Abstract
Renal cell carcinoma (RCC) is a common malignancy following kidney transplantation. We describe RCC risk and examine RCC risk factors among US kidney recipients (1987-2010). The Transplant Cancer Match Study links the US transplant registry with 15 cancer registries. Standardized incidence ratios (SIRs) were used to compare RCC risk (overall and for clear cell [ccRCC] and papillary subtypes) to the general population. Associations with risk factors were assessed using Cox models. We identified 683 RCCs among 116,208 kidney recipients. RCC risk was substantially elevated compared with the general population (SIR 5.68, 95%CI 5.27-6.13), especially for papillary RCC (SIR 13.3 vs. 3.98 for ccRCC). Among kidney recipients, RCC risk was significantly elevated for blacks compared to whites (hazard ratio [HR] 1.50) and lower in females than males (HR 0.56). RCC risk increased with prolonged dialysis preceding transplantation (p-trend<0.0001). Risk was variably associated for RCC subtypes with some medical conditions that were indications for transplantation: ccRCC risk was reduced with polycystic kidney disease (HR 0.54), and papillary RCC was increased with hypertensive nephrosclerosis (HR 2.02) and vascular diseases (HR 1.86). In conclusion, kidney recipients experience substantially elevated risk of RCC, especially for papillary RCC, and multiple factors contribute to these cancers.
Introduction
Approximately 17,000 kidney transplants were performed in the US in 2014, representing an increase of over 200% since 1988 (1,2). While kidney transplantation greatly improves survival and quality of life for patients with end-stage renal disease (ESRD), increased cancer risk is a major concern (3,4). Transplant recipients have an increased incidence of cancer compared to the general population (3-6). Excess risk is related to the need for immunosuppressive therapy to prevent rejection of the transplanted organ, and many of the subsequent cancers are caused by oncogenic viruses. Kidney recipients also have an approximate 6-fold increased incidence of renal cell carcinoma (RCC) (3,4,6), the most common urologic malignancy following kidney transplantation (7).
RCC comprises several major histologic subtypes including clear cell RCC (ccRCC, approximately 70% of cases in the general population), papillary RCC (10-15%), and chromophobe RCC (5%) (8,9). Each RCC subtype has distinct clinical and genetic characteristics, with ccRCC demonstrating a worse prognosis than other subtypes (8,9). Given the differences in presentation, RCC subtypes may also possess distinct etiologies.
Overall, established RCC risk factors in the general population include male sex, older age, African descent, excess body weight, cigarette smoking, and hypertension (10). ESRD patients treated with dialysis also have an elevated risk of RCC (11-13), which is at least in part related to the development of acquired cystic kidney disease (ACKD, a condition associated with kidney dysfunction that is characterized by the progressive development of multiple fluid-filled renal cysts) (14-16). At the time of kidney transplantation, the diseased native kidneys are usually left in situ, and most RCCs in kidney transplant recipients develop in the native kidneys rather than in the donor kidney (17).
To date, risk factors for RCC in kidney recipients have not been systematically assessed in a large population-based study. Some contributing factors may include the cause of underlying ESRD, other recipient characteristics, and specific immunosuppressive medications. Characteristics of kidney donors, such as donor type (deceased vs. living donor) or human leukocyte antigen (HLA) mismatch have rarely been assessed. Since kidney recipients are typically under heightened medical surveillance, the elevated RCC risk may also be partly due to detection bias, e.g., through screening or the use of computed tomography or ultrasound in medical evaluations. Surveillance would be expected to lead to detection of asymptomatic RCCs, so there would be an excess risk for local stage cancers and an absence of increased risk for cancers that are regionally advanced or metastatic (18,19).
Given the substantial burden of RCC in kidney transplant recipients, a better understanding of the risk factors for this cancer is needed to uncover carcinogenic mechanisms and identify ways to reduce morbidity and mortality. Previously we examined how kidney dysfunction, among a large population of US patients with ESRD, was associated with increased risk of kidney cancer (13). In the present study, we focus on examining the relationship of established and suspected RCC risk factors among kidney transplant recipients with a functioning graft. We further investigate whether associations between these examined risk factors and RCC differ by histologic subtype.
Methods and Materials
Study Population, Outcomes, and Risk factors
The Transplant Cancer Match (TCM) Study has been described in detail previously (www.transplantmatch.cancer.gov) (6). Briefly, the TCM Study links the Scientific Registry of Transplant Recipients (SRTR) with 15 population-based US cancer registries (located in California, Colorado, Connecticut, Florida, Georgia, Hawaii, Iowa, Illinois, Michigan, New Jersey, New York, North Carolina, Seattle (Washington), Texas, and Utah), which collectively provide ascertainment of cancers for 47% of the US transplant population (1987-2010). The SRTR contains information on all solid organ transplant recipients, including demographic characteristics, reason for transplantation, characteristics of organ donors, and initial immunosuppressive regimen.
Our study population consisted of kidney recipients in the TCM Study who received a transplant between 1987 and 2010. We restricted analysis to non-Hispanic whites, non-Hispanic blacks, Hispanics, and Asian/Pacific Islanders to allow comparison with general population cancer incidence. We further excluded subjects if they had received a previous transplant, received a multi-organ transplant, or had a previous kidney cancer diagnosis documented in the cancer registries.
Subjects were considered at risk for cancer beginning with transplantation or start of cancer registry coverage, whichever came last (95.9% of the cohort were followed from the time of transplantation, while the median time from transplantation to start of follow-up was 1.9 years for the 4.1% who entered late). Recipients were followed until development of RCC (as identified in cancer registries), death, failure of the transplanted kidney, subsequent transplant, loss to follow-up (i.e., no longer followed by transplant centers), or end of cancer registry coverage, whichever came first; all patients exited by December 31, 2010. We identified incident cases of RCC using International Classification of Diseases for Oncology Third Edition (ICD-O-3) topography code C64.9 and used with morphology codes to define subtypes: 8260 (papillary), 8310 (clear cell), 8312 (not otherwise specified), or 8316-18 (cyst-associated, chromophobe, sarcomatoid, and spindle cell). We performed analyses for RCC overall and separately for ccRCC and papillary RCC; examination of other RCC histologic subtypes was not feasible due to small numbers. For a subset of six cancer registries, we also searched clinical case abstracts and pathology reports for any mention of whether the RCC was in the donor or native kidney.
Recipient and donor characteristics were ascertained from the SRTR. For duration of dialysis prior to transplant (i.e., vintage), we ignored dialysis prior to 1972 (the year the US Congress approved Medicare reimbursement for dialysis) to truncate extreme values for vintage for a few recipients (20). We categorized the primary reason for kidney transplant into the following categories: glomerular diseases, diabetes mellitus, hypertensive nephrosclerosis, polycystic kidney disease, vascular diseases, tubular/interstitial diseases, congenital/familial/metabolic disorders, and other/unknown conditions.
Statistical analysis
We compared RCC risk in kidney recipients to the general population using standardized incidence ratios (SIRs). SIRs were calculated by dividing the number of observed cases by the number expected, based on general population rates specific to 5-year age group, sex, race/ethnicity, calendar year, and cancer registry. We estimated SIRs for RCC overall and for clear cell and papillary subtypes. We also assessed SIRs by tumor stage (local: tumor that is within the organ of origin; regional: tumor that extends beyond the limits of the organ of origin to surrounding tissues; distant: tumor that is metastatic; and unknown) and grade (low [grades I and II] and high [III and IV]). Based on the observed and expected counts, RCC cases in the transplant population were more likely to have a classified histologic subtype than cases in the general population. This difference could bias the SIRs for the specified subtypes upwards. We therefore adjusted for this difference by reducing the expected count for unspecified histologic subtypes and proportionately increasing the expected counts for the specified subtypes (see Table S1).
We used Cox proportional hazards models, with time since transplant as the time metric, to calculate hazard ratios (HRs) assessing risk factors for RCC overall, ccRCC, and papillary RCC. HRs relating primary reason for transplant to RCC incidence were evaluated using effect parameterization, which compares each category to the average of all categories combined (21). We assessed the proportional hazards assumption graphically and by adding interaction terms between each risk factor and time since transplant; no evidence of violations against proportionality was found. We selected a final model by simultaneously including all risk factors that were significant in univariate models, then removing variables that were no longer significant in the multivariate model. Final models varied for ccRCC and papillary RCC based on the variables that were significant.
We also graphically illustrate RCC risk as a function of time since transplant, using a flexible parametric procedure to model the hazard as a linear combination of cubic B-splines (22). This technique generates smaller mean square errors than non-parametric methods and provides smooth estimates without making strong parametric assumptions. Additionally, to describe the absolute risk of a transplant recipient developing RCC during follow-up while taking into consideration the competing risk of death, we depict cumulative incidence curves for RCC overall and for papillary and clear cell subtypes; only recipients followed from transplantation are included in this analysis. We conducted all analyses using SAS Version 9.2 (SAS Institute, Cary, NC, USA) and MATLAB. Statistical tests were determined significant at a two sided p-value<0.05 and were not adjusted for multiple comparisons.
Results
We assessed 116,208 kidney recipients followed for 595,352 person-years (median 4.2 years; range: 0.003-23.1) (Table 1). Nearly 60% were male, and the average age at transplant was 45 years. The majority of subjects were white (52%), and 30% had a normal body mass index (BMI 18.5-24.9 kg/m2; 17% had missing data on BMI). Prior dialysis was common (89%) with an average vintage of 2.7 years before transplant. The three most common indications for a kidney transplant were glomerular diseases (29%), diabetes mellitus (23%), and hypertension (18%). Approximately two-thirds of recipients (63%) received their kidney from a deceased donor.
Table 1.
Selected characteristics of kidney transplant recipients
| Characteristics | N | % 1 |
|---|---|---|
| Overall | 116,208 | 100.0 |
| Sex | ||
| Male | 69,377 | 59.7 |
| Female | 46,831 | 40.3 |
| Age at transplant, years | ||
| <30 | 19,696 | 17.0 |
| 30-<40 | 19,552 | 16.8 |
| 40-<50 | 25,881 | 22.3 |
| 50-<60 | 27,935 | 24.0 |
| 60+ | 23,144 | 19.9 |
| Calendar year of transplant | ||
| <1995 | 19,740 | 17.0 |
| 1995-<2000 | 26,360 | 22.7 |
| 2000-<2005 | 32,450 | 27.9 |
| 2005+ | 37,658 | 32.4 |
| Race | ||
| White | 60,064 | 51.7 |
| Black | 27,442 | 23.6 |
| Hispanic | 20,778 | 17.9 |
| Asian/Pacific Islander/Hawaiian | 7,924 | 6.8 |
| Body mass index (kg/m2)2 | ||
| 10-<18.5 (underweight) | 5,601 | 4.8 |
| 18.5-24.9 (normal) | 34,808 | 30.0 |
| 25-29.9 (overweight) | 31,580 | 27.2 |
| 30+ (obese) | 23,981 | 20.6 |
| Missing | 20,238 | 17.4 |
| Dialysis prior to transplant | ||
| No | 12,304 | 10.6 |
| Yes | 102,817 | 88.5 |
| Missing/Unknown | 1,087 | 0.9 |
| Duration of dialysis (vintage), years | ||
| 0 (no dialysis) | 12,304 | 10.6 |
| < 1 | 23,772 | 20.5 |
| 1-<2 | 23,476 | 20.2 |
| 2-<3 | 16,867 | 14.5 |
| 3+ | 38,702 | 33.3 |
| Missing/Unknown | 1,087 | 0.9 |
| Indication for transplant | ||
| Glomerular Diseases | 33,256 | 28.6 |
| Diabetes mellitus | 26,377 | 22.7 |
| Hypertensive Nephrosclerosis | 20,567 | 17.7 |
| Polycystic Kidney Disease | 10,680 | 9.2 |
| Tubular/Interstitial Diseases | 5,994 | 5.2 |
| Vascular Diseases | 4,513 | 3.9 |
| Congenital/Familial/Metabolic Disorders | 3,676 | 3.2 |
| Other/Unknown Conditions | 11,145 | 9.6 |
| Donor type | ||
| Living | 42,928 | 36.9 |
| Deceased | 73,280 | 63.1 |
Abbreviations: N- number; std- standard deviation
Frequencies may not sum to 100% due to rounding.
140 subjects with a body mass index <10kg/m2 or >100kg/m2 were assigned a missing value.
Patients exited the study for a variety of reasons including: RCC (N=683, 0.6%), death (N=18,022, 15.5%), graft failure (N=27,742, 23.9%), retransplantation (N=1,035, 0.9%), loss to follow-up by the SRTR (N=12,664, 10.9%), or end of cancer registry coverage (N=56,062, 48.2%). Differences in select patient characteristics by exit reason are displayed in Table S2. Patients who exited because of death were more likely to be older, white, have had dialysis prior to transplant, have diabetes mellitus as an indication for transplant, and receive a kidney from a deceased donor compared to patients who exited for other reasons. Patients who exited because of graft failure or retransplantation were more likely than other patients to be black, and they were also likely to have had prior dialysis and to have received a deceased donor kidney. Patients with glomerular disease were over-represented among patients who exited due to loss to follow-up by SRTR. Patients who exited due to the end of cancer registry coverage were transplanted in the most recent calendar years.
A total of 683 incident cases of RCC were identified among transplant recipients. By histology, clear cell, papillary, and chromophobe RCCs comprised 32%, 28%, and 2% of cases, respectfully, while 2% of cases had other specified histologic subtypes of RCC and 36% of cases had unspecified histologic subtypes. Overall, RCC risk was elevated 5.7-fold compared with the general population (SIR 5.68, 95% CI 5.27-6.13) (Table 2). Notably, the risk of papillary RCC was much more elevated than for ccRCC (SIR 13.3 vs. 3.98). Significantly elevated RCC risk was observed across all stage and grade categories, although higher SIRs were observed for local stage cancers (SIR 6.94) compared to regional stage (SIR 3.27) or distant stage cancers (SIR 2.97), and for low grade (SIR 6.69) versus high grade cancers (SIR 3.95). For each histologic subtype, the patterns according to stage and grade mirrored those for RCC overall.
Table 2.
Adjusted RCC SIR among kidney transplant recipients by subtype, stage, and grade
| RCC | Clear Cell RCC | Papillary RCC | Other specified subtype RCC | Unspecified subtype RCC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | SIR | 95%CI | Observed | SIR | 95%CI | Observed | SIR | 95%CI | Observed | SIR | 95%CI | Observed | SIR | 95%CI | |
| Overall | 683 | 5.68 | 5.27-6.13 | 219 | 3.98 | 3.47-4.55 | 191 | 13.3 | 11.5-15.3 | 29 | 3.67 | 2.46-5.27 | 244 | 5.68 | 4.98-6.43 |
| Stage | |||||||||||||||
| Local | 551 | 6.94 | 6.37-7.54 | 180 | 4.82 | 4.15-5.58 | 171 | 14.7 | 12.7-17.2 | 24 | 4.68 | 3.00-6.96 | 176 | 6.94 | 5.94-8.03 |
| Regional | 58 | 3.27 | 2.48-4.22 | 17 | 2.17 | 1.26-3.47 | 13 | 10.6 | 5.63-18.1 | 3 | 2.93 | 0.61-8.60 | 25 | 3.27 | 2.13-4.86 |
| Distant | 54 | 2.97 | 2.23-3.87 | 17 | 2.64 | 1.54-4.23 | 6 | 9.70 | 3.55-21.1 | 2 | 1.62 | 0.20-5.83 | 29 | 2.97 | 1.98-4.25 |
| Unknown | 20 | 4.15 | 2.54-6.41 | 5 | 4.61 | 1.50-10.8 | 1 | 2.77 | 0.10-15.5 | 0 | 0 | 0-20.5 | 14 | 4.15 | 2.25-6.91 |
| Grade | |||||||||||||||
| Low (I & II) | 342 | 6.68 | 6.00-7.43 | 147 | 5.29 | 4.47-6.22 | 96 | 12.6 | 10.2-15.4 | 14 | 4.48 | 2.45-7.50 | 85 | 6.68 | 5.35-8.28 |
| High (III & IV) | 145 | 3.95 | 3.33-4.65 | 40 | 2.20 | 1.57-2.99 | 50 | 13.8 | 10.3-18.2 | 5 | 2.17 | 0.70-5.05 | 50 | 3.95 | 2.92-5.19 |
| Unknown | 196 | 6.06 | 5.25-6.98 | 32 | 3.75 | 2.57-5.30 | 45 | 13.1 | 9.54-17.5 | 10 | 4.06 | 1.95-7.48 | 109 | 6.06 | 4.97-7.30 |
Abbreviations: CI- confidence interval; RCC- renal cell carcinoma; SIR- standardized incidence ratio
Among 96 RCC cases for which cancer registry data were available, 85 cases (89%) were in a native kidney, and only 11 (11%) were noted to arise in the donor kidney. The histologic subtypes of tumors in the donor kidney were: 55% (N=6) clear cell, 27% (N=3) papillary, 9% (N=1) chromophobe, and 9% (N=1) other/unspecified.
We assessed risk factors in univariate models to identify candidate variables to include in multivariate models (not shown). Table 3 shows risk factors that were significantly associated with at least one outcome (RCC, ccRCC, or papillary RCC) in multivariate models. Irrespective of histologic subtype, we observed reduced cancer risk among females compared to males (HRs 0.45-0.64). Risk increased with increasing age at transplant (RCC and ccRCC p-trends<0.0001, papillary RCC p-trend=0.01), and risk was higher following transplants in more recent calendar years (p-trends<0.0001). Compared to white recipients, Asians/Pacific Islanders had a 40% decreased risk while blacks had a 50% increase in risk for RCC; the increased risk among blacks was stronger for papillary RCC (HR 2.79).
Table 3.
RCC risk factors among kidney transplant recipients by subtype
| Risk Factors | RCC | Clear Cell RCC | Papillary RCC | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | % 1 | Controls | % 1 | HR2 | 95% CI2 | P-Trend2 | Cases | % 1 | Controls | % 1 | HR3 | 95% CI3 | P-Trend3 | Cases | % 1 | Controls | % 1 | HR4 | 95% CI4 | P-Trend4 | |
| Female sex 5 | 187 | 27.4 | 46,644 | 40.4 | 0.56 | 0.47-0.66 | 66 | 30.1 | 46,765 | 40.3 | 0.64 | 0.48-0.86 | 45 | 23.6 | 46,786 | 40.3 | 0.45 | 0.32-0.63 | |||
| Age at transplant, years 6 | |||||||||||||||||||||
| <30 | 26 | 3.8 | 19,670 | 17.0 | 1.00 | 7 | 3.2 | 19,689 | 17.0 | 1.00 | 12 | 6.3 | 19,684 | 17.0 | 1.00 | ||||||
| 30-<40 | 98 | 14.4 | 19,454 | 16.8 | 3.18 | 2.05-4.93 | 27 | 12.3 | 19,525 | 16.8 | 3.42 | 1.47-7.97 | 34 | 17.8 | 19,518 | 16.8 | 2.11 | 1.08-4.13 | |||
| 40-<50 | 180 | 26.4 | 25,701 | 22.3 | 4.66 | 3.05-7.11 | 50 | 22.8 | 25,831 | 22.3 | 5.13 | 2.28-11.54 | 58 | 30.4 | 25,823 | 22.3 | 2.81 | 1.47-5.35 | |||
| 50-<60 | 216 | 31.6 | 27,719 | 24.0 | 6.06 | 3.98-9.23 | 76 | 34.7 | 27,859 | 24.0 | 7.86 | 3.53-17.51 | 53 | 27.8 | 27,882 | 24.0 | 2.70 | 1.40-5.21 | |||
| 60+ | 163 | 23.9 | 22,981 | 19.9 | 6.59 | 4.29-10.15 | <0.0001 | 59 | 26.9 | 23,085 | 19.9 | 8.02 | 3.55-18.11 | <0.0001 | 34 | 17.8 | 23,110 | 19.9 | 2.64 | 1.32-5.27 | 0.01 |
| Calendar year of transplant 7 | |||||||||||||||||||||
| <1995 | 127 | 18.6 | 19,613 | 17.0 | 1.00 | 27 | 12.3 | 19,713 | 17.0 | 1.00 | 21 | 11.0 | 19,719 | 17.0 | 1.00 | ||||||
| 1995-<2000 | 205 | 30.0 | 26,155 | 22.6 | 1.37 | 1.08-1.74 | 57 | 26.0 | 26,303 | 22.7 | 1.96 | 1.18-3.25 | 62 | 32.5 | 26,298 | 22.7 | 3.42 | 1.95-6.02 | |||
| 2000-<2005 | 218 | 31.9 | 32,232 | 27.9 | 1.82 | 1.41-2.36 | 78 | 35.6 | 32,372 | 27.9 | 3.76 | 2.21-6.41 | 69 | 36.1 | 32,381 | 27.9 | 5.75 | 3.05-10.84 | |||
| 2005+ | 133 | 19.5 | 37,525 | 32.4 | 2.32 | 1.71-3.14 | <0.0001 | 57 | 26.0 | 37,601 | 32.4 | 6.12 | 3.36-11.14 | <0.0001 | 39 | 20.4 | 37,619 | 32.4 | 8.31 | 4.10-16.85 | <0.0001 |
| Race 8 | |||||||||||||||||||||
| White | 333 | 48.8 | 59,731 | 51.7 | 1.00 | 121 | 55.3 | 59,943 | 51.7 | 1.00 | 71 | 37.2 | 59,993 | 51.7 | 1.00 | ||||||
| Black | 221 | 32.4 | 27,221 | 23.6 | 1.50 | 1.24-1.80 | 39 | 17.8 | 27,403 | 23.6 | 0.71 | 0.48-1.04 | 98 | 51.3 | 27,344 | 23.6 | 2.79 | 1.98-3.92 | |||
| Hispanic | 103 | 15.1 | 20,675 | 17.9 | 0.99 | 0.79-1.24 | 48 | 21.9 | 20,730 | 17.9 | 1.24 | 0.88-1.75 | 18 | 9.4 | 20,760 | 17.9 | 0.71 | 0.42-1.21 | |||
| Asian/Pacific Islander/Hawaiian | 26 | 3.8 | 7,898 | 6.8 | 0.60 | 0.40-0.90 | 11 | 5.0 | 7,913 | 6.8 | 0.69 | 0.37-1.29 | 4 | 2.1 | 7,920 | 6.8 | 0.43 | 0.16-1.18 | |||
| Duration of dialysis (vintage), years 9 | |||||||||||||||||||||
| No | 40 | 5.9 | 12,264 | 10.6 | 1.00 | 19 | 8.7 | 12,285 | 10.6 | 1.00 | 10 | 5.2 | 12,294 | 10.6 | 1.00 | ||||||
| <1 | 114 | 16.7 | 23,658 | 20.5 | 1.26 | 0.87-1.81 | 33 | 15.1 | 23,739 | 20.5 | 0.88 | 0.50-1.56 | 33 | 17.3 | 23,739 | 20.5 | 1.40 | 0.69-2.86 | |||
| 1-<2 | 146 | 21.4 | 23,330 | 20.2 | 1.60 | 1.12-2.29 | 60 | 27.4 | 23,416 | 20.2 | 1.62 | 0.96-2.73 | 33 | 17.3 | 23,443 | 20.2 | 1.31 | 0.64-2.69 | |||
| 2-<3 | 79 | 11.6 | 16,788 | 14.5 | 1.23 | 0.84-1.81 | 24 | 11.0 | 16,843 | 14.5 | 0.90 | 0.49-1.66 | 29 | 15.2 | 16,838 | 14.5 | 1.51 | 0.73-3.15 | |||
| 3+ | 301 | 44.1 | 38,401 | 33.2 | 2.23 | 1.58-3.13 | 83 | 37.9 | 38,619 | 33.3 | 1.45 | 0.87-2.40 | 86 | 45.0 | 38,616 | 33.3 | 1.89 | 0.96-3.73 | |||
| Missing/Unknown | 3 | 0.4 | 1,084 | 0.9 | 0.51 | 0.16-1.64 | <0.0001 | 0 | 0.0 | 1,087 | 0.9 | --- | --- | 0.08 | 0 | 0.0 | 1,087 | 0.9 | --- | --- | 0.03 |
| Indication for transplant 10, 11 | |||||||||||||||||||||
| Glomerular Diseases | 202 | 29.6 | 33,054 | 28.6 | 1.24 | 1.05-1.47 | 65 | 29.7 | 33,191 | 28.6 | 1.21 | 0.90-1.61 | 52 | 27.2 | 33,204 | 28.6 | 1.29 | 0.91-1.82 | |||
| Diabetes mellitus | 106 | 15.5 | 26,271 | 22.7 | 0.77 | 0.62-0.94 | 48 | 21.9 | 26,329 | 22.7 | 0.98 | 0.71-1.36 | 24 | 12.6 | 26,353 | 22.7 | 0.77 | 0.49-1.19 | |||
| Hypertensive Nephrosclerosis | 192 | 28.1 | 20,375 | 17.6 | 1.55 | 1.29-1.86 | 50 | 22.8 | 20,517 | 17.7 | 1.26 | 0.92-1.73 | 69 | 36.1 | 20,498 | 17.7 | 2.02 | 1.43-2.86 | |||
| Polycystic Kidney Disease | 54 | 7.9 | 10,626 | 9.2 | 0.81 | 0.62-1.06 | 13 | 5.9 | 10,667 | 9.2 | 0.54 | 0.33-0.91 | 14 | 7.3 | 10,666 | 9.2 | 1.02 | 0.60-1.73 | |||
| Tubular/Interstitial Diseases | 20 | 2.9 | 5,974 | 5.2 | 0.68 | 0.46-1.02 | 7 | 3.2 | 5,987 | 5.2 | 0.71 | 0.36-1.39 | 4 | 2.1 | 5,990 | 5.2 | 0.59 | 0.24-1.44 | |||
| Vascular Diseases | 45 | 6.6 | 4,468 | 3.9 | 1.53 | 1.15-2.03 | 14 | 6.4 | 4,499 | 3.9 | 1.55 | 0.94-2.54 | 16 | 8.4 | 4,497 | 3.9 | 1.86 | 1.12-3.08 | |||
| Congenital/Familial/Metabolic Disorders | 9 | 1.3 | 3,667 | 3.2 | 0.81 | 0.45-1.46 | 4 | 1.8 | 3,672 | 3.2 | 1.11 | 0.46-2.69 | 2 | 1.1 | 3,674 | 3.2 | 0.57 | 0.16-1.99 | |||
| Other/Unknown Conditions | 55 | 8.1 | 11,090 | 9.6 | 0.99 | 0.76-1.28 | 18 | 8.2 | 11,127 | 9.6 | 1.00 | 0.64-1.57 | 10 | 5.2 | 11,135 | 9.6 | 0.77 | 0.43-1.40 | |||
| Induction with anti-IL2 antibodies12 | 137 | 20.1 | 26,207 | 22.7 | 0.96 | 0.78-1.17 | 43 | 19.6 | 26,301 | 22.7 | 0.82 | 0.57-1.18 | 55 | 28.8 | 26,289 | 22.7 | 1.51 | 1.07-2.13 | |||
| Induction with polyclonal antibodies13 | 159 | 23.3 | 30,314 | 26.2 | 0.99 | 0.82-1.19 | 66 | 30.1 | 30,407 | 26.2 | 1.36 | 1.01-1.84 | 42 | 22.0 | 30,431 | 26.2 | 0.93 | 0.64-1.34 | |||
| Female donor sex14 | 290 | 42.5 | 54,018 | 46.8 | 0.86 | 0.73-0.99 | 100 | 45.7 | 54,208 | 46.7 | 0.91 | 0.70-1.19 | 70 | 36.7 | 54,238 | 46.8 | 0.71 | 0.53-0.95 | |||
| Donor age, years 15, 16 | |||||||||||||||||||||
| <30 | 195 | 28.6 | 37,430 | 32.4 | 1.00 | 48 | 21.9 | 37,577 | 32.4 | 1.00 | 66 | 34.6 | 37,559 | 32.4 | 1.00 | ||||||
| 30-<40 | 141 | 20.6 | 23,403 | 20.3 | 1.26 | 1.01-1.57 | 48 | 21.9 | 23,496 | 20.3 | 1.61 | 1.08-2.41 | 38 | 19.9 | 23,506 | 20.3 | 1.01 | 0.67-1.50 | |||
| 40-<50 | 164 | 24.0 | 26,767 | 23.2 | 1.32 | 1.07-1.63 | 54 | 24.7 | 26,877 | 23.2 | 1.58 | 1.07-2.34 | 41 | 21.5 | 26,890 | 23.2 | 1.00 | 0.67-1.48 | |||
| 50-<60 | 130 | 19.0 | 19,668 | 17.0 | 1.49 | 1.19-1.87 | 45 | 20.6 | 19,753 | 17.0 | 1.80 | 1.19-2.72 | 38 | 19.9 | 19,760 | 17.0 | 1.37 | 0.91-2.06 | |||
| 60+ | 53 | 7.8 | 8,257 | 7.2 | 1.38 | 1.01-1.89 | 0.001 | 24 | 11.0 | 8,286 | 7.1 | 2.13 | 1.29-3.52 | 0.001 | 8 | 4.2 | 8,302 | 7.2 | 0.72 | 0.34-1.53 | 0.63 |
Abbreviations: CI- confidence intervals; HLA- human leukocyte antigen; HR- hazard rations; IL2- interleukin 2; mTOR- mammalian target of rapamycin; RCC- renal cell carcinoma
Frequencies may not sum to 100% due to rounding error.
Cox regression using time since transplant as time scale is adjusted for decade age at transplant (continuous), transplant year (<1995, 1995-<2000, 2000-<2005, 2005+), race, vintage (continuous years), primary reason for kidney transplant, donor sex and donor age (<30 vs. 30+ years).
Cox regression using time since transplant as time scale is adjusted for decade age at transplant (continuous), transplant year (<1995, 1995-<2000, 2000-<2005, 2005+), vintage (continuous years), primary reason for kidney transplant, polyclonal antibody induction therapy drugs and donor age (<30 vs. 30+ years).
Cox regression using time since transplant as time scale is adjusted for decade age at transplant (continuous), transplant year (<1995, 1995-<2000, 2000-<2005, 2005+), race, vintage (continuous years), primary reason for kidney transplant, IL2 induction therapy drugs and donor sex.. Cox regression models not adjusted for:
sex,
age at transplant,
transplant year,
race,
years of vintage,
primary reasons for kidney transplant,
Cox regression using EFFECT parameterization
IL2 induction therapy drug,
Poly induction therapy drug,
donor sex, or
donor age
Six non-cases had missing donor age information; mean donor age (38 years old) was imputed for the missing values.
RCC risk increased with increasing dialysis vintage (p<0.0001), with the trend more apparent for papillary RCC (p=0.03) than ccRCC (p=0.08). Compared to the average kidney recipient, recipients transplanted for diabetes mellitus had reduced risk of RCC (HR 0.77), and risk was increased for recipients with glomerular diseases (HR 1.24), hypertensive nephrosclerosis (HR 1.55), and vascular diseases (HR 1.53). For ccRCC, reduced risk was observed for recipients with polycystic kidney disease (HR 0.54). Strong associations with risk were seen for papillary RCC with hypertensive nephrosclerosis (HR 2.02) and vascular diseases (HR 1.86).
Induction therapy with polyclonal antibodies and interleukin 2 (IL2) antagonists was associated with elevated risks for ccRCC (HR 1.36) and papillary RCC (HR 1.51), respectively. Among recipients who received a kidney transplant from a female versus male donor, we observed reduced risk for RCC (HR 0.86), specifically for papillary RCC (HR 0.71). In contrast, an association of risk with increasing donor age was observed for RCC overall (p-trend 0.001) and ccRCC (p-trend 0.001). No significant associations with BMI, other induction or maintenance medications, HLA mismatch, cold ischemic time, and other donor characteristics (deceased vs. living donor, race, BMI) were observed (see adjusted HRs, Table S3).
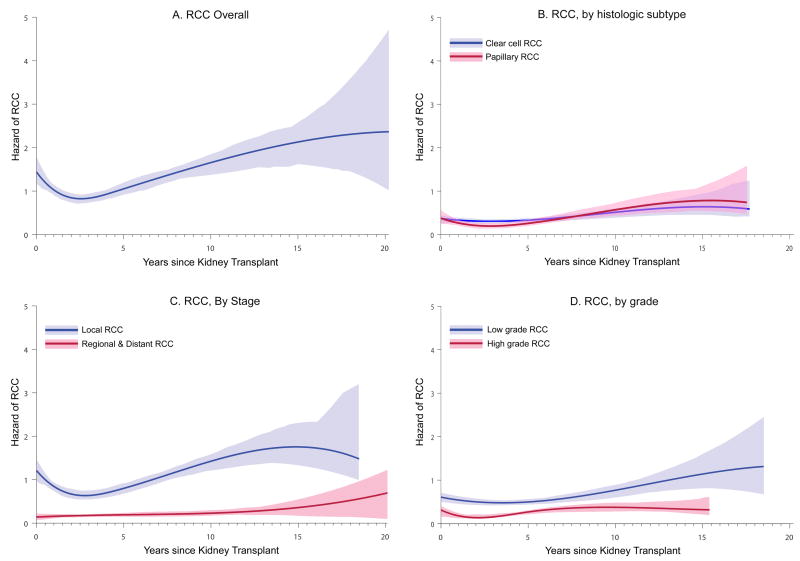
Figures 1 illustrates the risk of RCC as a function of time since transplant. For RCC overall (Figure 1A), risk followed a biphasic pattern: risk was high immediately after transplant, decreased to a minimum approximately 2.5 years after transplant, and then rose steadily over the following years. Similar results were observed for ccRCC and papillary RCC separately, although the biphasic pattern appeared stronger for papillary RCC (Figures 1B). Biphasic patterns were observed for localized RCC and for both low grade and high grade RCCs; risk for regional/distant RCC appeared somewhat constant over time (Figures 1C and 1D).
Figure 1. Hazard of RCC as a function of time since kidney transplant.
Results are shown for: (A) RCC overall, (B) according to histologic subtypes (clear cell and papillary RCC), (C) according to stage (local and regional/distant RCC), and (D) according to grade (low and high grade). Hazards were estimated using cubic B-splines. The vertical axis shows the hazard in units of “per 1000 person-years”. RCC, renal cell carcinoma.
Figure 2 illustrates the absolute risk of RCC as a function of time since transplant. At 5 years after transplant, 0.5% of recipients developed RCC, and 1.0% developed RCC by 10 years. By histology, very little difference in cumulative incidence was observed for ccRCC and papillary RCC during follow-up.
Figure 2. Cumulative incidence of RCC, overall and by histology.
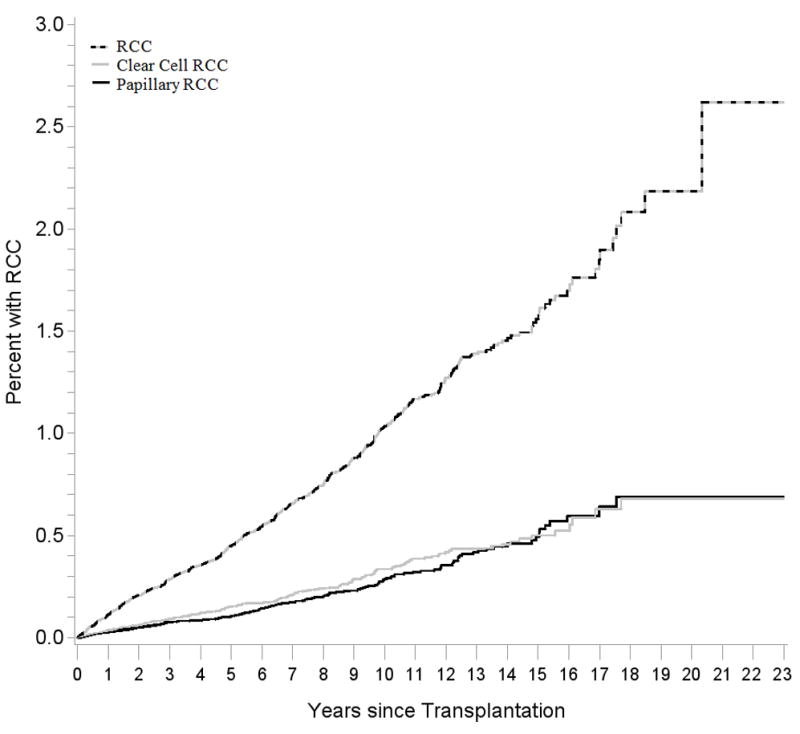
Results are shown for RCC overall (dashed black and gray line), clear cell RCC (gray line), and papillary RCC (black line). Note that the vertical axis is truncated and reaches a maximum of 3%. RCC, renal cell carcinoma.
Discussion
In our large population-based study of US kidney recipients, we observed a nearly 6-fold overall increased risk of RCC. Notably, a much greater elevation in risk was seen for the papillary RCC histologic subtype than for the clear cell subtype. RCC risk was highest in individuals who had had prolonged dialysis prior to transplantation, and it varied separately for ccRCC and papillary RCC according to the medical conditions that were the indication for transplantation. Based on our evaluation of a sample of cases, the great majority arose in a native kidney, with only 11% of the RCCs occurring in the donor kidney. We also observed a biphasic onset of RCC following transplant: a high risk immediately after transplant, a fall in risk until roughly 2.5 years after transplant, and then a subsequent gradual increase with time.
The 16-fold increase in risk for papillary RCCs among kidney recipients is noteworthy. In the general population, ccRCC and papillary RCC comprise about 70% and 10-15% of RCC cases, respectively (8). In contrast, recent studies indicate an overrepresentation of papillary RCCs among kidney recipients, typically reported to comprise more than 30% of cases (14,23-26). Most prior studies were small (i.e., they included fewer than 50 RCCs in recipients) and were based at single institutions (14,23,25,26). Our study, which is much larger, confirms that papillary RCCs are overrepresented in kidney transplant recipients (28% of our cases), and it provides the first SIR estimate quantifying the markedly elevated risk for this subtype.
The observed association with vintage, which was stronger for the papillary than clear cell subtype, suggests that processes that occur during dialysis contribute to RCCs that arise after kidney transplant. A large proportion of RCCs in dialysis patients are also classified as papillary RCCs (27). The biological mechanisms that contribute to the development of RCC in individuals with ESRD are believed to differ from those for RCCs that arise in the general population. For example, sporadic RCCs, which predominantly consist of the clear cell subtype, manifest genetic or epigenetic inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene in greater than 90% of cases (28), yet RCCs in ESRD patients typically do not exhibit this VHL mutation (29). In contrast, papillary tumors that arise in ESRD patients exhibit allelic duplications of chromosomes 7 and 17, accompanied by activation of the MET proto-oncogene on chromosome 7q (30).
Although kidney function typically improves soon after transplant, some early RCC tumors likely arise in the native kidney as a result of malignant transformation of renal cysts that had developed before transplantation as a part of ACKD (14-16). According to the National Institute of Diabetes and Digestive and Kidney Diseases, about 20% of patients who start dialysis have ACKD, 60-80% of patients on dialysis for four years develop ACKD, and 90% of patients with dialysis for at least eight years develop ACKD (31). Of patients with ACKD, roughly 10-20% develop RCCs (31,32), which tend to be large and multifocal (33). The high incidence of RCC among dialysis patients may be related to ACKD and effects of uremic toxins which may lead to DNA alterations (34), while the early decline following receipt of a kidney transplant, especially for papillary RCC, may be due to clearance of these toxins (13). Of note, there is also an elevated risk of late-onset kidney cancer, observed in our investigation and also in studies that included recipients of other transplanted organs (3,6), which is not well understood.
The absence of an increased risk of RCC among HIV-infected individuals suggests that the excess in transplant recipients is not a consequence of immune deficiency per se (35). Calcineurin inhibitors (i.e., cyclosporine and tacrolimus), which are widely used as maintenance immunosuppressive medications, exhibit nephrotoxic effects that result acutely in the reduction of glomerular filtration rate and, over time, in tubular atrophy and interstitial fibrosis (36). Calcineurin inhibitors and other maintenance medications (e.g., azathioprine) have additional biological effects that may promote the development of cancer (37). Although we did not see associations between specific maintenance immunosuppressive medications and RCC risk, it remains possible that their effects contributed to the increase in RCC, as most recipients were exposed to one calcineurin inhibitor or the other, and we were limited by our inability to assess dose and duration of use of these medications. Recipients of other transplanted organs, also on maintenance immunosuppressive medications, exhibit an elevated risk of kidney cancer, although it is not as high as seen in kidney recipients (6). Moreover, we observed increased risk related to induction therapy with polyclonal antibodies (for ccRCC) and IL2 antagonists (for papillary RCC), but the explanations for these findings are unclear.
The incidence of RCC has appeared to increase in the US and globally over the past few decades (10,38). Much of this increase is related to a rise in detection of asymptomatic small tumors, and up to 50% of RCC cases are speculated to be over-diagnosed (18,19,38). Incidentally detected tumors, on average, are smaller and diagnosed at a lower stage than symptomatic tumors (18,19). Because kidney recipients are closely followed by transplant providers, work-up for graft dysfunction, rejection, or other medical conditions may lead to incidental detection and perhaps over-diagnosis of RCC. These medical evaluations may be partly responsible for the excess risk of RCC observed in our study and others. Indeed, in our investigation, RCC risk, irrespective of histologic subtype, was greatest for local stage and low grade tumors. Incidental detection of RCCs may also explain the higher RCC risk observed in our study population following transplants in more recent calendar years. Importantly, however, the significantly elevated risks of advanced stage and high grade RCC imply that intensive medical surveillance does not entirely explain the excess.
Compared to the average kidney recipient in our population, patients with glomerular diseases, vascular diseases, and hypertensive nephrosclerosis as the indication for transplant had an elevated risk of RCC. Glomerular diseases, such as glomerulonephritis, IgA nephropathy, membranous nephropathy, focal glomerulosclerosis, and amyloidosis are common causes of ESRD (1) and have been linked in previous studies to increased RCC risk in transplant recipients (12,39). In glomerular diseases, loss of podocytes that envelope the glomerular capillaries can often lead to renal cysts, and several studies show that glomerular diseases are more common among ACKD patients than non-ACKD patients (40,41). Moreover, vascular diseases may increase RCC risk in ESRD patients by reducing blood flow to the kidney cortex. Arteriopathic renal diseases have previously been associated with increased kidney cancer risk in dialysis patients (11,12). In our study, vascular diseases were strongly associated with papillary RCC, but not ccRCC. Interestingly, glomerular and vascular changes are hallmarks of hypertensive nephrosclerosis (42). Because nearly all ESRD patients are hypertensive, determining whether hypertension is the cause or result of ESRD can be difficult. Nevertheless, hypertension is the second most common cause of ESRD and has been associated with increased RCC risk in the general population (10) and in ESRD patients (4,11).
It is less clear why patients with ESRD due to diabetes or polycystic kidney disease had a lower risk of RCC compared to other kidney recipients, although similar findings have been reported previously (11,43). It has been speculated that protective cellular mechanisms, triggered by the formation of cysts in patients with polycystic kidney diseases, may prevent malignant transformation (43). This hypothesis is supported by the observation that germline mutations in PKHDI, the gene responsible for autosomal recessive polycystic kidney, protect against colorectal cancer (44). Furthermore, nephrectomy at the time of transplantation may be more likely among recipients with polycystic kidneys than in other recipients, decreasing subsequent risk of RCC (43).
As expected, patterns of elevated RCC risk in our population were consistent with other known risk factors (10), including increasing age, male sex, and African descent. Additionally, we observed associations with donor age and donor male sex. Our finding for donor age, which has been observed previously (16), was adjusted for recipient age, suggesting that the variable is an independent RCC risk factor. Older and male donors may carry a higher risk of having pre-neoplastic kidney damage, but the relevance of this fact is uncertain, as only a minority of RCCs occurred in donor kidneys. The explanation for the observed association between donor sex and RCC is unclear. While the lack of association between elevated BMI and RCC may seem surprising at first, we note that our investigation was restricted to ESRD patients, and a low BMI in this population may be a marker of generally poor health (45).
Strengths of our study include its population-based design, large size, and representative sampling of the US transplant population. To our knowledge, this is the first study to provide SIR estimates for histologic subtypes of RCC among kidney transplant recipients, thereby quantifying the excess risk for papillary and clear cell RCC. Our ability to examine a range of RCC risk factors is an additional strength. Several limitations should also be noted. While we had a large number of RCC cases overall, analyses by histologic subtype may have been underpowered for some risk factors. We were unable to classify RCC subtype for approximately one-third of cases; however, the percentage of unknown tumor subtypes in our study is smaller than that in the general population. Also, we lacked data on smoking, which is a risk factor for RCC (10), and smoking may partly explain the elevated risks seen with hypertensive nephropathy and vascular disease. We also did not have data on the dose and duration of use for maintenance therapy drugs, which limits our ability to make firm conclusions about their effects. Since information on screening was unavailable, we were unable to assess whether RCC cases were incidentally or symptomatically diagnosed.
We examined risk factors for RCC in a cohort of transplant recipients who were also at risk of other competing outcomes, including graft failure, retransplantation, and death. A modest fraction of patients (10.9%) were lost to follow-up by the transplant centers reporting to the SRTR. As shown in Table S2, the characteristics of patients who exited for various reasons differed. Nonetheless, the HR estimates from our regression models, which measure the associations with risk factors for RCC, are correctly estimated under the assumption the hazard of RCC is independent of the hazard for the other outcomes conditional on the covariates in the models. Lastly, since we made multiple comparisons without adjustment, some associations may be due to chance. However, chance is an unlikely explanation for the strongly elevated risks shown in Table 2 or the most significant associations with RCC risk factors in Table 3.
Our estimates of absolute risk indicate that approximately 0.5% and 1.0% of kidney recipients are diagnosed with RCC within 5 and 10 years after transplant, respectively. A fraction of these cases were localized tumors and may represent over-diagnosis of cases detected incidentally through clinical evaluations or by screening efforts already in place in some transplant programs. Thus, it is difficult to assess whether additional screening for RCC should be directed at the overall kidney transplant population. Nonetheless, it will be important to evaluate these issues formally and determine whether certain subgroups of kidney recipients predicted to be at highest risk of RCC would benefit from targeted screening.
In summary, kidney transplant recipients experience a disproportionately greater incidence of RCC compared to the general population, and the elevation is substantially higher for papillary than for clear cell tumors. Our results indicate that RCC and its histologic subtypes are associated with complex multifactorial etiologies, involving elements related to demographics, medical indications for transplantation, and kidney dysfunction (e.g., as indicated by duration of prior dialysis). Additional studies that consider how clinical, environmental, and genetic factors modify cancer risk among kidney recipients are needed to identify the precise mechanisms involved in the malignant transformation and progression of RCC.
Supplementary Material
Table S1: Calculations used to estimate adjusted SIR among kidney transplant recipients by subtype, stage, and grade.
Table S2: Selected characteristics of kidney transplant recipients by exit reason.
Table S3: Multivariate models examining associations of kidney transplant recipient and donor characteristics in relation to RCC risk by subtype.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Cancer Institute. The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut, Georgia (Rana Bayakly), Hawaii (Michael Green), Iowa, Illinois (Lori Koch), Michigan, New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons). The authors also thank David Check (National Cancer Institute) for assistance in producing the figure.
The SRTR is currently operated under contract number HHSH250201000018C (Health Resources and Services Administration) by the Minneapolis Medical Research Foundation, Minneapolis, MN. During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (5U58/DP003931-02), New York (U58DP003879), North Carolina (U58DP000832) and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
Abbreviations used
- ACKD
acquired cystic kidney disease
- BMI
body mass index
- CI
confidence interval
- ESRD
end-stage renal disease
- HLA
human leukocyte antigen
- HR
hazard ratio
- ICDO
International Classification of Diseases for Oncology
- NIH
National Institutes of Health
- RCC
renal cell carcinoma
- SIR
standardized incidence ratio
- US
United States
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Disclaimer
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.National Kidney Foundation. [2015 Jul 10];ORGAN DONATION AND TRANSPLANTATION STATISTICS. [Internet] Available from: http://www.kidney.org/news/newsroom/factsheets/Organ-Donation-and-Transplantation-Stats.
- 2.U.S. Department of Health & Human Services. [2015 Jul 10];Scientific Registry of Transplant Recipients 2012 Annual Data Report. [Internet] Available from: http://srtr.transplant.hrsa.gov/annual_reports/2012/Default.aspx.
- 3.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: A UK registry audit. Am J Transplant. 2010;10:1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 4.Vajdic CM, McDonald SP, McCredie MRE, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatte T, Marberger M. Renal cell carcinoma of native kidneys in renal transplant patients. Curr Opin Urol. 2011;21:376–379. doi: 10.1097/MOU.0b013e32834962bf. [DOI] [PubMed] [Google Scholar]
- 8.Purdue MP, Moore LE, Merino MJ, Boffetta P, Colt JS, Kendra L, et al. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer. 2014;132:2640–2647. doi: 10.1002/ijc.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, et al. Common and Uncommon Histologic Subtypes of Renal Cell Carcinoma : Imaging Spectrum with Pathologic Correlation. Radiographics. 2006;26:1795–1807. doi: 10.1148/rg.266065010. [DOI] [PubMed] [Google Scholar]
- 10.Chow W-H, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14:288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart J, Buccianti G, Agodoa L, Gellert R, McCredie M, Lowenfels A, et al. Cancers of the Kidney and Urinary Tract in Patients on Dialysis for End-Stage Renal Disease: Analysis of Data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol. 2003;14:197–207. doi: 10.1097/01.asn.0000039608.81046.81. [DOI] [PubMed] [Google Scholar]
- 12.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 13.Yanik E, Clarke C, Snyder J, Pfeiffer R, Engels E. Variation in Cancer Incidence among End Stage Renal Disease Patients during Intervals of Kidney Function and Non-Function. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh A, Vathsala A. Native renal cysts and dialysis duration are risk factors for renal cell carcinoma in renal transplant recipients. Am J Transplant. 2011;11:86–92. doi: 10.1111/j.1600-6143.2010.03303.x. [DOI] [PubMed] [Google Scholar]
- 15.Russo P. End Stage and Chronic Kidney Disease: Associations with Renal Cancer. Front Oncol. 2012;2:1–7. doi: 10.3389/fonc.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst F, Jindal R, Graham L, Falta E, Elster E, Stackhouse G, et al. Incidence, predictors, costs, and outcome of renal cell carcinoma after kidney transplantation: USRDS experience. Transplantation. 2010;90:898–904. doi: 10.1097/TP.0b013e3181f30479. [DOI] [PubMed] [Google Scholar]
- 17.Leveridge M, Musquera M, Evans A, Cardella C, Pei Y, Jewett M, et al. Renal cell carcinoma in the native and allograft kidneys of renal transplant recipients. J Urol. 2011;186:219–223. doi: 10.1016/j.juro.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 19.Gudbjartsson T, Thoroddsen A, Petursdottir V, Hardarson S, Magnusson J, Einarsson G. Effect of incidental detection for survival of patients with renal cell carcinoma: results of population-based study of 701 patients. Urology. 2005;66:1186–1191. doi: 10.1016/j.urology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Morrison L. Medicare from A to D: What every nephrologist needs to know. Clin J Am Soc Nephrol. 2008;3:899–904. doi: 10.2215/CJN.02430607. [DOI] [PubMed] [Google Scholar]
- 21.University of California Institute for Digital Research and Education. [2015 Jul 10];FAQ, What is Effect Coding? [Internet] Available from: http://www.ats.ucla.edu/stat/mult_pkg/faq/general/effect.htm.
- 22.Rosenberg PS. Hazard function estimation using B-splines. Biometrics. 1995;51:874–887. [PubMed] [Google Scholar]
- 23.Klatte T, Seitz C, Waldert M, De Martino M, Kikic Z, Böhmig GA, et al. Features and outcomes of renal cell carcinoma of native kidneys in renal transplant recipients. BJU Int. 2010;105:1260–1265. doi: 10.1111/j.1464-410X.2009.08941.x. [DOI] [PubMed] [Google Scholar]
- 24.Gigante M, Neuzillet Y, Patard JJ, Tillou X, Thuret R, Branchereau J, et al. Renal cell carcinoma (RCC) arising in native kidneys of dialyzed and transplant patients: Are they different entities? BJU Int. 2012;110:E570–E573. doi: 10.1111/j.1464-410X.2012.11273.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsaur I, Obermüller N, Jonas D, Blaheta R, Juengel E, Scheuermann EH, et al. De novo renal cell carcinoma of native and graft kidneys in renal transplant recipients. BJU Int. 2011;108:229–234. doi: 10.1111/j.1464-410X.2010.09856.x. [DOI] [PubMed] [Google Scholar]
- 26.Végsö G, Toronyi É, Hajdu M, Piros L, Görög D, Deák PA, et al. Renal cell carcinoma of the native kidney: A frequent tumor after kidney transplantation with favorable prognosis in case of early diagnosis. Transplant Proc. 2011;43:1261–1263. doi: 10.1016/j.transproceed.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 27.Tickoo SK, dePeralta-Venturina MN, Harik LR, Worcester HD, Salama ME, Young AN, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. 2006;30:141–153. doi: 10.1097/01.pas.0000185382.80844.b1. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Beltran A, Montironi R, Egevad L, Caballero-Vargas MT, Scarpelli M, Kirkali Z, et al. Genetic profiles in renal tumors: Review Article. Int J Urol. 2010;17:6–19. doi: 10.1111/j.1442-2042.2009.02395.x. [DOI] [PubMed] [Google Scholar]
- 29.Hughson D, Schmidt L, Silva FG, Sandberg AA, Meloni M, Daugherty S. Renal cell carcinoma of end-stage renal disease: a histopathologic and molecular genetic study. J Am Soc Nephrol. 1996;7:2461–2468. doi: 10.1681/ASN.V7112461. [DOI] [PubMed] [Google Scholar]
- 30.Denton MD, Magee CC, Ovuworie C, Mauiyyedi S, Pascual M, Colvin RB, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: A pathologic analysis. Kidney Int. 2002;61:2201–2209. doi: 10.1046/j.1523-1755.2002.00374.x. [DOI] [PubMed] [Google Scholar]
- 31.National Institute of Diabetes and Digestive and Kidney Diseases. [2015 Jul 28];Acquired Cystic Kidney Disease. 2012 [Internet] Available from: http://www.niddk.nih.gov/health-information/health-topics/kidney-disease/acquired-cystic-kidney-disease/Pages/facts.aspx.
- 32.Grantham J, Nair V, Winklhofer F. Cystic diseases of the kidney. In: Brenner B, editor. B& RTK. 6. Philadelphia: W.B Saunders; 2000. pp. 1699–1730. [Google Scholar]
- 33.Crumley SM, Divatia M, Truong L, Shen S, Ayala AG, Ro JY. Renal cell carcinoma: Evolving and emerging subtypes. World J Clin Cases. 2013;1:262–275. doi: 10.12998/wjcc.v1.i9.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming S. Renal cell carcinoma in acquired cystic kidney disease. Histopathology. 2010;56:395–400. doi: 10.1111/j.1365-2559.2010.03492.x. [DOI] [PubMed] [Google Scholar]
- 35.Layman AB, Engels EA. Kidney and bladder cancers among people with AIDS in the United States. J Acquir Immune Defic Syndr. 2008;48:365–367. doi: 10.1097/QAI.0b013e31817ae5da. [DOI] [PubMed] [Google Scholar]
- 36.De Mattos A, Olyaei A, Bennett W. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–346. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 37.Guba M, Graeb C, Jauch KW, Geissler E. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77:1777–1782. doi: 10.1097/01.tp.0000120181.89206.54. [DOI] [PubMed] [Google Scholar]
- 38.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur Urol European Association of Urology. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Moudouni SM, Lakmichi A, Tligui M, Rafii A, Tchala K, Haab F, et al. Renal cell carcinoma of native kidney in renal transplant recipients. BJU Int. 2006;98:298–302. doi: 10.1111/j.1464-410X.2006.06267.x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson B, Jenkins D, Allan P, Winney R, Dick J, Wild S, et al. Acquired cystic disease of the kidney : an indication for renal transplantation? Br Med J (Clin Res Ed) 1986;293:1209–1210. doi: 10.1136/bmj.293.6556.1209-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fallon B, Williams R. Renal cancer associated with acquired cystic disease of the kidney and chronic renal failure. Semin Urol. 1989;7:228–236. [PubMed] [Google Scholar]
- 42.Hill G. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270. doi: 10.1097/MNH.0b013e3282f88a1f. [DOI] [PubMed] [Google Scholar]
- 43.Wetmore JB, Calvet JP, Yu ASL, Lynch CF, Connie J, Kasiske BL, et al. Polycystic Kidney Disease and Cancer after Renal Transplantation. 2014:1–7. doi: 10.1681/ASN.2013101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward CJ, Wu Y, Johnson RA, Woollard JR, Bergstralh EJ, Cicek MS, et al. Germline PKHD1 mutations are protective against colorectal cancer. Hum Genet. 2011;129:345–349. doi: 10.1007/s00439-011-0950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalantar-Zadeh K, Abbott K, Salahudeen A, Kilpatrick R, Horwich T. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Calculations used to estimate adjusted SIR among kidney transplant recipients by subtype, stage, and grade.
Table S2: Selected characteristics of kidney transplant recipients by exit reason.
Table S3: Multivariate models examining associations of kidney transplant recipient and donor characteristics in relation to RCC risk by subtype.