Abstract
Objective
To determine the strength of the surrogate-survival correlation for cancer drug approvals based on a surrogate.
Participants and Methods
We performed a retrospective study of the US Food and Drug Administration (FDA) database, with focused searches of MEDLINE and Google Scholar. Among cancer drugs approved based on a surrogate end point, we examined previous publications assessing the strength of the surrogate-survival correlation. Specifically, we identified the percentage of surrogate approvals lacking any formal analysis of the strength of the surrogate-survival correlation, and when conducted, the strength of such correlations.
Results
Between January 1, 2009, and December 31, 2014, the FDA approved marketing applications for 55 indications based on a surrogate, of which 25 were accelerated approvals and 30 were traditional approvals. We could not find any formal analyses of the strength of the surrogate-survival correlation in 14 out of 25 accelerated approvals (56%) and 11 out of 30 traditional approvals (37%). For accelerated approvals, just 4 approvals (16%) were made where a level 1 analysis (the most robust way to validate a surrogate) had been performed, with all 4 studies reporting low correlation (r≤0.7). For traditional approvals, a level 1 analysis had been performed for 15 approvals (50%): 8 (53%) reported low correlation (r≤0.7), 4 (27%) medium correlation (r>0.7 to r<0.85), and 3 (20%) high correlation (r≥0.85) with survival.
Conclusions
The use of surrogate end points for drug approval often lacks formal empirical verification of the strength of the surrogate-survival association.
The US Food and Drug Administration (FDA) may grant oncology drugs either accelerated (provisional) (AA) or traditional (full) (TA) marketing approval.1 Accelerated approvals are given based on a surrogate end point that is “reasonably likely to predict” true clinical efficacy, ie, survival or quality of life.2–4 Traditional approvals are granted when a drug demonstrates “a longer or better life or a favorable effect on an established surrogate for a longer or better life.”4 Surrogate end points, thus, play a prominent role in oncology drug approvals, with the strength of the surrogate end point guiding the pathway of approval.
When relying on surrogates to guide clinical and regulatory decisions, it is important that the surrogate-survival correlation is robust to avoid the approval of toxic drugs with no benefit. Bevacizumab received AA in 2008 based on data that it markedly improved progression-free survival (PFS).5 However, by 2011, that approval was withdrawn when multiple studies found that the drug did not improve overall survival (OS) and carried toxicity and that gains in PFS were smaller than initially appreciated.6 In retrospect, the approval and subsequent withdrawal of bevacizumab in metastatic breast cancer is not surprising given that multiple validation studies found that this specific surrogate-survival association is weak.7
The validation of surrogate end points in oncology is an increasingly important field, with different statistical methods used.8–12 We favor a clear and simple hierarchy to grade the strength of surrogate-survival correlations.7,13 In this model, level 3—the lowest level—requires the surrogate-survival correlation to be only biologically plausible. Level 2 and level 1 analyses require clinical data. Although level 2 analysis shows that the surrogate is associated with the final outcome across groups, level 1 analysis addresses the clinically relevant question of whether improving the surrogate end point is associated with improvements in survival across many randomized studies. Typically, regression analysis is performed in level 1 studies. The x coordinate reflects the change in surrogate end point, and the y coordinate reflects the change in final end point. Correlation coefficients (r) closer to 1 signify stronger associations. As such, the validation of surrogate-survival associations in oncology exists along an established hierarchy.
We set out to characterize the nature of FDA approvals in oncology from 2009 through 2014. Specifically, what percentages of approvals were accelerated and traditional? Among TAs, what percentage were made based on a surrogate end point? For all approvals granted on the basis of surrogates, what is the documented strength of the surrogate-survival association? Finally for drugs approved based on surrogates, have subsequent trials demonstrated improvements in survival or quality of life? In short, we set out to empirically describe the strength of evidence for 6 years of FDA cancer drug approvals.
METHODS
Data Source
The FDA provides a record of hematology and oncology drug approvals and safety notifications on their website (http://www.fda.gov/Drugs/InformationOnDrugs/ucm279174.htm) and in related links. Each relevant webpage was downloaded and is provided in the Supplemental Figure 1 (available online at http://www.mayoclinicproceedings.org). Further information for each approval was obtained from the Drugs@FDA website, which includes information regarding the approval of new oncology drugs as well as expanded indications for currently approved drugs, date of approval, basis of approval, and a summary of the clinical review that supported the approval.
Study Sample
We identified all oncology drugs approved by the FDA between January 1, 2009, and December 31, 2014, the last complete year at the time of this investigation. Oncology drugs were approved based on improvements in OS or one of the following surrogate end points: improvements in disease response rate (eg, hematologic, pathologic, or tumor response) or delay in progression (eg, improved PFS or recurrence-free survival). We included data on new oncology drugs and on new indications for previously approved oncology drugs.
End Points Extracted
We ascertained the total number of AAs and TAs. We noted the efficacy end point leading to approval. When drugs were approved on the basis of improvement in OS or quality of life—measures of patient-centered benefit—we performed no further investigation. When drugs were approved based on a surrogate end point, we investigated formal analyses of the surrogate-survival correlation and whether subsequent publications have found an OS benefit.
Literature Search
We sought to ascertain the strength of the surrogate-survival correlation. In other words, as the criteria for AA and TA based on surrogates are “reasonably likely to predict” and “established,” respectively, we sought to evaluate the practical meaning of these terms.
For each surrogate drug approval, we performed a focused review of the literature to identify available surrogate-survival association studies. Surrogate association studies are widely performed in oncology to assess the strength of the surrogate end points.14 These studies are often meta-analyses of randomized controlled trials conducted in the same setting as the particular indication of the drug approval. For example, if one wants to know whether PFS correlates with OS in metastatic castrate-resistant prostate cancer, one begins by collecting all randomized controlled trials in this setting. Then one plots whether the hazard ratio or change in PFS (x coordinate) predicts the hazard ratio or change in OS (y coordinate). Regression analysis is conducted across trials to demonstrate the general correlation between the surrogate and survival. For each specific surrogate drug approval identified, we performed a review of the literature to locate such analyses. Multiple searches were performed, and all the search terms used and databases searched are listed in Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org). Two CONSORT diagrams show (1) the number of articles retrieved and the percentage included for AAs and (2) AAs and TAs combined (Supplemental Figures 2 and 3, available online at http://www.mayoclinicproceedings.org).
Grading the Strength of Correlation
We scored the strength of correlation for level 1 studies based on a modification of criteria proposed by the Institute for Quality and Efficiency in Health Care,15 as we have done previously7: low correlation (r≤0.7), medium correlation (r>0.7 to r<0.85), and high correlation (r≥0.85). The specific cutoff points were adapted to function even when confidence intervals were not presented. If coefficients of determination (R2) were given instead of correlation coefficients (r), we calculated the r by taking the square root. Where multiple level 1 studies existed, the median r was used for scoring. We repeated the analysis using the best r, which did not materially change the results (data not shown). We could not score level 2 studies because a variety of analyses and reported measures were used.
Subsequent Publications
For all drugs approved on the basis of a surrogate end point, we performed a focused search of the published literature to identify subsequent publications that report whether the drug improved OS, as we have also done previously, albeit in a set of different years (2008–2012).16 We credited a drug for improving OS if that drug improved survival in any combination (even beyond a combination that received approval) or in any line of treatment (eg, if the drug was approved for second line but improved survival in first line, we would credit the drug as improving survival). The search terms and databases included are also given in Supplemental Table 2 (available online at http://www.mayoclinicproceedings.org).
RESULTS
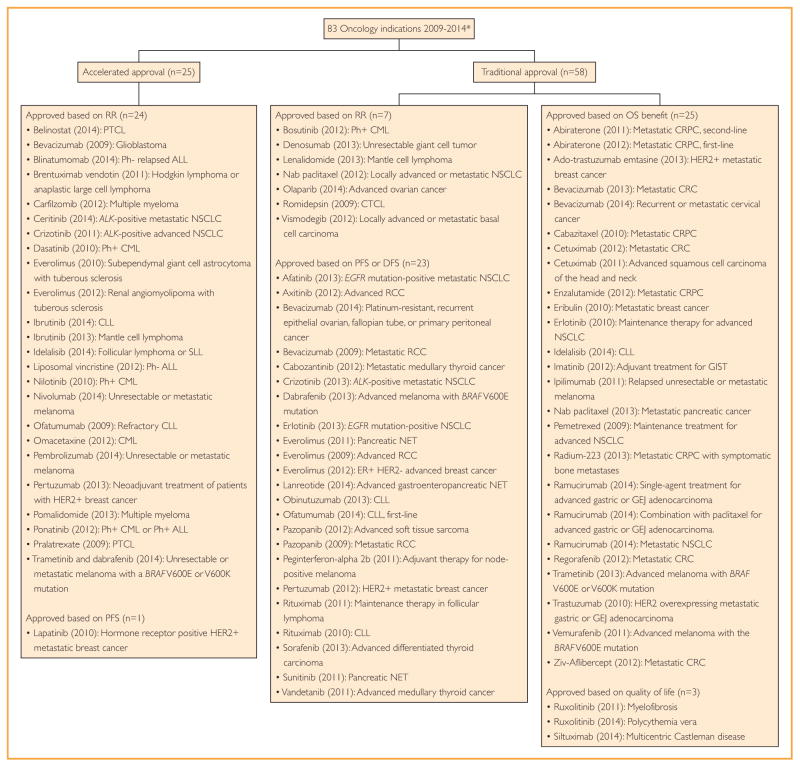
Between January 1, 2009, and December 31, 2014, the FDA approved marketing applications for 83 oncologic indications: 25 (30%) were AAs and 58 (70%) were TAs. Of the AAs, 24 (96%) were based on response rate (or duration of response) and 1 (4%) was based on PFS. Of the TAs, 28 (48%) were based on either improved OS or quality of life—patient-centered end points—and 30 (52%) were based on a surrogate end point, such as response rate (7 indications, 12% of TAs) or PFS or disease-free survival (23 indications, 40% of TAs). Figure 1 shows each approved drug, the year of approval, the indication for approval, the pathway for approval (traditional or accelerated), and the clinical end point supporting claims of efficacy at the time of approval.
FIGURE 1.
All indications receiving Food and Drug Administration marketing authorization for oncology drugs between 2009 and 2014. Approvals are grouped based on traditional or accelerated authorization and the efficacy end point met to garner approval. *Drugs approved based on bioequivalence (mercaptopurine (2014): ALL, asparaginase Erwinia chrysanthemi (2011): ALL) were removed from the analysis. ALL = acute lymphoblastic leukemia; CLL = chronic lymphocytic lymphoma; CML = chronic myeloid leukemia; CRC = colorectal carcinoma; CRPC = castration-resistant prostate cancer; CTCL = cutaneous T-cell lymphoma; DFS = disease-free survival; GEJ = gastroesophageal junction; GIST = gastrointestinal stromal tumor; HER2 = human epidermal growth factor receptor 2; NET = neuro-endrocine tumor; NSCLC = non-small cell lung cancer; OS = overall survival; PFS = progression-free survival; Ph = Philadelphia chromosome; PTCL = peripheral T-cell lymphoma; RCC = renal cell cancer; RR = response rate; SLL = small lymphocytic lymphoma.
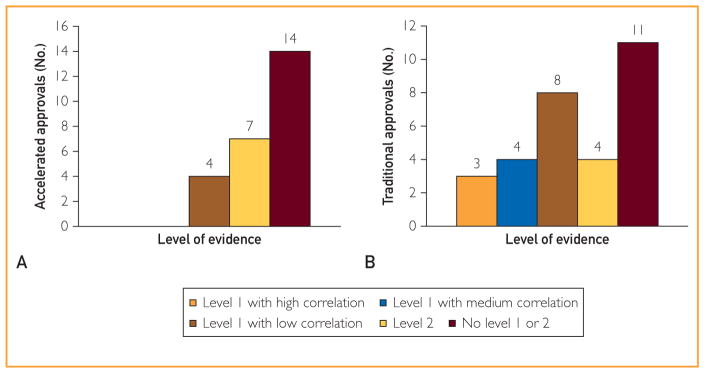
Accelerated approvals are made on the basis of a surrogate that is reasonably likely to predict true clinical efficacy. As shown in Figure 2, A, we could not find any formal analyses (studies that assessed level 1 or level 2 surrogacy) of the strength of the surrogate-survival correlation in 14 drug approvals (56%). In 7 instances (28%), a level 2 analysis (but not a level 1 analysis) could be found.17–22 The specific correlations established in those analyses are shown in Table 1. In 4 cases (16%), a level 1 analysis had been performed,24,25,28–33,41,42 with all studies reporting low correlation (r≤0.7).
FIGURE 2.
The strength of evidence between a surrogate-survival correlation for accelerated (A) and traditional (B) drug approvals based on surrogate end points. Level 1 studies were scored based on a modification to criteria proposed by the Institute for Quality and Efficiency in Health Care: low correlation (r≤0.7), medium correlation (r>0.7 to r<0.85), and high correlation (r≥0.85). No level 1 or 2 means that we could not identify a single association study in the literature. Where multiple level 1 studies exist, the median r was used for scoring.
TABLE 1.
Published Surrogate Correlation Studies for Accelerated Approvals
| Tumor type | Approved drug (year) | Basis of accelerated approval | Studies assessed level 2 surrogacy | Studies assessed level 1 surrogacy |
|---|---|---|---|---|
| Breast cancer | Pertuzumab (2013) | pCR | Kong et al,23 2011: OROS vs pCR=3.44, P<.00001 | Berruti et al,24 2014: rHR OS vs pCR=0.3 Cortazar et al,25 2014: rHR OS vs pCR=0.49 |
| Breast cancer | Lapatinib (2010) | PFS | Ciccarese et al,26 2007 (abstract): rOS vs DFS=0.77 Matsubara et al,27 2011: HROS vs PFS=0.93, P=.31 |
Hackshaw et al,28 2005: rHR OS vs TTP=0.75 Burzykowski et al,29 2008: rHR OS vs PFS=0.48 Miksad et al,30 2008: rHR OS vs. PFS=0.7 for anthracyclines Miksad et al,30 2008: rHR OS vs PFS=0.59 for taxanes Ng et al,31 2008: rdiff OS vs DFS=0.62 Sherrill et al,32 2008: rHR OS vs TTP=0.55 Beauchemin et al,33 2014: rdiff OS vs PFS/TTP=0.43 |
| Chronic lymphocytic leukemia | Ibrutinib (2014) Ofatumumab (2009) |
ORR | - | - |
| Chronic myeloid leukemia | Ponatinib (2012) Omacetaxine (2012) Dasatinib (2010) Nilotinib (2010) |
MCyR MaHR CCyR MMR |
Rosti et al,21 2003: rOS vs MCyR=0.66 Oriana et al,20 2013: P=.02 at 5 y, CCyR vs no CCyR (no report on coefficient) Jain et al,19 2013: P=.01 at 3 y, CCyR vs no CCyR |
- |
| Follicular lymphoma or small lymphocytic lymphoma | Idelalisib (2014) | ORR | - | - |
| Glioblastoma | Bevacizumab (2009) | ORR | Jaeckle et al,18 2008: P=.01 (no report on coefficient) | - |
| Hodgkin lymphoma and anaplastic large B cell | Brentuximab vedotin (2011) | ORR | - | - |
| Mantle cell lymphoma | Ibrutinib (2013) | ORR | - | - |
| Melanoma | Nivolumab (2014) Pembrolizumab (2014) Trametinib and dabrafenib (2014) |
ORR | - | - |
| Multiple myeloma | Pomalidomide (2013) Carfilzomib (2012) |
ORR | van de Velde et al,22 2007: P<.0001 (no report on coefficient) Gay et al,17 2011: OS HRCR vs PR =0.08, P<.001 |
- |
| Non-small cell lung cancer | Crizotinib (2011) Ceritinib (2014) |
ORR | Splinter,34 1991: rOS vs ORR=0.57 Paesmans et al,35 1997: rOS vs ORR=2.20, P<.0001 Sekine et al,36 1998: rOS vs ORR=0.504 Bruzzi et al,37 2007 (abstract): OS HRresponse vs. no response=0.50, P<.001 Tsujino et al,38 2009: rOS vs ORR=0.258 Mandrekar et al,39 2010: OS HRresponse vs no response=0.66, P=.009 Li et al,40 2012: rOS vs ORR=0.91 |
Blumenthal et al,41 2015: rHR OS vs ORR=0.3 Johnson et al,42 2006: rdiff OS vs ORR=0.4 |
| Ph— acute lymphoblastic leukemia | Blinatumomab (2014) | CR/reduction in MRD | - | - |
| Ph— acute lymphoblastic leukemia | Liposomal vincristine (2012) | CR/CRi | - | - |
| Peripheral T-cell lymphoma | Belinostat (2014) Pralatrexate (2009) |
ORR | - | - |
| Tuberous sclerosis with renal angiomyolipoma | Everolimus (2012) | ORR | - | - |
| Tuberous sclerosis with subependymal giant cell astrocytoma | Everolimus (2012) | ORR | - | - |
CCyR =complete cytogenetic response; CR =complete response; CRi =complete response with incomplete blood count recovery; DFS =disease-free survival; HR =hazard ratio; MaHR =major hematologic response; MCyR =major cytogenetic response; MMR =major molecular response; MRD =minimal residual disease; OR =odds ratio; ORR =objective response rate; OS =overall survival; pCR =pathologic complete response; PFS =progression-free survival; PR =partial response; TTP =time to progression.
When direct patient benefit has not been reported at the time of approval, TAs may be granted on the basis of an established surrogate for clinical benefit. Among 30 TAs based on a surrogate, we could not find any formal analyses of the strength of the surrogate-survival correlation in 11 instances (37%) (Figure 2, B). In 4 cases (13%), a level 2 analysis had been performed.19–21,43,44 The specific correlations established in those analyses are shown in Table 2. In 15 approvals (50%), a level 1 analysis had been performed to evaluate the strength of the surrogate-survival association, with 8 (53%) reporting low correlation (r≤0.7),28–33,41,42,45,49–53 4 (27%) reporting medium correlation (r>0.7 to r<0.85),56–59 and 3 (20%) reporting high correlation (r≥0.85) with survival.46 In only 7 of 30 TAs (23%) did a level 1 analysis demonstrate medium or high correlation with survival.46,56,57,59,60
TABLE 2.
Published Surrogate Correlation Studies for Traditional Approvals Based on a Surrogate
| Tumor type | Approved drug (year) | Basis of traditional approval | Studies assessed level 2 surrogacy | Studies assessed level 1 surrogacy |
|---|---|---|---|---|
| Basal cell carcinoma | Vismodegib (2012) | ORR | - | - |
|
| ||||
| Breast cancer | Pertuzumab (2012) | PFS | Ciccarese et al,26 2007 (abstract): rOS vs DFS=0.77 Matsubara et al,27 2011: HROS vs PFS=0.93, P=.31 |
Hackshaw et al,28 2005: rHR OS vs TTP=0.75 Burzykowski et al,29 2008: rHR OS vs PFS=0.48 Miksad et al,30 2008: rHR OS vs PFS=0.7 for anthracyclines Miksad et al,30 2008: rHR OS vs PFS=0.59 for taxanes Ng et al,31 2008: rdiff OS vs DFS=0.62 Sherrill et al,32 2008: rHR OS vs TTP=0.55 Beauchemin et al,33 2014: rdiff OS vs PFS/TTP=0.43 |
|
| ||||
| Chronic lymphocytic leukemia | Idelalisib (2014) Ofatumumab (2014) Obinutuzumab (2013) |
PFS | - | - |
|
| ||||
| Chronic myeloid leukemia | Bosutinib (2012) | MCyR Complete hematology response Overall hematologic response |
Rosti et al,21 2003: rOS vs MCyR=0.66 Oriana et al,20 2013: P=.02 at 5 y, CCyR vs no CCyR (no report on coefficient) Jain et al,19 2013: P=.01 at 3 y, CCyR vs no CCyR |
- |
|
| ||||
| Cutaneous T-cell lymphoma | Romidepsin (2009) | ORR | - | - |
|
| ||||
| Differentiated thyroid cancer | Sorafenib (2013) | PFS | - | - |
|
| ||||
| Follicular lymphoma | Rituximab (2011) | PFS | - | - |
|
| ||||
| Gastroenteropancreatic neuroendocrine tumor | Lanreotide (2014) Sunitinib (2011) for pancreatic NET Everolimus (2011) for pancreatic NET |
PFS | - | Singh et al,45 2014: rHR OS vs PFS/TTP=0.17 |
|
| ||||
| Giant cell tumor | Denosumab (2013) | ORR and DOR | - | - |
|
| ||||
| Mantle cell lymphoma | Lenalidomide (2013) | ORR and DOR | - | - |
|
| ||||
| Medullary thyroid carcinoma | Cabozantinib (2012) Vandetenib (2011) |
PFS | - | - |
|
| ||||
| Melanoma | Trametinib (2013) Dabrafenib (2013) Peginterferon-alpha 2b (2013) |
PFS | - | Flaherty et al,46 2014: rHR OS vs PFS=0.89 |
|
| ||||
| Non-small cell lung cancer | Nab paclitaxel (2012) | ORR | Splinter,34 1991: rOS vs ORR=0.57 Paesmans et al,35 1997: rOS vs ORR=2.20, P<.0001 Sekine et al,36 1998: rOS vs ORR=0.504 Bruzzi et al,37 2007 (abstract): OS HRresponse vs no response=0.50, P<.001 Tsujino et al,38 2009: rOS vs ORR=0.258 Mandrekar et al,39 2010: OS HRresponse vs no response=0.66, P=.009 Li et al,40 2012: rOS vs ORR=0.91 |
Blumenthal et al,41 2015: rHR OS vs ORR=0.3 Johnson et al,42 2006: rdiff OS vs ORR=0.4 |
| Crizotinib (2014) Afatinib (2014) Erlotinib (2013) |
PFS | Hayashi et al,47 2012: rPFS vs OS=0.43 Sekine et al,48 1999: rPFS vs OS=0.80 |
Blumenthal et al,41 2015: rHR OS vs PFS=0.28 Suzuki et al,49 2014: rHR OS vs PFS=0.41 Hayashi et al,50 2013: rdiff OS vs PFS=0.29 Hotta et al,51 2013: rHR OS vs PFS=0.37 Mauguen et al,52 2013: rHR OS vs DFS=0.96 Hotta et al,53 2009: rOS ratio vs TTP ratio=0.57 |
|
|
| ||||
| Ovarian cancer | Olaparib (2014) | ORR | Rose et al,44 2010: rOS vs ORR=0.56 | - |
|
| ||||
| Ovarian/fallopian/primary peritoneal cancer | Bevacizumab (2014) | PFS | Rose et al,44 2010: rOS vs PFS=0.66 | - |
|
| ||||
| Renal cell carcinoma | Axitinib (2012) Pazopanib (2009) Bevacizumab (2009) Everolimus (2009) |
PFS | Halabi et al,54 2014: HROS vs progression at 6 mo=2.8, P<.0001 Heng et al,55 2011: rOS vs PFS=0.66 |
Bria et al,56 2015: r12 mo OS vs 3 mo PFS=0.82, r12 mo OS vs 6 mo PFS=0.85 Johnson et al,57 2015: rdiff OS vs PFS=0.7 Petrelli and Barni,58 2013: rdiff OS vs PFS=0.36 Delea et al,59 2012: rdiff OS vs PFS/TTP=0.53 rHR OS vs PFS/TTP=0.79 |
|
| ||||
| Soft-tissue sarcoma | Pazopanib (2012) | PFS | Penel et al,43 2013: rOS vs PFS=0.4 | - |
CCyR =complete cytogenetic response; CRi =complete response with incomplete blood count recovery; DFS =disease-free survival; DOR =duration of response; HR =hazard ratio; MaHR =major hematologic response; MCyR =major cytogenetic response; MMR =major molecular response; MRD =minimal residual disease; NET =neuroendrocine tumor; ORR =objective response rate, OS =overall survival; pCR =pathologic complete response; PFS =progression-free survival; TTP =time to progression.
When an oncology drug is approved based on a surrogate, subsequent studies or longer follow-up for ongoing studies may report OS benefits. Table 3 summarizes the results of this review of subsequent publications for all marketing indications approved on the basis of a surrogate. Although 10 of 55 approvals (18%) were later found to carry an OS benefit, 15 (27%) were found not to improve OS. Most marketing approvals 30 of 55 (55%) remain untested. Traditional approvals based on surrogates were more likely not to show survival benefits than AAs (40% vs 12%; P=.02). The use of crossover occurred in 48% of these studies and did not vary among trials that found a survival advantage vs those that did not (4 of 10 [40%] vs 8 of 15 [53%]; P=.52).
TABLE 3.
Surrogate-Based Approvals for Which Subsequent Trials Report an OS Benefit or a Lack of Survival Benefit or for Which No Trials Exist Showing or Refuting a Survival Benefit
| Indication | Approvals (No. [%]) | ||
|---|---|---|---|
| Proven OS benefit | No OS benefit | OS benefit unknown | |
| Total (n=55) | 10 (18.2) | 15 (27.3) | 30 (54.5) |
| Accelerated approval (n=25) | 6 (24.0) | 3 (12.0) | 16 (64.0) |
| Traditional approval (n=30) | 4 (13.3) | 12 (40.0) | 14 (46.7) |
OS =overall survival.
Combining the results of the 2 analyses, we find that among 55 drugs approved on the basis of a surrogate, 10 (18.2%) have shown OS benefits, and another 2 (3.6%) have not shown OS benefits, but were approved on the basis of a high-strength correlation in a level 1 analysis. Thus, only 12 (21.8%) of 55 surrogate approvals were made on the basis of a strong surrogate-survival correlation or later showed OS benefits.
DISCUSSION
We found that most cancer drug approvals (55 of 83 [66%]) are based on a surrogate end point. Although the FDA grants TA based on established surrogate end points, this standard is lax. Only 3 of 30 such approvals (10%) have shown high correlation in a level 1 surrogate analysis, widely considered a prerequisite for clinical or regulatory decisions.15 Of concern, 11 of 30 TAs (37%) had no formal analyses of the surrogate-survival correlation. Accelerated approvals are granted on the basis of a surrogate that is reasonably likely to predict clinical benefit; but again, practically, this standard has not been enforced, with 56% of approvals (14 of 25) made without any formal analysis of surrogacy.
The frequent use of surrogate end points in FDA approval is paralleled by a rise in the use of these end points as the primary end point of clinical trials. Examining randomized controlled trials from 1974 through 2009 for non-small cell lung cancer, breast cancer, and colorectal cancer in 5 major journals, PFS increased in frequency as the primary end point of trials from 0% (1975–1984) to 20% (2005–2009).61 Sacher et al62 found, in an exhaustive look at trials for non-small cell lung cancer, that OS declined as the primary end point of lung cancer trials from 97% (1980–1990) to 96% (1991–2000) to 81% (2001–2010). This trend was accompanied by a rise in the PFS end point (a surrogate) as the primary end point. The improvement of a surrogate end point in oncology can be used to petition for drug approval4,63 and to expand clinical guidelines, which in many cases obliges insurers and Medicare to cover drugs. It is likely that the validation of surrogate end points in cancer would benefit from a formal set of guidelines, as was done decades ago for general statistical presentations in medicine.64
There are at least 2 potential reasons why surrogates may not correlate with OS for new oncology drugs. The first is that in contrast with OS, where the date of death is precise and can be ascertained for all patients, surrogates are prone to reader interpretation, measurement error, evaluation bias, and attrition bias.65,66 These artifacts may create spurious surrogate benefits. The second explanation is a biological one: a cancer drug with favorable surrogate effects may affect changes in tumor growth or aggressiveness or may increase off-target deaths.67–70 For these reasons, it is important that a surrogate is validated in a precise context (eg, whether disease-free survival correlates with OS for cytotoxic agents in the adjuvant treatment of colorectal cancer). Validation is performed for the unique combination of disease setting (adjuvant), tumor type (colorectal), class of agents (cytotoxic), and particular surrogate end point (disease-free survival).
Regulatory language appreciates these concerns and allows AA for surrogates reasonably likely to predict true efficacy and TA for surrogates that are established. Although there is clearly flexibility in this language, we believe that, at a minimum, the language implies some previous formal analysis of the surrogate-survival relationships. Yet, in 25 of 55 drug approvals based on a surrogate end point, we found no published analysis assessing the robustness of the surrogate-survival correlation. Although it is possible that the FDA has conducted internal and unpublished analyses in these cases, we consider that unlikely because the FDA has announced and published other analyses they have commissioned seeking to assess a surrogate.25 Without any formal analysis, we contend that it is incorrect to consider a surrogate as established.
Subsequent studies do not lend much clarity to surrogate approvals. In the present analysis, we found that less than half of the surrogate approvals (25 of 55, 45%) had a subsequent analysis of survival, and, when they did, such analyses concluded approximately 3 to 2 (15:10) that the drug did not improve survival. The present results regarding subsequent trials in the present data set are similar to the fate of drugs approved from 2008 through 2012, as we reported previously.16
A crossover design was used in 48% of these studies, and they did not vary among trials that found a survival advantage vs those that did not. As such, the present data provide further evidence to question the common narrative concerning crossover: that it masks OS benefits that truly exist.71,72 Alternative explanations include that crossover obscures the fact that survival benefits do not exist and prevents the ability to observe late toxicity. Moreover, despite crossover, some drugs have shown survival benefits.73
Finally, note that the sizable use of unvalidated (and altogether untested) surrogates to approve cancer drugs may further undermine the ability to conduct definitive trials of precision medicine. Once drugs are available, patients are naturally reluctant to participate in trials assessing their fundamental efficacy. Instead, we increasingly have to rely on case reports,74 a notoriously unreliable way to assess claims of efficacy.
There are several limitations to this analysis. Whether an end point is truly a surrogate or a measure of clinical benefit continues to be subject to debate in oncology.68 We considered improvements in patient-reported outcomes, quality of life, and OS to constitute a patient-centered benefit and improvements in all other outcomes as surrogate to this. Others may believe that under certain circumstances radiographic PFS becomes clinically meaningful. However, because this end point includes events that patients may not physically be aware of (radiographic progression), as such, we believe that our classification is technically accurate. Future research is needed to delineate the relationship between PFS and quality of life across tumor settings.
The use of follow-up studies remains inadequate among cancer drugs approved based on a surrogate, with 16 of 25 AAs and 14 of 30 TAs lacking a subsequent study reporting on OS. Given the recent nature of the drugs we investigated (2009–2014), it is likely that additional studies will become available in the future. However, this may be more true among AAs, where such postmarketing studies are a requirement for continued authorization, than among TAs, which often do not entail further postmarketing efficacy commitments. Thus, it is possible that some of the estimates change as future data become available. At the same time, it must be acknowledged that the FDA’s enforcement of postmarketing commitments has historically been poor75 and that future data will not change the known strength of surrogate-survival correlations at the time of approval, which remains the regulatory basis for approval.
Another limitation is that although the search strategy for surrogate-survival association studies involved the use of 2 search engines, it is possible that we missed such associational studies. However, previously we performed an exhaustive mixed-methods search for surrogate survival correlations,7 and, thus, we believe that we have captured most such papers that exist in the biomedical literature.
A final limitation—not necessarily of the study itself but of the topic that we are studying—is that surrogate validation studies likely have publication and selective reporting bias. Previous work7 has found that few level 1 validation studies (5 of 36) survey both published and unpublished trials, and when they do, they are able to retrieve and use data from only 51.5% of studies. Although this concern does not pertain to situations in which surrogate validation studies were absent in the present investigation, it suggests that in situations in which validation was present, the strength of correlation may be different based on a more comprehensive analysis using all trials conducted on a topic.
CONCLUSION
Most new cancer drugs are approved on the basis of surrogate end points. The standard for such approvals is that surrogates are reasonably likely to predict clinical efficacy or established in the case of AA and TA, respectively. We found that, practically, this standard is lax, with 56% and 37% of AAs and TAs, respectively, based on surrogates made without any formal analysis of the strength of the surrogate-survival correlation. Additional follow-up to existing trials or new trials were unlikely to be completed or to confirm survival benefits. The present study suggests that the use of surrogate end points for drug approval often lacks formal empirical verification. This practice should be reconsidered.
Supplementary Material
Acknowledgments
The views and opinions of the authors do not reflect the institutions with which they are affiliated.
Abbreviations and Acronyms
- AA
accelerated approval
- ALL
acute lymphoblastic leukemia
- CCyR
complete cytogenetic response
- CLL
chronic lymphocytic lymphoma
- CML
chronic myeloid leukemia
- CRC
colorectal carcinoma
- CR
complete response
- CRi
complete response with incomplete blood count recovery
- CRPC
castrate-resistant prostate cancer
- CTCL
cutaneous T-cell lymphoma
- DFS
disease-free survival
- DOR
duration of response
- FDA
Food and Drug Administration
- GEJ
gastroesophageal junction
- GIST
gastrointestinal stromal tumor
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- MaHR
major hematologic response
- MCyR
major cytogenetic response
- MMR
major molecular response
- MRD
minimal residual disease
- NET
neuroendrocine tumor
- NSCLC
non-small cell lung cancer
- OR
odds ratio
- ORR
objective response rate
- OS
overall survival
- pCR
pathologic complete remission
- PFS
progression-free survival
- Ph
Philadelphia chromosome
- PR
partial remission
- PTCL
peripheral T-cell lymphoma
- RCC
renal cell cancer
- RR
response rate
- SLL
small lymphocytic lymphoma
- TA
traditional approval
- TTP
time to progression
Footnotes
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Hirschfeld S, Pazdur R. Oncology drug development: United States Food and Drug Administration perspective. Crit Rev Oncol Hematol. 2002;42(2):137–143. doi: 10.1016/s1040-8428(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol. 2003;21(7):1404–1411. doi: 10.1200/JCO.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 3.Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 4.Sridhara R, Johnson JR, Justice R, Keegan P, Chakravarty A, Pazdur R. Review of oncology and hematology drug product approvals at the US Food and Drug Administration between July 2005 and December 2007. J Natl Cancer Inst. 2010;102(4):230–243. doi: 10.1093/jnci/djp515. [DOI] [PubMed] [Google Scholar]
- 5.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365(2):e3. doi: 10.1056/NEJMp1107201. [DOI] [PubMed] [Google Scholar]
- 7.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389–1398. doi: 10.1001/jamainternmed.2015.2829. [DOI] [PubMed] [Google Scholar]
- 8.Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1(1):49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11(2):167–178. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 10.Lassere MN, Johnson KR, Schiff M, Rees D. Is blood pressure reduction a valid surrogate endpoint for stroke prevention? an analysis incorporating a systematic review of randomised controlled trials, a by-trial weighted errors-in-variables regression, the surrogate threshold effect (STE) and the Biomarker-Surrogacy (BioSurrogate) Evaluation Schema (BSES) BMC Med Res Methodol. 2012;12:27. doi: 10.1186/1471-2288-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torri V, Simon R, Russek-Cohen E, Midthune D, Friedman M. Statistical model to determine the relationship of response and survival in patients with advanced ovarian cancer treated with chemotherapy. J Natl Cancer Inst. 1992;84(6):407–414. doi: 10.1093/jnci/84.6.407. [DOI] [PubMed] [Google Scholar]
- 12.Weir CJ, Walley RJ. Statistical evaluation of biomarkers as surrogate endpoints: a literature review. Stat Med. 2006;25(2):183–203. doi: 10.1002/sim.2319. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RS, Elston J. The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports. Health Technol Assess. 2009;13(8):iii, ix–xi, 1–50. doi: 10.3310/hta13080. [DOI] [PubMed] [Google Scholar]
- 14.Shi Q, Sargent DJ. Meta-analysis for the evaluation of surrogate endpoints in cancer clinical trials. Int J Clin Oncol. 2009;14(2):102–111. doi: 10.1007/s10147-009-0885-4. [DOI] [PubMed] [Google Scholar]
- 15.Institute for Quality and Efficiency in Health Care. [Accessed July 23, 2015];Validity of surrogate endpoints in oncology: executive summary. https://www.iqwig.de/download/A10-05_Executive_Summary_v1-1_Surrogate_endpoints_in_oncology.pdf. Published 2011. [PubMed]
- 16.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of us food and drug administration approvals. JAMA Intern Med. 2015;175(12):1992–1994. doi: 10.1001/jamainternmed.2015.5868. [DOI] [PubMed] [Google Scholar]
- 17.Gay F, Larocca A, Wijermans P, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117(11):3025–3031. doi: 10.1182/blood-2010-09-307645. [DOI] [PubMed] [Google Scholar]
- 18.Jaeckle K, Wu W, Kosel M, Flynn P, Buckner J. Correlation of response with survival endpoints in patients with newly diagnosed and recurrent glioblastoma (GBM) treated on prospective North Central Cancer Treatment Group (NCCTG) clinical trials. J Clin Oncol. 2008;26:2024. [Google Scholar]
- 19.Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121(24):4867–4874. doi: 10.1182/blood-2013-03-490128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oriana C, Martin H, Toby P, et al. Complete cytogenetic response and major molecular response as surrogate outcomes for overall survival in first-line treatment of chronic myelogenous leukemia: a case study for technology appraisal on the basis of surrogate outcomes evidence. Value Health. 2013;16(6):1081–1090. doi: 10.1016/j.jval.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Rosti G, Testoni N, Martinelli G, Baccarani M. The cytogenetic response as a surrogate marker of survival. Semin Hematol. 2003;40(2 suppl 2):56–61. doi: 10.1053/shem.2003.50042. [DOI] [PubMed] [Google Scholar]
- 22.van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92(10):1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 23.Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neo-adjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011;47(14):2084–2090. doi: 10.1016/j.ejca.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol. 2014;32(34):3883–3891. doi: 10.1200/JCO.2014.55.2836. [DOI] [PubMed] [Google Scholar]
- 25.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 26.Ciccarese M, Bria E, Cuppone F, et al. Disease-free survival (DFS) as surrogate end point for overall survival (OS) in adjuvant aromatase inhibitors (AIs) trials for breast cancer (BC): meta-analysis of 10 randomized clinical trials (RCTs) exploring the magnitude of the benefit. Paper presented at: American Society of Clinical Oncology (ASCO) 43rd Annual Meeting; June 1–5, 2007; Chicago, IL. [Google Scholar]
- 27.Matsubara Y, Sakabayashi S, Nishimura T, et al. Surrogacy of tumor response and progression-free survival for overall survival in metastatic breast cancer resistant to both anthracyclines and taxanes. Int J Clin Oncol. 2011;16(6):623–629. doi: 10.1007/s10147-011-0231-5. [DOI] [PubMed] [Google Scholar]
- 28.Hackshaw A, Knight A, Barrett-Lee P, Leonard R. Surrogate markers and survival in women receiving first-line combination anthracycline chemotherapy for advanced breast cancer. Br J Cancer. 2005;93(11):1215–1221. doi: 10.1038/sj.bjc.6602858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26(12):1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 30.Miksad RA, Zietemann V, Gothe R, et al. Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care. 2008;24(4):371–383. doi: 10.1017/S0266462308080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng R, Pond GR, Tang PA, MacIntosh PW, Siu LL, Chen EX. Correlation of changes between 2-year disease-free survival and 5-year overall survival in adjuvant breast cancer trials from 1966 to 2006. Ann Oncol. 2008;19(3):481–486. doi: 10.1093/annonc/mdm486. [DOI] [PubMed] [Google Scholar]
- 32.Sherrill B, Amonkar M, Wu Y, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008;99(10):1572–1578. doi: 10.1038/sj.bjc.6604759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beauchemin C, Cooper D, Lapierre ME, Yelle L, Lachaine J. Progression-free survival as a potential surrogate for overall survival in metastatic breast cancer. Onco Targets Ther. 2014;7:1101–1110. doi: 10.2147/OTT.S63302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Splinter TA. Response rate as criterium to evaluate chemotherapy in non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 1991;7(1):91–104. [Google Scholar]
- 35.Paesmans M, Sculier JP, Libert P, et al. Response to chemotherapy has predictive value for further survival of patients with advanced non-small cell lung cancer: 10 years experience of the European Lung Cancer Working Party. Eur J Cancer. 1997;33(14):2326–2332. doi: 10.1016/s0959-8049(97)00325-0. [DOI] [PubMed] [Google Scholar]
- 36.Sekine I, Kubota K, Nishiwaki Y, Sasaki Y, Tamura T, Saijo N. Response rate as an endpoint for evaluating new cytotoxic agents in phase II trials of non-small-cell lung cancer. Ann Oncol. 1998;9(10):1079–1084. doi: 10.1023/a:1008473003445. [DOI] [PubMed] [Google Scholar]
- 37.Bruzzi P, Sormani M, Tiseo M, Boni L, Rosell R, Ardizzoni A. Tumor response to chemotherapy as a surrogate endpoint of survival in advanced non-small cell lung cancer (NSCLC): results of an individual patients data meta-analysis. Paper presented at: American Society of Clinical Oncology (ASCO) 43rd Annual Meeting; June 1–5, 2007; Chicago, IL. [Google Scholar]
- 38.Tsujino K, Kawaguchi T, Kubo A, et al. Response rate is associated with prolonged survival in patients with advanced non-small cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol. 2009;4(8):994–1001. doi: 10.1097/JTO.0b013e3181a94a2f. [DOI] [PubMed] [Google Scholar]
- 39.Mandrekar SJ, Qi Y, Hillman SL, et al. Endpoints in phase II trials for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(1):3–9. doi: 10.1097/JTO.0b013e3181c0a313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Liu S, Gu H, Wang D. Surrogate end points for survival in the target treatment of advanced non-small-cell lung cancer with gefitinib or erlotinib. J Cancer Res Clin Oncol. 2012;138(11):1963–1969. doi: 10.1007/s00432-012-1278-z. [DOI] [PubMed] [Google Scholar]
- 41.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33(9):1008–1014. doi: 10.1200/JCO.2014.59.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson KR, Ringland C, Stokes BJ, et al. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: a meta-analysis. Lancet Oncol. 2006;7(9):741–746. doi: 10.1016/S1470-2045(06)70800-2. [DOI] [PubMed] [Google Scholar]
- 43.Penel N, Cousin S, Duhamel A, Kramar A. Activity endpoints reported in soft tissue sarcoma phase II trials: quality of reported endpoints and correlation with overall survival. Crit Rev Oncol Hematol. 2013;88(2):309–317. doi: 10.1016/j.critrevonc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Rose PG, Tian C, Bookman MA. Assessment of tumor response as a surrogate endpoint of survival in recurrent/platinum-resistant ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;117(2):324–329. doi: 10.1016/j.ygyno.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Wang X, Law CH. Association between time to disease progression end points and overall survival in patients with neuroendocrine tumors. Gastrointest Cancer Targets Ther. 2014;4:103–113. [Google Scholar]
- 46.Flaherty KT, Hennig M, Lee SJ, et al. Surrogate endpoints for overall survival in metastatic melanoma: a meta-analysis of randomised controlled trials. Lancet Oncol. 2014;15(3):297–304. doi: 10.1016/S1470-2045(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi H, Okamoto I, Morita S, Taguri M, Nakagawa K. Post-progression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol. 2012;23(6):1537–1541. doi: 10.1093/annonc/mdr487. [DOI] [PubMed] [Google Scholar]
- 48.Sekine I, Tamura T, Kunitoh H, et al. Progressive disease rate as a surrogate endpoint of phase II trials for non-small-cell lung cancer. Ann Oncol. 1999;10(6):731–733. doi: 10.1023/a:1008303921033. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki H, Hirashima T, Okamoto N, et al. Relationship between progression-free survival and overall survival in patients with advanced non-small cell lung cancer treated with anti-cancer agents after first-line treatment failure. Asia Pac J Clin Oncol. 2015;11(2):121–128. doi: 10.1111/ajco.12199. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi H, Okamoto I, Taguri M, Morita S, Nakagawa K. Post-progression survival in patients with advanced non-small-cell lung cancer who receive second-line or third-line chemotherapy. Clin Lung Cancer. 2013;14(3):261–266. doi: 10.1016/j.cllc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Hotta K, Suzuki E, Di Maio M, et al. Progression-free survival and overall survival in phase III trials of molecular-targeted agents in advanced non-small-cell lung cancer. Lung Cancer. 2013;79(1):20–26. doi: 10.1016/j.lungcan.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013;14(7):619–626. doi: 10.1016/S1470-2045(13)70158-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotta K, Fujiwara Y, Matsuo K, et al. Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(3):311–317. doi: 10.1097/JTO.0b013e3181989bd2. [DOI] [PubMed] [Google Scholar]
- 54.Halabi S, Rini B, Escudier B, Stadler WM, Small EJ. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic renal cell carcinoma. Cancer. 2014;120(1):52–60. doi: 10.1002/cncr.28221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heng DY, Xie W, Bjarnason GA, et al. Progression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapy. Cancer. 2011;117(12):2637–2642. doi: 10.1002/cncr.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bria E, Massari F, Maines F, et al. Progression-free survival as primary endpoint in randomized clinical trials of targeted agents for advanced renal cell carcinoma: correlation with overall survival, benchmarking and power analysis. Crit Rev Oncol Hematol. 2015;93(1):50–59. doi: 10.1016/j.critrevonc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Johnson KR, Liauw W, Lassere MN. Evaluating surrogacy metrics and investigating approval decisions of progression-free survival (PFS) in metastatic renal cell cancer: a systematic review. Ann Oncol. 2015;26(3):485–496. doi: 10.1093/annonc/mdu267. [DOI] [PubMed] [Google Scholar]
- 58.Petrelli F, Barni S. Surrogate end points and postprogression survival in renal cell carcinoma: an analysis of first-line trials with targeted therapies. Clin Genitourin Cancer. 2013;11(4):385–389. doi: 10.1016/j.clgc.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Delea TE, Khuu A, Heng DY, Haas T, Soulieres D. Association between treatment effects on disease progression end points and overall survival in clinical studies of patients with metastatic renal cell carcinoma. Br J Cancer. 2012;107(7):1059–1068. doi: 10.1038/bjc.2012.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrelli F, Pietrantonio F, Cremolini C, et al. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer. 2015;51(7):800–807. doi: 10.1016/j.ejca.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Kay A, Higgins J, Day AG, Meyer RM, Booth CM. Randomized controlled trials in the era of molecular oncology: methodology, biomarkers, and end points. Ann Oncol. 2012;23(6):1646–1651. doi: 10.1093/annonc/mdr492. [DOI] [PubMed] [Google Scholar]
- 62.Sacher AG, Le LW, Leighl NB. Shifting patterns in the interpretation of phase III clinical trial outcomes in advanced non-small-cell lung cancer: the bar is dropping. J Clin Oncol. 2014;32(14):1407–1411. doi: 10.1200/JCO.2013.52.7804. [DOI] [PubMed] [Google Scholar]
- 63.Amir E, Seruga B, Martinez-Lopez J, et al. Oncogenic targets, magnitude of benefit, and market pricing of antineoplastic drugs. J Clin Oncol. 2011;29(18):2543–2549. doi: 10.1200/JCO.2011.35.2393. [DOI] [PubMed] [Google Scholar]
- 64.Bailar JC, III, Mosteller F. Guidelines for statistical reporting in articles for medical journals: amplifications and explanations. Ann Intern Med. 1988;108(2):266–273. doi: 10.7326/0003-4819-108-2-266. [DOI] [PubMed] [Google Scholar]
- 65.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030–1033. doi: 10.1200/JCO.2011.38.7571. [DOI] [PubMed] [Google Scholar]
- 66.Shao T, Wang L, Templeton AJ, et al. Use and misuse of waterfall plots. J Natl Cancer Inst. 2014;106(12) doi: 10.1093/jnci/dju331. [DOI] [PubMed] [Google Scholar]
- 67.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8(4):210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miles D, Harbeck N, Escudier B, et al. Disease course patterns after discontinuation of bevacizumab: pooled analysis of randomized phase III trials. J Clin Oncol. 2011;29(1):83–88. doi: 10.1200/JCO.2010.30.2794. [DOI] [PubMed] [Google Scholar]
- 70.Moore TJ, Furberg CD. The safety risks of innovation: the FDA’s Expedited Drug Development Pathway. JAMA. 2012;308(9):869–870. doi: 10.1001/jama.2012.9658. [DOI] [PubMed] [Google Scholar]
- 71.Prasad V. Double-crossed: why crossover in clinical trials may be distorting medical science. J Natl Compr Canc Netw. 2013;11(5):625–627. doi: 10.6004/jnccn.2013.0077. [DOI] [PubMed] [Google Scholar]
- 72.Prasad V, Grady C. The misguided ethics of crossover trials. Contemp Clin Trials. 2014;37(2):167–169. doi: 10.1016/j.cct.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 74.Prasad V, Vandross A. Characteristics of exceptional or super responders to cancer drugs. Mayo Clin Proc. 2015;90(12):1639–1649. doi: 10.1016/j.mayocp.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 75.US Government Accountability Office. [Accessed July 25, 2015];New drug apporval: FDA needs to enhance its oversight of drugs approved on the basis of surrogate endpoints. http://www.gao.gov/new.items/d09866.pdf. Published September 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.