Abstract
Recent progress in population health at aggregate level, measured by life expectancy, has been accompanied by lack of progress in reducing the difference in health prospects between groups defined by social status. Cardiovascular disease is an important contributor to this undesirable situation. The stepwise gradient of higher risk with lower status is accounted for partly by social gradients in health behaviors. The psychosocial hypothesis provides a stronger explanation, based on social patterning of living and working environments and psychological assets that individuals develop during childhood. Three decades of research based on Whitehall II and other cohort studies provide evidence for psychosocial pathways leading to cardiovascular morbidity and mortality. Job stress is a useful paradigm because exposure is long term and depends on occupational status. Studies of social-biological translation implicate autonomic and neuroendocrine function among the biological systems that mediate between chronic adverse psychosocial exposures and increased cardiometabolic risk and cardiovascular disease incidence.
Introduction
Socioeconomic inequalities in cardiovascular (CVD) risk and other health problems are shown to persist in developed countries in study after study. ‘Status’, however defined, is associated with the incidence of CVD in societies where absolute poverty is rare, but hierarchies nevertheless remain. This widely known phenomenon is a challenge for policy makers and a major research question for biomedical and population health science.
The inverse relationship - high status, low CVD risk – may be thought of, at first sight, as obvious. Smoking and other vessel-damaging behaviors tend to cluster in lower social strata. However, there is another layer of understanding to penetrate that relates to what Michael Marmot calls the ‘causes of the causes’. Further questions then arise. Why do adverse health behaviors cluster as they do, and to what extent is the social gradient in heart attack and other CVD events explained by the familiar behavioral risk factors? The psychosocial perspective that will be developed in this article is that low social status constitutes a condition of chronic stress. (Brunner 1997) Social stresses may contribute to the social gradient in CVD in two ways: first as determinants of health behaviors, and second as risk factors for disease, independently of the effects of health behaviors.
The Jubilee Line of health inequality
London is an intensely rich city. It has an infrastructure that delivers clean water and energy and collects sewage and other waste efficiently and reliably. Housing quality is good, although in short supply and therefore expensive. The congestion zone reduces weekday traffic by charging drivers to enter the city center, and the air would be clean were it not for the cheating diesel engine manufacturers. Processed and fresh foods are available in abundance. Primary and secondary health care, as in the United Kingdom as a whole, is free at the point of use and the quality of the health care is consistently ranked highly in international league tables. In nineteenth or even twentieth century public health perspectives, London would be seen as an exemplary health-promoting place to live. It turns out however that when life expectancy is mapped at small area level, this key index of health reveals large differences between areas just a few 100s of meters apart.
The tube (subway) is the fastest way to travel around the city, and taking the Jubilee Line eastwards from Westminster, the wealthy nation’s democratic hub, a disturbing story of health inequality emerges. Male life expectancy at birth in 2004–08 was 78.5 (95% CI 75.5–81.6) years in Westminster. For every two stops along the line, life expectancy is one year lower. The gradient is similar among women. Six stations east we arrive at Canning Town, a poor district of London just next door to ultra-affluent London Docklands. In Canning Town, life expectancy was 73.6 (95% CI 71.9–75.2) years. (London Health Observatory/ Department of Health, http://www.lho.org.uk/viewResource.aspx?id=15463, accessed 5 Jan 2016) The loss of average life expectancy over a distance of 13 km is massive. A man retiring at 65 in Westminster will have some 14 years of life to look forward to. In Canning Town, his future is truncated by more than a third, to 8.6 years.
The social epidemiologist thinks about the causal explanation of these observations at area level in terms of composition and context. The health effects which are due to the characteristics of the people living in an area versus the effects of the area itself on the people living in it can be studied using a multilevel model which is able to separate compositional and contextual effects, if detailed data at both individual and area levels are available. Such work has not been done for residents along the Jubilee Line. We can infer that an effect of neighborhood per se does operate, because exposures such as air pollution, poor housing and walkability have been linked to excess CVD risk in many places using a variety of study designs, some of which controlled for the characteristics of individuals living in the study areas. (Diez Roux 2007) (Gill et al. 2011) (Avendano and Kawachi 2014) On the other hand, the impact of neighborhood is likely to be smaller in rich than in developing countries where the range of living conditions is considerably more extreme. (Subramanian et al. 2003)
The ‘Canning Town effect’ is partly the consequence of the local physical and social environment, and the ways in which local conditions differ from those in affluent Westminster. However, the evidence suggests that individual characteristics would explain the lion’s share of the life expectancy difference between these two places if the study was carried out. In many ways, individuals living in today’s rich city have their own personal, portable environment. This environment is both real and virtual, which is to say material and psychosocial. It is possible to travel from one side of the city to the other in about an hour. Urban living is highly geographically mobile. Yet health inequalities associated with education, occupation, wealth and income, particularly those due to CVD, remain stubbornly large.
Recent trends in life expectancy, cardiovascular disease rates and inequality
The instrumental aim of biomedical and life science is to make a contribution to human welfare, and it is worthwhile to consider the broad pattern of trends in population health as a yardstick of progress. More specifically, we can situate our thinking about the links between social factors and CVD on the firm foundations of recent trajectories in overall life expectancy, CVD mortality rates and social differences in those rates.
Life expectancy at birth has been increasing at the rate of some 3 months per year over recent decades. In 2012, a man could expect to live to 80 and a woman to 83 on average in England and Wales. These figures are some 7 years higher than in 1981, and forecast to increase at about the same rate in the short- to medium-term. (Bennett et al. 2015) Most of these recent and probable future gains are due to an increase in survival among people over 65. A combination of lifestyle improvement and risk factor lowering treatment (for blood pressure and cholesterol), and better health status on reaching age 65 appears to account for these rapid and remarkable changes. Decline in coronary heart disease mortality is an important component of the life expectancy gains. The rate fell by about 60% between the mid-1980s and mid-2000s, largely as a result of improving lipid and blood pressure levels and falling smoking rates, despite the constraining effect of rising adult adiposity. (Hardoon et al. 2012) (Bajekal et al. 2013) Such population-level trends in Britain are reflected in other rich countries.
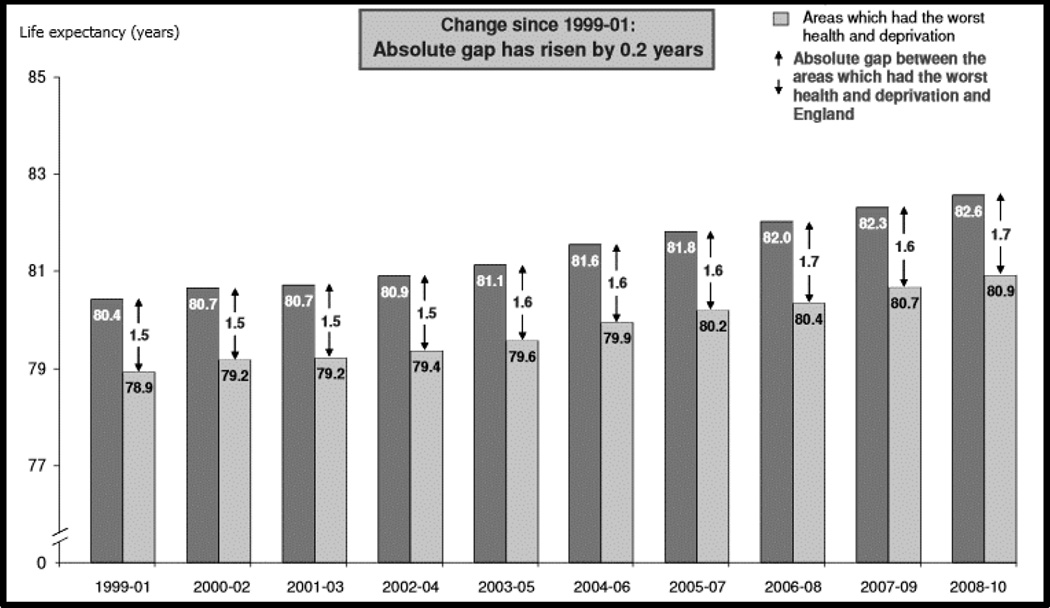
These overall health improvements are encouraging. The picture is less positive when we look at these key trends according to social and economic circumstances. The UK Department of Health monitors inequality in life expectancy at birth by comparing the trend for England as a whole with the trend in the worst 20% of local government areas, defined by mortality data in the mid-1990s and an index of multiple deprivation. The data series shows the absolute difference increased over the first decade of the millennium, by 0.2 years in women (Figure 1) and 0.1 year in men (data not shown). Both absolute and relative inequality measures help in understanding the size of inequality, in this case the evolving trend. In relative terms, inequality in life expectancy at birth over the period increased by 0.15 percentage points (7.9%) in women and decreased by 0.01 percentage points (0.2%) in men. In summary, there have been remarkable health gains in recent decades, but they are not evenly distributed across the population.
Figure 1.
Life expectancy at birth in England and areas with worst health and deprivation. Women, 1999–2010
Source: UK Department of Health, Mortality Monitoring Bulletin, Life expectancy and all-age-all-cause mortality, and mortality from selected causes, overall and inequalities, October 2011. https://www.gov.uk/government/publications/mortality-monitoring-bulletin-life-expectancy-and-all-age-all-cause-mortality-and-mortality-from-selected-causes-overall-and-inequalities-update-to-include-data-for-2010 (accessed 8 January 2016)
The data for England for the first decade of the 21st century show that social inequality in the headline measure of life expectancy at birth increased in women and was stable in men. A general pattern is illustrated in these data for one country: that good progress in population health at aggregate level can often be accompanied by lack of progress in reducing the difference in health prospects between groups defined by social status. This pattern is evident in the trend in coronary mortality as well as life expectancy in Great Britain (GB), among men and women less than 75 years old. (McCartney et al. 2012) Across the whole population of GB, as we saw above, coronary mortality has been declining in recent decades. However in relative terms, excess coronary mortality increased between 1994 and 2008 in the lowest compared to the highest area deprivation quintile (fifth) from 77% (95% CI 68%–86%) in 1994 to 132% (95% CI 114%–152%) in 2008 in women, and from 52% (95% CI 47%–57%) in 1994 to 84% (95% CI 76%–93%) in 2008 in men.
The Whitehall II study
Whitehall II is a follow-up study of chronic disease and aging which commemorated its 30th year in 2015. The study was set up in 1985 as a long-term prospective study similar to the Framingham study of CVD incidence, with the addition of a major inequality aspect that builds on a wider range of risk factor measurements. We have shown that the wider determinants (causes of the causes) such as low perceived sense of control over health and other important dimensions of life, together with biological and behavioral factors, are important in explaining the social gradient in CVD. Our evidence on the importance of material and psychosocial risk factors supports UK and international strategies on health inequalities. As we have seen in earlier sections of this article, aiming to reduce health inequalities is a challenging and long-term goal. The cohort, now 65–85 years of age, is increasingly valuable as a platform for studying age-related aspects of health with measures such as stiffness of the large arteries, physical and cognitive functioning. (Brunner et al. 2011)
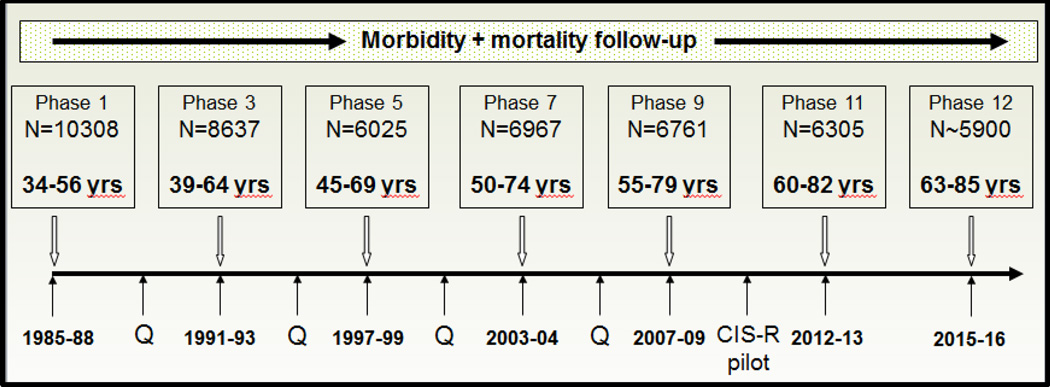
The study is an occupational cohort of men and women who were working in the offices of central government in 1985–88. Everyone in 20 London-based ministries and departments was invited to participate in a screening examination during working hours. The response rate was good (73%) and just as importantly, the majority of those entering the study (n=10308), and who remained alive after three decades and 11 contact phases, continue to participate (n=6305, Figure 2). (Marmot and Brunner 2005) The sample of men and women provided an ideal setting to test hypotheses in the relation to psychosocial risk factors including job stress, not only as risk factor for morbidity and mortality, but also to examine the extent to which individual risk factors, and groups of risk factors are able to explain the social gradient in CVD events. A special characteristic of the sample in this respect is that all participants had a basic level of job security and many remained in Civil Service employment until retirement. It turned out that contact could be maintained and the health of the cohort followed over decades. Research clinics were set up at 3–5 year intervals to carry out repeated cardiovascular examinations and to collect blood. Further, everyone in the study could be placed on a scale of six grades of employment defining income and status in a standard way across the 20 employing departments. This scale classifies participants in unskilled jobs including receptionists, filing clerks and messengers at the lower end, to those who have first class university degrees and considerable power to run their department and to influence public policy at the upper end. Along with this dimension of power and control, there is a steep income gradient made up of largely non-overlapping salary bands, which in 1987 ranged from £3061 to £62100.
Figure 2.
Whitehall II cohort study. Timeline, phases of contact, number of participants and age range.
Contact phases above the timeline are clinical examinations with questionnaire. Q refers to questionnaire-only contact phases. CIS-R pilot refers to the pilot study of a computerized version of Clinical Interview Schedule-Revised.
The first Whitehall study debunks executive stress
Whitehall II was so-called because it came after a first large cohort of British civil servants that had been recruited in the late 1960s. This study, of 17718 men, was set up to study CVD and respiratory disease from a narrow biomedical perspective. However, Michael Marmot and others took the opportunity to study socioeconomic status (SES) as a risk factor for coronary mortality in its own right, rather than to use it only as a confounding or nuisance variable. (Marmot et al. 1978) This study, which was based on accumulated mortality from coronary disease over 7.5 years, divided the men into a hierarchy of four groups according to their employment grade. Excess coronary mortality in the lower social strata was much larger in this study than in national vital statistics from the same period, with an odds ratio of 4.0 in office support staff compared to top administrators. The finding had considerable impact because at the time there was popular interest in the notion of executive stress, which linked increased heart attack risk with high rather than low social status. (Friedman et al. 1958) (BRADY et al. 1958)
The social gradient in coronary death and the risk factor gap
Mortality data in male civil servants in the first Whitehall study demonstrated not only the substantial inverse gradient in coronary death rates according to SES, but also made possible an analytic study to estimate the contribution of conventional risk factors to the social gradient in fatal events. (Marmot, Rose, Shipley, & Hamilton 1978) This analysis was an early application of multiple logistic regression in epidemiology, that used coronary mortality as the dependent variable and SES as the primary risk factor. Models omitting and then including potential explanatory risk factors such as smoking provide estimates of their respective contributions to the social gradient in coronary death, estimated by the extent of attenuation of the regression coefficient for employment grade. The explanatory risk factors were cholesterol, smoking, blood pressure, body mass index, physical activity and blood glucose. Interestingly, all these risk factors together accounted for 31% of the social gradient in events: fully 69% of the gradient was not explained.
Forty years on, finding this set of risk factors explained only a third social inequality coronary mortality would be less of a surprise than it was at the time. When the result was first interpreted, it was taken broadly at face value. Risk factors including early life exposures, diet and psychosocial factors were considered to be candidates for the missing variables needed to fill the gap in the statistical explanation. The importance of measurement imprecision was poorly recognized, and little attention was paid to potential change in behavior patterns over follow-up, and the greater explanatory power such information would bring to the epidemiological model. The consequence of these perspectives, when modelling was in its early days, was reinforcement of two ideas. First, and entirely reasonably, risk factors for coronary disease were not fully understood and further research on novel risk factors including ‘stress’ was needed. Second, again justifiably, known risk factors such as serum cholesterol might be important for stratifying individuals according to their coronary risk, but not so valuable for explaining differences, or inequalities, in risk between social strata. In terms of the distribution of risk, individual variation in cholesterol level was large within each stratum, but the mean cholesterol level differed little between strata. (Brunner et al. 1997)
Health behaviors and the social gradient
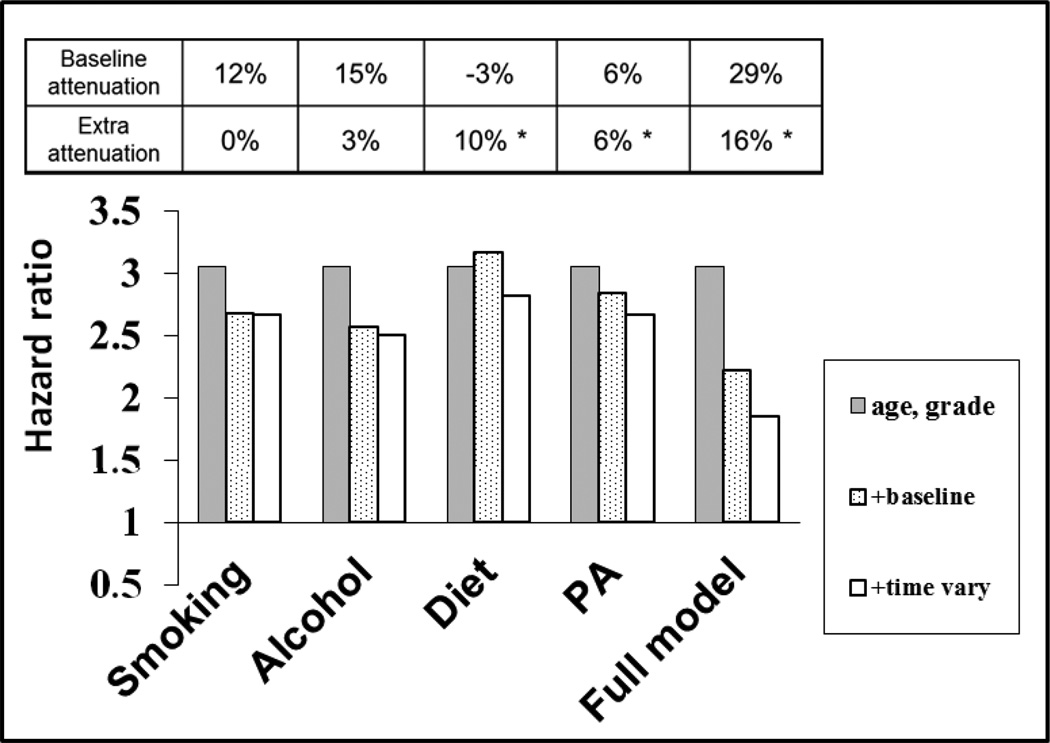
The impact of modelling change in health behavior, such as quitting smoking, rather than assuming behaviors remain constant over long periods was investigated in the Whitehall II study. (Stringhini et al. 2010) CVD mortality was collated between study baseline (1985–88) and 2009. Self-reports of smoking, drinking, diet pattern and physical activity habits were collected at the time of the baseline examination and three subsequent clinics at 5-yearly intervals (Figure 2). The statistical model utilized in the analysis allowed for time dependent exposure to the four behaviors of interest. As in the analysis based on the first Whitehall study, above, the mediating role of each health behavior was determined by the percentage reduction in the coefficient for SES after including the health behavior in model.
The extent to which each behavior accounted statistically for social inequality in CVD mortality differed considerably (Figure 3). Smoking explained some 12% of the social gradient in the baseline exposure model, and the attenuation was unchanged in the time-varying model. In contrast, the attenuations due to diet and physical activity increased significantly in the time-varying model compared to the baseline exposure model. The increase in attenuation due to the time-varying approach reflects the pattern of behavior change according to SES over the 1990s and 2000s. The relative decline in current smoking rates in the higher and lower employment grades was similar – about halving – over the period of follow-up, and thus for smoking the two modelling approaches produce the same attenuation estimate. Contrastingly, an unhealthy dietary pattern almost disappeared in the higher employment grades over the follow-up whereas positive dietary change was more limited in the lower grades.
Figure 3.
Socioeconomic gradient in CVD mortality and health behavioral explanations, Whitehall II study. The analysis compares models assuming baseline behavior continues unchanged with models in which behavior can vary over the follow-up time.
Redrawn from Stringhini S, Sabia S, Shipley M et al JAMA 2010;303:1159-66.
All models are adjusted for sex. The grey bars show the hazard ratio for cardiovascular disease mortality for the lowest compared with the highest socioeconomic status (3.05, 95% CI 1.94–4.78). The dotted bars show the hazard ratio additionally adjusted for one or all health behaviors at baseline. The open bars show the hazard ratio with further adjustment for health behavior(s) at 5, 10 and 15 years of follow-up. * increase in attenuation p<0.05 compared to the baseline adjusted model.
Together, the four health behaviors in question were important mediators in the model for the social gradient on CVD mortality. The combined attenuating effect in the time-varying Cox model was 45%, about half again the attenuation in the baseline exposure model (29%). It would be reasonable to consider that if the exposure measurements had been more precise, the explained proportion of the social gradient in events might exceed 50%. Indeed, in a similar analysis of metabolic syndrome components and health behaviors, with height included in the set of mediating variables as a marker of the effects of early life, 62% of the social gradient in major coronary events was accounted for. (Marmot et al. 2008b)
Causes of the causes
Research on the size of contribution of health behaviors to the social gradient in CVD, and other lifestyle-related diseases, has evolved in the past 40 years. We have seen that the unexplained part of the gradient has become smaller as a consequence of using better science: both the evidence and statistical methods have become more sophisticated. Understanding the implications of the findings is a challenge for researchers and policy makers alike. We know that knowledge of the harms caused by smoking and other health-damaging behaviors (and the benefits of health-promoting behaviors such as sufficient physical activity) translates only slowly and incrementally into behavior change. (Rees et al. 2013) (Jepson et al. 2010) In order to reduce health inequality, first the potentially regressive effect of health promotion that is taken up more readily in the higher social strata needs to be avoided. (Jepson, Harris, Platt, & Tannahill 2010) Second, programs that have a progressive effect must be identified and implemented. This second step is difficult in part because health science has focused largely on research on disease causation and mechanisms (the science of discovery) rather than on increasing the evidence on cost-effective and equitable interventions (the science of delivery) which could deliver substantial health gain. (Catford 2009)
Another fundamental question that has stimulated research on the wider causes of social inequalities in CVD concerns the role of ‘stress’. Stress is a difficult concept because of the range of contexts where it is invoked. Stress can refer to the external stimulus, or to the internal response. Stress can be primarily a psychological issue (one person’s stress is another’s stimulation) or a physiological phenomenon (Frank’s blood pressure stress response is large). Often, we think about the stressed individual. The psychosocial hypothesis is an alternative paradigm which places individuals in their socioeconomic setting, paying attention to psychological demands which arise as a result of position in the social hierarchy, and also to the resources available which may provide resilience or coping ability to that individual. (Pearlin and Schooler 1978)
We showed for example that financial insecurity is a risk factor for weight gain in women in the Whitehall II study. (Conklin et al. 2014) The likelihood of weight gain of 5 kg or more over 11 years of follow-up was associated with financial insecurity in women (OR 1.45 (95%CI 1.05–2.01)) but not in men. The study illustrates two important features of the psychosocial hypothesis. The psychosocial stressor exhibited a social gradient: persistent financial difficulties were more often reported in the lower employment grades. The effect of financial insecurity remained after adjustment for SES, consistent with a psychosocial interpretation such that subgroups within socioeconomic strata were vulnerable to the effects of financial insecurity on risk of weight gain. This interpretation is further supported because adjustments for health behaviors smoking, dietary pattern, alcohol consumption, and moderate and vigorous physical activity as well as SES did not abolish the association. Why was the influence of financial insecurity on body weight evident in women but not men? Cultural norms about attractiveness and body shape are strong for women but not men. We can speculate that women may express their vulnerability to financial hardship through loss of control of their body weight.
Social-biological translation
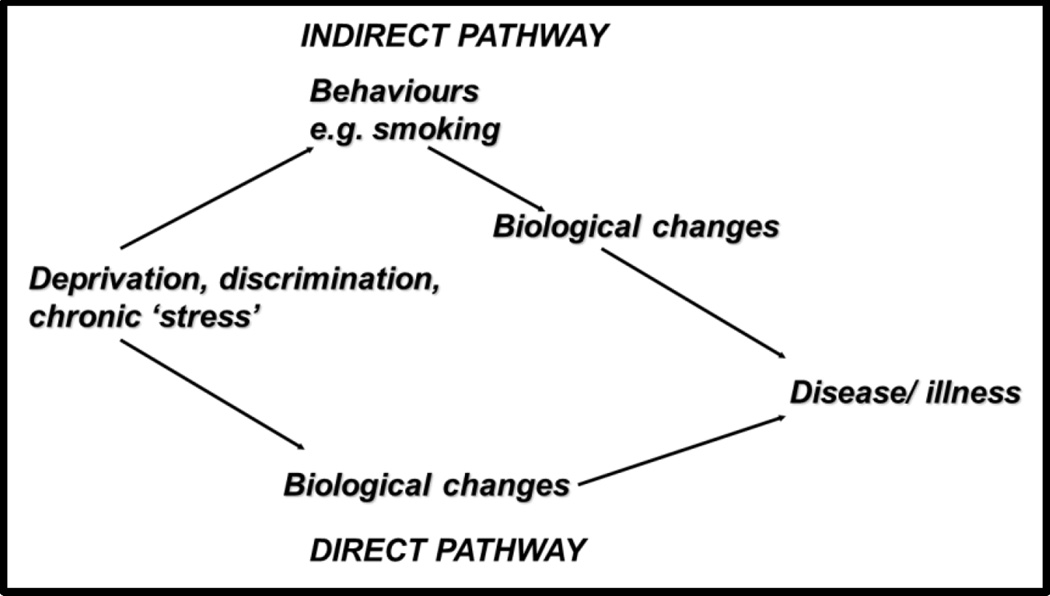
Conklin’s weight change study illustrates a pathway involving ‘causes of the causes’ that link upstream exposures to adverse health outcomes. Thinking about this pathway, it is clear that financial insecurity and other psychosocial factors must work on some biological substrate in order to exert their influence on health. Here the biological change was measured as weight change, and mechanistically we would like to know what the nature of the link between financial insecurity and weight change might be, and whether it involves behavior change (Figure 4). We might hypothesize that psychoneuroendocrine mechanisms were involved (Brunner et al. 2002), in the sense that adjustments for four relevant health behaviors did not attenuate the prospective association between financial insecurity and weight gain, consistent with a ‘direct pathway’. If adjustment for health behaviors had reduced or abolished the association, an ‘indirect pathway’ would be supported by the findings.
Figure 4.
Schematic diagram of pathways linking psychosocial factors to disease. The indirect pathway involves one or more health behaviors. The direct pathway does not involve health behaviors in the usual sense, and instead is dependent on autonomic, neuroendocrine, inflammatory and/or other psychobiological mechanisms.
Redrawn from E Brunner ‘Stress mechanisms in coronary heart disease’, in Stress and the Heart, eds: Stansfeld SA and Marmot MG, BMJ Books 2002.
Psychosocial factors and cardiovascular disease
Apart from financial insecurity, several psychosocial factors have been implicated in the social gradient in CVD. We have seen above that epidemiological research shows health behaviors play an important role in the gradient. Further, that the gap has narrowed between the statistically ‘explained’ and ‘unexplained’ parts of this gradient as epidemiological research has improved techniques for measuring and modelling exposures over decades or more. Nevertheless, there remains an overriding need to understand why the inverse social gradient in CVD remains the general pattern in rich countries, even if further careful research abolishes the unexplained part of the SES effect on CVD risk altogether. An important guiding idea here is brought to light in the difference between inequality and inequity. Health inequality refers to differences between groups, and is purely a descriptive label. On the other hand, health inequity adds the sense that the difference is both unfair and avoidable.
The idea of inequity underlies much of the thinking in public health. In the nineteenth century, pioneers such as Chadwick and Farr in London and Virchow in Berlin were painfully aware of the links between poverty, impoverished living and working conditions and bad health. Then, the focus was on physical living conditions: sanitary and housing reform, regulation of work and the food supply. Today, the new public health movement draws attention to inequity as a determinant of poor health and short life expectancy, arising from the twenty-first century problem of relative rather than absolute poverty. (Marmot et al. 2006;Marmot et al. 2008a) This review is not the place to discuss the political philosophy of inequality, but it will be understandable that psychological concepts including power, control and sense of unfairness, and psychosocial resources such as social support and self-esteem are correlated with education, income, and social and occupational status. Evidence is accumulating that such psychosocial factors are risk factors for cardiovascular disease. (De Vogli et al. 2007;Marmot, Wilkinson, & (eds) 2006)
Job stress and CVD
Work is a major part of many lives. Work often has positive impacts on the individual, family and society, however it may also have a downside for health. In the UK and other post-industrial countries, manual work has become less important, and non-manual work in offices and shops replaced factory occupations. For many decades, corresponding to this historic trend in working lives, occupational health research has shifted focus away from physical and chemical exposures, towards the potential effects that the psychological and social experience of the work environment may have on mental and physical health. It is no surprise that heavy manual work takes its toll on health and life expectancy – quite literally wearing the body out, by exceeding its capacity to repair itself. The contemporary experience of work hazards may be more subtle, but evidence has grown that supports concerns that work-related exposures such as repetitive, poorly paid and insecure jobs may be risk factors for coronary and other physical disease, as well as poor mental health.
The modern era of occupational health research began in the 1970s, located mainly in Nordic countries and the USA. Work pressure, skill use, decision making and rewards were seen as vital aspects of the work experience. There are at least two important models of job stress currently employed in population-scale health research, both of which take a psychosocial approach to defining exposure. Space does not allow discussion of the effort-reward imbalance model, (Siegrist et al. 1990) however it is worthwhile to note that there is evidence for distinct effects on coronary disease and perceived general health of the two conceptualizations of work stress. (Bosma et al. 1998;Ostry et al. 2003)
The first developed was the demand-control model which considers the combination of two dimensions to define a highly differentiated set of work experiences: (1) demands and (2) control, or decision-making authority and autonomy. In this scheme, low demands and high control constitutes low strain work, low demands and low control is passive work, high demands and high control is active work, and high demands and low control is high strain work. (Karasek et al. 1981) The system can be applied to manual as well as non-manual occupations. Thus, work on a production line and in a ticket office would be classed as high strain work, where behavior is highly regulated by external events for many hours at a time. At the other end of the spectrum, lab science is likely to be a low strain occupation, because external demands are not intense and autonomy may be high for much of the working week.
Early studies tested the demand-control model as a risk factor for CVD at individual and at group level. At group level, job stress levels can be assigned to occupations based on questionnaire, and the association between job stress and disease indicators can then be determined by ranking occupations according to mean stress exposure. (Karasek, Baker, Marxer, Ahlbom, & Theorell 1981) Replication studies followed, showing only a weak association between high strain work and CVD risk. The researchers modified their hypothesis by adding a third dimension of social support at work. (Johnson and Hall 1988) Findings supported the view that the adverse health effect of job stress was greatest in workers who lacked support from colleagues. The demand-control model was tested in the Whitehall II study, as a part of the research on social inequality in CVD. In this analysis, men and women were followed-up for 11 years after they had completed the job characteristics questionnaire. Incidence of verified major coronary heart disease was highest in those reporting high strain work, and low job control and high job demands were both associated with increased risk. (Kuper and Marmot 2003)
Findings such as those from Whitehall II stimulated vigorous debate between narrower biomedical and broader social perspectives on primary prevention of CVD. The debates concerned questions of causality. Was stress in general and job stress in particular ‘truly’ causal, or a marker of low SES and risky health behaviors? (Macleod et al. 2002) If stress was a risk factor for CVD, what was the mechanism if not the indirect, behavioral pathway (Figure 4)? (Marmot, Shipley, Hemingway, Head, & Brunner 2008b) Why bother trying to reduce stress exposures for untested benefit when we have statins and blood pressure lowering medication to intervene, cost-effectively, on proximal disease processes? (Mihaylova et al. 2006) (Law et al. 2009) It turned out, when the prospective data on job strain and incident coronary events were pooled and meta-analysed, that stress is an important cause (Hazard ratio 1.23, 95% CI 1.10–1.37). (Kivimaki et al. 2012) This huge study provided 1.5 million person-years of experience with 2358 new coronary cases and, after controlling for socioeconomic status and behavioral and biomedical risk factors, found the proportion of disease due to job stress, although less than that due to smoking, was 3.4%.
Stress mechanisms
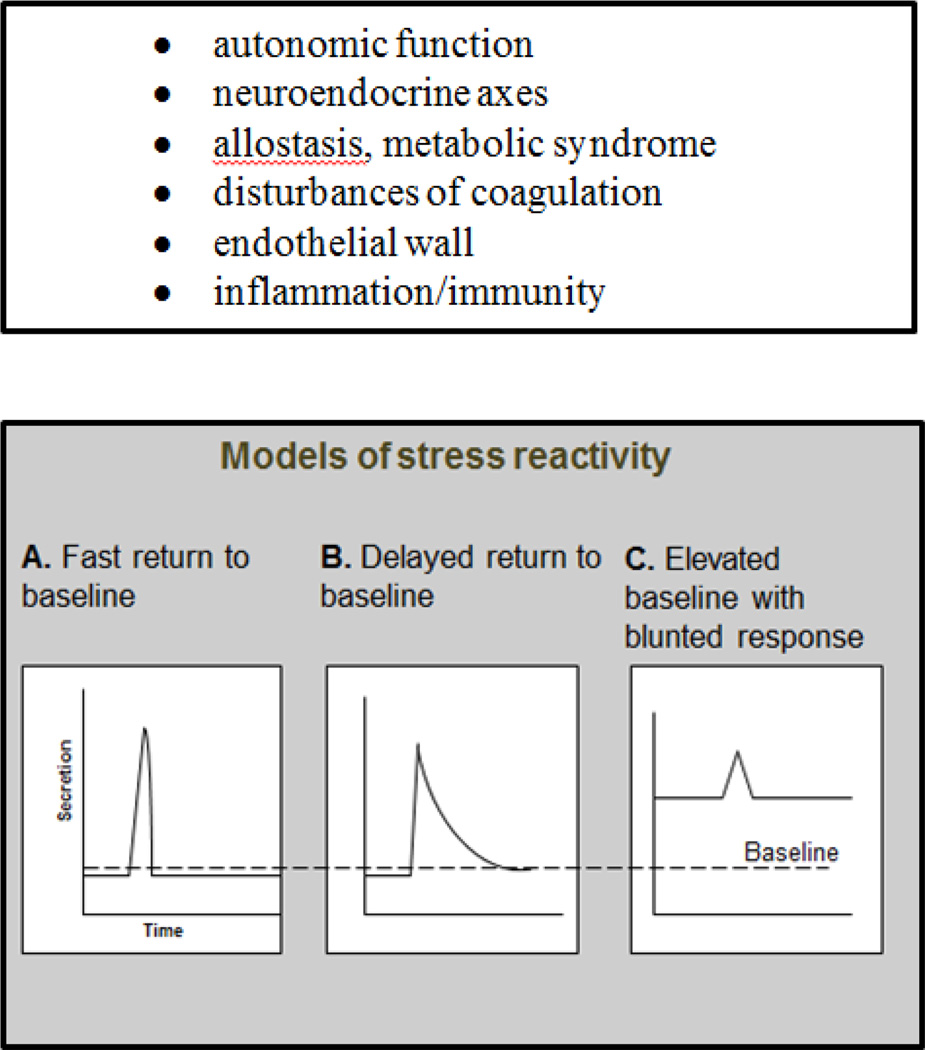
This large and definitive pooling study offers epidemiologic evidence for a direct effect of chronic stress on incident CVD that is independent of established risk factors. Along with this evidence, mechanistic studies examining a range of candidate biological pathways support the plausibility of direct effects of stress on cardiovascular risk (Figure 4). A feature of many of these studies, regardless of biological focus, is the idea that chronic stress may change short term reactivity and baseline resting set points in autonomic, neuroendocrine or metabolic pathways (Figure 5). Such changes in response to a repeated stressor can be seen as adaptions to the environment. Brain resilience appears to be central in this adaptive process, contributing to individual variation in capability to return to healthy basal levels or failing this, to make unhealthy biological adaptions. (McEwen et al. 2015) Infant and childhood living circumstances and early adverse experiences such as physical abuse and emotional neglect are among the determinants of resilience, when brain plasticity is at its greatest. There is evidence such influences may extend back to fetal exposures. (O'Donnell et al. 2013) The chances of exposure to such adverse developmental experiences, with later health effects, are increased by low childhood SES but they are clearly not limited to these groups. (Giesinger et al. 2014)
Figure 5.
Candidate mediating biological systems linking chronic stress with increased cardiovascular risk and schematic models of changed short term stress reactivity
Redrawn from E Brunner and M Marmot, Social organization, stress, and health, in Social determinants of health, eds Marmot M and Wilkinson RG, 2nd edition, Oxford University Press, 2006.
Links between stress biology and the metabolic syndrome, and thus increased risk of vascular disease, have been demonstrated in the Whitehall II study. (Brunner, Hemingway, Walker, Page, Clarke, Juneja, Shipley, Kumari, Andrew, Seckl, Papadopoulos, Checkley, Rumley, Lowe, Stansfeld, & Marmot 2002) A subset of male civil servants gave blood and 24 hour urine samples on a working day. Men with metabolic syndrome, compared to controls, produced more norepinephrine and cortisol, had higher levels of serum interleukin-6, higher heart rate and lower heart rate variability (Table 1). The temporal sequence of the associations with metabolic syndrome is unclear, however the case-control study encouraged an added cortisol component to the research, measuring cortisol secretion in six saliva samples collected between waking and bedtime on test day, for further study of neuroendocrine function and CVD risk. One important hypothesis tested was that disturbance in the diurnal rhythm predicted cardiovascular mortality. More than 4000 men and women were followed for six years. The secretion pattern on and after waking was not associated with cardiovascular mortality, but the shallower the daytime slope and the higher the bedtime level, the greater was the risk of CVD death. (Kumari et al. 2011) This was a specific finding: no index of cortisol diurnal rhythm was associated with risk of non-CVD death.
Table 1.
Prospective case-control study of metabolic syndrome. Association of autonomic, adrenocortical and inflammatory analytes with caseness. Whitehall II substudy.
| Analyte | Controls n=152 |
MetSyn cases n=29 |
Standardized difference * |
Difference P |
|---|---|---|---|---|
| mean(95%CI) | ||||
| Urinary normetanephrine (µg/day) | 177 (151–207) | 233 (185–293) | 0.45 | 0.02 |
| Urinary cortisol (mg/day) | 6.31 (5.2–7.7) | 8.90 (6.6–12) | 0.49 | 0.03 |
| Heart rate variability (SDRR) | 42.5 (36–49) | 32.3 (24–40) | −0.72 | 0.006 |
| Heart rate (beat/min) | 64.5 (60–69) | 72.3 (67–78) | 0.61 | 0.002 |
| Serum interleukin-6 (pg/mL) | 1.10 (09–1.3) | 1.90 (1.5–2.4) | 0.89 | 0.001 |
difference in SD units; MetSyn, metabolic syndrome; SDRR, SD of RR interval
Source: Brunner et al, Circulation 2002;106:2659–65.
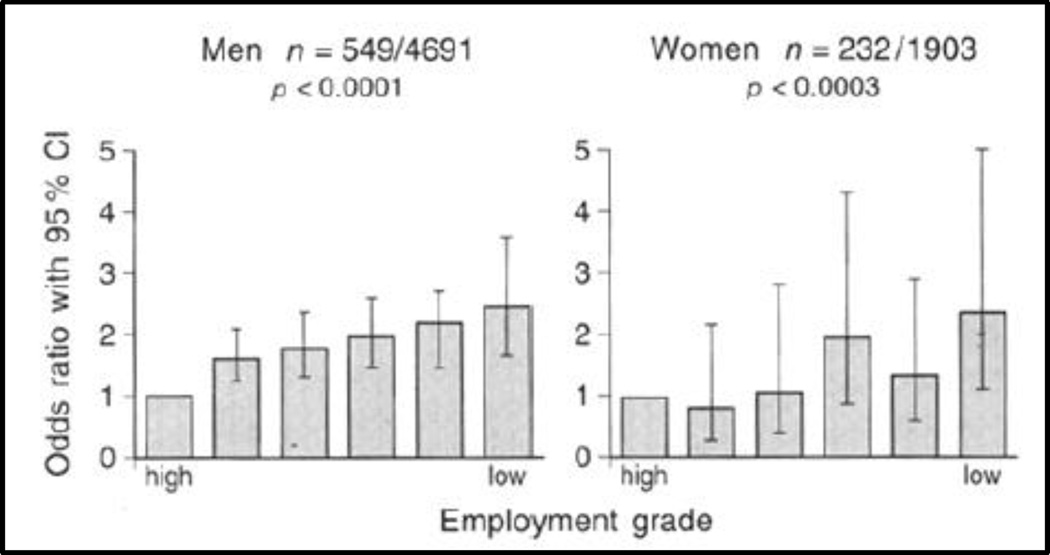
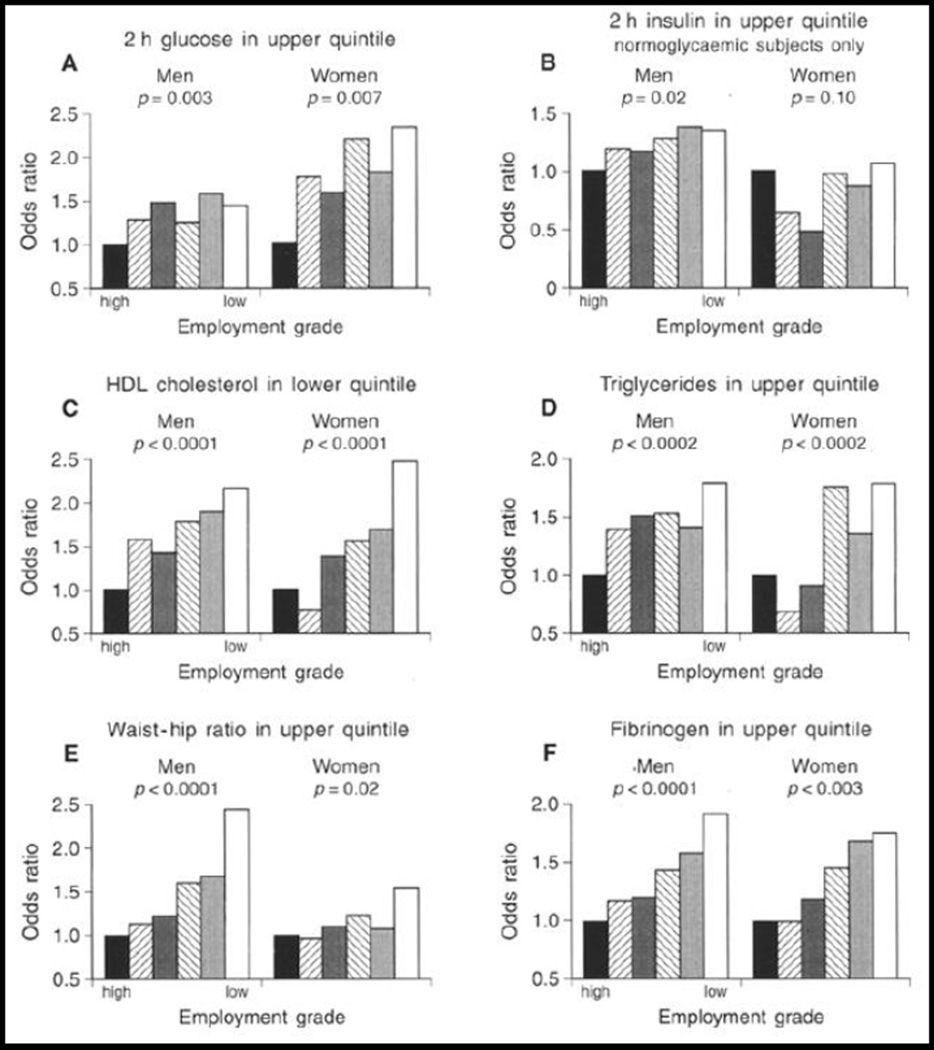
Adults with metabolic syndrome have modified autonomic and neuroendocrine function. (Landsberg et al. 1991) (Landsberg 1992) Further, those with an elevated nighttime baseline in cortisol secretion have double the risk of CVD death. Additional evidence for a chronic stress mechanism leading to increased CVD risk comes from prospective studies in the Whitehall II cohort of the effects of chronic work stress on risks of developing metabolic syndrome and obesity over some 15 years. During follow up, men and women were classified according to the number of survey waves, to a maximum of four, at which they reported high job strain and lack of support from coworkers or supervisors, defined as isostrain. (Johnson & Hall 1988) Employees reporting isostrain on 3–4 occasions were more than twice as likely as those who did not report job stress to develop metabolic syndrome, after controlling for age, SES and health behaviors. (Chandola et al. 2006) Similar findings were obtained using central obesity and body mass index as outcomes. (Brunner et al. 2007) These population-scale studies of office-based employees provide strong evidence that chronic psychosocial stress is linked with increased CVD risk with a dose-response effect (Figures 6 and 7).. (Brunner, Marmot, Nanchahal, Shipley, Stansfeld, Juneja, & Alberti 1997)
Figure 6.
Odds ratios for metabolic syndrome by employment grade. Adjusted for age, and menopausal status in women. P values are test for trend across grade categories.
Source: Diabetologia, Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study, 40, 1997, 1346, EJ Brunner et al. With permission of Springer.
Figure 7.
Odds ratios for occupying the top quintile of anthropometric and biochemical variables by employment grade. Adjusted for age, and menopausal status in women. P values are test for trend across grade categories.
Source: Diabetologia, Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study, 40, 1997, 1345, EJ Brunner et al. With permission of Springer.
Social status, chronic stress and cardiovascular morbidity
The line of reasoning developed in this review is that low social status constitutes a condition of chronic stress. The psychosocial perspective positions individuals in their social hierarchy and considers how psychological and cultural experience shapes health expectancies, starting in infancy and continuing through adult life. Material circumstances and health behaviors are vital aspects of population health. However, a useful explanation – for prevention - of social inequality in cardiovascular risk and other important health conditions involves a deeper understanding about why the social gradient in risk has remained, and stubbornly refuses to decline, across societies where absolute poverty is rare or absent. Knowledge of the harms associated with smoking and other adverse health behaviors is universal, but psychological experience and resources to act on that information, as we have seen in the work-based examples above, are not uniform across social strata.
Conclusion
This review has presented epidemiologic evidence about direct and indirect pathways from chronic stress to cardiovascular morbidity and mortality. An early and striking finding made in Whitehall II was simply an analysis of the prevalence of metabolic syndrome and its components according to employment grade in the middle-aged men and women participating in the study. (Brunner, Marmot, Nanchahal, Shipley, Stansfeld, Juneja, & Alberti 1997) This analysis shows clearly the social gradient in metabolic syndrome which underlies the social gradient in CVD. It further illustrates that the stepwise social gradients in biological risk are seen in glucose metabolism, coagulation and central obesity. Although the patterns by social strata are not entirely uniform, the overall picture consistently points to a dose-response process of social-biological translation. The wider question is whether the evidence of preventable inequity in cardiovascular risk will be translated into effective policy action.
HIGHLIGHTS.
‘Social factors and cardiovascular morbidity’ for NBR Special Issue ‘Stress, Behavior and the Heart’
Perspective piece that considers the role of stress in population health
Content spans from headline administrative data on life expectancy and CVD mortality trends to the diurnal trend on cortisol secretion in healthy adults
In an accessible way, presents research in social and psychosocial epidemiology for lab scientists without making assumptions about familiarity with the methods
Presents recent and key findings from the Whitehall II study, including
Socioeconomic gradient in CHD and cardiometabolic risk factors
Empirical study of role of smoking and other behaviors in the socioeconomic gradient in incident CVD mortality
Findings on job stress and CHD
Nested case-control study of autonomic, neuroendocrine and inflammatory factors as risk factors for metabolic syndrome
Acknowledgments
Funding
The author’s research is supported by British Heart Foundation (RG/13/2/30098), British Medical Research Council (K013351), US National Heart, Lung, and Blood Institute (R01HL036310), US National Institute on Aging (R01AG013196) and European Commission Framework 7 Moodfood Collaborative Project (613598).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avendano M, Kawachi I. Why do Americans have shorter life expectancy and worse health than do people in other high-income countries? Annual Reveiw Public Health. 2014;35:307–325. doi: 10.1146/annurev-publhealth-032013-182411. available from: PM:24422560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajekal M, Scholes S, O'Flaherty M, Raine R, Norman P, Capewell S. Unequal trends in coronary heart disease mortality by socioeconomic circumstances, England 1982–2006: an analytical study. PLoS. One. 2013;8(3):e59608. doi: 10.1371/journal.pone.0059608. available from: PM:23527228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JE, Li G, Foreman K, Best N, Kontis V, Pearson C, Hambly P, Ezzati M. The future of life expectancy and life expectancy inequalities in England and Wales: Bayesian spatiotemporal forecasting. Lancet. 2015;386(9989):163–170. doi: 10.1016/S0140-6736(15)60296-3. available from: PM:25935825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H, Peter R, Siegrist J, Marmot MG. Two alternative job stress models and the risk of coronary heart disease. Am. J. Publ. Health. 1998;88:68–74. doi: 10.2105/ajph.88.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY JV, PORTER RW, CONRAD DG, MASON JW. Avoidance behavior and the development of gastroduodenal ulcers. J Exp. Anal. Behav. 1958;1:69–72. doi: 10.1901/jeab.1958.1-69. available from: PM:13872361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EJ. Socioeconomic determinants of health: Stress and the biology of inequality. British Medical Journal. 1997;314:1472–1476. doi: 10.1136/bmj.314.7092.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol. 2007;165(7):828–837. doi: 10.1093/aje/kwk058. available from: PM:17244635. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GDO, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, Alberti KGMM. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 1997;40(11):1341–1349. doi: 10.1007/s001250050830. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Shipley MJ, Witte DR, Singh-Manoux A, Britton AR, Tabak AG, McEniery CM, Wilkinson IB, Kivimaki M. Arterial stiffness, physical function, and functional limitation: the Whitehall II Study. Hypertension. 2011;57(5):1003–1009. doi: 10.1161/HYPERTENSIONAHA.110.168864. available from: PM:21444833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catford J. Advancing the 'science of delivery' of health promotion: not just the 'science of discovery'. Health Promotion International. 2009;24(1):1–5. doi: 10.1093/heapro/dap003. available from: PM:19171666. [DOI] [PubMed] [Google Scholar]
- Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. British Medical Journal. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin AI, Forouhi NG, Brunner EJ, Monsivais P. Persistent financial hardship, 11-year weight gain, and health behaviors in the Whitehall II study. Obesity.(Silver. Spring) 2014;22(12):2606–2612. doi: 10.1002/oby.20875. available from: PM:25155547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vogli R, Brunner E, Marmot MG. Unfairness and the social gradient of metabolic syndrome in the Whitehall II Study. Journal of Psychosomatic Research. 2007;63:413–419. doi: 10.1016/j.jpsychores.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Neighborhoods and health: where are we and were do we go from here? Rev. Epidemiol. Sante Publique. 2007;55(1):13–21. doi: 10.1016/j.respe.2006.12.003. available from: PM:17320330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Rosenman RH, Carrol V. Changes in the serum cholesterol and blood clotting time in men subjected to cyclic variation of occupational stress. Circulation. 1958;17:852–861. doi: 10.1161/01.cir.17.5.852. [DOI] [PubMed] [Google Scholar]
- Giesinger I, Goldblatt P, Howden-Chapman P, Marmot M, Kuh D, Brunner E. Association of socioeconomic position with smoking and mortality: the contribution of early life circumstances in the 1946 birth cohort. Journal of Epidemiology and Community Health. 2014;68(3):275–279. doi: 10.1136/jech-2013-203159. available from: PM:24249001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill EA, Curl CL, Adar SD, Allen RW, Auchincloss AH, O'Neill MS, Park SK, Van Hee VC, Diez Roux AV, Kaufman JD. Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Prog. Cardiovasc. Dis. 2011;53(5):353–360. doi: 10.1016/j.pcad.2011.02.001. available from: PM:21414470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoon SL, Morris RW, Whincup PH, Shipley MJ, Britton AR, Masset G, Stringhini S, Sabia S, Kivimaki M, Singh-Manoux A, Brunner EJ. Rising adiposity curbing decline in the incidence of myocardial infarction: 20-year follow-up of British men and women in the Whitehall II cohort. Eur. Heart J. 2012;33(4):478–485. doi: 10.1093/eurheartj/ehr142. available from: PM:21653562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson RG, Harris FM, Platt S, Tannahill C. The effectiveness of interventions to change six health behaviours: a review of reviews. BMC. Public Health. 2010;10:538. doi: 10.1186/1471-2458-10-538. available from: PM:20825660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JV, Hall EM. Job strain, work place social support, and cardiovascular disease: a cross-sectional study of a random sample of the Swedish working population. Am. J. Public Health. 1988;78:1336–1342. doi: 10.2105/ajph.78.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasek R, Baker D, Marxer F, Ahlbom A, Theorell T. Job decision latitude, job demands and cardiovascular disease: a prospective study of Swedish men. Am. J. Public Health. 1981;71:694–705. doi: 10.2105/ajph.71.7.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Nyberg ST, Batty GD, Fransson EI, Heikkila K, Alfredsson L, Bjorner JB, Borritz M, Burr H, Casini A, Clays E, De BD, Dragano N, Ferrie JE, Geuskens GA, Goldberg M, Hamer M, Hooftman WE, Houtman IL, Joensuu M, Jokela M, Kittel F, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Kumari M, Madsen IE, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Rugulies R, Salo P, Siegrist J, Singh-Manoux A, Suominen SB, Vaananen A, Vahtera J, Virtanen M, Westerholm PJ, Westerlund H, Zins M, Steptoe A, Theorell T. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380(9852):1491–1497. doi: 10.1016/S0140-6736(12)60994-5. available from: PM:22981903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 2011;96(5):1478–1485. doi: 10.1210/jc.2010-2137. available from: PM:21346074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Marmot M. Job strain, job demands, decision latitude, and the risk of coronary heart disease within the Whitehall II study. Journal of Epidemiology and Community Health. 2003;57(2):147–153. doi: 10.1136/jech.57.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg L. Hyperinsulinemia: possible role in obesity-induced hypertension. Hypertension. 1992;19(1 Suppl):I61–I66. doi: 10.1161/01.hyp.19.1_suppl.i61. available from: PM:1730456. [DOI] [PubMed] [Google Scholar]
- Landsberg L, Troisi R, Parker D, Young JB, Weiss ST. Obesity, blood pressure, and the sympathetic nervous system. Annals of Epidemiology. 1991;1(4):295–303. doi: 10.1016/1047-2797(91)90040-j. available from: PM:1669511. [DOI] [PubMed] [Google Scholar]
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. British Medical Journal. 2009;338:b1665. doi: 10.1136/bmj.b1665. available from: PM:19454737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod J, Davey Smith G, Heslop P, Metcalfe C, Carroll D, Hart C. Psychological stress and cardiovascular disease: empirical demonstration of bias in a prospective observational study of Scottish men. British Medical Journal. 2002;324:1247–1251. doi: 10.1136/bmj.324.7348.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Wilkinson RG, editors. Social Determinants of Health. 2nd. Oxford: OUP; 2006. [Google Scholar]
- Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008a;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. available from: PM:18994664. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Brunner EJ. Cohort Profile: The Whitehall II study. Int. J. Epidemiol. 2005;34(2):251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Rose G, Shipley M, Hamilton PJS. Employment grade and coronary heart disease in British civil servants. Journal of Epidemiology and Community Health. 1978;32:244–249. doi: 10.1136/jech.32.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG, Shipley MJ, Hemingway H, Head J, Brunner EJ. Biological and behavioural explanations of social inequalities in coronary heart disease: the Whitehall II Study. Diabetologia. 2008b;51:1980–1988. doi: 10.1007/s00125-008-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney D, Scarborough P, Webster P, Rayner M. Trends in social inequalities for premature coronary heart disease mortality in Great Britain, 1994–2008: a time trend ecological study. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2011-000737. available from: PM:22710128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray J, Nasca C. Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiol. Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. available from: PM:25506601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20,536 people. British Medical Journal. 2006;333(7579):1145. doi: 10.1136/bmj.38993.731725.BE. available from: PM:17098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, O'Connor TG. Prenatal maternal mood is associated with altered diurnal cortisol in adolescence. Psychoneuroendocrinology. 2013;38(9):1630–1638. doi: 10.1016/j.psyneuen.2013.01.008. available from: PM:23433748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry AS, Kelly S, Demers PA, Mustard C, Hertzman C. A comparison between the effort-reward imbalance and demand control models. BMC. Public Health. 2003;3:10. doi: 10.1186/1471-2458-3-10. available from: PM:12636876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Schooler C. The structure of coping. J. Health Soc. Behav. 1978;19(1):2–21. available from: PM:649936. [PubMed] [Google Scholar]
- Rees K, Dyakova M, Ward K, Thorogood M, Brunner E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD002128.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist J, Peter R, Junge A, Cremer P, Seidel D. Low status control, high effort at work and ischemic heart disease: prospective evidence from blue-collar men. Soc Sci Med. 1990;31:1127–1134. doi: 10.1016/0277-9536(90)90234-j. [DOI] [PubMed] [Google Scholar]
- Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, Singh-Manoux A. Association of socioeconomic position with health behaviors and mortality. Journal of the American Medical Association. 2010;303(12):1159–1166. doi: 10.1001/jama.2010.297. available from: PM:20332401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Delgado I, Jadue L, Kawachi I, Vega J. Income inequality and self rated health: an analysis from a contextual perspective in Chile. Rev. Med Chil. 2003;131(3):321–330. available from: PM:12790083. [PubMed] [Google Scholar]