Abstract
A seamless tube is a very narrow-bore tube that is composed of a single cell with an intracellular lumen and no adherens or tight junctions along its length. Many capillaries in the vertebrate vascular system are seamless tubes. Seamless tubes also are found in invertebrate organs, including the Drosophila trachea and the C. elegans excretory system. Seamless tube cells can be less than a micron in diameter, and they can adopt very simple “doughnut-like” shapes or very complex, branched shapes comparable to those of neurons. The unusual topology and varied shapes of seamless tubes raise many basic cell biological questions about how cells form and maintain such structures. The prevalence of seamless tubes in the vascular system means that answering such questions has significant relevance to human health. In this review, we describe selected examples of seamless tubes in animals and discuss current models for how seamless tubes develop and are shaped, focusing particularly on insights that have come from recent studies in Drosophila and C. elegans.
1. What are seamless tubes?
Organs are composed of tubes with different sizes and shapes that are specialized for their particular functions [1]. Most tubes are composed of polarized epithelial or endothelial cells that have an apical surface facing the lumen and a basal surface facing other tissues, and that are linked together by adherens junctions and tight junctions. Branched organs, such as the mammalian vascular system, lung, and kidney, typically consist of centrally located, larger-bore tubes that transport fluids or gas over long distances, and progressively narrower tubes at the periphery that exchange nutrients and waste with nearby tissues.
Seamless tubes are very narrow-bore tubes that are only one cell in diameter, such that the apical domain and lumen are intracellular (Fig. 1). As their name implies, such tubes do not have adherens or tight junctions along their length, although they do have such junctions where they connect to other tubes. Seamless tubes are found in the vertebrate vascular system (Fig. 1A, B) [2] and in many invertebrate organs and glia [3–7], as well as in plants (e.g. pollen tubes [8]). Many insights into how seamless tubes are built and shaped have come from studies of such tubes in the Drosophila trachea (Fig. 1C) and C. elegans excretory system (Fig. 1D), where imaging is possible at the necessary single-cell resolution, and the power of forward genetics has been applied to identify relevant molecular mechanisms.
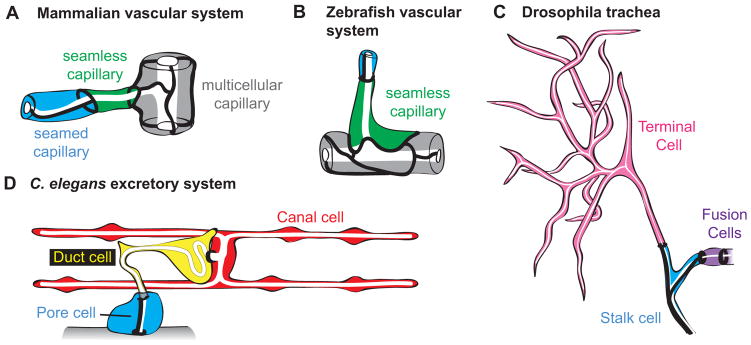
Figure 1. Examples of seamless tubes.
(A, B) Vertebrate capillaries are a mix of multicellular, seamed unicellular and seamless tubes. Drawings of adult rat and embryonic zebrafish capillaries based on [2] and [15]. (C) The Drosophila trachea contains two seamless tube types, terminal cells and fusion cells. Drawing of third instar larval branch tip based on [31]. (D) The C. elegans excretory system contains two seamless tube types, the canal cell and duct. Drawing of early L1 larval excretory system based on [41] and [84].
2. Examples of seamless tubes
2.1. Seamless tubes in the vertebrate vascular system
Unicellular seamed and seamless tubes make up a sizeable proportion of the capillaries in the vertebrate microvasculature. Decades ago, serial section transmission electron microscopy (TEM) studies of tissues from rats and other mammals revealed many capillaries with only a single cell surrounding the lumen [2, 9, 10]. Some such capillaries had an autocellular junction or “seam” that sealed the tube, but others had no darkly staining junctional material between the lumen and the plasma membrane, and were termed “seamless” (Fig. 1A). Many of these tubes were found at anastomoses or branch points in the capillary network. More recently, seamless tubes have been observed by confocal microscopy in the mouse and zebrafish vasculature (Fig. 1B) [11, 12]. In zebrafish, where live imaging has been possible, some seamless tubes are transient, developmental precursors to larger multicellular tubes or to pruned vessels [13–16]. However, in mammals, TEM studies suggested that as many as 30% – 50% of the tubes in capillary beds of adults can be seamless tubes [10].
Not surprisingly, capillary defects are implicated in various cardiovascular diseases. These include common ailments such as hypertension [17], coronary microvascular disease (MVD), a leading cause of heart attacks, especially in women [18] and cerebral Small Vessel Disease (SVD), a leading contributor to age-related dementia and stroke [19]. Loss or abnormal structure of capillary beds is also observed in Mendelian syndromes such as Hereditary Hemorrhagic Telangiecstasia (HHT) [20], Cerebral Cavernous Malformation (CCM) [21], and familial SVD syndromes like CADASIL [19] (Table 1). Genes involved in these Mendelian syndromes point to dysregulation of TGFβ signaling, vesicle trafficking, cytoskeletal organization and extracellular matrix (ECM) as key factors in capillary malfunction (Table 1).
Table 1.
Mendelian diseases associated with capillary abnormalities
| Disease | Affected Gene Product(s) | OMIM entries |
|---|---|---|
| Cerebral cavernous malformation (CCM) | CCM1/Krit1 CCM2/malcavernin CCM3/PDCD10 |
OMIM: 116860; 603284; 603285 |
| Hereditary Hemorrhagic Telangiecstasia (HHT) | HHT1/Endoglin HHT2/ALK1/ACVRL1 HHT5/GDF2/BMP9 |
OMIM: 187300; 600376; 615506 |
| Juvenile Polyposis with Hereditary Hemorrhagic Telangiecstasia (JP/HHT) | SMAD4 | OMIM: 175050 |
| Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) | Notch3 | OMIM: 125310 |
| Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) | HTRA1 serine protease | OMIM: 600142 |
| COL4A1/A2-related angiopathies | COL4A1 COL4A2 |
OMIM: 607595; 614519 |
| Retinal vasculopathy with cerebral leukodystrophy (RVCL) | TREX1 exonuclease | OMIM: 192315 |
Due to their small size and density, mammalian capillaries are difficult to visualize in vivo. Therefore it is not known if the pathology of these diseases specifically involves seamless tubes. However, some aspects of capillary development can be modeled using human umbilical cord endothelial cells (HUVECs) grown in a 3D collagen matrix [22]. CCM1 and CCM2 are required for intracellular lumen formation in such assays [23, 24]. A zebrafish model of CCM1 [23] and invertebrate models of CCM3 [25, 26] also show seamless tube defects, supporting a connection between capillary diseases and seamless tubes.
2.2. Seamless tubes in the Drosophila tracheal system
The Drosophila trachea is a branched respiratory organ with ~1600 cells and several different types of tube topologies [6, 27]. The largest tubes are multicellular. Smaller branches contain seamed unicellular tubes, called stalk cells. At the tips of most branches are two seamless tube types: fusion cells and terminal cells (Figs. 1C, 2A). Fusion cells are very short, simple toroids that undergo anastomosis to connect different branches in the network [28, 29]. Anastomosis fuses the apical membrane but does not remove junctions, so fusion cells have ring-shaped adherens junctions at both ends, where they connect to stalk cells and to each other (Fig. 1C). Terminal cells form complex, highly branched structures with narrow, lumenized processes that are closed at their tips and contact target tissues for gas exchange [6, 30] (Fig. 1C). A terminal cell has a ring-shaped adherens junction at its base, where it connects to a stalk cell, and sometimes a small stretch of autocellular junction (Fig. 2B).
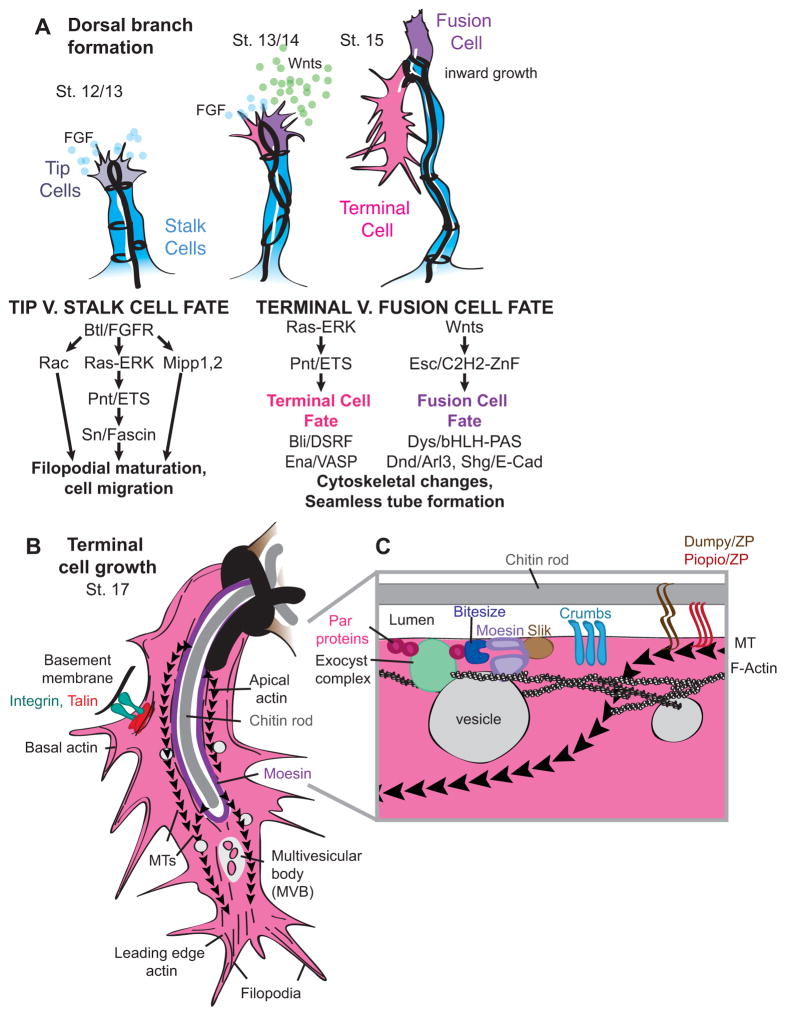
Figure 2. Seamless tube formation and growth in the Drosophila tracheal system.
(A) Developmental timeline for one dorsal branch [6] and roles of FGF and Wnt signaling. Heavy black lines represent junctions. FGF signaling promotes tip cell identity (gray) [31] and expression or activity of Singed/Fascin [37], Mipp 1 and 2 [34], and Rac [36], which promote filopodia maturation leading to tip cell migration and outgrowth. The FGFR target Pointed (Pnt) promotes terminal cell identity (pink) [6, 31] and expression of the DSRF transcription factor for tube morphogenesis [106]. DSRF upregulates Enabled (Ena) to promote actin filament elongation [35]. Wnt signaling promotes Escargot (Esc) expression and fusion cell identity (purple) [33]. Fusion cells express the transcription factor Dysfusion (Dys) and upregulate Deadend (Dnd)/Arl3 and Shotgun/E-cadherin to promote anastomosis [71, 72, 107, 108]. The terminal cell turns ventrally and builds lumen starting at the stalk cell interface [35]. The fusion cell extends filopodia dorsally in search of its partner fusion cell [28]. (B) Structure and organization of a growing terminal cell. Multivesicular bodies are present near the leading edge at later stages [55]. Actin is found along the apical and basal membranes and at the leading edge [35, 79]. Microtubules are nucleated along the apical membrane, and extend toward the leading edge [35, 50, 94]. The (−) end directed MT motor dynamin is required for lumen growth [50]. Moesin organizes actin at the apical membrane [80]. Integrins link the basal cell membrane to the basal ECM [45]. (C) Model for terminal cell apical domain organization. Bitesize and Crumbs recruit Moesin to the apical membrane [53, 80], where it is phosphorylated and activated by Slik [38]. Moesin then recruits actin to promote apical-directed trafficking. PAR proteins recruit the exocyst complex for vesicle docking [48]. A chitin rod determines lumen diameter [86, 87, 97, 98]. The ZP proteins Dumpy (Dpy) and Piopio (Pio) may link the chitin rod to the apical membrane and cytoskeleton [60, 93–95].
Fibroblast Growth Factor Receptor (FGFR) signaling promotes tip cell vs. stalk cell identity and terminal cell identity [31, 32] (Fig. 2A). Wnt signaling promotes fusion cell identity [33] (Fig. 2A). Signaling leads to many changes in cytoskeletal organization [34–38] (Fig. 2A), and these cytoskeletal changes may be important for the ultimate formation of seamless tubes.
2.3. Seamless tubes in the C. elegans excretory system
The excretory system of C. elegans is a simple osmoregulatory organ that contains just three tandemly-connected unicellular tubes, two of which are seamless [4, 39] (Fig. 1D). The H-shaped canal cell has four long, seamless, lumenized branches or “canals” that are closed at their tips [40]. The canals merge within the cell body in the head of the worm, where the cell makes a ring-shaped junction to the duct. The duct is a smaller seamless tube with an asymmetric and looping shape [41]. The duct empties into the seamed pore tube, which connects to the outside environment for fluid excretion. The duct has ring-shaped apical junctions at both ends, where it connects to the canal cell and pore.
Epidermal Growth Factor Receptor (EGFR) signaling promotes duct vs. pore cell identity [42], and specifically promotes duct tube seamlessness via upregulation of the fusogen AFF-1 [41] (F. Soulavie and M. Sundaram, unpublished data) (Fig. 3A). Notch signaling and a series of asymmetric cell divisions promote canal cell identity [43].
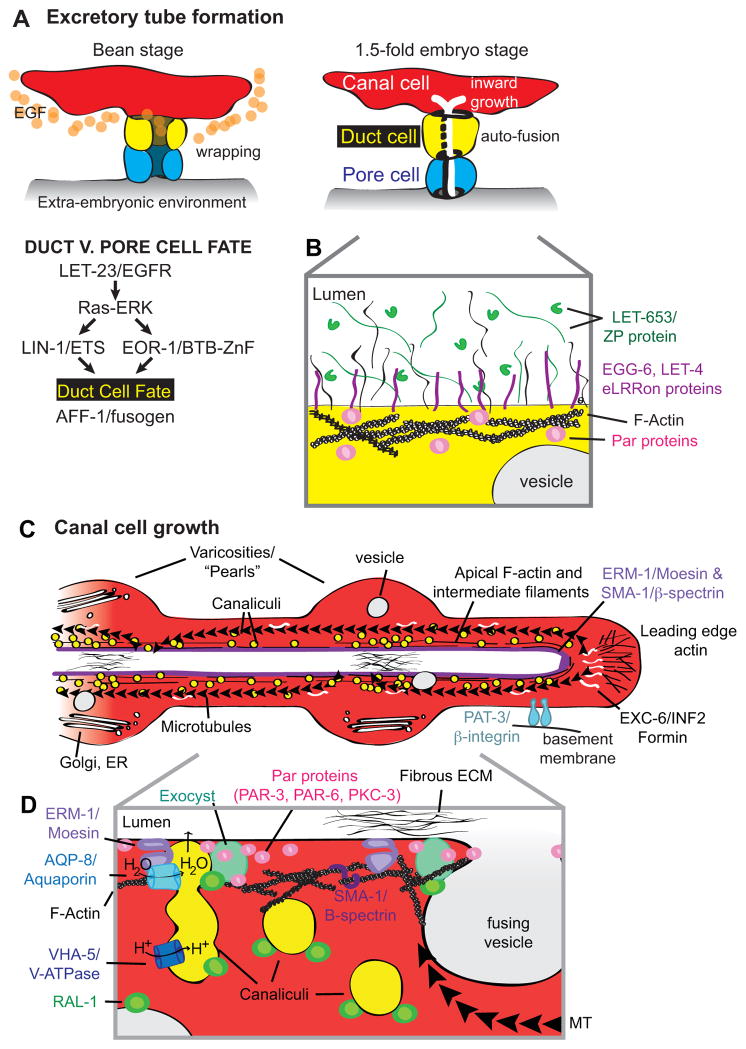
Figure 3. Seamless tube formation and growth in the C. elegans excretory system.
(A) Developmental timeline and duct fate specification by EGF signaling [42]. The canal cell expresses LIN-3/EGF. EGF-Ras-ERK signaling acts via LIN-1/Ets and EOR-1/BTB-Zinc finger transcription factors to promote the duct fate and a top, canal-proximal position. The other cell adopts the pore fate. The duct and pore cells epithelialize and wrap themselves into tubes. Both the duct and pore initially form auto-junctions, but the duct expresses AFF-1 and auto-fuses [41]. The canal cell lumen originates at the duct-canal junction and grows inward. (B) Model for luminal matrix organization in the duct cell. During embryogenesis, the duct lumen is filled with a fibrous ECM containing the ZP and mucin-like protein LET-653 (H. Gill, J. Cohen and M. Sundaram, unpublished data). The eLRRon proteins LET-4 and EGG-6 may interact with one or more components of this matrix [49]. (C) Structure and organization of a growing canal arm. Canalicular vesicles are plentiful along the apical region; other vesicle types are present more sparsely [4, 59]. Cytoplasmic swellings, or varicosities, form periodically along the canal arms during growth or recovery from osmotic stress, and canaliculi preferentially dock to the apical membrane in these regions [74]. There are two major pools of actin: apical and at the leading edge [59, 70]. Intermediate filaments also line the apical membrane [74]. MTs are nucleated primarily at the leading edge, where they are linked to actin via the formin EXC-6 [70], and extend back toward the cell body. The kinesin motor promotes lumen growth [70]. ERM-1/moesin and SMA-1/β-spectrin recruit and stabilize actin at the apical membrane [40, 59]. Integrins link the basal cell membrane to the basal ECM [46]. A fibrous apical ECM is sometimes observed [40]. (D) Model for apical membrane addition and lumen growth in the canal cell [47, 59, 74]. Canalicular vesicles contain the V-ATPase and the aquaporin AQP-8; they dock at the apical membrane to allow water influx and, potentially, membrane addition. Other vesicles may also contribute apical membrane. ERM-1 recruits an actin coat to vesicles and binds AQP-8 to promote canaliculi docking. RAL-1 on vesicles promotes fusion by interacting with apical PAR proteins to recruit the exocyst.
3. Mechanisms of Seamless Tube Formation
3.1. Polarization
Seamless tube formation requires the cell to establish an unusual pattern of apical-basal polarity, with the apical domain inside the cell. The initial cues that establish such polarity are still unclear. Basal ECM factors may provide cues, since growing HUVEC cells in a 3D collagen matrix promotes intracellular lumen formation, and this requires integrin-ECM interactions [44]. Although integrins can affect terminal cell and canal tube shape [45, 46], they are not essential for polarization and intracellular lumen formation. In vivo, seamless tubes form in contexts where a cell makes ring-shaped adherens junctions on either one or two sides (Figs. 1,2A, 3A, 4C), so neighboring tubes might provide a polarizing cue. Several apical polarity proteins localize to apical domains of developing seamless tubes [47–51]. PAR-6 and PKC (but not Bazooka/PAR-3 or Crumbs) are required for lumen growth in the terminal cell [48, 52, 53].
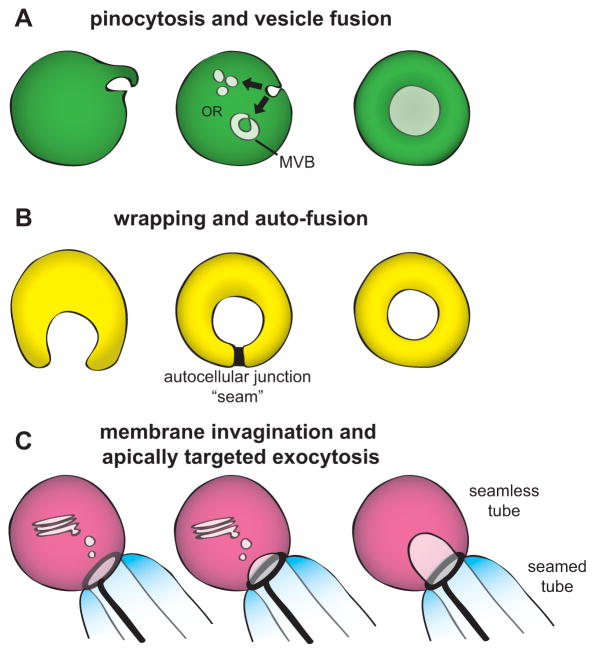
Figure 4. Models for seamless tube formation.
(A) Pinocytosis. (B) Wrapping and auto-fusion. (C) Membrane invagination and apically directed exocytosis. See text for details and references.
Once polarity has been established to nucleate lumen formation, intracellular vesicle trafficking must direct apical membrane and other relevant molecules to the appropriate region for lumen growth. Both endocytic and exocytic trafficking pathways have been implicated in seamless tube formation and morphogenesis, as discussed below.
3.2. Pinocytosis
One mechanism for seamless tube formation involves pinocytosis (“cell drinking”) or macropinocytosis (“cell gulping”), related types of membrane ruffling-associated endocytosis in which the cell internalizes basal plasma membrane to form internal vesicles [54]. These vesicles are proposed to coalesce to form the intracellular apical domain, which then connects to other tubes via anastomosis [11, 22, 44, 55]. This mechanism of tube formation is sometimes called “cell hollowing” (Fig. 4A).
The pinocytic model is based primarily on observations of cultured HUVEC or other mammalian endothelial cells [22]. The cells form large intracellular vacuoles that can be labeled with an exogenously applied dextran-fluorescein tracer, indicating some form of fluid phase uptake [44]. Vesicles have been observed moving from a basal site of origin to the intracellular vacuole [22]. Molecules required for vacuole formation include several regulators of macropinocytosis, including Rac and Pak [56, 57].
In vivo evidence to support the pinocytic model has been more limited. Various large intracellular vacuoles or multivesicular bodies (MVBs) have been detected near the growing lumen of vertebrate capillaries [9, 11], Drosophila terminal cells [55] and the C. elegans canal cell [4, 58], and have been speculated to derive from pinocytosis and contribute to the apical membrane. However, the origin of these vacuoles has not been determined definitively. Apical markers are generally not observed in any large or abundant vesicular compartments in wild-type animals, suggesting that the seamless lumen does not form from the coalescence of pre-established apical compartments [35, 59]. Rather, membrane may acquire apical characteristics only at late stages of vesicle targeting to the lumen.
Molecular regulators of endocytosis have varied effects on seamless tube growth in vivo. Rab11, a marker of recycling endosomes [54], promotes lumen growth in the terminal and canal cells [26, 48]. Early endocytic regulators, such as Shibire/Dynamin, Rab5, Vps45 and Rabenosyn-5, negatively regulate apical growth in terminal cells [53].
3.3. Wrapping and auto-fusion
A second mechanism for seamless tube formation is wrapping and auto-fusion (Fig. 4B). In this mechanism, a cell first forms a seamed tube of defined lumen diameter by wrapping around ECM [60] or a cellular scaffold [5] and forming an autocellular junction. The tube then eliminates its seam by membrane fusion. This mechanism is used by C. elegans pharyngeal valve cells [5] and the excretory duct (Fig. 3A) [41]. Conceptually, wrapping and auto-fusion is similar to macropinocytosis, except that it involves fusion rather than fission of a large segment of membrane, an extracellular scaffold, and the formation of a transient adherens junction; it is not known if similar molecular controls underlie the two mechanisms.
Discovery of this mechanism in C. elegans was enabled by identification of the relevant fusogens [61, 62]. EFF-1 and AFF-1 are transmembrane proteins with structural, but not primary sequence, homology to viral class II fusogens [63, 64]. Homotypic interactions between fusogens on apposing plasma membranes mediate cell-cell fusion to generate many syncytial tissues in the worm [62, 65]. In eff-1 or aff-1 mutants, normally seamless tubes such as the duct now have a seam, revealing that these fusogens can also mediate tube auto-fusion [5, 41].
Wrapping and auto-fusion also generate some seamless tubes in the zebrafish vasculature [15] and in mammalian Madin-Darby Canine Kidney (MDCK) epithelial cells grown around silicone pegs [66], suggesting this mechanism may be widely used. MDCK auto-fusion requires cytoskeletal and trafficking regulators such as Rac, Rho, Cdc42 and the Arp2/3 complex [67], but the relevant fusogen is unknown.
3.4. Membrane invagination and apically-directed exocytosis
A third mechanism for seamless tube formation is membrane invagination coupled with apically-directed exocytosis (Fig. 4C). Evidence for this mechanism comes from studies of Drosophila fusion and terminal cells, the C. elegans canal cell, and anastomosing zebrafish vascular cells. In all of these tube types, lumen formation initiates at an intercellular junction with another tube (Figs. 2A, 3A) [13, 14, 41]. Lumen then grows inward in a manner dependent on F-actin and/or microtubules (MTs), which appear to provide routes for motor-directed vesicle trafficking [35, 50, 59, 68–70]. Where examined, lumen growth requires the exocyst [47, 48, 71, 72], a complex that tethers secretory vesicles to the plasma membrane for subsequent SNARE-dependent fusion [73]. Models for cytoskeletal organization and trafficking in the terminal cell and canal cell are shown in Figs. 2B–C, 3C–D. See [29] for a recent review of fusion cell formation
A current challenge is to elucidate the specific pathways and classes of vesicles that deliver apical membrane and luminal cargos. A striking feature of the canal cell is the presence of abundant 50–100 nm tubulo-vesicles, termed canaliculi, which fill the canal cytoplasm adjacent to the lumen (Fig. 3C–D) [4]. The origin of canaliculi is unknown, but canaliculi can dock with the apical membrane and may contribute membrane and/or water to drive lumen expansion (Fig. 3D) [47, 59, 74]. Going forward, live imaging will be an important tool for understanding the origin and role of canaliculi and the various other classes of vesicles that have been observed.
3.5. Combinatorial models
Although different seamless tubes may form by different mechanisms, the three major models discussed above are not mutually exclusive. It is likely that both endocytic and Golgi-derived exocytic vesicles contribute to the growing apical domain of most seamless tubes, although their relative importance could vary.
Within a single tube cell, initial lumen nucleation and subsequent lumen growth could also proceed by different mechanisms. For example, the excretory duct tube continues to grow extensively after initial wrapping and auto-fusion (Fig 1D, 3A) [41]. Several trafficking-related genes are required only for later outgrowth and maintenance of the terminal cell or canal cell, but not for initial tube formation [25, 26, 75, 76]. Terminal cells might nucleate lumen via a wrapping-like mechanism, since some have a small stretch of auto-junction near the cell’s base (Fig. 2B), which usually disappears as the cells mature [6, 25, 52].
4. Seamless tube shaping and maintenance
Seamless tubes exhibit varied shapes, including very elongated and branched shapes, as exemplified by terminal cells and the canal cell. The absence of junctions along seamless tubes presents special challenges for tube shaping and maintenance. In multicellular tubes and planar epithelia, junctions play important roles in polarity, trafficking, cytoskeletal organization and in generating and transmitting forces for tissue shaping [77, 78]. In seamless tubes, junctions are likely important during initial tube formation (see above), but as the tubes elongate further away from the junction, they must rely on other, junction-independent mechanisms for shaping and strength.
4.1 Apical vs. basal trafficking
The amount of apical vs. basal outgrowth in a seamless tube will affect its shape. For example, fusion cells generate relatively little apical membrane compared to basal membrane [68]; as a result, the lumen and its associated cytoskeleton may be under tension and exert a pulling force on the attached stalk cells [29], leading to a “finger in a balloon” topology [27]. On the other hand, duct apical outgrowth surpasses basal outgrowth, leading to the characteristic looped path of the lumen in the cell (Fig. 1D) [4, 41]. Molecular mechanisms that restrain or promote apical vs. basal growth in these cells are not yet known.
In the terminal and canal cells, apical and basal outgrowth are closely coordinated; the basal domain grows out first, and the apical domain follows shortly behind [35, 74]. Actin and MT filaments at the leading edge appear to link the apical and basal membranes to coordinate trafficking activity and hold the lumen in place [35, 45, 70, 79]. Defects in branch outgrowth and lumen outgrowth are often coupled, but some mutations disrupt this coordination, and lead to long, convoluted lumens out of register with the basal membrane, or to multiple separate and disorganized lumens. Such mutants affect cytoskeletal organization and/or specific trafficking regulators, such as Rab35 or Cdc42, that may direct vesicles to appropriate locations for membrane fusion [38, 50, 70, 75, 80–82].
Continuous endocytosis and recycling of apical membrane are important for lumen shaping and maintenance. In terminal cells, mutations in early endocytic regulators or in the CCM3-STRIPAK complex cause an over-accumulation of apical membrane markers and result in focal dilations and cysts [25, 53]. In the canal cell, CCM3-STRIPAK and the EXC-1/5/9 pathway both regulate CDC-42 activity to affect recycling endosome trafficking for apical membrane maintenance [26, 75].
Puzzlingly, CCM3 promotes apical growth in the canal cell [26], but inhibits apical growth in terminal cells [25]. Another discrepancy is in the role of the vacuolar ATPase (V-ATPase). The V-ATPase is a proton pump involved in vesicle acidification; it plays multiple roles in vesicle trafficking, fusion and lysosomal degradation [83]. Both C. elegans and Drosophila V-ATPase mutants accumulate excess MVBs, consistent with a trafficking defect, but the V-ATPase restrains lumen growth in the canal cell [55, 81, 82, 84], yet promotes lumen growth in terminal cells [52, 55]. The mechanistic basis for these discrepancies is not known, but they could reflect differences in MT orientation (Figs. 2C, 3D), differences in junction organization at the cell base [25], or differences in the types of vesicles that contribute to the lumen of each cell [55].
4.2. The apical cytoskeleton and the apical ECM
The cytoskeleton is a major determinant of cell shape, and some effects of manipulating actin or MTs in seamless tubes may be due to changes in structural properties of cytoskeletal support networks [40]. The canal apical cytoskeleton also contains intermediate filaments [74], which generally provide mechanical strength to tissues [85].
Most developing tubes secrete a mix of fibril-forming and gel-forming molecules into their lumens, and studies in the fly and worm showed that this apical ECM influences lumen shape in tubes of all sizes [49, 86–88]. Zona Pellucida (ZP)-domain containing proteins are common constituents of these matrices [89]. Interestingly, the capillary disease-associated ZP protein HHT1/endoglin (Table 1) is a component of the endothelial luminal matrix [90, 91]. The apical ECM can affect signaling [92] and may be physically linked to the cytoskeleton [93–96], but its mechanisms of action and effects on seamless tubes are just beginning to be explored.
In the fly trachea, a transient luminal ECM containing chitin and ZP proteins is present throughout tube formation and morphogenesis (Fig. 2B, C), but is then cleared during cuticle maturation (reviewed in [86, 87]). Mutants for Expansion, a gene required for chitin deposition, revealed large luminal dilations in terminal cells, but not fusion cells, suggesting differential importance of the luminal ECM in shaping these two seamless tube types [97, 98]. Mutations in the receptor tyrosine phosphatases Ptp4E and Ptp10D [99] or the lipid transport protein Mtp [100] result in similar terminal cell cysts, suggesting signaling and lipids might also affect matrix organization.
In the C. elegans excretory duct and pore cells, a transient luminal ECM is also present throughout tube formation and morphogenesis, but then cleared during cuticle maturation [49, 101]. One component of the luminal ECM is the secreted ZP protein LET-653 [102] (Fig. 3B), which is critical for maintaining duct lumen integrity during elongation (H. Gill, J. Cohen and M. Sundaram, unpublished data). Mutants for the lipocalin LPR-1 [41], or for the extracellular leucine-rich repeat only (eLRRon) transmembrane proteins LET-4 or EGG-6 [49], exhibit duct defects very similar to those of let-653 mutants, and may also affect matrix organization (Fig. 3B).
The developing canal cell also contains a luminal matrix of unknown composition and function [40] (Fig 3C). The canal cell does not make cuticle and does not express LET-653 (H. Gill, J. Cohen and M. Sundaram, unpublished data), but does express a different ZP protein, DYF-7 [103].
4.3. The seamless-seamed tube junction
Seamless tubes often connect to seamed tubes at their origin (Fig. 4C). At such a connection point, a ring-shaped junction shared by two different cells is contacted by a linear autocellular junction. The topology of this junction is similar to that of a tricellular junction, in which three linear junctions converge at a single point. Specific proteins localize to, and stabilize, tricellular junctions [104, 105]. Whether these proteins localize to or play a role in the stability of seamless-seamed tube junctions remains to be determined. Interestingly, a number of mutations, including those affecting Drosophila CCM3-STRIPAK, specifically affect lumen shape and integrity near a seamless-seamed tube junction [25, 41, 49](H. Gill, J. Cohen and M. Sundaram, unpublished data), suggesting this region is particularly fragile, and that the pathology of human capillary diseases might involve such junctions.
5. Summary and future challenges
Seamless tubes are found in many multicellular organisms, including mammals. The formation and maintenance of these tiny tubes present unique challenges. Recent studies in zebrafish, flies and worms have made great strides in visualizing the processes that build seamless tubes and in identifying specific genes required for their shaping and integrity. Cells can form seamless tubes through macropinocytosis, wrapping and auto-fusion, or apically directed exocytosis, but many questions remain about these mechanisms. What cues trigger inside/outside polarity in seamless tubes? Do endocytic and/or exocytic vesicles nucleate and expand the lumen? What are the relevant trafficking pathways involved? How are apical and basal growth coordinated for tube shaping? Through what mechanisms does the luminal ECM affect tube formation, shaping and maintenance? Finally, why do seamless tubes exist? Are they stronger and less leaky than seamed tubes of comparable diameter? Does the absence of junctions free them to adopt more complex shapes?
Ultimately, a very important challenge is to relate the findings in model systems to human microvascular diseases. Do such diseases specifically disrupt the formation or maintenance of seamless tubes or do they affect narrow tubes more generally? In model systems, it is possible to identify genetic manipulations that ameliorate unicellular tube defects; could such manipulations suggest strategies for treating microvascular disease?
Acknowledgments
We thank Amin Ghabrial, John Murray, Janis Burkhardt and members of our laboratory for helpful discussions and comments on the manuscript. M.S. was supported by grants from NIH (R01GM58540) and NSF (1257879).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 2.Wolff JR, Bar T. ‘Seamless’ endothelia in brain capillaries during development of the rat’s cerebral cortex. Brain Research. 1972;41:17–24. doi: 10.1016/0006-8993(72)90613-0. [DOI] [PubMed] [Google Scholar]
- 3.Freeman MR. Drosophila Central Nervous System Glia. Cold Spring Harbor perspectives in biology. 2015 doi: 10.1101/cshperspect.a020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson FK, Albert PS, Riddle DL. Fine structure of the C. elegans secretory-excretory system. J Ultrastruct Res. 1983;82:156–71. doi: 10.1016/s0022-5320(83)90050-3. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen JP, English K, Tenlen JR, Priess JR. Notch signaling and morphogenesis of single-cell tubes in the C. elegans digestive tract [see comment] Developmental Cell. 2008;14:559–69. doi: 10.1016/j.devcel.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 7.Shaham S. Glial development and function in the nervous system of Caenorhabditis elegans. Cold Spring Harbor perspectives in biology. 2015;7:a020578. doi: 10.1101/cshperspect.a020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geitmann A. How to shape a cylinder: pollen tube as a model system for the generation of complex cellular geometry. Sexual plant reproduction. 2010;23:63–71. doi: 10.1007/s00497-009-0121-4. [DOI] [PubMed] [Google Scholar]
- 9.Dyson SE, Jones DG, Kendrick WL. Some observations on the ultrastructure of developing rat cerebral capillaries. Cell Tissue Res. 1976;173:529–42. doi: 10.1007/BF00224312. [DOI] [PubMed] [Google Scholar]
- 10.Bar T, Guldner FH, Wolff JR. “Seamless” endothelial cells of blood capillaries. Cell & Tissue Research. 1984;235:99–106. doi: 10.1007/BF00213729. [DOI] [PubMed] [Google Scholar]
- 11.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. [see comment] Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 12.Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140:3051–61. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 13.Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21:1942–8. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Lenard A, Ellertsdottir E, Herwig L, Krudewig A, Sauteur L, Belting HG, et al. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev Cell. 2013;25:492–506. doi: 10.1016/j.devcel.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Lenard A, Daetwyler S, Betz C, Ellertsdottir E, Belting HG, Huisken J, et al. Endothelial Cell Self-fusion during Vascular Pruning. PLoS Biol. 2015;13:e1002126. doi: 10.1371/journal.pbio.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu JA, Castranova D, Pham VN, Weinstein BM. Single-cell analysis of endothelial morphogenesis in vivo. Development. 2015;142:2951–61. doi: 10.1242/dev.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murfee WL, Schmid-Schonbein GW. Chapter 12. Structure of microvascular networks in genetic hypertension. Methods Enzymol. 2008;444:271–84. doi: 10.1016/S0076-6879(08)02812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean J, Cruz SD, Mehta PK, Merz CN. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nature reviews Cardiology. 2015;12:406–14. doi: 10.1038/nrcardio.2015.72. [DOI] [PubMed] [Google Scholar]
- 19.Haffner C, Malik R, Dichgans M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1038/jcbfm.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. European journal of human genetics: EJHG. 2009;17:860–71. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draheim KM, Fisher OS, Boggon TJ, Calderwood DA. Cerebral cavernous malformation proteins at a glance. J Cell Sci. 2014;127:701–7. doi: 10.1242/jcs.138388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacharidou A, Stratman AN, Davis GE. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cells, tissues, organs. 2012;195:122–43. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Rigamonti D, Badr A, Zhang J. Ccm1 regulates microvascular morphogenesis during angiogenesis. Journal of vascular research. 2011;48:130–40. doi: 10.1159/000316851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nature Medicine. 2009;15:177–84. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y, Eng M, Ghabrial AS. Focal defects in single-celled tubes mutant for Cerebral cavernous malformation 3, GCKIII, or NSF2. Dev Cell. 2013;25:507–19. doi: 10.1016/j.devcel.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lant B, Yu B, Goudreault M, Holmyard D, Knight JD, Xu P, et al. CCM-3/STRIPAK promotes seamless tube extension through endocytic recycling. Nature communications. 2015;6:6449. doi: 10.1038/ncomms7449. [DOI] [PubMed] [Google Scholar]
- 27.Uv A, Cantera R, Samakovlis C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13:301–9. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 28.Samakovlis C, Manning G, Steneberg P, Hacohen N, Cantera R, Krasnow MA. Genetic control of epithelial tube fusion during Drosophila tracheal development. Development. 1996;122:3531–6. doi: 10.1242/dev.122.11.3531. [DOI] [PubMed] [Google Scholar]
- 29.Caviglia S, Luschnig S. Tube fusion: making connections in branched tubular networks. Semin Cell Dev Biol. 2014;31:82–90. doi: 10.1016/j.semcdb.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Jarecki J, Johnson E, Krasnow MA. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell. 1999;99:211–20. doi: 10.1016/s0092-8674(00)81652-9. [DOI] [PubMed] [Google Scholar]
- 31.Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–9. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 32.Schottenfeld J, Song Y, Ghabrial AS. Tube continued: morphogenesis of the Drosophila tracheal system. Curr Opin Cell Biol. 2010;22:633–9. doi: 10.1016/j.ceb.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caviglia S, Luschnig S. The ETS domain transcriptional repressor Anterior open inhibits MAP kinase and Wingless signaling to couple tracheal cell fate with branch identity. Development. 2013;140:1240–9. doi: 10.1242/dev.087874. [DOI] [PubMed] [Google Scholar]
- 34.Cheng YL, Andrew DJ. Extracellular Mipp1 Activity Confers Migratory Advantage to Epithelial Cells during Collective Migration. Cell reports. 2015 doi: 10.1016/j.celrep.2015.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gervais L, Casanova J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr Biol. 2010;20:359–66. doi: 10.1016/j.cub.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 36.Lebreton G, Casanova J. Specification of leading and trailing cell features during collective migration in the Drosophila trachea. J Cell Sci. 2014;127:465–74. doi: 10.1242/jcs.142737. [DOI] [PubMed] [Google Scholar]
- 37.Okenve-Ramos P, Llimargas M. Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis. Development. 2014;141:929–39. doi: 10.1242/dev.103218. [DOI] [PubMed] [Google Scholar]
- 38.Ukken FP, Aprill I, JayaNandanan N, Leptin M. Slik and the receptor tyrosine kinase Breathless mediate localized activation of Moesin in terminal tracheal cells. PLoS One. 2014;9:e103323. doi: 10.1371/journal.pone.0103323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson FK, Riddle DL. Functional study of the C. elegans secretory-excretory system using laser microsurgery. J Exptl Zool. 1984;231:45–56. doi: 10.1002/jez.1402310107. [DOI] [PubMed] [Google Scholar]
- 40.Buechner M, Hall DH, Bhatt H, Hedgecock EM. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Developmental Biology. 1999;214:227–41. doi: 10.1006/dbio.1999.9398. [DOI] [PubMed] [Google Scholar]
- 41.Stone CE, Hall DH, Sundaram MV. Lipocalin signaling controls unicellular tube development in the Caenorhabditis elegans excretory system. Dev Biol. 2009;329:201–11. doi: 10.1016/j.ydbio.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdus-Saboor I, Mancuso VP, Murray JI, Palozola K, Norris C, Hall DH, et al. Notch and Ras promote sequential steps of excretory tube development in C. elegans. Development. 2011;138:3545–55. doi: 10.1242/dev.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskowitz IP, Rothman JH. lin-12 and glp-1 are required zygotically for early embryonic cellular interactions and are regulated by maternal GLP-1 signaling in Caenorhabditis elegans. Development. 1996;122:4105–17. doi: 10.1242/dev.122.12.4105. [DOI] [PubMed] [Google Scholar]
- 44.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Experimental Cell Research. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 45.Levi BP, Ghabrial AS, Krasnow MA. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development. 2006;133:2383–93. doi: 10.1242/dev.02404. [DOI] [PubMed] [Google Scholar]
- 46.Hedgecock EM, Culotti JG, Hall DH, Stern BD. Genetics of cell and axon migrations in Caenorhabditis elegans. Development. 1987;100:365–82. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- 47.Armenti ST, Chan E, Nance J. Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell. Dev Biol. 2014;394:110–21. doi: 10.1016/j.ydbio.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones TA, Nikolova LS, Schjelderup A, Metzstein MM. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev Biol. 2014;390:41–50. doi: 10.1016/j.ydbio.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancuso VP, Parry JM, Storer L, Poggioli C, Nguyen KC, Hall DH, et al. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development. 2012;139:979–90. doi: 10.1242/dev.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schottenfeld-Roames J, Ghabrial AS. Whacked and Rab35 polarize dynein-motor-complex-dependent seamless tube growth. Nat Cell Biol. 2012;14:386–93. doi: 10.1038/ncb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waaijers S, Ramalho JJ, Koorman T, Kruse E, Boxem M. The C. elegans Crumbs family contains a CRB3 homolog and is not essential for viability. Biology open. 2015;4:276–84. doi: 10.1242/bio.201410744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francis D, Ghabrial AS. Compensatory branching morphogenesis of stalk cells in the Drosophila trachea. Development. 2015;142:2048–57. doi: 10.1242/dev.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schottenfeld-Roames J, Rosa JB, Ghabrial AS. Seamless tube shape is constrained by endocytosis-dependent regulation of active Moesin. Curr Biol. 2014;24:1756–64. doi: 10.1016/j.cub.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 55.Nikolova LS, Metzstein MM. Intracellular lumen formation in Drosophila proceeds via a novel subcellular compartment. Development. 2015;142:3964–73. doi: 10.1242/dev.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. Journal of Cell Science. 2002;115:1123–36. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 57.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. Journal of Cell Science. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 58.Berry KL, Bulow HE, Hall DH, Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. [see comment] Science. 2003;302:2134–7. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 59.Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, et al. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat Cell Biol. 2013;15:143–56. doi: 10.1038/ncb2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jazwinska A, Ribeiro C, Affolter M. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nature Cell Biology. 2003;5:895–901. doi: 10.1038/ncb1049. [DOI] [PubMed] [Google Scholar]
- 61.Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, et al. The type I membrane protein EFF-1 is essential for developmental cell fusion. Developmental Cell. 2002;2:355–62. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 62.Sapir A, Choi J, Leikina E, Avinoam O, Valansi C, Chernomordik LV, et al. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans. [see comment] Developmental Cell. 2007;12:683–98. doi: 10.1016/j.devcel.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Vargas J, Krey T, Valansi C, Avinoam O, Haouz A, Jamin M, et al. Structural basis of eukaryotic cell-cell fusion. Cell. 2014;157:407–19. doi: 10.1016/j.cell.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Zeev-Ben-Mordehai T, Vasishtan D, Siebert CA, Grunewald K. The full-length cell-cell fusogen EFF-1 is monomeric and upright on the membrane. Nature communications. 2014;5:3912. doi: 10.1038/ncomms4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, et al. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Developmental Cell. 2006;11:471–81. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Sumida GM, Yamada S. Self-contact elimination by membrane fusion. Proc Natl Acad Sci U S A. 2013;110:18958–63. doi: 10.1073/pnas.1311135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sumida GM, Yamada S. Rho GTPases and the downstream effectors actin-related protein 2/3 (Arp2/3) complex and myosin II induce membrane fusion at self-contacts. J Biol Chem. 2015;290:3238–47. doi: 10.1074/jbc.M114.612168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gervais L, Lebreton G, Casanova J. The making of a fusion branch in the Drosophila trachea. Dev Biol. 2012;362:187–93. doi: 10.1016/j.ydbio.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 69.Phng LK, Gebala V, Bentley K, Philippides A, Wacker A, Mathivet T, et al. Formin-mediated actin polymerization at endothelial junctions is required for vessel lumen formation and stabilization. Dev Cell. 2015;32:123–32. doi: 10.1016/j.devcel.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 70.Shaye DD, Greenwald I. The disease-associated formin INF2/EXC-6 organizes lumen and cell outgrowth during tubulogenesis by regulating F-actin and microtubule cytoskeletons. Dev Cell. 2015;32:743–55. doi: 10.1016/j.devcel.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Jiang L, Rogers SL, Crews ST. The Drosophila Dead end Arf-like3 GTPase controls vesicle trafficking during tracheal fusion cell morphogenesis. Developmental Biology. 2007;311:487–99. doi: 10.1016/j.ydbio.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kakihara K, Shinmyozu K, Kato K, Wada H, Hayashi S. Conversion of plasma membrane topology during epithelial tube connection requires Arf-like 3 small GTPase in Drosophila. Mech Dev. 2008;125:325–36. doi: 10.1016/j.mod.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Liu J, Guo W. The exocyst complex in exocytosis and cell migration. Protoplasma. 2012;249:587–97. doi: 10.1007/s00709-011-0330-1. [DOI] [PubMed] [Google Scholar]
- 74.Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat Cell Biol. 2013;15:157–68. doi: 10.1038/ncb2662. [DOI] [PubMed] [Google Scholar]
- 75.Mattingly BC, Buechner M. The FGD homologue EXC-5 regulates apical trafficking in C. elegans tubules. Dev Biol. 2011;359:59–72. doi: 10.1016/j.ydbio.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghabrial AS, Levi BP, Krasnow MA. A systematic screen for tube morphogenesis and branching genes in the Drosophila tracheal system. PLoS Genet. 2011;7:e1002087. doi: 10.1371/journal.pgen.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1185–9. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 78.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–42. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oshima K, Takeda M, Kuranaga E, Ueda R, Aigaki T, Miura M, et al. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 2006;16:1531–7. doi: 10.1016/j.cub.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 80.JayaNandanan N, Mathew R, Leptin M. Guidance of subcellular tubulogenesis by actin under the control of a synaptotagmin-like protein and Moesin. Nature communications. 2014;5:3036. doi: 10.1038/ncomms4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liegeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. Journal of Cell Biology. 2006;173:949–61. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liegeois S, Benedetto A, Michaux G, Belliard G, Labouesse M. Genes required for osmoregulation and apical secretion in Caenorhabditis elegans. Genetics. 2007;175:709–24. doi: 10.1534/genetics.106.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cotter K, Stransky L, McGuire C, Forgac M. Recent Insights into the Structure, Regulation, and Function of the V-ATPases. Trends Biochem Sci. 2015;40:611–22. doi: 10.1016/j.tibs.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hahn-Windgassen A, Van Gilst MR. The Caenorhabditis elegans HNF4alpha Homolog, NHR-31, mediates excretory tube growth and function through coordinate regulation of the vacuolar ATPase. PLoS Genet. 2009;5:e1000553. doi: 10.1371/journal.pgen.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J Biol Chem. 2015;290:17145–53. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luschnig S, Uv A. Luminal matrices: an inside view on organ morphogenesis. Exp Cell Res. 2014;321:64–70. doi: 10.1016/j.yexcr.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Zuo L, Iordanou E, Chandran RR, Jiang L. Novel mechanisms of tube-size regulation revealed by the Drosophila trachea. Cell Tissue Res. 2013;354:343–54. doi: 10.1007/s00441-013-1673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bulik DA, Robbins PW. The Caenorhabditis elegans sqv genes and functions of proteoglycans in development. Biochim Biophys Acta. 2002;1573:247–57. doi: 10.1016/s0304-4165(02)00391-4. [DOI] [PubMed] [Google Scholar]
- 89.Plaza S, Chanut-Delalande H, Fernandes I, Wassarman PM, Payre F. From A to Z: apical structures and zona pellucida-domain proteins. Trends Cell Biol. 2010;20:524–32. doi: 10.1016/j.tcb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 90.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–51. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 91.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv: European journal of physiology. 2007;454:345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 93.Dong B, Hannezo E, Hayashi S. Balance between apical membrane growth and luminal matrix resistance determines epithelial tubule shape. Cell reports. 2014;7:941–50. doi: 10.1016/j.celrep.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 94.Brodu V, Baffet AD, Le Droguen PM, Casanova J, Guichet A. A developmentally regulated two-step process generates a noncentrosomal microtubule network in Drosophila tracheal cells. Dev Cell. 2010;18:790–801. doi: 10.1016/j.devcel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 95.Ray RP, Matamoro-Vidal A, Ribeiro PS, Tapon N, Houle D, Salazar-Ciudad I, et al. Patterned Anchorage to the Apical Extracellular Matrix Defines Tissue Shape in the Developing Appendages of Drosophila. Dev Cell. 2015;34:310–22. doi: 10.1016/j.devcel.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annual review of biomedical engineering. 2014;16:505–32. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iordanou E, Chandran RR, Yang Y, Essak M, Blackstone N, Jiang L. The novel Smad protein Expansion regulates the receptor tyrosine kinase pathway to control Drosophila tracheal tube size. Dev Biol. 2014;393:93–108. doi: 10.1016/j.ydbio.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moussian B, Letizia A, Martinez-Corrales G, Rotstein B, Casali A, Llimargas M. Deciphering the genetic programme triggering timely and spatially-regulated chitin deposition. PLoS Genet. 2015;11:e1004939. doi: 10.1371/journal.pgen.1004939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeon M, Zinn K. Receptor tyrosine phosphatases control tracheal tube geometries through negative regulation of Egfr signaling. Development. 2009;136:3121–9. doi: 10.1242/dev.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baer MM, Palm W, Eaton S, Leptin M, Affolter M. Microsomal triacylglycerol transfer protein (MTP) is required to expand tracheal lumen in Drosophila in a cell-autonomous manner. J Cell Sci. 2012;125:6038–48. doi: 10.1242/jcs.110452. [DOI] [PubMed] [Google Scholar]
- 101.Page AP, Johnstone IL. The cuticle. Wormbook. 2007:1–15. doi: 10.1895/wormbook.1.138.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones SJ, Baillie DL. Characterization of the let-653 gene in Caenorhabditis elegans. Molecular & General Genetics. 1995;248:719–26. doi: 10.1007/BF02191712. [DOI] [PubMed] [Google Scholar]
- 103.Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137:344–55. doi: 10.1016/j.cell.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue barriers. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Byri S, Misra T, Syed ZA, Batz T, Shah J, Boril L, et al. The Triple-Repeat Protein Anakonda Controls Epithelial Tricellular Junction Formation in Drosophila. Dev Cell. 2015;33:535–48. doi: 10.1016/j.devcel.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 106.Gervais L, Casanova J. The Drosophila homologue of SRF acts as a boosting mechanism to sustain FGF-induced terminal branching in the tracheal system. Development. 2011;138:1269–74. doi: 10.1242/dev.059188. [DOI] [PubMed] [Google Scholar]
- 107.Jiang L, Crews ST. Dysfusion transcriptional control of Drosophila tracheal migration, adhesion, and fusion. Mol Cell Biol. 2006;26:6547–56. doi: 10.1128/MCB.00284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee M, Lee S, Zadeh AD, Kolodziej PA. Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development. 2003;130:5989–99. doi: 10.1242/dev.00806. [DOI] [PubMed] [Google Scholar]