoPh & SAD & GAD | 1 | |||
| SoPh & SAD & SpecPh | 1 | |||
| SoPh & GAD & SpecPh | 1 | |||
| Self - reported SCARED total | 25.26 (10.62) | 9.82 (7.60) | ||
| Parent - reported SCARED total | 36.79 (14.52) | 4.82 (5.57) | ||
| Self - reported LSAS total | 65.33 (25.23) | 19.62 (21.19) | ||
| Mean Age (years) | 28.98 (8.37) | 12.08 (2.93) | 28.78 (5.59) | 13.03 (2.87) |
| Age range (years) | 19.25 – 50.42 | 8.08 –17.67 | 21.42 – 39.67 | 8.08 – 17.50 |
| Number Female (% of sample) | 17 (85.0) | 9 (47.4) | 10 (45.5) | 11 (55.0) |
| 2 WASI sub-scales IQ | 115.75 (10.71) | 107.83 (12.22) | 116.85 (12.41) | 110.67 (9.94) |
Note. GAD – Generalized anxiety disorder, SoPh – Social phobia, SAD – Separation anxiety, PD – Panic disorder, SpecPh – Specific phobia, SCARED - Self-Report for Childhood Anxiety Related Emotional Disorder, LSAS – Liebowitz Social Anxiety Scale, PARS – Pediatric Anxiety Rating Scale, SES – Socioeconomic status. 2 WASI sub-scales are vocabulary and matrix reasoning.
Procedures
Two tasks were used: a visual scene and an emotional word task. Participants completed both tasks on the same day with a 2–3 minutes break between them. During this short break they were told that they could rest their eyes, stretch in their chairs and move around in the room. They were not offered to leave the room, and only did so when they specifically requested to. The order of the two tasks was randomly determined.
Instruments and apparatus
Eye movements and pupil diameter were recorded from the left pupil using a remote mounted infra-red eye tracking camera with a sampling rate of 240 Hz and 0.25 ° spatial resolutions (Applied Science Laboratories; ASL Inc., Bedford, MA). A chin rest was used to minimize movement and to ensure an invariable distance of 60 cm from the monitor throughout testing. A nine-point calibration procedure was performed prior to the first task and testing occurred under standard fluorescent lighting conditions. Calibration between tasks was completed if significant drift was detected. Stimuli for both tasks were displayed visually on a 16″, 1000 Hz computer monitor located directly in front of participants.
The Visual Scene Task (VST)
The visual scene task (VST) included 58 trials that consisted of a 500ms fixation cross, followed by a 5000ms presentation of three images (Figure 1A). Image triads consisted of color photographs, displayed on a black 80×60mm background creating a visual angle of 5.725 ° and 7.628 ° respectively, from the International Affective Picture System (IAPS; (Lang et al., 2005)) supplemented with items similar in content from publically-available media. The center image was always a neutral scene, and was flanked on the right and left by images depicting threatening or rewarding scenes. On each trial, one of the two threat or reward images was embedded in a social interaction (e.g. a threatening person or a friendly person), while the other did not (e.g. a threatening animal or a piece of candy). The picture categories differed in terms of valence ratings (M = 3.28, SD = 0.67, for the negative stimuli content; M = 4.86, SD = 0.39, for neutral stimuli content; and M = 7.5, SD = 0.50, for positive picture content) (Lang et al., 2005). These scores represent ratings that range from 1 extremely unpleasant to 9 extremely pleasant with 5 as the middle point. The location of the social and non-social images in each triad was counterbalanced across the stimulus set, and the order of each triad was randomized across subjects. However, the pictures that formed each triad were maintained across subjects to control for differences in color, brightness, contrast, and complexity (e.g number of items in each stimulus). After completing the task, participants were asked to rate how they felt about the images for valence on a scale of 1- most positive to 5 – most negative.
Figure 1.
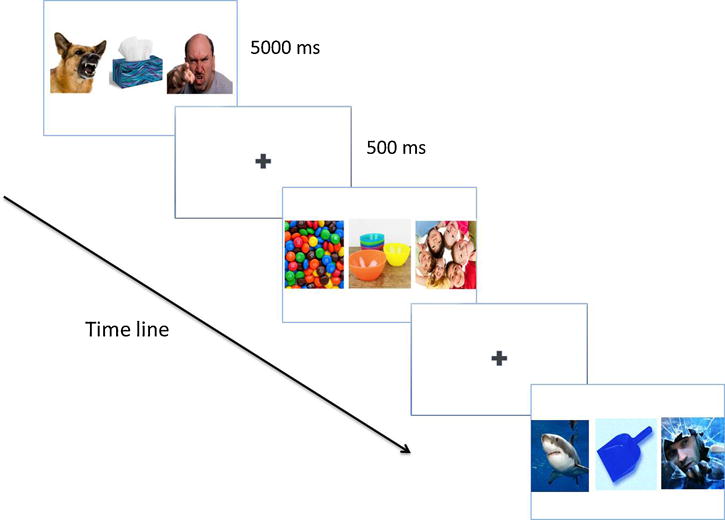
Visual Scene Task (VST) (A) and Negative Words Task (NWT) (B)
The task consisted of 36 threat trials and 22 reward triads presented in random order. More threat than reward trials were included, to assess the possible difference between threat of bodily harm and humiliation (supplementary analysis). Because no such difference emerged, analyses collapsed across this variable. Participants were instructed to look at the fixation that marked the beginning of each trial, but that once the pictures appeared, they were free to look anywhere on the screen. They were also told that some of the pictures might make them feel uncomfortable and that they could choose not to look at any particular picture they did not want to see, provided they kept their gaze on the screen.
Negative Words Task (NWT)
The negative words task (NWT) consisted of 45 trials of negative social (n = 20; e.g. lonely, bully), negative non-social (n = 20; e.g. poison, hurt), and neutral (n = 5; e.g. plate, paper) words that were presented both visually (word displayed on a computer screen) and aurally (over headphones). Spoken words were recorded to maintain consistency across participants and linked to visual word onset. Each trial was initiated by a fixation cross (1000ms) followed by a single word (10 seconds). The fixation and words were shown in gray Courier New font, size 36, on a plain black background creating a visual angle of approximately 3.341° (Figure 1B). Words were selected from standardized affective vocabulary list that was generated by children and adolescents (Neshat-Doost et al., 1999) based on their similarity in length and developmentally appropriate level of comprehension.
In the NWT, participants were told that on each trial they would view and hear a word, and then be asked to rate the word on a four point scale: 1-“no feelings about the word”; 2-“do not like the word”; 3-“really do not like the word”; 4-“hate the word”. Subjects were instructed to look at the fixation cross that marked the beginning of each trial but they were free to look anywhere on the screen during presentation of the words. Prior to the beginning of the task and while reading the instructions, the experimenter confirmed that participants were able to use their left hand to press the “1” and “2” keys and their right hand to press the “3” and “4” keys without looking at the keyboard.
Each task was optimized for one purpose (e.g., either eye gaze or pupil diameter), and the procedures that were used to optimize one of the measures would interfere with measurement of the other. Therefore, two independent variables reflecting two different attention-cognition processes were assessed: 1) attention during stimuli presentation, measured by fixation gaze and; 2) immediate and prolonged physiological response to threats, measured by pupil diameter change. To optimize the acquisition of these two measures, two different tasks were designed. For example, in the NWT, to minimize artifact that could affect pupil size (such as differences in lighting, complexity and fixation patterns), no colored images were used, the screen was black and the words were presented in a grey font. A similar task was previously used with depressed children (Silk et al., 2008). Furthermore, in the NWT task participants had no visual stimuli on which to fixate so patterns of fixation would be relatively meaningless.
Data analysis
The Visual Scene Task (VST)
ASL software was used to calculate fixations (Applied Science Labs, Bedford MA). Visual fixations were defined as a gaze maintained within 1.00 degrees of visual angle lasting at least 100 ms. Three primary analyses of fixation patterns were performed for each triad: (1) Dwell time for each image of the triad that was computed as the cumulative duration of visual fixations; (2) Probabilities of first fixation direction; (3) Latency for first fixation. Similar to previous studies, dwell time is an index for later threat related processes whereas probabilities of first fixation and latencies for first fixation reflect threat vigilance (Shechner et al., 2013). The three dependent measures were subject to a four-way repeated measures ANOVA with valence (threat, reward), and social content (social, non-social) as within subjects factors; and anxiety group (anxious, non-anxious) and age (youth, adults) as between subjects factors. Given the unequal numbers of males and females across the groups, all the ANOVAs were conducted while controlling for gender.
In a secondary analysis, cumulative fixation durations from the VST were transformed into bias scores (neutral – threats), and Pearson correlations were used to assess the relations to anxiety (SCARED scores). Finally, participants’ valence ratings were analyzed using four-way repeated measures ANOVA with Valence (threat, reward) X Social content (social, non-social) X Age (youth, adults) X Anxiety (anxious, non-anxious). These behavioral ratings were further used to examine possible correlation with attention bias scores.
Negative Words Task (NWT)
Pupil diameter across the task was calculated by ASL software (Applied Science Labs, Bedford MA). Similar to previous studies, trials missing more than 50% of data points due to blinks were excluded (Silk et al., 2007). Missing data points from trials that were maintained for analysis were interpolated from surrounding points in time series similar to method reported in previous studies (Granholm et al., 1996; Silk et al., 2007). In order to reduce data (240 Hz camera generated 2,400 data points/trial) we divided each of the 10 second long trials into 20 time bins of 500 ms, and computed the average the pupil diameter for each bin (Shechner, 2013 #277). To account for individual differences in normative pupil dilation, stimulus-linked dilation is reported as percent change from baseline dilation measured during the 500 ms prior to stimulus onset for each trial. Primary analysis of pupil dilation data consisted of a repeated measures ANOVA with Social content (social, non-social) X Time (20 time bins) X Anxiety (anxious, non-anxious) X Age (youth, adults) as factors.
Maximum amplitude change in dilation for each trial was also calculated for each participant as the difference between baseline and peak pupil diameter. Pearson correlations were used to measure the association between mean maximum pupil diameter change and both age and anxiety as continuous variables. Pearson correlation was also used to assess the association between maximum pupil change and mean fixation duration to threat related stimuli (based on performance in the VST). Finally, Participants’ ratings of the negative words were analyzed using a three-way repeated measures ANOVA with Social content (social, non-social) X Age (youth, adults) X Anxiety (anxious, non-anxious).
Results
The four-factor ANOVA (Valence-by-Social content-by-Age group-by-Anxiety group) for dwell time in the VST and for pupil change in the NWT failed to yield anxiety-by-age interactions. Hence, four sets of results are presented: 1) Effects of anxiety (collapsed across age group) for each task; 2) Effects of age for each task (collapsed across diagnosis group); 3) First fixation probabilities and latencies for the VST; 4) Overall effects for each task, and cross-task correlations.
1. Effects of Anxiety
VST
The four factor ANOVA conducted on the cumulative fixation duration in the VST revealed a three way interaction between Valence, Social content and Anxiety group, F(2, 162) = 3.46, p = .034, partial η2 = .04 (Figure 2). Means and standard deviation are reported in Table 2. After collapsing across age, follow-up decomposition contrasts revealed a significant Valence-by-Social content interaction in patients, F(2, 76) = 3.13, p = .049, partial η2 = .08, but not healthy participants, F(2, 92) = 1.11, p = .333. In patients, cumulative fixation duration was significantly lower for the non-social threat than neutral stimuli, t(38) = −2.38, p = .023; a similar trend was observed for the social threat versus neutral stimuli, t(38) = −1.89 p = .066. No such differences occurred in healthy subjects. As predicted these results indicate that anxious, but not non-anxious subjects avoid fixating on threat related stimuli. No categorical effects were found for the reward triad.
Figure 2.
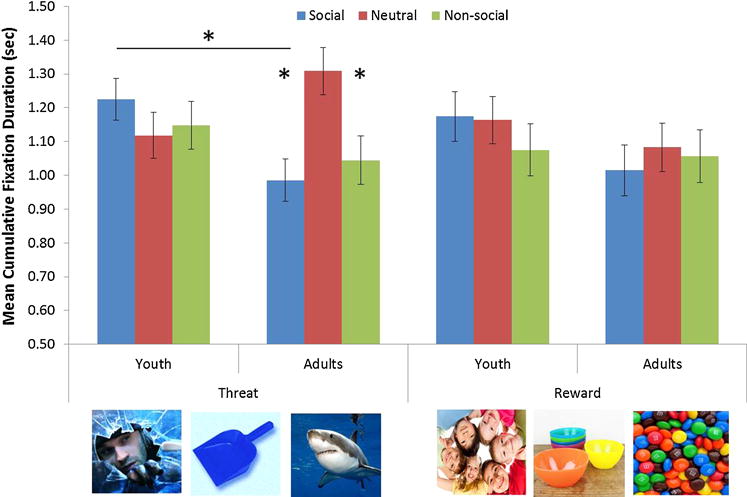
The effect of valance, social content and anxiety on mean cummulative fixation duration
Table 2.
Means and (standard deviations) of dwell time in the VST for anxiety and age
| Threat | Reward | |||||
|---|---|---|---|---|---|---|
| Social | Neutral | Non-social | Social | Neutral | Non-social | |
| Main effects | ||||||
| Anxious | 1.10 (0.34) |
1.27 (0.49) |
1.06 (0.40) |
1.07 (0.40) |
1.07 (0.36) |
1.11 (0.56) |
| Non-anxious | 1.14 (0.47) |
1.17 (0.44) |
1.15 (0.50) |
1.14 (0.54) |
1.19 (0.52) |
1.04 (0.44) |
| Youth | 1.23 (0.45) |
1.11 (0.36) |
1.15 (0.49) |
1.18 (0.55) |
1.16 (0.36) |
1.08 (0.42) |
| Adults | 1.00 (0.34) |
1.33 (0.51) |
1.06 (0.43) |
1.03 (0.38) |
1.10 (0.55) |
1.07 (0.57) |
In a supplementary follow up analysis we used a continuous measure of anxiety (indexed by SCARED - parents/child combined) to determine the extent to which avoidance measures were linearly related to anxiety severity. Unfortunately, comparable continuous measures were not obtained on adults so this follow up analysis was restricted to the youth. To assess the relationship between attention and anxiety symptoms across this broader spectrum of expression, we used correlation analyses to relate SCARED score and threat bias scores (cumulative fixation duration to threat vs neutral stimuli). A significant positive correlation, r = .38, p = .003, emerged. Thus, among youth, a significant linear relationship was found between the number of anxiety symptoms and the degree of avoidance of threatening stimuli.
NWT
No main or interaction effects for anxiety were found in the primary ANOVA (Time-by-Social content-by-Age Group-by- Anxiety Group) or follow-up contrasts for pupil dilation during NWT. However, secondary analyses among youth demonstrated that maximal change in pupil dilation was positively correlated with the continuous index of anxiety (SCARED-parent/child combined score, r = .371, p = .006). Thus, youths with higher levels of anxiety had greater maximal change in pupil dilation following emotional word presentation.
A secondary ANOVA for valence of participant ratings revealed a two way interaction between Stimuli ratings and Anxiety group, F(1,71) = 5.961, p = .017, partial η2 = .08. Anxious participants rated social words more negatively than non-social words, t(31) = 3.937, p <.001, whereas no such difference emerged in the non-anxious group, t(42) = .766, p = .448. These effects did not differ as a function of age.
2. Effects of Age
VST
The four factor ANOVA (Valence-by-Social content-by-Age group-by-Anxiety group) conducted on cumulative fixation duration revealed a three way interaction between age group, valence and social content, F(2, 162) = 4.10, p = .018, partial η2 = .05 (Figure 3). Means and standard deviation are reported in Table 2. After collapsing across diagnosis groups, follow-up contrasts to decompose this interaction revealed two primary effects: First, for threatening stimuli, an age by social content effect was found F(2, 164) = 8.46, p < .001, partial η2 = .09. Specifically, among adults, but not youth, there was a significant increase in cumulative fixation duration on neutral, relative to both social threat, t(40) = −3.27, p = .002 and non-social threat t(40) = −2.33, p = .025. The second effect was an increase in cumulative fixation duration on social threat stimulus in youth compared with adults, t(84) = 2.71, p = .008. No differences were found in the comparison of cumulative fixation durations on non-social threats between adults and youth, t(84) = 0.95, p = .346. No differences were found among any stimulus category for the reward triads.
Figure 3.
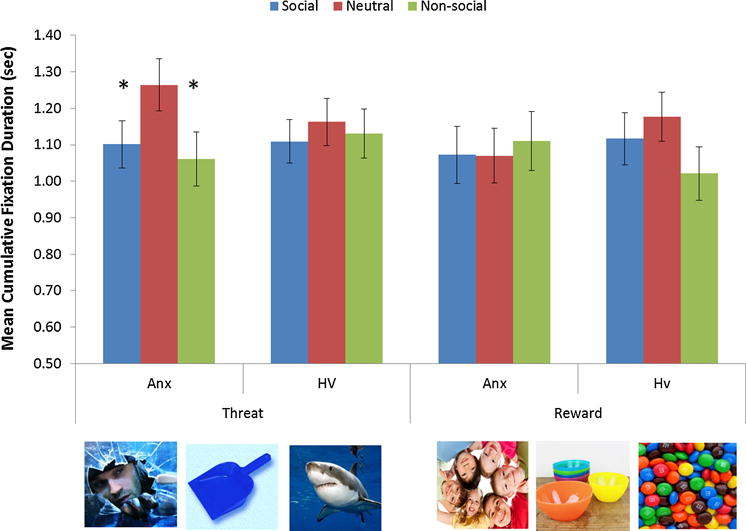
The effect of valance, social content and age on mean cumulative fixation duration
Finally, we compared cumulative fixation patterns with participants’ post-task ratings of the images. For each individual the mean rating for the threatening images was correlated with the overall mean threat bias score as described above. Across all subjects, a significant positive correlation was found (r = .346, p = .001), indicating that individuals who had a strong self-reported dislike for images also displayed a fixation bias away from negative images. However this pattern appeared to be driven primarily by the adults. A linear regression to predict fixation bias scores with post task ratings of the images, a dummy variable (Adults), and an interaction term computed as the multiplication of post task ratings of the images X Adults, indicated that this interaction was significant (β = 2.66, p = .002). When this correlation was examined for each age group separately, the significant relationship only held up for the adults (r = .501, p < .001) and not for the youth (r = .153, p > .31) (Fig 4).
Figure 4.
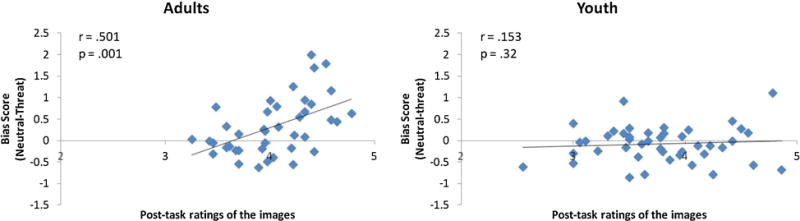
Correlation between post-task ratings of the images and threat bias scores
NWT
The 4 factor ANOVA (Time-by-Social content-by-Age Group-by- Anxiety Group) conducted on change in pupil diameter revealed two significant age related effects. A main effect of age, F(1, 77) = 4.75, p = .03, partial η2 = .06, was driven from greater overall pupil dilation change in adults than in youth. In addition, a two way interaction emerged for Time-by-Age group, F(19, 1463) = 1.86, p = .013, partial η2 = .03, which was driven by a greater peak response in the adult groups than the children (Fig 5).
Figure 5.
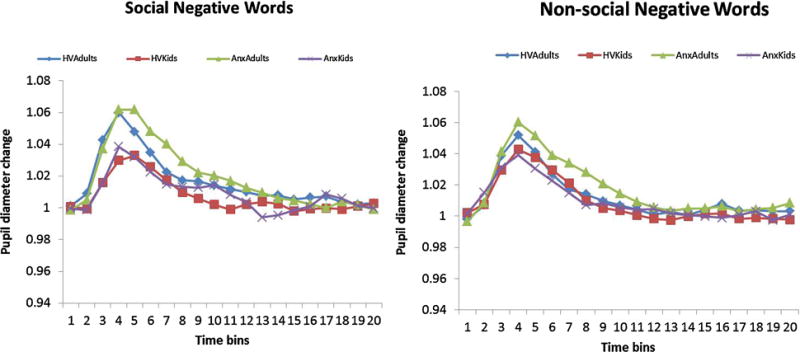
Pupil diameter change over time for social and non-social negative words
No interactions were found on either task between age and anxiety groups.
3. Probabilities of first fixation direction and Latency for first fixation
No main effects or interactions emerged for the four-factor ANOVA on Probabilities of first fixation direction (all ps > .146).
Similarly, no main effects of interactions emerged for the analysis on latency for first fixation (all ps > .132).
4. General Task Effects
VST
The ANOVA conducted on participants’ post-task ratings of the stimuli yielded a highly significant main effect of Valence, F(1, 81) = 499.79, p < .001, partial η2 = .86. All participants disliked social threats (M = 3.77, SD = 0.65) and non-social threats (M = 3.96, SD = 0.55) and liked social rewards (M = 1.72, SD = 0.44) and non-social reward (M = 1.98, SD = 0.59); this did not vary as a function of either age or anxiety group. Social content did not influence the valance ratings of threatening or rewarding images.
NWT
A highly significant time effect was found in the ANOVA on pupil dilation following the negative word presentation, F(19, 1463) = 4.38, p < .001, partial η2 = .05, indicating a significant change in pupil dilation induced by word presentation. No differences emerged as a function of social content.
Correlation between the two tasks
Across all subjects in the study, there was a negative relationship between cumulative fixation duration on threat related stimuli during the VST and maximum pupil reactivity to threat words during the NWT, r = −.318, p = .004. Specifically, avoidance of threatening visual stimuli was associated with greater physiological pupil change.
Discussion
The aim of the current study was to examine the effects of anxiety and age on attention allocation and physiological responsiveness to threats and rewards. Although there were main effects of both age and anxiety, no interactions emerged. First, when examining attention allocation, indexed by voluntary fixation on negative and neutral images, both anxiety and age were associated with avoidance of negative scenes but no group differences emerged in early stages of the trials indicating similar vigilance to evocative stimuli. Second, physiological reactivity, indexed by greater pupil dilation in response to negative words in a separate task, was associated with diminished attention allocation. Greater anxiety symptoms among youth were associated with more avoidant attention allocation and greater physiological responsiveness to threat across both studies. Of note, stimulus ratings for both tasks and the strong effect of pupil change after stimulus onset in the NWT suggest these novel eye tracking paradigms represent valid methods for study of emotional responding in these populations.
In line with our first hypothesis, anxiety was associated with greater avoidance of aversive stimuli. Anxious participants avoided threats more than the non-anxious participants, regardless of whether anxiety was treated as a categorical or continuous measure with SCARED scores. However, no group differences emerged in early stages of the trial indicating similar vigilance to negative stimuli. Threat avoidance is a core feature of anxiety disorders and has been reported in previous eye tracking studies with both anxious adults (for example, Rink & Becker, 2006) and anxious children (for example, (In-Albon et al., 2010)). Similar to the current design, both prior studies used a paradigm that employed passive viewing, and highly potent stimuli presented for a relatively long duration (4 – 60 seconds). Taken together, these data clearly indicate greater avoidance of threat in the anxious group. Moreover, measures of attention (fixation patterns) and autonomic arousal (pupil dilation) were correlated across individuals. Greater physiological response to negative words was associated with greater avoidance of threats in the visual scene task. This cross-task correlation suggests a mechanism by which negative affective states could affect visual attention allocation, or vice versa.
In line with our second hypothesis, age had a significant effect on the visual fixation response to threatening images. Although no age related difference was found in self-reported ratings of stimuli, when collapsed across anxiety, adults avoided looking at the negative images, whereas youth did not. This suggests distinct patterns of attention may be allocated to threatening contexts across development. Because stimuli were displayed for a relatively long time (5 sec), and because no age differences emerged in vigilance to threats, visual avoidance in adults may reflect an attempt to minimize the aversive impact of these stimuli. Thus, under passive viewing conditions adults may engage emotion regulation strategies that youth do not. Emotion regulation strategies range from attentional control processes directed to shift attention away from threatening stimuli to processes aimed to change the affective valence of the situation ({Gross, 1998 #742; Ochsner, 2005 #746}). And indeed, recent studies have demonstrated that attention to threats could be modulated by emotional regulation strategies (i.e. threat reappraisal) ((Adam et al., 2014). When considered in light of the fact that brain regions typically implicated in attention monitoring and executive control (Amso and Scerif, 2015; Crone, 2009; Gogtay et al., 2004; Luna et al., 2010; Paus, 2005) develop relatively late in maturation, and given the well-established age differences in emotional regulation strategies (i.e. {Blanchard-Fields, 2008 #747; John, 2004 #755}), these differences may well reflect a diminished capacity to actively regulate emotions in youth. This interpretation is still speculative and should be examined in future studies.
In contrast to our initial prediction adults had a greater change in pupil dilation than youth in response to threatening words. Pupil dilation serves as an index of autonomic arousal which could be a reflection of both affective arousal and cognitive effort (Granholm et al., 1996; Siegle et al., 2003; Siegle et al., 2011). Specifically, the pupil becomes more dilated in response to stimuli that require greater cognitive processing (Siefle, Steinhauer, Carter & Thasem 2003), longer memory use (Granholm, Asarnow, Sarkin, Dykes, 1996), interpretation of more difficult materials (Steinhauer & Hakerman, 1992), and it is also correlated with stimuli emotional intensity (Beatty, 1982a; Janisse, 1974; Siegle, Granholm, Ingram & Matt, 2001). While our findings may indicate that the negative words elicited greater affective arousal in the adults, they may reflect greater cognitive efforts – perhaps as a means of emotion regulation. Thus, early detection of, or heightened reactivity to, threat may engage top-down mechanisms that promote emotion regulation via attention monitoring and executive control. This may be particularly relevant for concurrent emotion regulation, as no age differences emerged for valence ratings that were typically provided later in the 10-sec stimulus presentation in the NWT or following eye tracking for the VST. However, it is also possible that the enhanced response in adults reflected an increased potency of social rejection in adults relative to adolescents. Further work is clearly needed to isolate the mechanisms that support these age-related differences in both attention allocation and physiological reactivity.
Main effects for both anxiety and age on threat avoidance – and no interaction between these factors, and suggest that each factor has an independent contribution to attention allocation. Thus, age and anxiety should be considered as important yet separate factors in models of attention deployment. For example, attention differences that vary as a function of age may stem from different neural substrates than attention differences that vary as a function of anxiety. While both contribute to attention response they don’t interact with each other. In general, adults may use attention allocation as a means of regulating emotional impact of aversive stimuli more than adolescents do. This may reflect a heightened vulnerability of youth to the impact of strongly aversive events. However, in addition to this developmental trend, anxiety is associated with avoidance of negative stimuli – in both adults and youth. The positive correlation between anxiety levels and threat avoidance among youth suggests that although attention avoidance is generally not performed in youth, anxiety levels could expedite the development of this behavior. Of note, lack of high order interaction could also result from inadequate sample size. And indeed, observed power for the four-way interaction was low (observed power = 0.198).
Taken together, it is reasonable to assume that learning to shift attention away from threats results from the interaction between the aversive potency of stimuli and the development of relevant brain areas like prefrontal and parietal cortices. In non-anxious individuals this process develops gradually with age, as individuals have the opportunity to engage with a variety of experiences, some of which are aversive. In anxious children, who usually perceive even minor threats as dangerous and aversive, this process may start earlier. For them, failure to engage with threatening stimuli as a coping strategy, may result in limited opportunities to have experience with a wide array of experiences and ultimately result in a more limited and less adaptive behavioral repertoire.
From a clinical perspective the present data may have relevance for attention training treatment and vulnerability to anxiety inducing experiences. Age related differences in attention may be an important issue to consider in therapeutics. Degree of control over attention may be moderated by maturation level and anxiety level. This initial result warrants further exploration of this potentially important developmental difference and its implication in attention based clinical interventions.
Findings and their interpretation should be considered in light of some methodological limitations. The eye tracking task that was used in the study did not require participants to identify a target but rather to passively view the stimuli on the screen. Hence, we cannot dissociate behavioral tendency from capability. In other words it is possible that if we instructed participants to disengage attention from aversive images, the age effects would disappear. Nonetheless, even in the absence of data that speak to capability, understanding that age plays an important role in the way individuals deploy attention under naturalistic conditions is an important insight and should be considered in light of emotion regulation strategies and attention modification therapies. Another limitation is the wide age range and the different anxiety subtypes of participants. In order to understand exactly how attention develops and how this maturation differs in healthy and anxious individuals it future studies should utilize more homogeneous samples stratified, rather than dichotomized, by age. However, findings from continuous measures suggest a linear relationship with both age and anxiety severity. Future research should further explore these two factors by means of using disorder specific stimuli and more distinct age groups and larger sample sizes. Another limitation is related to the definition of social and non-social stimuli. In line with the long debate in the scientific community regarding the specific definition of social and non-social stimuli, we have used somewhat arbitrary definitions of social and non-social stimuli. In the VST “social” was operationalized as any picture that included at least one person while the non-social pictures did not. In the NWT social negative words described negative social situations/consequences whereas the non-social negative words described adversities unrelated to social interactions. Interpretation of the current result should be in light of the specificity of these definitions. Finally, the instructions read to the participants clearly stated that they could look away from stimuli “if they made them feel uncomfortable”. Although this explicit instruction could have had some effect on participants’ gaze patterns, it is important to remember that in studies examining anxious and youth populations, such instruction are important to reduce unnecessary burden the often times result in high attrition rates.
Highlights.
Both age and anxiety level influence visual avoidance of aversive images.
Anxiety may hasten development of adult-like avoidance.
Adults have larger pupil dilation to negative words than adolescents.
Self-reported aversive intensity correlates with patterns of visual avoidance in adults only.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health. First author (TS) is supported by the Marie Curie Career Integration Grant (PCIG13-GA-2013-618534) and the Israel Science Foundation grant (1377/14).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All co-authors report no conflict of interests.
References
- Adam R, Schonfelder S, Forneck J, Wessa M. Regulating the blink: Cognitive reappraisal modulates attention. Front Psychol. 2014;5:143. doi: 10.3389/fpsyg.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amso D, Scerif G. The attentive brain: insights from developmental cognitive neuroscience. Nat Rev Neurosci. 2015;16:606–619. doi: 10.1038/nrn4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y. Research review: Attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry. 2010;51:859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is Adolescence a Sensitive Period for Sociocultural Processing? Annual Review of Psychology. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Crone EA. Executive functions in adolescence: inferences from brain and behavior. Dev Sci. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Gamble AL, Rapee RM. The time-course of attentional bias in anxious children and adolescents. Journal of Anxiety Disorders. 2009;23:841–847. doi: 10.1016/j.janxdis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33:457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- In-Albon T, Kossowsky J, Schneider S. Vigilance and Avoidance of Threat in the Eye Movements of Children with Separation Anxiety Disorder. Journal of Abnormal Child Psychology. 2010;38:225–235. doi: 10.1007/s10802-009-9359-4. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction Technical Report A-6. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod C, Mathews A, Tata P. Attentional Bias in Emotional Disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, de Bono J, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behav Res Ther. 1997;35:297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neshat-Doost HT, Moradi AR, Taghavi MR, Yule W, Dalgleish T. The development of a corpus of emotional words produced by children and adolescents. Personality and Individual Differences. 1999;27:433–451. [Google Scholar]
- Paus T. Mapping brain development and aggression. Can Child Adolesc Psychiatr Rev. 2005;14:10–15. [PMC free article] [PubMed] [Google Scholar]
- Seefeldt WL, Kramer M, Tuschen-Caffier B, Heinrichs N. Hypervigilance and avoidance in visual attention in children with social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2014;45:105–112. doi: 10.1016/j.jbtep.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Perez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, Leibenluft E, Pine DS. Attention biases, anxiety, and development: toward or away from threats or rewards? Depress Anxiety. 2012;29:282–294. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Britton JC, Leibenluft E, Pine DS, Nelson EE. Attention bias of anxious youth during extended exposure of emotional face pairs: an eye-tracking study. Depress Anxiety. 2013;30:14–21. doi: 10.1002/da.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biol Psychiatry. 2011;69:726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, Siegle GJ. Pupillary and reactivity to emotional information in child adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. Vol 65. [DOI] [PubMed] [Google Scholar]
- Waters AM, Kokkoris LL, Mogg K, Bradley BP, Pine DS. The time course of attentional bias for emotional faces in anxious children. Cognition & Emotion. 2010;24:1173–1181. [Google Scholar]


