Abstract
Aim:
This study was accomplished to test raw milk and certain dairy products sold in local markets of Qena, Egypt, for the presence of Campylobacter coli and Campylobacter jejuni.
Materials and Methods:
A total of 150 samples of raw milk, kareish cheese, and yoghurt (50 samples each) were subjected first to enrichment in Bolton broth at 42°C for 2 days under a microaerobic condition, subsequently campylobacter blood free selective agar plates were cultured and incubated in the same condition of the broth. Based on the morphological and biochemical themes of the growing colonies, it was further classified into Campylobacter spp. The identified isolates were later affirmed by polymerase chain reaction using primers that were designed to locate hipO genes in C. jejuni and glyA in C. coli.
Results:
Of the total 150 examined samples of raw milk and soft cheese samples; 37 (24.6%) samples were contaminated with Campylobacter spp. C. jejuni was dominating in this study in 20%, 14%, and 8% of the examined raw milk, kareish cheese, and yoghurt samples, respectively. No sample harbored C. coli.
Conclusion:
Campylobacter spp. could be detected in 24.6% of the investigated samples. C. jejuni isolated from 14% of the total tested samples, while C. coli could not be detected from the examined samples. Campylobacter spp. is rampant in the areas of poor hygienic conditions making products made from raw milk of public health hazard.
Keywords: Campylobacter coli, Campylobacter jejuni, dairy products, multiplex polymerase chain reaction, raw milk
Introduction
Campylobacteriosis is a massed description for zoonotic diseases that caused by the bacterial genus Campylobacter which is accounted as a leading human food-borne pathogen and it is currently considered to be the main cause of bacterial gastroenteritis worldwide [1,2]. Campylobacter spp. initiated 7.5 million disability-adjusted life years in the study carried out by the Global Burden of Disease in 2010, it overtopped Shigella (7.1 million) and enterotoxigenic Escherichia coli (6.9 million) [3].
About 20 species are members of the Campylobacter genus, of these; Campylobacter jejuni and Campylobacter coli are responsible for most of the infections caused by this bacterium [4,5]. Campylobacter spp., mainly C. jejuni and C. coli induce enteric diseases that vary from a watery, nonbloody, non-inflammatory diarrhea to a severe inflammatory diarrhea with abdominal pain, fever, and malaise [5]. However, Guillain-Barré syndrome (GBS), which is a serious neurological disease with symptoms that include flaccid paralysis, Reiter’s syndrome or reactive arthritis may appear as serious postinfection sequelae [6-9]. The Campylobacter infection’s epidemiology in developed countries is significantly different to that in the developing world. In developing countries, Campylobacter enteritis has no preference for seasonality; in contrast, campylobacteriosis epidemics occur in summer and autumn in developed countries [1,10].
A number of transmission means have been blamed to the transmission of Campylobacter spp. to human, including consumption or handling of food as raw or underdone poultry or meat, raw milk and milk products [11]. Dairy products are predetermined as the main source of Campylobacter infection to human, as it ranked the first among food associated with Campylobacteriosis outbreaks [12,13]. This study aimed to explore the incidence of Campylobacter spp. in some dairy products with special concentration on C. jejuni and C. coli as a pathogen of major public health importance.
Materials and Methods
Ethical approval
Ethical approval is not required to pursue this type of study.
Design of study
This study was conducted within September 2014-February 2015 in the Department of Food Hygiene and Control, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt.
Samples collection
A total of 150 samples of raw milk, kareish cheese, and yoghurt (50 samples each) were collected from local markets and street vendors in Qena city, Egypt. These samples were transferred to laboratory directly to be examined for the presence of C. jejuni and C. coli.
Isolation of Campylobacter spp. from samples
The preparation of the samples and isolation of Campylobacter spp. from the examined samples was done according to FDA [14]. The pH of the samples was adjusted to 7.5±0.2, and then centrifugation of 50 g portion at 20,000 ×g for 40 min was attained. Supernatant was discarded and pellets were dissolved in 10 ml Bolton broth (supplemented with Bolton broth Selective Supplement and Laked Horse Blood, Oxoid) and then was transmitted to 90 ml enrichment broth and incubated at 42°C for 48 h in an anaerobic jar containing a gas generating Kit (Oxoid). The Campylobacter blood free selective agar (mCCDA-Preston, Oxoid) which was supplemented with CCDA selective supplement (Oxoid), were then streaked with a loopful of each enrichment broth, and subsequently, incubated at 42°C for 48 h under microaerobic condition. From 2 to 3 presumptive Campylobacter colonies were purified on Columbia blood agar (containing 7% defibrinated sheep blood)without supplement. About 100 Campylobacter isolates were submitted to Gram-stain, oxidase, catalase, inability to grow aerobically at 25°C, hippurate hydrolysis and resistance to naladixic acid and cephalothin to exclude Campylobacter spp. except C. jejuni and C. coli.
Identification of C. jejuni and C. coli using multiplex polymerase chain reaction (mPCR)
From the biochemically confirmed Campylobacter isolates, 9 strains were selected to be submitted to PCR. From the tested strains, there were 4 suspected strains (one strain was giving a light grayish color in hippurate hydrolysis test, and the other 3 strains were suspected to be sensitive to nalidixic acid).
DNA extraction
DNA extraction from isolates was operated using the QIAamp DNA Mini kit (Qiagen, Germany, GmbH) with remodeling of the manufacturer’s recommendations. In brief, 200 µl of the sample suspension was incubated with 10 µl of proteinase K and 200 µl of lysis buffer at 56°C for 10 min. Following the incubation, 200 µl of 100% ethanol was added to the lysate. The sample was thereafter washed and centrifuged according to the manufacturer’s recommendations. Nucleic acid was eluted with 100 µl of elution buffer afforded with the kit.
Multiplex Polymerase Chain Reaction (mPCR)
mPCR was used to confirm Campylobacter isolates according to Wang et al. [15]. Primers used were supplied from Metabion (Germany). The used primers were intended to identify hipO genes in C. jejuni and glyA in C.coli. The primer sequences used are presented in Table-1.
Table-1.
Sequences of the oligonucleotide primers.
| Target agent | Target gene | Primers sequences (5′-3′) | Amplified segment (bp) | Reference |
|---|---|---|---|---|
| C. jejuni | hipO | ACTTCTTTATTGCTTGCTGC | 126 | Wang et al., 2002 |
| GCCACAACAAGTAAAGAAGC | ||||
| C. coli | glyA | GTAAAACCAAAGCTTATCGTG | 323 | |
| TCCAGCAATGTGTGCAATG |
C. coli=Campylobacter coli, C. jejuni=Campylobacter jejuni.
PCR amplification and analysis of the PCR products.
The PCR mixture reaction (50 µl) consisted of 25 µl of EmeraldAmp Max PCR Master Mix (Takara, Japan), 1 µl of each primer of 20 pmol concentrations, 9 µl of water, and 12 µl of DNA template. Amplification of DNA was accomplished with 35 cycles of the following: Primary denaturation at 94°C for 10 min, annealing at 55°C for 30 s and extension at 72°C for 30 s with a final extension time of 72°C for 7 min (Table-1) in an Applied Biosystem 2720 thermal cycler. The products of PCR were separated by electrophoresis on 1.5% agarose gel (Applichem, Germany, GmbH) in 1 x TBE buffer at room temperature using gradients of 5 V/cm. For gel analysis, 30 µl of the products were loaded in each gel slot. A 100 bp DNA Ladder (Qiagen, Germany, GmbH) was used to determine the fragment sizes. The gel was photographed by a gel documentation system (Alpha Innotech, Biometra), and the data were analyzed through computer software.
Results and Discussion
Results illustrated in Table-2 revealed that Campylobacter spp. were detected in 24.6% of the examined samples. No C. coli could be recovered from the samples, while C. jejuni could be isolated from 20% 14%, and 8% of raw milk, kareish cheese, and yoghurt samples, respectively. C. jejuni is now thought to be a major promoting agent of GBS and it is associated with several pathogenic profiles of GBS, axonal subtypes following the contagion may be more severe [16]. The infective dosage of C. jejuni is considered to be small, as human feeding studies submit that about 400-500 bacteria may produce illness in some persons, while in others, greater numbers are required [17].
Table-2.
Incidence of Campylobacter spp. in the examined samples.
| Samples | Number samples | No. (%) | |||
|---|---|---|---|---|---|
| Campylobacter spp. | C. jejuni | C. coli | Other Campylobacter spp. | ||
| Raw milk | 50 | 11 (22) | 10 (20) | 0 (0) | 1 (2) |
| Kareish cheese | 50 | 17 (34) | 7 (14) | 0 (0) | 10 (20) |
| Yogurt | 50 | 9 (18) | 4 (8) | 0 (0) | 5 (10) |
| Total | 150 | 37 (24.6) | 21 (14) | 0 (0) | 16 (10.6) |
C. coli=Campylobacter coli, C. jejuni=Campylobacter jejuni.
The presence of Campylobacter spp. in raw milk may be contributed to contamination during milking process from the farm environment through feces [18], or after milking due to poor hygienic conditions during storage and handling of milk plus the major role played by workers in accelerating the incidence of the Campylobacter through cross contamination. Lower results recorded by Barakat et al. [19] who isolated C. jejuni from 4.4% of the investigated samples, while Yang et al. [17] obtained higher results as they could isolate C. jejuni from 26% of the examined raw milk samples. On the contrary, Muehlherr et al. [20] and Haghi et al. [21] could not isolate C. jejuni from milk samples and Gergs [22] isolated C. coli from 3% of the samples. Kareish cheese is one of the soft cheeses that are made from raw cow’s or buffaloes’ milk in farmers’ houses, so raw milk is a potential source of kareish cheeses contamination [23,24]. The usage of raw milk in the manufacturing of kareish cheese and the unhygienic conditions of preparation, processing, handling, storage, and selling methods explains the existence of highest positive samples among kareish cheese samples. The incidence of C. jejuni in this study is higher than Barakat et al. [19], who could isolate C. jejuni in 6.7% of the samples, while El-Sharoud [25] could not detect Campylobacter spp. in the examined kareish cheese samples. Contrary to our results Mina and Thanaa [26] isolated C.coli from kareish cheese samples.
The lowest incidence of Campylobacter spp. was found in yoghurt and this may have resulted from the low pH which hinders survival and growth of Campylobacter spp. [25]. The obtained result is closely related to those obtained by Aygun and Pehlivanlar [27] who could isolate C. jejuni from 6% of the samples while Barakat et al. [19] detected C. jejuni in a higher percent of the examined samples (13.4%). Like El-Sharoud [25], no C. coli could be determined in the inspected samples.
Substandard hygienic and sanitary condition and the tight closeness to animals in developing countries all backup the easy and recurrent attainment of any enteric pathogen including Campylobacter. Campylobacteriosis is deeply endemic in developing countries [10]. The major provenances of human infections are environmental pollution and foods. The results showed in this study display raw milk and dairy products as a mean of Campylobacter transmission.
Nowadays, in developing countries, as rule raw milk is boiled before being fed to babies, children, and other family members to protect them from fatal milk-borne infections, but still, there is a potential threat from consumption artisanal products made from raw milk. Taylor et al. [13] notified that Campylobacter outbreaks are much associated with contaminated dairy products as they found that dairy products were implicated in 65 (29%) out of 225 Campylobacter initiated foodborne outbreak in the US. Campylobacteriosis is a pediatric disease in developing countries as it has been stated that 60,000 per 100,000 children below 5 years of age are distressed by Campylobacter infections. In general, developing countries including Egypt do not have internal superintending recording system for Campylobacter foodborne outbreaks; therefore, incidence values expressed in the form of the number of patient cases for a population do not exist. Most evaluations of incidence in developing countries are collected from laboratory-based surveillance of pathogens responsible for diarrhea [28].
The isolate that gave the weak reaction of the hippurate hydrolysis was confirmed to be C. jejuni using multiple PCR (Figure-1). That weak reaction, caused by that C. jejuni strain, may be resulted from using low bacterial concentration in the test. Nakari et al. [29] found that 32% of the 145 strains that gave the negative reaction in the standardized hippurate test turned out to be C.jejuni by PCR and 9 of these strains were responsible for an outbreak. These situations proved that phenotypic tests should be reinforced by the molecular method for the authoritative recognition of C. jejuni and C. coli; hence making the epidemiological statistics on the infections caused by Campylobacter spp. is more authentic.
Figure-1.
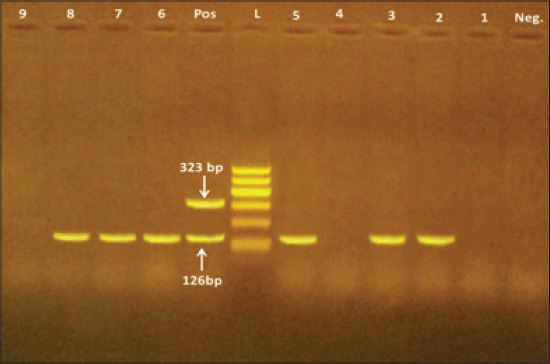
Multiplex-polymerase chain reaction of Campylobacter jejuni and Campylobacter coli strains isolates from raw milk, Kareish cheese and yoghurt samples. Lane (POS): Positive control. Lane (Neg): Negative control. Lane (L): 100 bp ladder as DNA marker. Lanes 2, 3, 5, 6, 7, 8 are positive for C. jejuni only. Lanes 1, 4, 9 are negative for both C. jejuni and C. coli. Lane 2: The strain gave a weak hippurate reaction.
Conclusion
Campylobacter spp. occurred in 24.6% of the examined samples. About 56.7% of the isolated strains were identified as C. jejuni and no C. coli could be detected in the samples using culture and PCR methods. The high incidence of Campylobacter spp. in this study could be contributed to the unhygienic condition applied during production, and storage and also to the warm weather which help the microorganism to grow and multiply and also it shows the need for the increase awareness of the farmers and the small producers for the hygienic precautions during production.
Authors’ Contributions
MAE conceived, designed the study, drafted and revised the manuscript. KGAH and MAE collected and analyzed samples. Both authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to all staff members of the Food Hygiene and Control Department, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt for their technical help.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Platts-Mills J.A, Kosek M. Update on the burden of Campylobacter in developing countries. Curr. Opin. Infect. Dis. 2014;27(5):444–450. doi: 10.1097/QCO.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei B, Cha S.Y, Yoon R.H, Kang M, Roh J.H, Seo H.S, Lee J.A, Jang H.K. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from retail chicken and duck meat in South Korea. Food Control. 2016;62:63–68. [Google Scholar]
- 3.Murray C.J, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Gharst G, Oyarzabal O.A, Hussain S.K. Review of current methodologies to isolate and identify Campylobacter spp. From foods. J. Microbiol. Methods. 2013;95:84–92. doi: 10.1016/j.mimet.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Vencia W, Nogarol C, Bianchi D.M, Gallina S, Zuccon F, Adriano A, Gramaglia M, Decastelli L. Validation according to ISO 16140: 2003 of a commercial real-time PCR-based for detecting Campylobacter jejuni C. coli, and C. lari in foods. Int. J. Food Microbiol. 2014;177:78–80. doi: 10.1016/j.ijfoodmicro.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M.J, Engberg J. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I, Szymanski C.M, Blaser M.J, editors. Campylobacter. Washington, DC: ASM Press; 2008. pp. 99–121. [Google Scholar]
- 7.Smith J. Campylobacter jejuni infection during pregnancy: Long-term consequences of associated bacteremia, Guillain-Barre syndrome, and reactive arthritis. J. Food Prot. 2002;65(4):696–708. doi: 10.4315/0362-028x-65.4.696. [DOI] [PubMed] [Google Scholar]
- 8.Connor B.A, Riddle M.S. Review post-infectious sequelae of travelers’ Diarrhea. J. Travel Med. 2013;20:303–312. doi: 10.1111/jtm.12049. [DOI] [PubMed] [Google Scholar]
- 9.Ganan M, Silvan J.M, Carrascosa A.V, Martinez-Rodriguez A.J. Review, alternative strategies to use antibiotics or chemical products for controlling Campylobacter in the food chain. Food Control. 2012;24:6–14. [Google Scholar]
- 10.Coker K.O, Isokpehi R.D, Bolaji N, Thomas B.N, Amisu K.O, Obit C.L. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 2002;8:237–243. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain I, Mahmood M.S, Akhtar M, Khan A. Prevalence of Campylobacter species in meat, milk and other food commodities in Pakistan. J. Food Microbiol. 2007;24:219–222. doi: 10.1016/j.fm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Painter J.A, Hoekstra R.M, Ayers T, Tauxe R.V, Braden C.R, Angulo F.J, Griffin P.A. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998-2008. Emerg. Infect. Dis. 2013;19:407–415. doi: 10.3201/eid1903.111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor E.V, Herman K.M, Ailes E.C, Fitzgerald C, Yoder J.S, Mahon B.E, Tauxe R.V. Common source outbreaks of Campylobacter infection in the USA. Epidemiol. Infect. 2013;141:987–996. doi: 10.1017/S0950268812001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA. Campylobacter. Bacteriological Analytical Manual. 2001. [Accessed on 21-08-2016]. Available from: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm072616.htm .
- 15.Wang G, Clark C.G, Taylor T.M, Pucknell C, Barton C, Price L, Woodward D.L, Rodgers F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. Fetus. J. Clin. Microbiol. 2002;40:4744–4747. doi: 10.1128/JCM.40.12.4744-4747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyati K.K, Nyati R. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barre syndrome: An update. Biomed. Res. Int. 2013;2013:852195. doi: 10.1155/2013/852195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, Jiang Y, Huang K, Zhu C, Yin Y. Application of real-time PCR for quantitative detection of Campylobacter jejuni in poultry, milk and environmental water. FEMS Immunol. Med. Microbiol. 2003;38:265–271. doi: 10.1016/S0928-8244(03)00168-8. [DOI] [PubMed] [Google Scholar]
- 18.Callon C, Gilbert F.B, Cremoux R.D, Montel M.C. Application of variable numbers of tandem repeat analysis to determine the origin of S. aureus contamination from milk to cheese in goat cheese farms. Food Control. 2008;19:143–150. [Google Scholar]
- 19.Barakat A.M.A, Mona M.S, El Fadaly H.A.A, Nagwa S.R, Nashwa O.K, Eman R.H, Kotb M.H.R, Zeinab M.S, Girh A, Dalia M.S, Mona S.Z. Zoonotic hazards of campylobacteriosis in some areas in Egypt. Life Sci. J. 2015;12(7):9–14. [Google Scholar]
- 20.Muehlherr J.E, Zweifel C, Corti S, Blanco J.E, Stephan R. Microbiological quality of raw bulk-tank milk in Switzerland. J. Dairy Sci. 2011;86(12):3849–3856. doi: 10.3168/jds.S0022-0302(03)73992-7. [DOI] [PubMed] [Google Scholar]
- 21.Haghi F, Zeighami H, Naderi G, Samei A, Roudashti S, Bahari S, Shirmast P. Detection of major food-borne pathogens in raw milk samples from dairy bovine and ovine herds in Iran. Small Rumin. Res. 2015;131:136–140. [Google Scholar]
- 22.Gergs A.E. Thesis, (M. Sc) Egypt: Faculty of Veterinary Medicine, Assiut University; 2004. The Zoonotic Importance of Campylobacter Infection in Man and Animals. [Google Scholar]
- 23.Robison R.K. Dairy Microbiology. 2nd ed. London, New York: Chapman and Hall; 1990. [Google Scholar]
- 24.André M.C.D, Campos M.R.H, Borges L.J, Kipnis A, Pimenta F.C, Serafini A.I.B. Comparison of Staphylococcus aureus isolates from food handlers, raw bovine milk and minas frescal cheese by antibiogram and pulsed field gel electrophoresis following SmaI digestion. Food Control. 2008;19:200–207. [Google Scholar]
- 25.El-Sharoud W.M. Prevalence and survival of Campylobacter in Egyptian dairy products. Food Res. Int. J. 2009;42(55-6):622–626. [Google Scholar]
- 26.Mina H, Thanaa N. Thesis, (M.V.Sc) Egypt: Faculty of Veterinary Medicine, Assiut University; 2004. Occurrence of Campylobacter species in milk and some milk products. [Google Scholar]
- 27.Aygun O, Pehlivanlar S. Campylobacter and Listeria species in the raw milk and dairy products in Antakya, Tyrkey. Food Control. 2006;17(8):676–679. [Google Scholar]
- 28.Epps S.V.R, Harvey R.B, Hume M.E, Phillips T.D, Anderson R.C, Nisbet D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health. 2013;10:6292–6304. doi: 10.3390/ijerph10126292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakari U.M.A, Puhakka A, Siitonen A. Correct identification and discrimination between Campylobacter jejuni and C. coli by a standardized hippurate test and species-specific polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:513–518. doi: 10.1007/s10096-008-0467-9. [DOI] [PubMed] [Google Scholar]


