Abstract
We have demonstrated that the combination of bioactive components generated by fish oil (containing n-3 polyunsaturated fatty acids) and fermentable fiber (leading to butyrate production) act coordinately to protect against colon cancer. This is the result, in part, to an enhancement of apoptosis at the base of the crypt throughout all stages (initiation, promotion and progression) of colon tumorigenesis. Since mitochondria are key organelles capable of regulating the intrinsic apoptotic pathway and mediating programmed cell death, we investigated the effects of diet on mitochondrial function by measuring mucosal cardiolipin composition, mitochondrial respiratory parameters and apoptosis in isolated crypts from the proximal and distal colon. C57BL/6 mice (n=15 per treatment) were fed one of two dietary fats (corn oil and fish oil) and two fibers (pectin and cellulose) for 4 wk in a 2 × 2 factorial design. In general, diet modulated apoptosis and the mucosal bioenergetic profiles in a site-specific manner. The fish/pectin diet promoted a more proapoptotic phenotype, e.g., increased proton leak (P-interaction = 0.002), compared to corn/cellulose (control) only in the proximal colon. With respect to the composition of cardiolipin, a unique phospholipid localized to the mitochondrial inner membrane where it mediates energy metabolism, fish oil feeding indirectly influenced its molecular species with a combined carbon number of C68 or greater, suggesting compensatory regulation. These data indicate that dietary fat and fiber can interactively modulate the mitochondrial metabolic profile and thereby potentially modulate apoptosis and subsequent colon cancer risk.
Keywords: bioenergetic profile, cardiolipin, colon cancer, phospholipid molecular species, lipidomics
Introduction
There is evidence from epidemiological and observational studies indicating that the consumption of fiber, which increases butyrate levels in the lumen of the colon, is chemoprotective against colorectal cancers (Bingham et al., 2003; Sebastian and Mostoslavsky, 2014). In contrast, several systematic reviews have challenged the premise that dietary fiber reduces colon cancer risk, fueling a debate regarding the role of fermentable fiber as a chemoprotective agent (Alberts et al., 2000; Ben et al., 2014). In order to address this apparent conundrum, our laboratory has focused on elucidating the molecular mechanisms underlying the chemoprotective effects of fiber.
Apoptotic cell death is generally considered protective, because it selectively eliminates damaged cells that can contribute to carcinogenesis. However, in certain contexts, non-targeted cell death in the presence of residual DNA damage can promote cell proliferation and generate defective progenitor cells, which is potentially a tumor-promoting event (Hua et al., 2012). We have previously demonstrated that the chemoprotective effect of dietary fish oil, enriched in n-3 polyunsaturated fatty acid (PUFA), is greatly enhanced with the addition of pectin (a highly fermentable fiber) (Chang et al., 1997; Vanamala et al., 2008). This protective effect is mediated in part by the up-regulation of targeted apoptotic deletion of damaged cells containing DNA adducts during tumor initiation (Hong et al., 2000; Chapkin et al., 2002) and spontaneous apoptosis during promotion (Davidson et al., 2004). In addition, it has been recently demonstrated that the fish oil/pectin diet favorably modulates the expression of genes involved in apoptosis and cell cycle regulation throughout all stages of tumorigenesis (Cho et al., 2011). This is significant because the induction of apoptosis represents a protective cellular mechanism against cancer (Qiu et al., 2010; Zhang et al., 2010).
With respect to the molecular targets of n-3 PUFA and fermentable fiber, mitochondria are key organelles capable of regulating the intrinsic apoptotic pathway and mediating cell death (Schug and Gottlieb, 2009). Mounting evidence suggests that dietary fish oil may prime colonocytes for butyrate-induced apoptosis by enhancing the unsaturation of mitochondrial phospholipids (Hong et al., 2002; Ng et al., 2005; Stanley et al., 2012). This is consistent with the fact that cardiolipin, the specific phospholipid of mitochondrial membranes, plays multiple key roles in the regulation of mitochondrial metabolism (Ren et al., 2014). Unfortunately, the combined effects of dietary fat and fiber on mucosal cardiolipin and mitochondrial respiratory parameters have not been rigorously addressed to date. To investigate the chemoprotective combinatorial effects of dietary fish oil (n-3 PUFA) and pectin (butyrate), we have combined mass spectrometry-based mitochondrial lipidomics profiling with a novel metabolomics model system to measure bioenergetic profiles (BEP) in isolated crypts from the mouse proximal and distal colon.
Materials and Methods
Animals and diets
Age-matched Male C57BL6 mice (n=15 per treatment, 6–8 weeks old) were fed semi-purified diets containing 15% fat and 6% fiber by weight. The diets differed with respect to the source of lipid, corn oil (enriched in n-6 PUFA) vs fish oil (enriched in n-3 PUFA); and source of fiber, cellulose (insoluble, poorly-fermentable fiber) vs pectin (soluble, highly fermentable fiber). The four dietary groups were corn oil/cellulose (CC), corn oil/pectin (CP), fish oil/cellulose (FC), and fish oil/pectin (FP). The dietary fatty acid composition of the semi-purified diets is shown in Supplemental Table 1. All procedures adhered to the guidelines approved by Public Health Service and the Institutional Animal Care and Use Committee at Texas A&M University. Animals were euthanized at the end of a 4 wk feeding period. Colons were dissected, rinsed in PBS, and fixed in 4% paraformaldehyde for 4 h as previously described (Fan et al., 2011). In addition, for metabolic analyses, 2 cm sections of distal and proximal colon were removed and crypts isolated as previously described (Fan et al., 2015). The remaining colonic mucosa was scraped, snap frozen in liquid nitrogen pending membrane phospholipid analysis.
Cardiolipin analysis
Cardiolipin molecular species were analyzed by high-performance liquid chromatography (HPLC) tandem mass spectrometry, as previously described (Houtkooper et al., 2009). Briefly, lipids were extracted using a modified Folch method (Folch et al., 1957). Lipid extracts were assayed using a straight-phase HPLC system, and cardiolipin composition analysis was performed using an on-line electrospray tandem mass spectrometer. Mass spectra of cardiolipin and monolysocardiolipin molecular species were obtained by continuous scanning from m/z 380 to m/z 1100 (Houtkooper et al., 2009).
Measurement of intestinal apoptosis
Apoptotic cells were enumerated in paraformaldehyde-fixed sections from the distal and proximal colon, respectively, using a terminal deoxynucleotidyl transferase (TUNEL) labeling kit (Trevigen) as previously described (Fan et al., 2011). The number of apoptotic cells was recorded in at least 100 well-oriented crypts per mouse.
Mitochondrial bioenergetic analysis
Seahorse XF 24-well cell culture plates were precoated with 50 µL/well of 10× diluted Matrigel (BD Bioscience) in buffer free seahorse XF medium (Seahorse Bioscience, North Billerica, MA), supplemented with 17.5 mM glucose, 2 mM Glutamax, 1 mM sodium pyruvate, 100 U/mL penicillin and 0.1 mg/mL streptomycin, pH 7.4, at 4°C overnight. Pre-coated plates were warmed to room temperature 30 min prior to crypt plating. Colonic crypts isolated by EDTA dissociation and mechanical dislodge as described previously (Fan et al., 2014), were immediately seeded into plates at a concentration of 250 crypts/50 µL medium. Plates were incubated in a CO2 free incubator at 37°C for 30 min to allow crypts to adhere to the pre-coated wells. XF 24-well cell culture plates were then transferred to the XF24 Extracellular Flux Analyzer. Hydrated cartridges containing optimal concentration titrated mitochondrial mediators, oligomycin (2 µM), FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone; 0.5 µM), and rotenone (5 µM) were injected at timed intervals into the sample wells, and the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were monitored continuously (Fan et al., 2015).
Statistical analysis
Data were analyzed using the two-way analysis of variance and the results from treatments showing significant overall changes were subjected to post-hoc Tukey’s test with significance at P < 0.05. Data were tested for normality by D’Agostino-Pearson omnibus normality test, and are presented as means ± SE. Analyses were conducted by using the Prism 6 program (GraphPad Software, Inc., La Jolla, CA).
Results
Dietary fat and fiber source alter colonic cardiolipin mass and composition
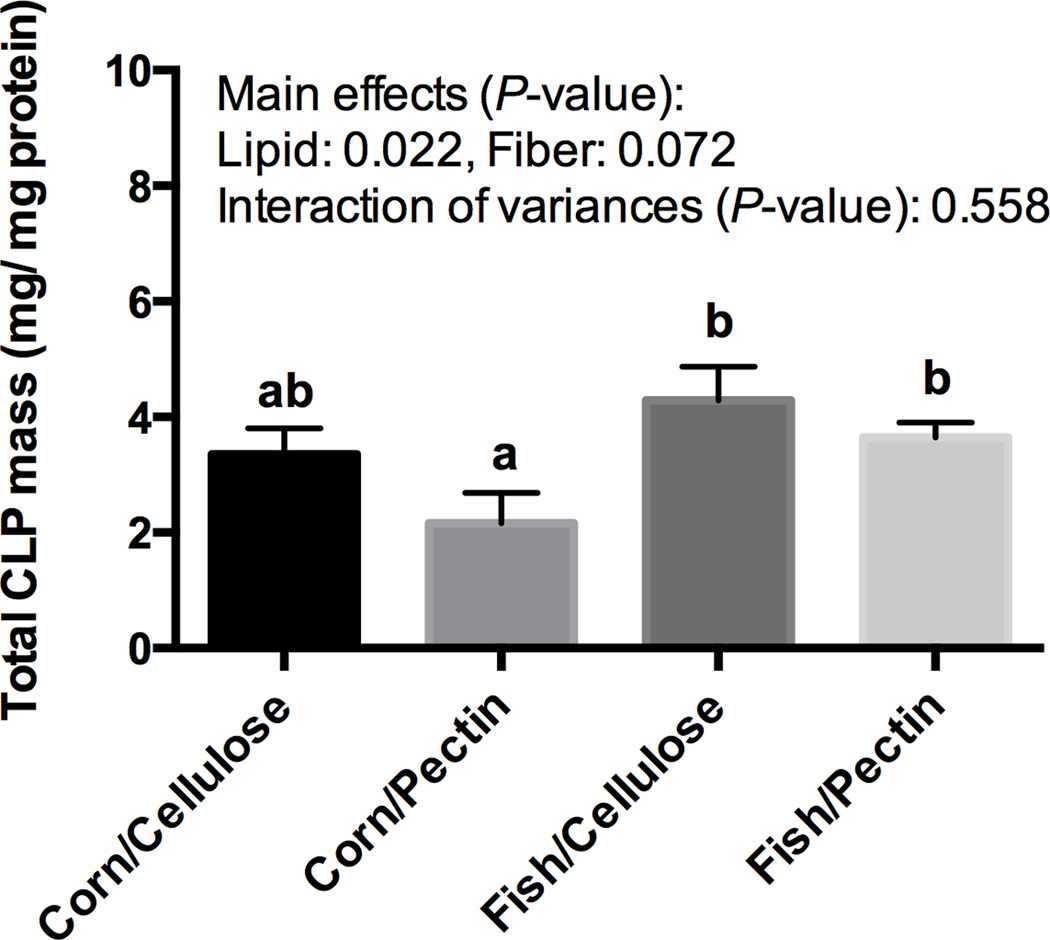
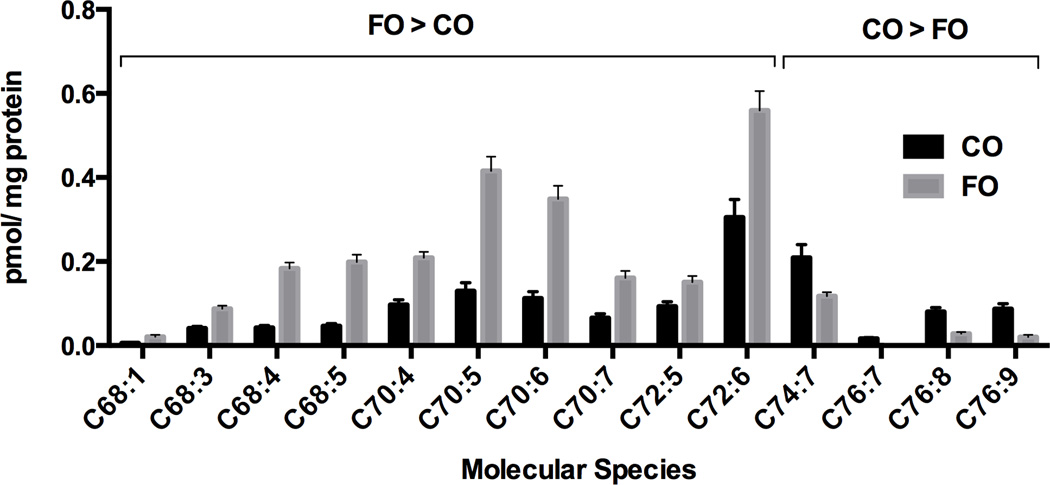
There were no significant differences (P > 0.05) in body weight gain across all diets (data not shown), suggesting that there was no difference in energy intake. Since changes in cardiolipin content can affect mitochondrial mediated respiration and apoptosis, we initially assessed the impact of dietary fat and fiber combination on colonic epithelial cell cardiolipin content using a mass spectrometry-based lipidomics platform. As shown in Figure 1, fish oil feeding enhanced (P < 0.05) mitochondrial cardiolipin levels, with a trend (P = 0.07) associated with the fiber (cellulose > pectin) treatment. We subsequently measured the composition and level of cardiolipin molecular species. In total, 27 cardiolipin molecular species were detected (Supplemental Table 2). Interestingly, the fatty acid composition of cardiolipin was not directly affected by the lipid composition of the diet (Supplemental Table 1), as noted by the lack of n-3 PUFA, e.g., 20:5n-3, 22:5n-3 or 22:6n-3, incorporation following fish oil feeding (fish/cellulose and fish/pectin vs corn/cellulose and corn/pectin). However, fish oil feeding profoundly modulated cardiolipin molecular species (Figure 2). For example, the abundance of C68:1, C68:3, C68:4, C68:5, C70:4, C70:5, C70:6, C70:7, C72:5 and C72:6 was increased. In contrast, the abundance of larger mass species C74:7, C76:7, C76:8 and C76:9 were all reduced in response to the incorporation of fish oil into the diet. Although long chain n-3 PUFA (20:5n-3, 22:5n-3, and 22:6n-3) were not directly incorporated into cardiolipin molecular species, they were associated with other membrane phospholipids, including phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, phosphatidyglycerol, but not phosphatidylserine (Supplemental Tables 3–7).
Figure 1. Dietary fat and fiber modulate cardiolipin mass.
Mice were fed one of four semi-purified diets, varying only in the type of fat (15%, w/w) (corn oil vs fish oil) and fiber (6%, w/w) (cellulose vs pectin) for 4 weeks. Mice were subsequently killed and colonic mucosa isolated by scraping. Three mouse colons were pooled per sample, and the fatty acyl composition of cardiolipin molecular species was quantified by mass spectrometry. Data are presented as total cardiolipin mass per mg protein (mean ± SE, n=5). Means not sharing a common letter are significantly different (P < 0.05).
Figure 2. Fatty acyl composition of cardiolipin molecular species distribution is modulated by dietary fat source.
See Figure 1 for legend detail. Individual molecular species are reported as pmol/mg protein (mean ± SE, n=10). Abbreviation: FO, fish oil diet; CO, corn oil diet. Fatty acid nomenclature describes (the number of carbon atoms in the fatty acid backbone):(the number of double bonds).
Levels of apoptosis in the proximal and distal colon are enhanced by dietary fish oil and pectin combination
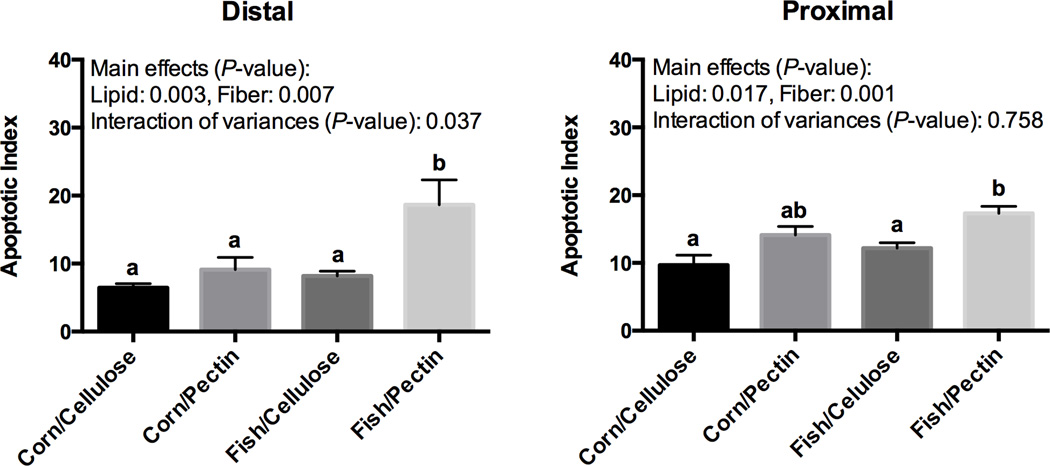
With respect to apoptosis, the combination of fish oil and fermentable fiber (pectin) significantly promoted apoptosis in both the distal and proximal colon. However, the interaction effect of fat × fiber was only seen in the distal colon (P = 0.037) (Figure 3), with no interaction evident in the proximal colon, although there were main effects of fat (P = 0.017) and fiber (P = 0.001).
Figure 3. Effect of dietary fat and fiber combination on mouse distal and proximal colonocyte apoptosis.
Mice were fed one of four semi-purified diets for 4 weeks. See Figure 1 legend for details. Distal and proximal colon were removed, fixed, and embedded in Parafilm™. Apoptotic index is expressed as the total number of apoptotic cells per 100 crypts (Mean ± SE, n=15). Means not sharing a common letter are significantly different (P < 0.05).
Mitochondrial bioenergetic profiles are altered by dietary fat and fiber in a site-specific manner
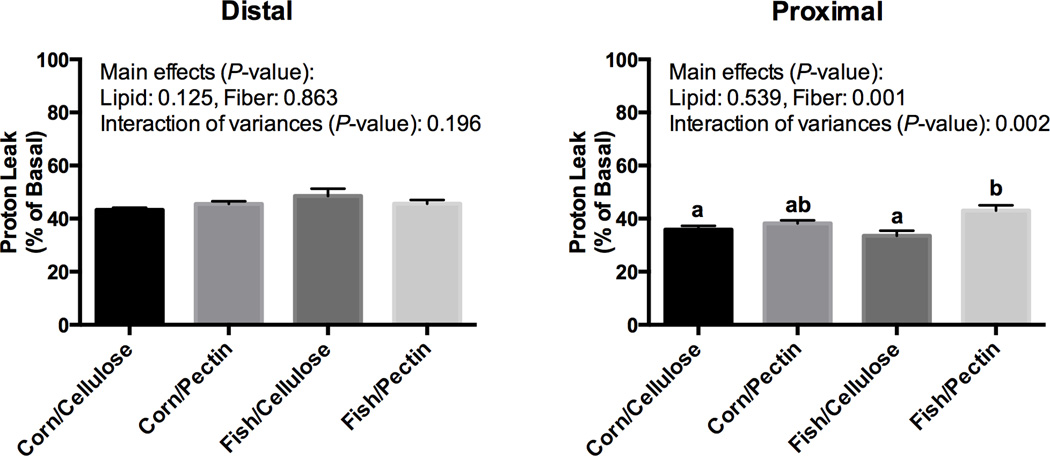
To further investigate the role of mitochondrial metabolism in relation to the chemoprotective combinatorial effects of dietary n-3 PUFA and fermentable fiber, we developed a novel metabolomics model system to measure the bioenergetic profile (BEP) in isolated primary colonic crypts from mice fed the experimental diets. This is an important advancement, because it allows one to monitor epithelial cell BEP in a physiologically relevant setting. For example, we quantified bioenergetic profiles in intact crypts containing various cell types as opposed to single types or isolated mitochondria from fractionated cells. A representative quantitative measure of colonic epithelial BEP using the Seahorse Extracellular Flux Analyzer is shown in Supplemental Figure 1. In contrast to experiments with isolated mitochondria, respiration is sustained in these conditions by respiratory substrates provided by cellular metabolism. Despite the disaggregation of crypts over the 3 h incubation period, the oxygen consumption rate (OCR) was fairly stable (slight decrease over time), indicating that the cells were metabolically active. Cell viability was also measured in parallel using a Live/Dead assay, with ~90% viability at both 30 min and 3.5 h time points (data not shown). Using pooled data from all dietary groups, OCR, a measure of mitochondrial activity (174.8 ± 14.8 vs 121.7 ± 10.4 pmol/min/250 crypts, n=20) and ECAR, a measure of glycolytic flux (9.6±0.5 vs 7.4 ± 0.4 mpH/min/250 crypts, n=20) were significantly higher (P < 0.05) in the distal vs. proximal colon, respectively. Interestingly, diet differentially affected OCR in the proximal vs distal colon (Table 2). Specifically, dietary fat and fiber interactively modulated OCR and ECAR only in the proximal colon (P-interaction = 0.001 and 0.07 for OCR and ECAR, respectively). In this analytical system, the proton leak value is defined as the OCR attributed to all processes which allow ion movement across the mitochondrial inner membrane. For this analysis, proton leak data represent the difference in OCR following oligomycin and rotenone challenge (Supplemental Figure 2). To our surprise, the fish/pectin diet enhanced the proton leak related OCR only in the proximal colon (P-interaction = 0.002) (Figure 4). Additional respiration parameters, such as maximum respiratory capacity and reserved respiratory capacity, indicators of mitochondrial function for assess/predict the ability of cells to manage and overcome stress, are shown in Supplemental Figure 3. The similar apoptotic and proton leak profiles in the proximal colon suggests that the two phenotypes may be linked.
Table 2.
Dietary effects on basal respiration of colonic crypts.
| CC | CP | FC | FP | P-interaction | Corn | Fish | P-lipid | Cellulose | Pectin | P-fiber |
|---|---|---|---|---|---|---|---|---|---|---|
| Distal Colon | ||||||||||
| OCR (pmol/min) 181±28 |
144±30 | 172±30 | 199±34 | 0.655 | 176±20 | 173±23 | 0.913 | 164±20 | 185±22 | 0.472 |
| ECAR (mpH/min) 9.1±1.9 |
10.0±1.0 | 8.5±1.4 | 10.8±1.7 | 0.747 | 9.6±1.0 | 9.7±1.1 | 0.934 | 8.8±1.2 | 10.4±0.9 | 0.307 |
| ECAR/OCR 0.04±0.01 |
0.05±0.01 | 0.05±0.01 | 0.04±0.01 | 0.531 | 0.04±0.01 | 0.05±0.01 | 0.519 | 0.04±0.01 | 0.05±0.01 | 0.352 |
| Proximal Colon | ||||||||||
| OCR (pmol/min) 76±7a |
175±24b | 88±6a | 139±18b | 0.001 | 128±17 | 115±12 | 0.528 | 82±5a | 157±15b | 0.001 |
| ECAR (mpH/min) 5.1±0.5a |
9.5±1.6b | 7.0±0.8ab | 7.6±1.2ab | 0.070 | 7.4±1.0 | 7.3±0.7 | 0.920 | 6.1±0.5a | 8.6±1.0b | 0.037 |
| ECAR/OCR 0.05±0.01 |
0.04±0.01 | 0.05±0.01 | 0.04±0.01 | 0.707 | 0.05±0.01 | 0.05±0.01 | 0.562 | 0.05±0.01 | 0.04±0.01 | 0.378 |
Data are reported as mean ± SEM (n=15).
Superscripts with different letters indicate a significant difference (P<0.05).
CC, Corn/Cellulose; CP, Corn/Pectin; FC, Fish/Cellulose; FP, Fish/Pectin; OCR, oxygen consumption rate; ECAR, extracellular acidification rate.
Figure 4. Mouse mitochondrial respiration associated proton leak is enhanced by fish oil and fermentable fiber feeding in the proximal colon.
Following consumption of the experimental diets for 4 wks, isolated distal and proximal colonic crypts were immediately cultured and mitochondrial bioenergetic profiles were measured using the Seahorse XF24 Flux system as described in the Methods section. Proton leak is defined as the OCR attributed to all processes which allow ion movement across the mitochondrial inner membrane, and was measured as the difference in OCR following oligomycin and rotenone challenge. Data are normalized to the original basal OCR level (before challenging with mitochondrial inhibitors) (Mean ± SE, n=15). Means not sharing a common letter are significantly different (P < 0.05).
Discussion
Colorectal cancer continues to pose a serious health problem in the U.S, affecting 6% of the U.S. population. Our previous dietary studies have demonstrated that the protective effect of fish oil-derived n-3 PUFA with respect to colon tumor development is enhanced when a highly fermentable fiber, pectin, rather than a poorly fermentable fiber, cellulose, is added to the diet (Chang et al., 1997; Vanamala et al., 2008; Cho et al., 2011). This protective effect is mediated, in part, by the upregulation of targeted deletion of DNA adducts damaged cells throughout all stages of tumor development (Crim et al., 2008; Cho et al., 2011). This is an important consideration given the varied biologic, biochemical and metabolic roles of dietary fat and fiber subtypes with respect to colon cancer risk (Chapkin et al., 2007; Crim et al., 2008; Sebastian and Mostoslavsky, 2014).
We and others have demonstrated that n-3 PUFA may uniquely prime colonocytes for short chain fatty acid (SCFA)-induced apoptosis by enhancing the unsaturation of mitochondrial phospholipids (Hong et al., 2002; Ng et al., 2005; Stanley et al., 2012). Although we did not measure SCFA levels in this study, our group has previously documented luminal SCFA levels in stool from rats fed identical diets, i.e., 6% dietary fiber (cellulose or pectin), and have shown significant changes in SCFA concentrations among the different groups (Zoran et al., 1997a). Despite the fact that butyrate may not be the main SCFA in the colonic lumen, compared to acetate and propionate, butyrate is highly correlated with indices of cell proliferation (Lupton and Kurtz, 1993), and its role in histone deacetylation, apoptosis, and colon cancer is well established (Goncalves and Martel, 2013; Donohoe et al., 2014; Bordonaro and Lazarova, 2015).
Among the various membrane phospholipids, cardiolipin, a diphosphatidylglycerol lipid, is found almost exclusively in the inner mitochondrial membrane, and is essential for the optimal function of numerous enzymes involved in mitochondrial energy metabolism (Schug and Gottlieb, 2009; Ren et al., 2014). The de novo synthesis of cardiolipin in mitochondria is followed by a unique remodeling process, in which the parent phospholipid undergoes cycles of deacylation and reacylation (Yamashita et al., 2014). With respect to the four distinct acyl chains in cardiolipin, the potential for complexity of its molecular species is enormous, although in most animal tissues, linoleic acid (18:2n-6) is preferentially enriched in the cardiolipin pool (Schlame 1993, Schenkel and Bakovic, 2014). Our novel data clearly demonstrate that the fatty acyl composition of the colonic crypt inner mitochondrial membrane cardiolipin pool does not directly reflect the presence of long chain n-3 PUFA in the diet, but that the fatty acid composition of the molecular species is influenced by the fat source. These findings suggest that the colonic phospholipid remodeling mechanisms involving deacylation-reacylation and transacylation via tafazzin (Schlame, 2013; Ren et al., 2014) actively exclude long chain n-3 PUFA. This is consistent with recent substrate specificity findings, where the transacylation activity of tafazzin was highest for linoleoyl groups, and negligible for arachidonoyl groups in Drosophila (Xu 2006) and n-3 PUFA in rat liver (Stavrovskaya et al., 2013). Interestingly, acyl-CoA:lysocardiolipin acylytansferase 1 (ALCAT1) has been reported to play an important role in cardiolipin remodeling (Yamashita et al., 2014). ALCAT1 has been implicated in mitochondrial dysfunction and susceptibility to diet-induced obesity (Li et al., 2010) and its overexpression in C2C12 cells selectively increased docosahexaenoic acid (DHA, 22:6n3)-containing cardiolipin species, while enhancing mitochondrial membrane potential, oxygen consumption rate, and proton leak. Although we did not observe the direct incorporation of dietary DHA into cardiolipin molecular species, we did see a significant increase in mitochondrial proton leak in the fish/pectin treatment in comparison to corn/cellulose in the proximal colon. These data suggest the potential role of ALACT1 in this modulation. Future studies are needed in order to elucidate the contribution of ALACT1 with regard to the role of diet in colon cancer prevention. With regard to the data shown in Table 1, fish oil feeding modulated cardiolipin composition in an indirect manner. This is in contrast to other phospholipid classes associated with mitochondria, e.g., ethanolamine, choline, and inositol-containing glycerophospholipids, which readily incorporate dietary n-3 PUFA (Chapkin et al., 2002; Hong et al., 2002) (Supplemental Tables 3–5). Since glycerophosphoethanolamine (PE) has been reported to play a role in the integrity and organization of the mitochondrial inner membrane through an intricate mechanism involving proteins modulating cristae morphogenesis (Osman et al., 2009), the direct incorporation of n-3 PUFA into PE suggests a potential modulatory role of dietary fat and fiber on colonic mitochondrial function.
Table 1.
Colonic cardiolipin molecular species distribution from mice fed different fat and fiber combinations.
| Molecular Species* |
Fatty Acyl Chain Composition** (pmol/mg) | Corn/Cellulose | Corn/Pectin | Fish/Cellulose | Fish/Pectin |
|---|---|---|---|---|---|
| C68:1 | (C16:0)2/(C18:0)1/(C18:1)1 | 6±1a | 4±1a | 17±3b | 25±9b |
| C68:3 |
(C14:0)1/(C16:1)1/(C18:1)2, (C16:1)1/(C16:0)1/(C18:1)2 (C16:1)2/(C18:0)1/(C18:1)1 |
49±7a | 33±5a | 89±13b | 87±7b |
| C68:4 | (C16:1)2/(C18:1)2 | 44±8a | 41±8a | 194±27b | 174±8b |
| C68:5 | (C16:1)2/(C18:1)1/(C18:2)1 | 55±8a | 37±9a | 218±33b | 180±8b |
| C70:4 |
(C16:1)1/(C18:1)3, (C16:0)1/(C18:1)2/(C18:2)1 (C16:1)1/(C18:0)1/(C18:1)1/(C18:2)1 |
114±14a | 81±18a | 210±27b | 207±16b |
| C70:5 | (C16:0)1/(C18:1)1/(C18:2)2, (C16:1)1/(C18:1)2/(C18:2)1 | 155±27a | 104±27a | 433±65b | 399±29b |
| C70:6 |
(C16:0)1/(C16:1)1/(C18:1)1/(C20:4)1 (C16:1)1/(C18:1)1/(C18:2)2, (C16:0)1/(C18:2)3 |
135±20a | 89±21a | 392±53b | 306±25b |
| C70:7 |
(C16:1)1/(C18:2)3, (C16:1)2/(C18:2)1/(C20:3)1 (C16:1)2/(C18:1)1/(C20:4)1 |
77±13a | 53±12a | 182±29b | 140±14b |
| C72:5 | (C18:0)1/(C18:1)1/(C18:2)2, (C18:1)3/(C18:2)1 | 115±13ab | 71±11a | 163±24b | 138±18b |
| C72:6 |
(C18:1)2/(C18:2)2, (C16:0)1/(C18:1)1/(C18:2)1/(C20:3)1 (C16:0)1/(C18:1)2/(C20:4)1, (C16:0)1/(C18:0)1/(C18:1)1/(C20:5)1 |
363±54ab | 248±59a | 586±83b | 533±47b |
| C74:7 | (C18:1)2/(C18:2)1/(C20:3)1, (C18:1)3/(C20:4)1 | 265±39b | 153±36a | 125±19a | 110±8a |
| C76:7 | (C18:0)1/(C18:1)1/(C20:3)2 | 18±3b | 14±4b | 0±0a | 0±0a |
| C76:8 |
(C16:0)1/(C18:1)1/(C20:3)1/(C22:4)1, (C16:0)1/(C18:0)1/(C20:4)1/(C22:4)1 |
97±7b | 63±17b | 31±7a | 26±5a |
| C76:9 |
(C16:0)1/(C18:1)1/(C20:4)1/(C22:4)1, (C18:1)2/(C18:2)1/(C22:5)1 |
110±14b | 63±18b | 8±7a | 12±9a |
Only statistical significant different species are shown.
A complete set of data is reported in the supplemental table 2 (mean ± SE, n=5).
Values with different superscripts are significantly different (P<0.05).
Fatty acid nomenclature describes (the number of carbon atoms in the fatty acid backbone):(the number of double bonds).
Putative fatty acyl chain compositions were assigned based on known molecular species.
Since disease susceptibility of the colon exhibits an anatomical bias with respect to dietary risk factors and chemoprevention (Mladenova et al., 2011; Hjartaker et al., 2013; Parr et al., 2013), the effect of nutritional combinations on intestinal biology is typically assessed in both the proximal and distal colon. Due to the low level of apoptosis in non-carcinogen treated mice, the apoptotic index is expressed as the total number of apoptotic cells per 100 crypts as previously reported (Davidson et al., 2012), instead of per crypt column. Since higher levels of apoptosis are considered protective against colon cancer (Qiu et al., 2010; Zhang et al., 2010), our dietary fish oil and pectin apoptosis data are consistent with previous reports indicating a reduction in colonic tumors (Chang et al., 1997). Although additional studies are needed to determine why dietary fat and fiber interactively modulate apoptosis and mitochondrial proton leak in a site-specific manner, it is not altogether unexpected based on the fact that the proximal and distal colon exhibit distinct gene-specific methylation profiles, transcriptional profiles, and molecular and clinical characteristics (Deng et al., 2008; Triff et al., 2013; Kaz et al., 2014). These differences may explain why subsite-specific responses to diet are observed (Hjartaker et al., 2013). Regional differences in colonic-mucosa associated microbiota have also been recently described (Hu et al., 2010), which could alter the spectrum of SCFA generated via the fermentation of fiber and thereby modulate the induction of apoptosis along bowel subsites. Although dietary fiber effects on luminal butyrate levels in the rodent colon are similar in the proximal and distal colon (Zoran et al., 1997b), the alteration of membrane FA composition by n-3 PUFA may create a permissive environment for butyrate to modulate mucosal physiology. For example, it has been reported that the highest butyrate oxidation rate occurs in the proximal colon (Topping and Clifton, 2001). Therefore, we propose that the enhanced level of butyrate oxidation in the proximal colon, in combination with the ability of n-3 PUFA to perturb mitochondrial membrane lipid composition, may promote a proton leak related apoptotic phenotype (Ott et al., 2007; Green et al., 2014). This unique dietary site-specific protective effect in the proximal colon may have clinical relevance, in view of the increased incidence of right-sided (proximal) colon cancers and a decreased incidence of rectosigmoid tumors (Cucino et al., 2002; Rabeneck et al., 2003; Lee et al., 2015).
We have previously shown that DHA increases mitochondrial membrane lipid oxidation (Ng et al., 2005), and that the addition of butyrate further potentiates the dissipation of membrane potential which contributes to the induction of apoptosis in colonocytes (Ng et al., 2005). To further investigate the role of mitochondrial metabolism in relation to the chemoprotective combinatorial effects of dietary n-3 PUFA and fermentable fiber, we developed a novel metabolomics model system to measure the BEP in isolated primary colonic crypts from mice fed the experimental diets. This is an important advancement, because it allows one to monitor epithelial cell BEP in a physiologically relevant setting. Since mitochondrial bioenergetics can be perturbed by changes in cardiolipin abundance and composition (Ren et al., 2014), we anticipated that parameters of mitochondrial respiration would also be modulated by diet. The significantly higher levels of OCR and ECAR in the distal colon may be explained by an increase in mitochondrial numbers, larger mitochondria, or enhanced function (Faris et al., 2014). The higher basal respiration profile observed in the distal colon is consistent with a recent report indicating that ATPase gene expression is higher in the distal colon vs. proximal colon (Triff et al., 2013). Based on our apoptosis findings, we hypothesized that the metabolic profile in intact crypts isolated from fish oil and pectin fed mice would be altered in a manner consistent with a more proapoptotic phenotype, e.g., increased proton leak (Ott et al., 2007; Green et al., 2014). Indeed, proximal colonic crypts from mice fed the fish oil/pectin combination diet exhibited an increased proton leak (Figure 4), which was associated with an induction of apoptosis (Figure 3). These observations are consistent with previous findings indicating that mitochondrial proton leak can contribute to the induction of apoptosis (Ott et al., 2007; Green et al., 2014).
The results of this study are consistent with recent evidence that the pescovegetarian dietary pattern is highly protective against all forms of colorectal cancer (Orlich et al., 2015). Given the critical nature of apoptosis in cancer prevention, and the fact that inhibition of apoptosis is an integral component in the initiation and progression of cancer, it is imperative to elucidate the precise mechanisms by which dietary fish oil and fermentable fiber reduce colon tumorigenesis. Our results add to a growing body of evidence that fish oil alters colonocyte mitochondrial membrane composition and function, thereby creating a permissive environment for apoptosis induced by fiber fermentation products, such as butyrate.
Supplementary Material
Acknowledgments
This work was supported in part by the American Institute for Cancer Research and NIH grants R35CA197707 and P30ES023512.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- Ben Q, Sun Y, Chai R, Qian A, Xu B, Yuan Y. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology. 2014;146:689–699. e686. doi: 10.1053/j.gastro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- Bordonaro M, Lazarova DL. CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer. World J Gastroenterol. 2015;21:8238–8248. doi: 10.3748/wjg.v21.i27.8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Hong MY, Fan YY, Davidson LA, Sanders LM, Henderson CE, et al. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- Cho Y, Kim H, Turner ND, Mann JC, Wei J, Taddeo SS, et al. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J Nutr. 2011;141:1029–1035. doi: 10.3945/jn.110.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, Chapkin RS, et al. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis. 2008;29:1415–1420. doi: 10.1093/carcin/bgn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Goldsby JS, Callaway ES, Shah MS, Barker N, Chapkin RS. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta. 2012;1822:1600–1607. doi: 10.1016/j.bbadis.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Kakar S, Tanaka H, Matsuzaki K, Miura S, Sleisenger MH, et al. Proximal and distal colorectal cancers show distinct gene-specific methylation profiles and clinical and molecular characteristics. Eur J Cancer. 2008;44:1290–1301. doi: 10.1016/j.ejca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Davidson LA, Callaway ES, Goldsby JS, Chapkin RS. Differential effects of 2- and 3-series E-prostaglandins on in vitro expansion of Lgr5+ colonic stem cells. Carcinogenesis. 2014;35:606–612. doi: 10.1093/carcin/bgt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Davidson LA, Callaway ES, Wright GA, Safe S, Chapkin RS. A bioassay to measure energy metabolism in mouse colonic crypts, organoids, and sorted stem cells. Am J Physiol Gastrointest Liver Physiol. 2015;309:G1–G9. doi: 10.1152/ajpgi.00052.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Ran Q, Toyokuni S, Okazaki Y, Callaway ES, Lupton JR, et al. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev Res (Phila) 2011;4:1267–1274. doi: 10.1158/1940-6207.CAPR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris R, Fan YY, De Angulo A, Chapkin RS, deGraffenried LA, Jolly CA. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential for murine CD4(+) T cell metabolic activation. Biochim Biophys Acta. 2014;1842:1475–1482. doi: 10.1016/j.bbalip.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14:994–1008. doi: 10.2174/1389200211314090006. [DOI] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjartaker A, Aagnes B, Robsahm TE, Langseth H, Bray FLarsen IK. Subsite-specific dietary risk factors for colorectal cancer: a review of cohort studies. J Oncol. 2013;2013:703854. doi: 10.1155/2013/703854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, et al. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- Houtkooper RH, Rodenburg RJ, Thiels C, van Lenthe H, Stet F, Poll-The BT, et al. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal Biochem. 2009;387:230–237. doi: 10.1016/j.ab.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang Y, Lichtenstein L, Tao Y, Musch MW, Jabri B, et al. Regional differences in colonic mucosa-associated microbiota determine the physiological expression of host heat shock proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1266–G1275. doi: 10.1152/ajpgi.00357.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaz AM, Wong CJ, Dzieciatkowski S, Luo Y, Schoen RE, Grady WM. Patterns of DNA methylation in the normal colon vary by anatomical location, gender, and age. Epigenetics. 2014;9:492–502. doi: 10.4161/epi.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol. 2015;41:300–308. doi: 10.1016/j.ejso.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010;12:154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton JR, Kurtz PP. Relationship of colonic luminal short-chain fatty acids and pH to in vivo cell proliferation in rats. J Nutr. 1993;123:1522–1530. doi: 10.1093/jn/123.9.1522. [DOI] [PubMed] [Google Scholar]
- Mladenova D, Daniel JJ, Dahlstrom JE, Bean E, Gupta R, Pickford R, et al. The NSAID sulindac is chemopreventive in the mouse distal colon but carcinogenic in the proximal colon. Gut. 2011;60:350–360. doi: 10.1136/gut.2010.208314. [DOI] [PubMed] [Google Scholar]
- Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, et al. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlich MJ, Singh PN, Sabate J, Fan J, Sveen L, Bennett H, et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med. 2015;175:767–776. doi: 10.1001/jamainternmed.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Haag M, Potting C, Rodenfels J, Dip PV, Wieland FT, et al. The genetic interactome of prohibitins: coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol. 2009;184:583–596. doi: 10.1083/jcb.200810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Parr CL, Hjartaker A, Lund E, Veierod MB. Meat intake, cooking methods and risk of proximal colon, distal colon and rectal cancer: the Norwegian Women and Cancer (NOWAC) cohort study. Int J Cancer. 2013;133:1153–1163. doi: 10.1002/ijc.28101. [DOI] [PubMed] [Google Scholar]
- Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010;107:20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabeneck L, Davila JA, El-Serag HB. Is there a true "shift" to the right colon in the incidence of colorectal cancer? Am J Gastroenterol. 2003;98:1400–1409. doi: 10.1111/j.1572-0241.2003.07453.x. [DOI] [PubMed] [Google Scholar]
- Ren M, Phoon CK, Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res. 2014;55:1–16. doi: 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Schlame M. Cardiolipin remodeling and the function of tafazzin. Biochim Biophys Acta. 2013;1831:582–588. doi: 10.1016/j.bbalip.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Mostoslavsky R. Untangling the fiber yarn: butyrate feeds Warburg to suppress colorectal cancer. Cancer Discov. 2014;4:1368–1370. doi: 10.1158/2159-8290.CD-14-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2012;15:122–126. doi: 10.1097/MCO.0b013e32834fdaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrovskaya IG, Bird SS, Marur VR, Sniatynski MJ, Baranov SV, Greenberg HK, et al. Dietary macronutrients modulate the fatty acyl composition of rat liver mitochondrial cardiolipins. J Lipid Res. 2013;54:2623–2635. doi: 10.1194/jlr.M036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Triff K, Konganti K, Gaddis S, Zhou B, Ivanov I, Chapkin RS. Genome-wide analysis of the rat colon reveals proximal-distal differences in histone modifications and proto-oncogene expression. Physiol Genomics. 2013;45:1229–1243. doi: 10.1152/physiolgenomics.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Hayashi Y, Matsumoto N, Nemoto-Sasaki Y, Oka S, Tanikawa T, et al. Glycerophosphate/Acylglycerophosphate acyltransferases. Biology (Basel) 2014;3:801–830. doi: 10.3390/biology3040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464:1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran DL, Barhoumi R, Burghardt RC, Chapkin RS, Lupton JR. Diet and carcinogen alter luminal butyrate concentration and intracellular pH in isolated rat colonocytes. Nutr Cancer. 1997a;27:222–230. doi: 10.1080/01635589709514530. [DOI] [PubMed] [Google Scholar]
- Zoran DL, Turner ND, Taddeo SS, Chapkin RS, Lupton JR. Wheat bran diet reduces tumor incidence in a rat model of colon cancer independent of effects on distal luminal butyrate concentrations. J Nutr. 1997b;127:2217–2225. doi: 10.1093/jn/127.11.2217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.