Abstract
Aim:
A cross-sectional study was conducted to determine the seroprevalence of bovine brucellosis among cattle slaughtered at three municipal abattoirs of Gombe State, Nigeria.
Materials and Methods:
A total of 200 blood samples collected from slaughtered cattle of different breeds (Sokoto Gudali - 50, White Fulani - 102, Red bororo – 34, and Crossbreeds - 14), sex (males - 19 and females - 181), and from different locations (Billiri - 30, Yamaltu Deba – 50, and Gombe - 120) were screened for brucellosis using rose bengal plate test (RBPT), serum agglutination test (SAT), and microtiter agglutination test (MAT).
Results:
Of the 200 serum samples analyzed, 7 (3.5%), 10 (5.0%) and 18 (9.0%) were positive by RBPT, SAT and MAT, respectively. The results showed no statistically significant association between sex and seropositivity to bovine brucellosis. However, seropositivity of bovine brucellosis was higher in females than in males. Similarly, no statistically significant association was observed between breed and occurrence of bovine brucellosis. Moreover, the prevalence of brucellosis was higher in Sokoto Gudali as compared with the other breeds. Based on the study locations, higher seroprevalence was observed in animals screened from Billiri as compared with those from other locations (p<0.05).
Conclusion:
The presence of Brucella abortus antigen in the sera of slaughtered cattle in Gombe state poses a significant public health risk. Therefore, it is important to carry out further epidemiological studies on fulani herdsmen and cattle herds in the study area, in order to explore the risk factors associated with the occurrence and perpetuation of brucellosis among cattle herds, ascertain the prevalence and status of the disease among both farms and nomadic herds.
Keywords: bovine brucellosis, Gombe state, microtiter agglutination test, Nigeria, rose bengal plate test, serum agglutination test
Introduction
Bovine brucellosis is a disease with a significant economic and public health importance due to losses incurred as a result of infertility in animals and extensive chronic morbidity in humans [1]. In Nigeria, bovine brucellosis is a major animal health problem affecting the growth of the cattle industry. It remains a significant disease in animals and humans worldwide and an important cause of reproductive failure such as abortion in cows and sterility in bulls [2-4]. Bovine brucellosis still remains the most widespread form of brucellosis even though reported incidences and prevalence of the disease showed that it varies from country to country. The geographical distribution of the disease is limited. However, it is still a major problem in livestock industry in the Asia, Africa, Latin American, and the Mediterranean regions [5,6].
The disease is transmitted through contamination of feed and water with discharges from infected animals, aerosols, contaminated equipment, fetal fluids, and placental discharges. Infection can also be transmitted via break in the integrity of the skin and mucous membrane [7]. In endemic areas, humans get infected with brucellosis through close contact with infected animals or after birth content of animals, consumption of unpasteurized milk or milk products obtained from either dairy cows, sheep or goats [8,9]. Over the past decades, factors - such as socioeconomic, sanitary, political, and international travel - have greatly impacted on the epidemiology of brucellosis. The occurrence of brucellosis is worldwide, except in Australia, Cyprus, Canada, Norway, New Zealand, Finland, Denmark, and Sweden where the etiologic agent of bovine brucellosis has been eradicated [10].
Bovine brucellosis is responsible for huge economic losses in animal production. This is manifested as abortions, reduced milk yield, and delayed conception; in addition to its zoonotic and public health threat [9]. For instance, an estimated loss of 600 million US dollar due to bovine brucellosis was reported in Latin America. While in the US, an estimated loss due to abortion and reduced milk production was 400 million US dollars [10]. Economic losses due to brucellosis in Nigeria are as a result of abortion, reduced milk production, sterility in bulls, and loss of man-hour in people infected with Brucella abortus [11].
Brucellosis is endemic in Nigeria; and the prevalence of bovine brucellosis varies significantly between herds located in the same area, with an estimated seroprevalence in cattle ranging from as low as 0.2% to about 80%. The prevalence of brucellosis in institutional and dairy farms, abattoir surveys and ranches in southern Nigeria ranges from 3.7% to 48.8%. However, the prevalence was low in herd of cattle belonging to the traditional nomadic Fulani than those in the farms and ranches. Similarly, the prevalence of 32.2% was recorded in a herd of cattle belonging to a prison farm. In Northern Nigeria, 25.3% and 19.5% of milk and serum samples were, respectively, reported positive for bovine brucellosis [11].
The occurrence of the disease is influenced by the use of common grazing areas, herding animals of different breeds, age and sex together. Other factors reported to facilitate the occurrence of bovine brucellosis includes, season of the year, lactation, and pregnancy [7,12]. The occurrence of brucellosis in animals has been reported to be a factor limiting the growth and success of livestock in Nigeria [11]. Serological diagnosis of brucellosis in livestock using a well-standardized kit specifically designed for B. abortus is considered an important component of disease surveillance and eradication. These tests are also available for use in other livestock other than cattle [13,14]. Despite studies on bovine brucellosis in northeastern Nigerian states such as Yobe, Borno, Bauchi, Adamawa, and Taraba, only a few were conducted in Gombe state and therefore the need for this study.
It is important, therefore, to adopt the use of effective control measures through early and accurate diagnosis of the disease aimed at reducing the prevalence of the disease to boost production. This study was designed to investigate the seroprevalence of bovine brucellosis in three Municipal abattoirs of Gombe state, Northeastern Nigeria.
Materials and Methods
Ethical approval
The study to determine the seroprevalence of bovine brucellosis was designed and performed according to the global standard guidelines to care and use of experimental animals described by Ochei and Kolhatkar [15]. Approval was duly obtained from the Institutional Ethics and Research Committee, Faculty of Veterinary Medicine, University of Maiduguri.
Study area
Gombe state is located in the northeastern geopolitical zone of Nigeria (Figure-1). It shares a common boundary line with Borno, Adamawa, Bauchi, Yobe, and Taraba state along the expansive savannah zone. The state is situated at latitude 9°30’ and 12°30’N and longitudes 8°45’ and 11°45’E. It has an area of 20,265 km2 and a population of about 2,353,000 people (NPC, 2006). The state has two different climatic conditions, the dry season ranging from November to March and the rainy season spanning from April to October. It has an average rainfall of 850 mm (https://en.wikipedia.org/wiki/Gombe_State).
Figure-1.
Map of Nigeria showing Gombe state (Source: Google maps).
Sample collection
A total of 200 blood samples were obtained from slaughtered cattle in the municipal abattoirs located in the three agro-ecological zones of Gombe state (Figure-2). Of these, about 91%, 51% and 60% were, respectively, females, males, White fulani breeds and sampled from Gombe metropolis council (Table-1). About 10 mL each of blood was aseptically collected in a well-labeled plain tube without anticoagulant, from the anterior jugular vein of cows slaughtered in these abattoirs. The blood was kept in a transport container and allowed to clot. The sera were collected in 2 mL cryovials after centrifugation at 3000 ×g for 5 min and stored at −20°C.
Figure-2.
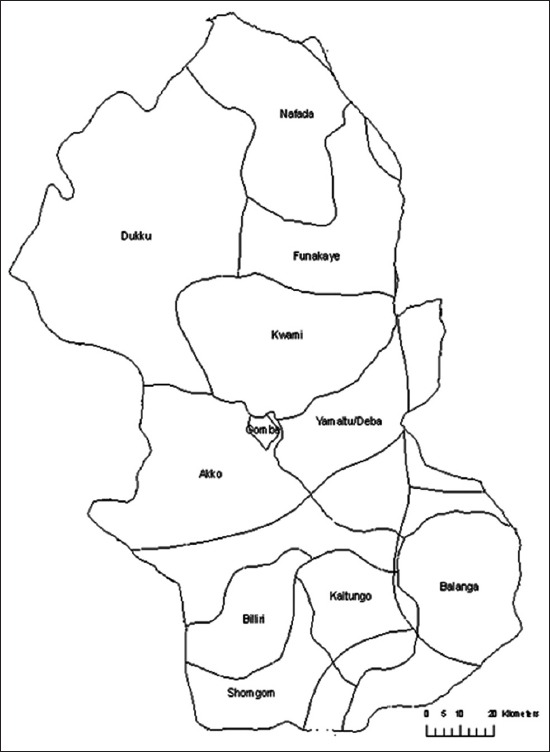
Map of Gombe state showing the three municipal abattoirs.
Table-1.
Baseline characteristics of slaughtered cattle sampled from the three municipal abattoirs in Gombe state, Northeastern Nigeria.
| Variables | Categories | Frequency (%) |
|---|---|---|
| Sex | Male | 19 (9.5) |
| Female | 181 (90.5) | |
| Breeds | Sokoto Gudali | 50 (25) |
| White Fulani | 102 (51) | |
| Red Bororo | 34 (17) | |
| Cross breed | 14 (7) | |
| Location | Billiri | 30 (15) |
| Yamaltu Deba | 50 (25) | |
| Gombe | 120 (60) | |
| Overall | 200 (100) |
Serological reaction
Blood sera was analyzed for B. abortus antibodies using rose bengal plate test (RBPT), serum agglutination test (SAT), and microtiter agglutination test (MAT) as described by Adamu et al. [7] and Gómez et al. [16]. Positive and negative reactions were recorded based on the presence or absence of agglutination reaction. The samples were considered positive for infection with brucellosis if the antigen-antibody titer is three-fold (1.40) or more (one part of the antigen and four parts of the antibody). Antigen for SAT and RBPT standard B. abortus antigen Surry KT15 3NB (New Haw Weybridge, UK.) and S99 CVL (Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, India) were used. The preparation of cocktail was assessed by titration and carried out according to the standard guidelines recommended by the World Organization for Animal Health (OIE) [17]. For SAT, Brucella antigen was prepared in a 1:50 dilution in 0.85% saline solution. A 1:10 to 1:1280 double dilution of serum was carried in tubes containing saline solutions. Appropriate titers of high, low, and negative sera were used as controls. Tubes containing 0.5 ml of diluted serum were mixed with equal amount of 1:50 dilution of Brucella antigen and then incubated at 37°C overnight.
Statistical analysis
Factors considered to assess their association with seroprevalence of bovine brucellosis include sex, geographical location, and breeds. The results were considered statistically significant at p<0.05. Chi-square test using SPSS version 22.0 (SPSS Chicago, USA), was used to determine the strength of association between the seroprevalence of bovine brucellosis and risk factors.
Results and Discussion
Bovine brucellosis is considered as one of the most significant bacterial zoonosis hindering the development of the dairy industry in Nigeria. The disease is endemic in many African countries, Asia, Middle East, Central and South America. It is prevalent in areas where effective control programs have not yielded much progress. To this effect, a cross-sectional study was conducted to determine the seroprevalence of bovine brucellosis in three municipal abattoirs in Gombe state. To achieve this, three serological test, namely RBPT, SAT, and MAT were used to screen for cattle sera. The result obtained showed that 7 (3.5%), 10 (5.0%) and 18 (9.0%) of the cattle sera analyzed were positive by RBPT, SAT and MAT, respectively (Table-1). Statistically, no significant difference was observed between the seropositivity of the disease and the use of RBPT and SAT as a diagnostic tool in screening for bovine brucellosis. However, a statistically significant difference was observed between the seropositivity of the disease and the sensitivity of MAT in detecting antibodies to B. abortus in the sera screened for bovine brucellosis
The seroprevalence of bovine brucellosis as reported in this study was lower than the seroprevalence of bovine brucellosis reported in Yobe (34.0%), Adamawa (36.6%), and Bauchi state (5.4% and 3.9%) respectively [7]. Similarly, in southern Nigeria, Cadmus et al. [18] reported a seroprevalence of 6% in 2004, 6.17% in 2005 and 5.31% in 2006, which was higher than the seroprevalence of bovine brucellosis reported in this study. Furthermore, in another study, Cadmus et al. [19] reported a seroprevalence of 8.6% in three cattle production system in southwestern Nigeria. This difference in the seroprevalence of the disease could be attributed to the difference in breeds, sensitivity of test kits, seasonal variation, farming system, and sample size. In addition, lower rates of bovine brucellosis was reported in this study than those reported in other African countries such as Ethiopia (4.9%) [2], Central African Republic (4.9%) [20], Eritrea (4.2%) [21], Chad (7%) [22], Jordan (6.5%) [23], and Cameroon (8.4%) [24]. In Eastern Sudan, Gumaa et al. [12] reported seroprevalence of 2.15%, after sampling 2500 serum samples collected from sheep. The difference in prevalence could be due to the difference in breeds, geographical location, sample size, serological techniques, and inter-laboratory variation. Similarly, Junaidu et al. [25] also reported a much higher (19.5%) seroprevalence of bovine brucellosis after sampling 1,547 serum samples in Sokoto, Northwestern Nigeria. In another study, Zubairu et al. [26] reported a seroprevalence of 21.3% in cattle sera collected in Taraba state, North-eastern Nigeria. This disparity in the seroprevalence of bovine brucellosis in different parts of the country is in congruent with the report of Mai et al. [11]. Where the authors reported that the prevalence of bovine brucellosis varies between animals in the same agro-pastoral zone. Even though the seroprevalence of brucellosis reported in this study was lower using RPBT and SAT, it was however higher with MAT (Table-2).
Table-2.
Seroprevalence of bovine brucellosis among slaughtered cattle in Gombe state, Northeastern Nigeria.
| Variables | Number sampled | Number of positive animals (%) | ||
|---|---|---|---|---|
| RBPT | MAT | SAT | ||
| Sex | ||||
| Male | 19 | 0 (0) | 1 (5.3) | 0 (0) |
| Female | 181 | 7 (3.9) | 17 (9.4) | 10 (5.5) |
| Breeds | ||||
| Sokoto Gudali | 50 | 3 (6.0) | 8 (16.0) | 4 (8.0) |
| White Fulani | 102 | 3 (2.9) | 9 (8.8) | 5 (4.9) |
| Red Bororo | 34 | 1 (2.9) | 1 (2.9) | 1 (2.9) |
| Cross breeds | 14 | 0 (0) | 0 (0) | 0 (0) |
| Locations | ||||
| Billiri | 30 | 2 (6.7) | 3 (10.0) | 4 (13.3) |
| Yamaltu Deba | 50 | 2 (4.0) | 6 (12.0) | 3 (6.0) |
| Gombe | 120 | 3 (2.5) | 9 (7.5) | 3 (2.5) |
| Overall | 200 | 7 (3.5) | 18 (9.0) | 10 (5.0) |
RBPT=Rose bengal plate test, MAT=Microtiter agglutination test, SAT=Serum agglutination test
In this study, the seropositivity of bovine brucellosis was higher in Sokoto gudali and White fulani as compared with Red bororo and crossbreeds. However, there was no statistically significant association between breeds and occurrence of brucellosis in cattle (p>0.05) (Table-2). This finding is in congruent with the report of Junaidu et al. [24], where the authors reported a higher prevalence of bovine brucellosis in Sokoto Gudali (29.59%) breeds. In addition, even though the proportion of bovine brucellosis was higher in females than in males, the difference was not statistically significant (p>0.05) (Table-2). This is because female cows remain the locale of infection, which helps to spread the disease from one animal to another, either through lactation or during mating. Pregnancy and lactation were reported to enhance susceptibility to infection. The growth of virulent strains of Brucella organism was reported to be stimulated more in females because of the presence of higher volume of D-erythritol normally found fetal tissue than in testes and seminal vesicle [24]. These thoughts provide proofs as to the different rates of seroprevalence of bovine brucellosis observed in this study.
The risk of bovine brucellosis is not only restricted to the animal husbandry alone but also represent significant zoonotic implications characterized by debilitating and severe complications in humans [1]. Serological studies have shown that bovine brucellosis is a common problem in many grazing zone in Nigeria [7]. From reports, it was observed that there was variation in the prevalence of bovine brucellosis in many countries, the surveillance strategies adopted for the control and prevention of the disease is generally very poor. Purchase of infected animals as replacement cattle, interaction with wildlife, free movement of animals by nomads, change in climatic conditions, deforestation, and the system of animal production, regulatory issues and demographic factors were considered as likely factors that increase the spread of bovine brucellosis. Other factors include sharing of bulls between farmers, the practice of free range grazing and movement as a result of trade have greatly increased the risk of exposure to brucellosis in cattle [7].
Conclusion
The presence of Brucella abortus antibodies in the sera of cattle slaughtered in the municipal abattoirs of Gombe state poses a significant public health risk. Therefore, it is imperative to design an effective control and preventive measures aimed at reducing the spread of brucellosis in cattle and subsequent exposure to humans in the study area.
Authors’ Contributions
Authors SMJ and NNA conceived and designed the study. AAB and TMH contributed in literature review and data analysis. AOA conducted sampling from the abattoir. SMJ and AAB drafted the first manuscript. NNA and TMH revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors’ wishes to acknowledge the staff of Veterinary Public Health Laboratory, Department of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria, for their cooperation throughout the period of this research study. We are equally grateful to all the technical staff and abattoir workers/butchers of all the three municipal abattoirs, Gombe state for their cooperation throughout the sampling process.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Gwida M, El-Ashker M, Melzer F, El-Diasty M, El-Beskawy M, Neubauer H. Use of serology and real time PCR to control an outbreak of bovine brucellosis at a dairy cattle farm in the Nile Delta region, Egypt. Irish Vet. J. 2016;69(1):1. doi: 10.1186/s13620-016-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mekonnen H, Kalayou S, Kyule M. Serological survey of bovine brucellosis in barka and arado breeds (Bos indicus) of Western Tigray, Ethiopia. Prev. Vet. Med. 2010;94(1):28–35. doi: 10.1016/j.prevetmed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Gul S.T, Khan A, Ahmad M, Hussain I. Seroprevalence of brucellosis and associated hemato-biochemical changes in Pakistani horses. Pak. J. Agric. Sci. 2013;50:745–750. [Google Scholar]
- 4.Al y S, Sprague L, Neubauer H. New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev. Sci. Tech. 2013;32(1):177–188. doi: 10.20506/rst.32.1.2204. [DOI] [PubMed] [Google Scholar]
- 5.Corbel M.J. Brucellosis: An overview. Emerg. Infect. Dis. 1997;3(2):213. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumi B, Firdessa R, Yamuah L, Sori T, Tolosa T, Aseffa A, Schelling E. Seroprevalence of Brucellosis and Q-Fever in Southeast Ethiopian pastoral livestock. J. Vet. Sci. Med. Diagn. 2013;2(1):1–5. doi: 10.4172/2325-9590.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamu S, Atsanda N, Tijjani A, Usur A, Sule A, Gulani I. Epidemiological study of bovine brucellosis in three senatorial zones of Bauchi State, Nigeria. Vet. World. 2016;9(1):48–52. doi: 10.14202/vetworld.2016.48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakhtullah F.P, Shahid M, Khan M.A, Raqeebullah S.G, Wazir I. Prevalence of brucellosis in cow Bos primigenius (Tarus) in Bannu and Lakki Marwat Districts of Khyber Pakhtunkhwa. IJAVMS. 2014;8(1):12–20. [Google Scholar]
- 9.Aznar M, Linares F, Cosentino B, Sago A, La Sala L, León E, Perez A. Prevalence and spatial distribution of bovine brucellosis in San Luis and La Pampa, Argentina. BMC Vet. Res. 2015;11(1):209. doi: 10.1186/s12917-015-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seleem M.N, Boyle S.M. Sriranganathan N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010;140(3):392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Mai H.M, Irons P.C, Kabir J, Thompson P.N. A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Vet. Res. 2012;8(1):1. doi: 10.1186/1746-6148-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumaa M, Osman H, Omer M, El Sanousi E, Godfroid J, Ahmed A. Seroprevalence of brucellosis in sheep and isolation of Brucella abortus biovar 6 in Kassala State, Eastern Sudan. Rev. Sci. Tech. Off. Int. Epiz. 2014;33:957–965. doi: 10.20506/rst.33.3.2333. [DOI] [PubMed] [Google Scholar]
- 13.Blasco J.M, Molina-Flores B. Control and eradication of Brucella melitensis infection in sheep and goats. Vet. Clin. N. Am. Food Anim. Pract. 2011;27(1):95–104. doi: 10.1016/j.cvfa.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Minas A. Control and eradication of brucellosis in small ruminants. Small Rumin. Res. 2006;62(1):101–107. [Google Scholar]
- 15.Ochei J, Kolhatkar A. Medical Laboratory Science, Theory and Practice. Vol. 1. New Delhi, India: McGraw-Hill; 2000. pp. 12–13. [Google Scholar]
- 16.Gómez M.C, Nieto J.A, Rosa C, Geijo P, Escribano M.A, Muñoz A, Lopez C. Evaluation of seven tests for diagnosis of human brucellosis in an area where the disease is endemic. Clin. Vaccine Immunol. 2008;15(6):1031–1033. doi: 10.1128/CVI.00424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manual O.T. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris: Office International des Epizooties; 2008. Bovine brucellosis; p. 3. [Google Scholar]
- 18.Cadmus S, Adesokan H, Adedokun B, Stack J. Seroprevalence of bovine brucellosis in trade cattle slaughtered in Ibadan, Nigeria, from 2004-2006. J. South Afr. Vet. Assoc. 2010;81(1):50–53. doi: 10.4102/jsava.v81i1.96. [DOI] [PubMed] [Google Scholar]
- 19.Cadmus S.I, Alabi P.I, Adesokan H.K, Dale E.J, Stack J.A. Serological investigation of bovine brucellosis in three cattle production systems in Yewa Division, South-Western Nigeria. J. South Afr. Vet. Assoc. 2013;84(1):E1–E6. doi: 10.4102/jsava.v84i1.217. [DOI] [PubMed] [Google Scholar]
- 20.Nakoune E, Debaere O, Koumanda-Kotogne F, Selekon B, Samory F, Talarmin A. Serological surveillance of brucellosis and Q fever in cattle in the Central African Republic. Acta Trop. 2004;92(2):147–151. doi: 10.1016/j.actatropica.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Omer M, Skjerve E, Holstad G, Woldehiwet Z, Macmillan A. Prevalence of antibodies to Brucella spp. in cattle, sheep, goats, horses and camels in the State of Eritrea;influence of husbandry systems. Epidemiol. Infect. 2000;125(2):447–453. doi: 10.1017/s0950268899004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, Tanner M, Zinsstag J. Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev. Vet. Med. 2003;61(4):279–293. doi: 10.1016/j.prevetmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Al-Majali A.M, Talafha A.Q, Ababneh M.M, Ababneh M.M. Seroprevalence and risk factors for bovine brucellosis in Jordan. J. Vet. Sci. 2009;10(1):61–65. doi: 10.4142/jvs.2009.10.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayemi P, Webb E.C, Nsongka M.V, Unger H, Njakoi H. Prevalence of Brucella abortus antibodies in serum of Holstein cattle in Cameroon. Trop. Anim. Health Prod. 2009;41(2):141–144. doi: 10.1007/s11250-008-9184-8. [DOI] [PubMed] [Google Scholar]
- 25.Junaidu A, Oboegbulem S, Salihu M. Serological survey of Brucella antibodies in breeding herds. J. Microbiol. Biotechnol. Res. 2011;1:60–65. [Google Scholar]
- 26.Zubairu A, Ardo M, Mai H. Seroprevalence of ruminant brucellosis in three selected local government areas of Taraba state. Sokoto J. Vet. Sci. 2014;12(1):51–56. [Google Scholar]


