Abstract
Background
An attractive hypothesis about how the brain learns to keep its motor commands accurate is centered on the idea that the cerebellar cortex associates error signals carried by climbing fibers with simultaneous activity in parallel fibers. Motor learning can be impaired if the error signals are not transmitted, are incorrect, or are misinterpreted by the cerebellar cortex. Learning might also be impaired if the brain is overwhelmed with a sustained barrage of meaningless information unrelated to simultaneously appearing error signals about incorrect performance.
Methods
We test this concept in subjects with syndrome of oculopalatal tremor (OPT), a rare disease with spontaneous, irregular, roughly pendular oscillations of the eyes thought to reflect an abnormal, synchronous, spontaneous discharge to the cerebellum from the degenerating neurons in the inferior olive. We examined motor learning during a short-term saccade adaptation paradigm.
Results
We found a unique pattern of disturbed adaptation in subjects with OPT, discrete compared to the abnormal learning from direct involvement of the cerebellum. Both fast (seconds) and slow (minutes) timescales of learning were impaired. We suggest that the spontaneous, continuous, synchronous output from the inferior olive prevents the cerebellum from receiving the error signals it needs for appropriate motor learning.
Conclusion
The important message from this study is that impaired motor adaptation and resultant dysmetria is not the exclusive feature of cerebellar disorders, but it also highlights disorders of the inferior olive and its connections to the cerebellum.
Keywords: saccade, dysmetria, oscillations, nystagmus, cerebellum, inferior olive
Introduction
Changes in our environment, injury to the central or peripheral motor system, and inherent variability of central motor commands can all compromise the accuracy of movements. Fortunately our brain has evolved mechanisms for monitoring and adjusting motor performance. Relatively short latency internal feedback loops compensate for the inevitable variations in central motor commands, while persistent inaccuracies due to central or peripheral disruptions are corrected by enduring adaptive changes to the motor plan [1, 2].
One hypothesis regarding how this adaptation is achieved emphasizes the role of a neural network consisting of the inferior olive, cerebellar cortex, and the deep cerebellar nuclei. Movement errors lead to a change in the phase of synchronous sub-threshold oscillations in the membrane potential of inferior olivary neurons [3, 4]. The inferior olive discharge, carrying the error signal, is transmitted to the Purkinje neurons via the climbing fibers (CF) and also to the deep cerebellar nuclei via CF collaterals. Simultaneously parallel fibers (PF) relay an internal copy of the performed movement. The Purkinje neurons compare the activity of CF to that of PF. Once an error is detected the change in the activity of Purkinje neuron alters ongoing movement by inducing plasticity in their target neurons in the deep cerebellar nuclei [5-15].
Motor learning evolves on multiple timescales [16-19]. The fast timescale process rapidly learns from errors, but also forgets quickly. Simultaneous slow timescale adapts gradually in response to persistent systematic errors, but is relatively resistant to forgetting. Together, these two processes enable the motor system to learn robustly when challenged by a consistent movement perturbation.
As one would expect, adaptation to inaccurate motor behavior is impaired if there are disruptions within the learning circuits. For example, the subjects with structural abnormalities in the cerebellar cortex adapt poorly [19-23]. Fast learning process is predominantly impaired in cerebellar subjects, but their slow learning timescale is relatively unaffected [19, 24]. Such dissociation suggests prominent role of cerebellum to regulate trial-to-trial movement corrections, while other brain regions facilitate long-term learning.
We hypothesized that spontaneous, non-error-driven output from the inferior olive will interfere with the motor learning by disrupting or masking appropriate errors signals destined for the cerebellum. We test this concept in a unique “disease model”, the syndrome of oculopalatal tremor (OPT), featuring pathologically synchronized, spontaneous discharge from the inferior olive. Such abnormal activity is the delayed consequence of a lesion in the brainstem or cerebellar outflow tracts that causes a breach in the Guillain-Mollaret triangle (connections from the inferior olive to the deep cerebellar nuclei, and back to the inferior olive with fibers running in the central tegmental tract after passing near the red nucleus) [25]. We predict that motor learning should be impaired at multiple time-scales such that subjects with OPT do not correct errors in motor accuracy, and instead show persistent dysmetria.
Methods
Ten healthy subjects (age range: 25 - 65) and three subjects with OPT (age range: 38 - 62) participated in the study. Clinical features are outlined in table 1. The ethics committee at The Johns Hopkins University approved the study protocol. The subjects signed written informed consent. Eye movements were recorded using a scleral search coils calibrated in a magnetic field (Skalar Medical, Delft, The Netherlands; Chronos Vision, Berlin, Germany) [26]. Data were acquired at 1000 Hz with an angular resolution of 0.1°. Subjects sat in a dark room in a stationary chair. A bite bar was used to prevent head movements. Targets were presented using a laser dot, 2 mm in diameter, rear-projected onto a screen 1 m in front of the subject.
Table 1.
Clinical diagnosis, demographics, and magnetic resonance imaging (MRI) findings of patients with oculopalatal tremor.
| Patient | Age/Gender | Diagnosis | Duration of symptoms | Character of eye oscillations | MRI findings | Pertinent medication profile |
|---|---|---|---|---|---|---|
| P1 | 62/F | Idiopathic OPT | 24 months | Pure torsional, regular, binocular | Mild T2 hyperintensity involving the bilateral medullary olives. Multiple dilated perivascular spaces involving the mid brain and pons. |
Clonazepam (0.5 mg twice a day) |
| P2 | 38/F | Brainstem hemorrhage | 12 months | Torsional and vertical, irregular, binocular | Post-surgical encephalomalacia just to the left of midline of the caudal cerebellum. High signal intensity in both inferior olives, but slightly more prominent on the left. |
None |
| P3 | 48/M | Brainstem hemorrhage due to pontine-mesencephalic vascular malformation | 10 months | Torsional and vertical, irregular, binocular | Encephalomalacia in the medial aspect of the right midbrain tegmentum extending along the ventral aspect of the cerebral aqueduct inferiorly to the left dorsal pons to the level of the brachium pontis. Hemosiderin deposition in the 4th ventricular floor, along tentorium and cerebral peduncles. Increased T2 signal and enlargement of both inferior olivary nuclei. |
Clonazepam (1 mg every night), memantine (10 mg three times a day) |
Experimental protocol
Each experiment was divided in two sessions – gain-increasing adaptation and gain-decreasing adaptation. Each session, comprised of open-loop and adaptation trials, was performed at least one month apart (Figure 1).
Figure 1.
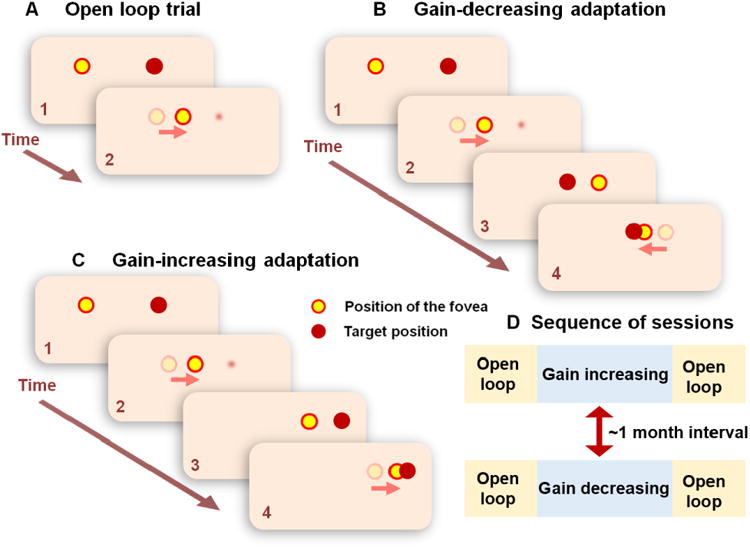
Summary of the experimental protocol for saccade adaptation. Throughout the experiment the subject is seated in a dark room. (A) Open-loop trial: 1) During this trial the target turns on 2) as soon as the subject starts moving the eyes towards the target (red arrow) the target turns off. Open-loop trials were obtained before and after saccade adaptation paradigms. (B) Gain-decreasing saccade adaptation paradigm: 1) The target (T1) turns on, 2) as soon as the subject starts to move their eyes towards the target, it turns off. 3) Once the eyes reach the estimated location of T1, another target (T2) turns on, but 2.5 degrees closer to the previous position of the eyes, introducing an error signal. 4) As a result the subject makes a corrective saccade to T2. (C) Gain-increasing saccade adaptation paradigm: This is analogous to the gain-decreasing saccade adaptation paradigm. However, during steps 3 and 4, T2 appears 2.5 degrees farther away from the previous position of the eyes, inducing an error signal to make larger saccades.
Open-loop trials
Sets of open-loop trials assessed changes in saccade gain as an index of the level of adaptation (Figure 1A). During an open-loop trial, the target shifted 10° away from the current position, either to the right or left. The onset of the saccade triggered the disappearance of the target; hence, the eyes landed in darkness. Five hundred milliseconds after the end of primary saccade, a new fixation target appeared at the current location of the eye. The 500 ms delay provided sufficient separation between trials to minimize any additional changes in saccade gain as there was no error feedback immediately after the movement [27]. Open-loop trials allowed for an estimate of the initial motor command that drove the primary saccade [28].
Adaptation trials
The adaptation paradigm contained six learning blocks each consisting of 60 double-step saccade-adaptation trials except in the first block. The first block consisted of 30 trials with no double step, to measure saccade gain to fixed targets, followed by 30 adaptation trials. The same paradigm was used in all OPT and healthy subjects. In control subjects, there were only four blocks for the gain-decreasing adaptation paradigm. These data were still representative of typical gain-decreasing saccade adaptation because all control subjects reached an asymptote in their learning before the fourth block, and therefore were not expected to show any changes with additional training.
Each adaptation trial began with a random fixation time ranging from 1-2 seconds and then the target (T1) jumped 10° to an eccentric position. The onset of the primary saccade in response to T1 was detected when the eye exited a 3° window centered on the fixation target. At this time, T1 was extinguished and another target (T2) appeared a fixed distance away from T1 along the horizontal midline (a double-step trial). In the gain-decreasing paradigm, the target was shifted backward (towards the initial fixation point) by 25% (2.5°), bringing the destination 7.5° away from the location of the initial fixation target (Fig. 1B). In the gain-increasing paradigm, the target was shifted farther by 25% (2.5°), moving the destination 12.5° away from the initial fixation target (Fig. 1C). The double-step trials were repeated to induce learning.
Each adaptation block was separated by a rest period of about 30 seconds. During the rest period, subjects remained in the dark with their eyes closed.
Analysis
Eye positions were analyzed offline with interactive software programmed in MATLAB™ (The MathWorks, Natick, MA). The software was designed to select the start and end of the primary saccade using a 15°/s velocity threshold. Saccades associated with blinks were discarded from the analysis. Corrective saccades were not analyzed. Kinematics (amplitude, peak velocity, peak acceleration, and peak deceleration) and timing parameters (latency, duration, time-to-peak velocity, time-to-peak acceleration, and time-to-peak deceleration) were measured. The gain for each trial was calculated as the size of the primary saccade divided by the size of the initial target step. Each block was divided in first 20 trials and last 20 trials. We compared parameters of first and last 20 trials in each block for measuring rapid timescale of adaptation. We compared last 20 trials of the preceding block and first 20 trials of the following block for comparison of retention. For such comparisons we did paired t-test.
Results
Ten healthy subjects (age range: 25 - 65) and three subjects with OPT (age range: 38 - 62) participated in the study. As expected all OPT subjects had irregular disconjugate 2Hz pendular oscillations of the eyes[29]. Table 1 outlines the clinical summary and MRI findings. All subjects had the characteristic radiological sign of inferior olive hypertrophy on MRI. In addition one subject had prominent perivascular spaces around the aqueduct but these were thought to be a normal variant; one subject had mild encephalomalacia in the midbrain tegmentum, and one subject had a suboccipital craniectomy with a small post-operative lesion to the left of the posterior cerebellar vermis. The lesion was not in regions of the cerebellar cortex known to affect eye movements.
Saccade adaptation in healthy subjects
Healthy subjects appropriately modified saccade amplitudes in response to the intra-saccadic target steps during saccade adaptation paradigms. Figure 2A,B illustrate the learning response of a normal subject in the gain-increasing and gain-decreasing adaptation paradigms. The ratio of actual and desired saccade amplitude (saccade gain) increased by the end of gain-increasing saccade adaptation when compared to the beginning of the paradigm (Figure 2A). Saccade amplitudes became larger (Figure 2C, pre-adaptation amplitude 9.1° ± 0.7°; post-adaptation amplitude: 10.0° ± 0.7°, red trace; t-test: p < 0.01). Increase in saccade amplitude resulted in a gain change from 0.9 ± 0.07 pre-adaptation to 1.0 ± 0.07 post-adaptation. This change was achieved by a tendency to increase saccade duration without changing peak velocity (Figure 2E, pre-adaptation saccade duration 39.5 ± 1 ms; post-adaptation saccade duration 44.5 ± 5.1 ms).
Figure 2.
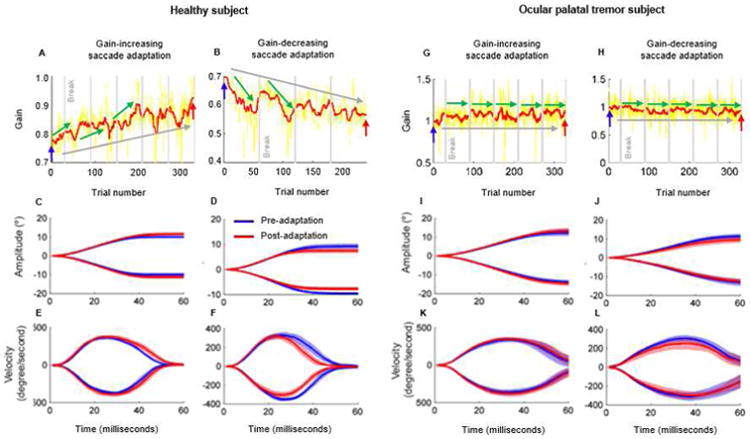
Example of saccade adaptation in one healthy subject during gain-increasing (A) and gain decreasing (B) saccade adaptation paradigm. In panels A,B gain of saccades (actual eye movement/desired eye movement) is plotted against the trial number. Yellow trace depicts actual value of gain, while red trace is moving average. Green arrows depict saccade adaptation over a short timescale; grey arrow depicts adaptation over a longer timescale. Grey vertical lines depict break times. Panels G, H depict same phenomenon in the subject with ocular palatal tremor. Panels C-F depict eye position and eye velocity in healthy subjects. Panels I-L depict eye position and velocity in the subject with ocular palatal tremor. The blue lines depict the mean values of parameters before adaptation; the red lines depict mean values after adaptation. Lighter shades of blue and red around the lines represent the standard deviation. Positive values of position and velocity are for rightward saccades, negative values are for leftward saccades.
Saccade gain decreased after the gain-decreasing paradigm (Figure 2B). Saccade amplitudes were significantly reduced (Figure 2D, pre-adaptation amplitude 8.8° ± 0.3°; post-adaptation amplitude 7.6° ± 0.3°; t-test, p < 0.01). The gain reduced from 0.9 ± 0.03 pre-adaptation to 0.7 ± 0.03 post-adaptation. The gain reduction was achieved by decreasing the peak velocity (Figure 2F, pre-adaptation velocity 350.4°/s ± 30.7 °/s; post-adaptation velocity 337.5 °/s ± 33.3 °/s; p < 0.01) and keeping the same duration of the adapted saccades (pre-adaptation duration 37.9 ms ± 2.1 ms; post-adaptation duration 37.5 ± 3.3 ms; t-test, p = 0.7). These results are consistent with previous observations [30-34].
Impaired motor learning in disorder of inferior olive
We suggest that spontaneous, non-error-driven output from the inferior olive will also interfere with saccade adaptation by disrupting or masking appropriate error signals destined for the cerebellum. It is also possible that a “sick” inferior olive is not able to generate an accurate error-signal to drive adaptation. We examine these proposals in a unique “disease model”, the syndrome of oculopalatal tremor (OPT), which features a pathologically synchronized, excessive, spontaneous discharge from the inferior olive. Such abnormal activity is the delayed consequence of a lesion in the brainstem or the cerebellar outflow tracts that cause a breach in the Guillain-Mollaret triangle (fibers from the dentate nuclei passing through the red nucleus and then in the central tegmental tract to the contralateral inferior olive) [25, 35, 36].
Figure 2G,H illustrate that gain-increasing and gain-decreasing saccade adaptation was impaired in a representative OPT subject. Saccade amplitude, gain, peak velocity, and duration before the gain-increasing adaptation are 10.1 ± 3.2°, 1.0 ± 0.03, 287.0°/s ± 95.1°/s, and 58.0 ± 5.8 ms, respectively (blue traces in Figure 2 I,K). These values did not significantly change after the gain-increasing paradigm (11.2° ± 3.7° (t-test, p=0.4), 1.1 ± 0.04, 287.0 °/s ± 95.1 °/s (t-test, p=0.1) and 286.9 ms ± 111.9 ms (t-test, p=0.9) (red traces in Figure 2I,K). Saccade amplitude, peak velocity, and duration also are unchanged after the gain-decreasing adaptation paradigm (Figure 2 J,L; pre-adaptation: amplitude 11.5 ° ± 1.6°, gain 1.2 ± 0.02, velocity 236.5 °/s ± 74.4°/s, duration 126.9 ± 115.7 ms; post-adaptation: amplitude 11.5 ° ± 1.6°, velocity 219.6°/s ± 64.3°/s, and duration 136.6 ms ± 112.4 ms; p-values for t-test while comparing amplitude: 0.1; gain: 0.1; velocity: 0.1; and duration: 0.3). These results were consistent across all three OPT subjects. Box and whisker plots in Figure 3 summarize data from gain-increasing and gain-decreasing adaptation for rightward and leftward saccades from all OPT subjects, except subject # 3 for whom only leftward saccades were analyzed because his rightward saccades were pathologically slowed. Subject#3 elected to participate in only one experiment session, hence only the gain-increasing paradigm could be performed.
Figure 3.
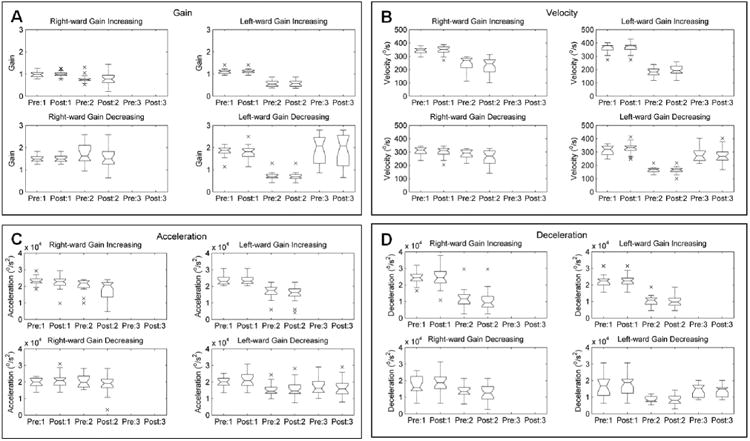
Summary of saccade adaptation over a slow timescale in the three OPT subjects. Comparisons of tested parameters are sorted in individual subplots (A: gain; B: velocity; C: acceleration; D: deceleration). Each panel in a given subplot depicts experiment condition as labeled. Each box and whisker plot in a given subplot depicts a summary of all saccade trials in one subject during one session. The horizontal line in the center of each box and whisker plot depicts the median value, the notch-length is the 95% confidence interval, the length of the box depicts the interquartile distance, and the distance between the whiskers includes the range of the data analyzed. The values of parameter tested on the y-axis were compared between the box and whisker plots labeled ‘Pre’ and ‘Post’. The plots labeled ‘Pre’ depict corresponding values prior to saccade adaptation, while the plots labeled ‘Post’ depict the values after saccade adaptation. Numbers succeeding ‘Pre’ or ‘Post’ represent individual subject, i.e. Pre:1 means before saccade adaptation in Subject #1. The overlapping notches between the two plots suggest that the difference between the corresponding medians is not significant with 95% confidence (p>0.05). Each panel depicts one parameter in four conditions - gain-increasing and gain-decreasing adaptation for rightward and leftward saccades. Saccade velocity, saccade amplitude (gain), peak-acceleration, peak-deceleration, were compared. None of these parameters were affected by saccade adaptation. We could only record gain-decreasing saccade adaption paradigm in Subject # 3. Furthermore, subject # 3 had slowed rightward saccades; hence only leftwards saccades were analyzed.
Our results show a lack of motor learning in OPT, which we attribute to abnormal function of the inferior olive. However, there are potential confounds. OPT subjects have more baseline variability in saccade amplitudes than do healthy subjects (amplitude standard deviation OPT = 3.18°; healthy subjects = 0.72°). This could be due to their underlying lesion or to the oscillations per se masking small amounts of learning. It is also possible that pendular oscillations of the eyes and consequent retinal slip could induce a fluctuating error signal that might also interfere with the ability of the cerebellum to adapt its saccades. Against this explanation, two of our OPT subjects had predominantly torsional nystagmus with a relatively small horizontal component, which would not interfere with accurate detection of horizontal post saccadic errors. Impairment of saccade adaptation in these two subjects was comparable to the other. It is also possible that baseline dysmetria masked adaptation that had occurred in subjects with OPT. Therefore we first classified saccades in each direction as hypermetric or hypometric at their baseline and then looked at the effects of the adaptation paradigm. Regardless of the particular adaptation paradigm in which they were tested, we still found that saccade adaptation was impaired (Table 2).
Table 2.
Summary of adaptation response after sorting saccades through hypermetric (gain-down) and hypometric (gain-up) adaptation.
| Parameter | Preadaptation | Post-adaptation | p | Pre-adaptation | Post-adaptation | p |
|---|---|---|---|---|---|---|
| Hypermetric (gain-down) | Hypometric (gain-up) | |||||
| Slow timescale of learning | ||||||
| Amplitude | 13.1 ± 0.8 | 11.9 ± 1.9 | 0.2 | 8.8 ± 3.3 | 9.5 ± 3.2 | 0.2 |
| Peak Velocity | 282.7 ± 83.6 | 268.8 ± 91.8 | 0.3 | 244.9 ± 90.3 | 245.5 ± 93.4 | 0.9 |
| Duration | 113.1 ± 98.7 | 121.3 ± 98.5 | 0.3 | 53.0 ± 8.4 | 63.7 ± 9.4 | 0.2 |
| Fast timescale of learning | ||||||
| Amplitude | 12.6 ± 1 | 11.6 ± 0.8 | <0.01 | 7.9 ± 2.7 | 8.6 ± 2.6 | 0.01 |
| Peak Velocity | 277.3 ± 79.7 | 266.6 ± 78.6 | <0.01 | 220.6 ± 71.6 | 233.5 ± 70.7 | 0.05 |
| Duration | 114.3 ± 84.1 | 112.5 ± 85.0 | 0.5 | 54.6 ± 8.2 | 56.7 ± 8.8 | 0.5 |
| Effects of breaks on retention | ||||||
| Amplitude | 8.71 ± 2.91 | 8.21 ± 3.03 | 0.16 | 11.67 ± 1.25 | 12.62 ± 1.10 | <0.0 1 |
| Peak Velocity | 239.82 ± 75.29 | 230.98 ± 84.72 | 0.33 | 265.75 ± 79.18 | 275.54 ± 81.31 | <0.0 1 |
| Duration | 54.4 ± 7.2 | 55.2 ± 7.5 | 0.85 | 113.0 ± 86.3 | 115.9 ± 83.6 | 0.33 |
| Time to Peak Deceleration | 51.4 ± 3.7 | 47.1 ± 4.5 | <0.01 | 75.3 ± 35.3 | 80.2 ± 41.3 | 0.30 |
Timescales of motor learning in OPT
Motor learning evolves on multiple timescales [16-19, 32, 37]. The fast timescale process rapidly learns from errors (green arrows in Figure 2A,B depict fast time scale of learning), but also rapidly forgets what it learned during breaks between trials (grey lines), hence, there is rapid unlearning [18, 32, 37-39]. At the same time, there is a slower timescale process that learns gradually in response to persistent systematic errors, but is relatively resistant to forgetting (grey arrow in Figure 2A,B). Together, these two processes enable the motor system to respond robustly when challenged by a consistent movement perturbation. Hypothetically, conditions that impair motor learning can affect the fast or the slow, or both learning processes. Therefore we examined whether the subjects with OPT could exhibit motor learning at any timescale even though they did not show any overall adaptation. Fig. 2G-L indicate the absence of learning on slow timescales in OPT subjects. The results suggest two possibilities. OPT subjects have a small amount of fast-timescale learning, but still no ultimate change in gain due to lack of retention. Alternate explanation is that OPT subjects cannot learn even on a fast timescale.
We distinguished among these possibilities by examining the fast timescale of learning within single adaptation blocks and measured the retention of learned responses across set breaks. Across each adaptation session there was no systematic change in saccade gain as qualitatively depicted for one OPT subject in Fig. 2G,H. This was true for rightward or leftward directed saccades during gain-increasing and gain-decreasing paradigms in OPT subjects. Saccades within blocks in each direction (rightward or leftward) were then classified as hypometric or hypermetric according to the average post-saccadic retinal error immediately following the primary saccade. In this manner we account for the target double-step, which occurs during the primary saccade, but ignore any corrective movements that may be made once the post-saccadic error can be observed. For example, during the gain-increase adaptation paradigm, saccade dysmetria was calculated relative to a 12.5° target step, and each block was classified according to the direction of this dysmetria (hypometric or hypermetric). We would expect hypometric saccades to exhibit gain increase and hypermetric saccades to exhibit gain decrease, regardless of the direction of the intra-saccadic target step.
We measured the amount of learning that occurred within individual blocks, and compared these changes across all blocks for all OPT subjects. For both hypometric and hypermetric saccades, small but significant adaptation was observed along with the expected changes in kinematics and timing parameters (Figure 4 and table 2). When patients produced hypermetric saccades, a small but statistically significant amount of learning occurred over the fast timescale: saccade amplitudes significantly decreased within a single adaptation block (paired t-test, p < 0.01), resulting from a decline in peak velocity (paired t-test, p < 0.01) with no compensatory change in the duration of saccades (paired t-test, p > 0.20). However, such learned responses were not retained across set breaks between successive blocks, as saccade gain increased following the rest break (Table 2; paired t-test, p<0.001). Note, these gain changes might also be the consequence of short-term fatigue [19, 21]. However, when saccades were hypometric, they exhibited a consistent gain increase within a single block (paired t-test, p < 0.01), resulting from an increase in peak velocity (paired t-test, p = 0.05) and a significant delay of the time of peak deceleration (paired t-test, p < 0.01). It appears therefore that fast timescale of learning was only marginally preserved in OPT subjects.
Figure 4.
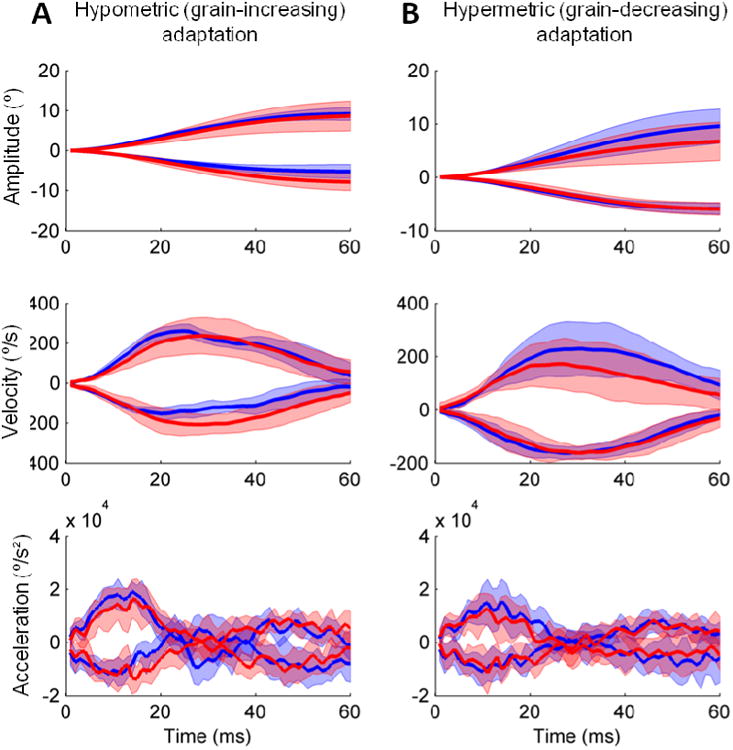
Example of the fast timescale of saccade adaptation in one OPT subject. Average amplitude (eye position), velocity, and acceleration across 20 saccades (10 in each direction) are compared at the beginning and end of a single adaptation block. The blue lines depict mean values at the beginning of the block; red lines depict values at the end of the block. Lighter shades of blue and red around the lines represent standard deviations. Positive values of position and velocity depict rightward saccades (which are also hypermetric in this example), while the negative values are for leftward saccades (hypometric).
Discussion
Motor learning relies on error signals to restore movement accuracy. The inferior olive provides error signals to the Purkinje cells via CF. An attractive hypothesis is that a change in the activity of Purkinje neurons promotes learning when these error signals are combined with the internal copy of the performed movement transmitted via mossy and parallel fibers (PF) [5, 6, 12, 13, 15, 40]. Previous studies using saccade adaptation paradigms have shown that subjects with structural or functional abnormalities in the cerebellum adapt poorly [19-23, 41]. Lesions of the medial-posterior cerebellum impair adaptation of both stimulus-driven (as in our study here) and self-triggered saccades; whereas selective deficits of self-triggered saccade adaptation are seen with lesions of the supero-anterior vermis [42]. Impaired adaptation of saccade gain and dysmetria is also a feature of structural lesions disrupting cerebellar inflow, especially the inferior olive to cerebellar input [43]. Experimental studies have suggested that motor learning begins in the cerebellar cortex, and that the deep cerebellar nuclei consolidate the learned responses [44]. Degenerative or focal lesions of the cerebellar cortex are associated with disinhibition and consequent hyperactivity of the deep cerebellar nuclei. Hence impaired adaptation from structural lesions in the cerebellar cortex could be due to the combination of the lack of function of Purkinje neurons and excessive activity in the deep nuclei.
We demonstrate a novel mechanism for learning impairment in the subjects with OPT where the inferior olive sends spontaneous signals to the cerebellar cortex masking the presence of meaningful error information. All our OPT subjects had impaired motor learning in fast and slow time-scales during both gain-increasing and gain-decreasing saccade adaptation paradigms. Such pattern contrasts with impaired learning in cerebellar disease where long time scales are preserved [19, 21, 24, 39]. These distinctions in adaptation patterns may be due to fundamental differences between the mechanisms that lead to impaired motor learning. In subjects with lesions in the cerebellar cortex, presumably adequate error signals from the inferior olive are not acted upon to produce learning. In contrast, in subjects with OPT the continuous spontaneous discharge interferes with the ability of the inferior olive to pass meaningful error signals to the cerebellum. One may also speculate that the “signal-to-noise ratio” for errors would be so low that the cerebellum could not support long-term motor learning. Instead, when such “noisy” signals were consistently repeated, as in a saccade adaptation paradigm, minimal learning could occur but would be rapidly extinguished during short breaks between adaptation blocks. This hypothesis is consistent with our results that OPT subjects have a remnant of intact learning on fast timescales, but no long-term learning after an entire adaptation session. In some instances there is unilateral inferior olive hypertrophy. We speculate, in such instances, there is still low signal-to-noise ratio for the error signal to evoke motor learning at the cerebellum. We further speculate that repetitive “noisy” output form unilaterally impaired inferior olive might still evoke cerebellar maladaptation causing coarse eye oscillations in OPT.
We recognize that the dysmetria and variability of saccade amplitudes, inherent to the pathology of OPT, could affect the interpretation of saccade adaptation paradigms. Such movement deficits could mask or exaggerate the effects of saccade adaptation. For example, an OPT subject with hypermetric saccades at baseline might have little gain-increase adaptation if their hypermetric primary saccades align with the location of the shifted target. Thus, it was important to exaggerate errors in both directions and provide distinct learning signals in at least one condition. Furthermore, by examining hypometric or hypermetric saccades as classified with respect to the final post-saccadic retinal error (that is, following the adaptive target double-step), we could examine whether subjects responded to such errors appropriately regardless of how the target was displaced. This approach also allowed us to take in to account the effects of the spontaneous oscillations of the eyes on the ability to adapt.
It is also possible that pendular oscillations of the eyes and consequent retinal slip could induce a fluctuating error signal that might also interfere with the ability of the cerebellum to learn. Against this idea, two of our subjects (subject 1, and 2) had a slow and predominantly torsional nystagmus with a relatively small horizontal component which would not interfere with detection of horizontal post saccadic errors and saccade dysmetria. Nevertheless saccade adaptation was still impaired in both of these subjects, and the nature of impairment was comparable in all subjects. Our results were also comparable with other studies of motor adaptation in subjects with inferior olive lesions in which eye-blink conditioning or arm pointing, for example, were used. Classical eye-blink conditioning was impaired in subjects with OPT[45-47]. Ours is the first study examining saccade adaptation and time dependent patterns of impaired motor learning in a disorder of inferior olive. An important message from this study is that impaired motor learning or dysmetria suggests not only cerebellar disorders but also disorders of the inferior olive and its connections to the cerebellum.
Acknowledgments
Authors are thankful to Dr. John Leigh for critical comments, and suggestions. This research was selected for the American Academy of Neurology, 2015 Alliance Founders Award. Grants from Gustavus Louis Pfeiffer Foundation (DSZ and AGS), Dystonia Medical Research Foundation (AGS), and NIH EY001849 (DSZ) supported this work. Dr.Optican received NIH/NEI intramural support. The authors have no conflict of interest.
Reference List
- 1.Robinson DA. Editorial: How the oculomotor system repairs itself. Invest Ophthalmol. 1975;14:413–5. [PubMed] [Google Scholar]
- 2.Kommerell G, Olivier D, Theopold H. Adaptive programming of phasic and tonic components in saccadic eye movements. Investigations of patients with abducens palsy. Invest Ophthalmol. 1976;15:657–60. [PubMed] [Google Scholar]
- 3.Leznik E, Makarenko V, Llinas R. Electrotonically mediated oscillatory patterns in neuronal ensembles: an in vitro voltage-dependent dye-imaging study in the inferior olive. J Neurosci. 2002;22:2804–15. doi: 10.1523/JNEUROSCI.22-07-02804.2002. doi:2002626222/7/2804[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci U S A. 2002;99:449–54. doi: 10.1073/pnas.012604899012604899[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–70. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M. Cerebellar control of the vestibulo-ocular reflex--around the flocculus hypothesis. Annu Rev Neurosci. 1982;5:275–96. doi: 10.1146/annurev.ne.05.030182.001423. [DOI] [PubMed] [Google Scholar]
- 7.Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Person AL, Raman IM. Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron. 2010;66:550–9. doi: 10.1016/j.neuron.2010.04.024. doi:S0896-6273(10)00295-3[pii]10.1016/j.neuron.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina JF. A recipe for bidirectional motor learning: using inhibition to cook plasticity in the vestibular nuclei. Neuron. 2010;68:607–9. doi: 10.1016/j.neuron.2010.11.011. doi:S0896-6273(10)00927-X[pii]10.1016/j.neuron.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElvain LE, Bagnall MW, Sakatos A, du Lac S. Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron. 2010;68:763–75. doi: 10.1016/j.neuron.2010.09.025. doi:S0896-6273(10)00767-1[pii]10.1016/j.neuron.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies JR, Porrill J, Dutia M, Dean P. Synaptic plasticity in medial vestibular nucleus neurons: comparison with computational requirements of VOR adaptation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 13.Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–27. doi: 10.1523/JNEUROSCI.4953-09.2010. doi:30/10/3715[pii]10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima Y, Soetedjo R, Fuchs AF. Effect of inactivation and disinhibition of the oculomotor vermis on saccade adaptation. Brain Res. 2011;1401:30–9. doi: 10.1016/j.brainres.2011.05.027. doi:S0006-8993(11)00912-7[pii]10.1016/j.brainres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soetedjo R, Fuchs AF. Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci. 2006;26:7741–55. doi: 10.1523/JNEUROSCI.4658-05.2006. doi:26/29/7741[pii]10.1523/JNEUROSCI.4658-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci. 2007;10:779–86. doi: 10.1038/nn1901. doi:nn1901[pii]10.1038/nn1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey MR. Synaptic mechanisms of sensorimotor learning in the cerebellum. Curr Opin Neurobiol. 2011;21:609–15. doi: 10.1016/j.conb.2011.06.011. doi:S0959-4388(11)00120-6[pii]10.1016/j.conb.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. doi:05-PLBI-RA-0791R2[pii]10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–9. doi: 10.1523/JNEUROSCI.3115-09.2009. doi:29/41/12930[pii]10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19:10931–9. doi: 10.1523/JNEUROSCI.19-24-10931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–44. doi: 10.1111/j.1460-9568.2007.05996.x. doi:EJN5996[pii]10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- 22.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83:2047–62. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- 23.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–31. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- 24.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–84. doi: 10.1152/jn.00822.2009. doi:00822.2009[pii]10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillain GMP. Deux cas de myoclonies synchrones et rhythmées vélo-pharyngo-laryngo-oculo-diaphragmatiques. Le problèm anatomique et physio-pathologique de ce syndrome. 1931:545–66. [Google Scholar]
- 26.Robinson DA. A Method of Measuring Eye Movement Using a Scleral Search Coil in a Magnetic Field. IEEE Trans Biomed Eng. 1963;10:137–45. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- 27.Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Brain Res Cogn Brain Res. 2002;13:41–52. doi: 10.1016/s0926-6410(01)00088-x. doi:S092664100100088X[pii] [DOI] [PubMed] [Google Scholar]
- 28.Ethier V, Zee DS, Shadmehr R. Spontaneous recovery of motor memory during saccade adaptation. J Neurophysiol. 2008;99:2577–83. doi: 10.1152/jn.00015.2008. doi:00015.2008[pii]10.1152/jn.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaikh AG, Hong S, Liao K, Tian J, Solomon D, Zee DS, Leigh RJ, Optican LM. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain. 2010;133:923–40. doi: 10.1093/brain/awp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straube A, Deubel H. Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vision Res. 1995;35:3451–8. doi: 10.1016/0042-6989(95)00076-q. doi:0042-6989(95)00076-Q[pii] [DOI] [PubMed] [Google Scholar]
- 31.Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci U S A. 2008;105:7309–14. doi: 10.1073/pnas.0706032105. doi:0706032105[pii]10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci. 2008;28:13929–37. doi: 10.1523/JNEUROSCI.3470-08.2008. doi:28/51/13929[pii]10.1523/JNEUROSCI.3470-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnier F, Lappe M. Differences in intersaccadic adaptation transfer between inward and outward adaptation. J Neurophysiol. 2011;106:1399–410. doi: 10.1152/jn.00236.2011. doi:jn.00236.2011[pii]10.1152/jn.00236.2011. [DOI] [PubMed] [Google Scholar]
- 34.Abrams RA, Dobkin RS, Helfrich MK. Adaptive modification of saccadic eye movements. J Exp Psychol Hum Percept Perform. 1992;18:922–33. doi: 10.1037//0096-1523.18.4.922. [DOI] [PubMed] [Google Scholar]
- 35.Lapresle J, Hamida MB. The dentato-olivary pathway. Somatotopic relationship between the dentate nucleus and the contralateral inferior olive. Arch Neurol. 1970;22:135–43. doi: 10.1001/archneur.1970.00480200041004. [DOI] [PubMed] [Google Scholar]
- 36.Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105:223–69. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- 37.Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci. 2008;28:2804–13. doi: 10.1523/JNEUROSCI.5300-07.2008. doi:28/11/2804[pii]10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian J, Ethier V, Shadmehr R, Fujita M, Zee DS. Some perspectives on saccade adaptation. Ann N Y Acad Sci. 2009;1164:166–72. doi: 10.1111/j.1749-6632.2009.03853.x. doi:NYAS03853[pii]10.1111/j.1749-6632.2009.03853.x. [DOI] [PubMed] [Google Scholar]
- 39.Criscimagna-Hemminger SE, Shadmehr R. Consolidation patterns of human motor memory. J Neurosci. 2008;28:9610–8. doi: 10.1523/JNEUROSCI.3071-08.2008. doi:28/39/9610[pii]10.1523/JNEUROSCI.3071-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–66. doi: 10.1152/jn.90526.2008. doi:90526.2008[pii]10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panouilleres MT, Miall RC, Jenkinson N. The role of the posterior cerebellum in saccadic adaptation: a transcranial direct current stimulation study. J Neurosci. 2015;35:5471–9. doi: 10.1523/JNEUROSCI.4064-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi KD, Kim HJ, Cho BM, Kim JS. Saccadic adaptation in lateral medullary and cerebellar infarction. Exp Brain Res. 2008;188:475–82. doi: 10.1007/s00221-008-1375-z. [DOI] [PubMed] [Google Scholar]
- 43.Waespe W, Baumgartner R. Enduring dysmetria and impaired gain adaptivity of saccadic eye movements in Wallenberg's lateral medullary syndrome. Brain. 1992;115(Pt 4):1123–46. [PubMed] [Google Scholar]
- 44.Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–77. doi: 10.1016/j.neuroscience.2005.12.035. doi:S0306-4522(05)01487-9[pii]10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 45.Deuschl G, Toro C, Valls-Sole J, Hallett M. Symptomatic and essential palatal tremor. 3. Abnormal motor learning. J Neurol Neurosurg Psychiatry. 1996;60:520–5. doi: 10.1136/jnnp.60.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain : a journal of neurology. 1996;119(Pt 4):1183–98. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- 47.Gauthier GM, Hofferer JM, Hoyt WF, Stark L. Visual-motor adaptation. Quantitative demonstration in patients with posterior fossa involvement. Arch Neurol. 1979;36:155–60. doi: 10.1001/archneur.1979.00500390073008. [DOI] [PubMed] [Google Scholar]


