Abstract
Recent data has indicated that Zn can modulate serotonergic function through the 5-HT1A receptor (5-HT1AR); however, the exact mechanisms are unknown. In the present studies, radioligand binding assays and behavioural approaches were used to characterize the pharmacological profile of Zn at 5-HT1ARs in more detail. The influence of Zn on agonist binding to 5-HT1ARs stably expressed in HEK293 cells was investigated by in vitro radioligand binding methods using the agonist [3H]-8-OH-DPAT. The in vivo effects of Zn were compared with those of 8-OH-DPAT in hypothermia, lower lip retraction (LLR), 5-HT behavioural syndrome and the forced swim (FST) tests. In the in vitro studies, biphasic effects, which involved allosteric potentiation of agonist binding at sub-micromolar Zn concentrations and inhibition at sub-millimolar Zn concentrations, were found. The in vivo studies showed that Zn did not induce LLR or elements of 5-HT behavioural syndrome but blocked such effects induced by 8-OH-DPAT. Zn decreased body temperature in rats and mice; however, Zn failed to induce hypothermia in the 5-HT1A autoreceptor knockout mice. In the FST, Zn potentiated the effect of 8-OH-DPAT. However, in the FST performed with the 5-HT1A autoreceptor knockout mice, the anti-immobility effect of Zn was partially blocked. Both the binding and behavioural studies suggest a concentration-dependent dual mechanism of Zn action at 5-HT1ARs, with potentiation at low dose and inhibition at high dose. Moreover, the in vivo studies indicate that Zn can modulate both presynaptic and postsynaptic 5-HT1ARs; however, Zn’s effects at presynaptic receptors seem to be more potent.
Keywords: Zn, Serotonin, 5-HT1A, Autoreceptor, Depression, Binding, Behavioural studies
Introduction
Zinc (Zn) is an essential trace element that is required for proper brain function [1]. Zn modulates neuronal excitability, plays an important role in synaptic plasticity and can function as a signalling molecule [2, 3]. Recent data have indicated that disturbances in Zn homeostasis are involved in the aetiology of some neurological disorders. Several studies, including both preclinical and clinical studies, showed that Zn deficiency leads to the development of depression [4–6] and that Zn supplementation improves the effectiveness of standard antidepressant treatment [7, 8]. One of the possible mechanisms involved in Zn antidepressant activity is the modulation of the serotonergic system through 5-HT1A and 5-HT2A receptors [9, 10]. Zn was found to enhance the effect of citalopram and fluoxetine in the forced swim test (FST) in mice, and pretreatment with an inhibitor of serotonin synthesis, p-chlorophenylalanine (pCPA), blocked the antidepressant-like effect observed in the FST [10]. Moreover, the fact that rats show an increase in the swimming but not the climbing parameter in the FST following Zn administration indicates (according to the observation of Detke et al. [11]) the involvement of the serotonin pathway in the effects of Zn in the FST [10]. Additionally, it was found that the antidepressant-like effect of Zn in the FST in mice was blocked by the 5-HT1AR antagonist WAY-100635, which suggested that the modulation of the serotonergic system by Zn is mediated mostly through the 5-HT1AR [10].
Further evidence for the postulated direct link between Zn and 5-HT1AR were recently provided by Tena-Campos et al. [12] who showed the impact of Zn homeostasis on the balance between monomers/heterodimers of 5-HT1A and galanin 1 receptor (GalR1). The latter protein was indicated as being involved in major depressive disorder by modulating 5-HT1A functionality via specific heterodimerization process [12].
The direct modulatory effect of Zn at the 5-HT1AR was also reported by Barrondo and Salles, who described negative allosteric modulatory properties of Zn ions against antagonist ([3H]-WAY-100635) and agonist ([3H]-8-OH-DPAT) binding at 5-HT1ARs in cortical membranes isolated from the rat brain [13]. It should be noted, however, that the changes observed for [3H]-8-OH-DPAT (i.e. a decrease of dissociation constant (K D) and B max values were demonstrated through saturation experiments only and were complex and difficult to interpret. Moreover, although studies using native tissue have strong biological significance, membranes prepared from rat cerebral cortex contain other receptors that can be targeted by [3H]-8-OH-DPAT (i.e. serotonin 5-HT7 receptors and α-2 adrenergic receptors) [14, 15], which might create an additional level of complexity in the interactions of Zn at 5-HT1ARs. As allosteric modulation is strongly probe-dependent [16], evaluation of the effects of Zn on agonist binding is of primary importance to its action observed in vivo. Thus, we performed in vitro radioligand experiments to gain further mechanistic insight into the nature of Zn interactions at 5-HT1ARs. In the present study, HEK293 cells expressing 5-HT1A (5-HT1A) receptors were used, which provides a homogeneous system to study effects solely attributable to the 5-HT1AR subtype. The influence of Zn ions on agonist binding was investigated by saturation, competition and both association and dissociation kinetic studies using [3H]-8-OH-DPAT, a 5-HT1AR agonist.
Due their localization, neuronal 5-HT1ARs are divided into two different classes: the 5-HT1A autoreceptors located on the soma and dendrites of serotonergic neurons in the raphe nucleus and the heteroreceptors expressed postsynaptically in the prefrontal cortex (PFC), amygdala and hippocampus [17, 18]. The activation of 5-HT1AR has been shown to induce a number of behavioural responses, including lower lip retraction (LLR), flat body posture (FBP), forepaw treading (FT), and a decrease in body temperature [19–23]. The ability of compounds to induce or block these behaviours is commonly used to differentiate and characterize their activity at pre- or postsynaptic 5-HT1ARs. Therefore, in addition to the binding assays, we assessed the ability of Zn to produce LLR, FBP and FT in rats. We also studied both the capacity of Zn to induce hypothermia and the activity of Zn in the FST in rats as well as in wild-type and 5-HT1A autoreceptor knockout mice.
Methods
Receptor Binding Studies
Drugs
[3H]-8-OH-DPAT (135.2 Ci/mmol) was purchased from PerkinElmer and (R)-(+)-8-OH-DPAT, 5-HT and ZnCl2 were obtained from Sigma-Aldrich.
Expression of the Gene for the Human 5-HT1AR
The full-length human 5HTR1A complementary DNA (cDNA), which was cloned into the mammalian expression vector pcDNA3.1(+), was obtained from the Missouri S&T cDNA Resource Center (www.cdna.org). The receptor cDNA was stably transfected into human embryonic kidney cells (HEK293, ATCC) with the use of Lipofectamine 2000 (Invitrogen). A clone yielding a high expression level of 5-HT1AR was selected during preliminary experiments, including RT-PCR and Western blot analysis.
Cell Culture and Preparation of Cell Membranes
HEK293 cells with stable expression of 5-HT1AR were maintained at 37 °C in a humidified atmosphere with 5 % CO2 and were grown in Dulbecco’s Modified Eagle Medium (Lonza Ltd.) containing 10 % dialysed foetal bovine serum (Lonza Ltd.) and 500 μg/ml G418 sulphate (Sigma-Aldrich). For membranes preparations, the cells were subcultured in 150 cm2 flasks, grown to 90 % confluence, washed twice with phosphate buffered saline (PBS) prewarmed to 37 °C and pelleted by centrifugation (200 g for 5 min) in PBS containing 0.1 mM EDTA and 1 mM dithiothreitol. Prior to membrane preparation, the pellets were stored at −80 °C.
Preparation of Membranes for Radioligand Binding Assays
Cell pellets were thawed and homogenized in 20 volumes of 50 mM Tris–HCl buffer (pH 7.7) containing 0.1 mM EDTA and 10 mM MgCl2, using an Ultra Turrax tissue homogenizer. The pellets were then centrifuged twice at 35,000 g for 20 min at 4 °C, with incubation for 15 min at 37 °C in between centrifugations. Membranes were aliquoted in tubes. Membrane protein concentrations were determined using the Pierce™ Coomassie (Bradford) Protein Assay Kit, with bovine serum albumin (BSA) as a standard.
Radioligand Binding Assays
[3H]-8-OH-DPAT was used as a selective 5-HT1AR agonist. The affinity (K D) and maximal number of binding sites (B max) were measured by saturation binding experiments over a radioligand concentration range of 0.1–14 nM. The affinity shift was determined by measuring the K D obtained in saturation binding assays performed in the absence and presence of six concentrations of Zn (0.01–5 mM). Competition studies were performed with 2.5 nM of [3H]-8-OH-DPAT in the presence of various concentrations of orthosteric agonist serotonin in the absence and presence of Zn at two concentrations (10 and 500 μM). Non-specific binding was estimated in the presence of 10 μM 5-HT. The incubation buffer consisted of 50 mM Tris–HCl (pH 7.7), 10 mM MgCl2, 10 mM pargyline and 0.1 % ascorbic acid. Radioligand binding assays were performed by incubating 30 μg of protein of the membrane suspension in 96-well microtitre plates for 60 min at room temperature with shaking, in a total volume of 200 μl. The binding reactions were stopped by filtration through GF/C Unifilter plates using a harvester (PerkinElmer). The plate filters were dried, and 20 μl of Ultima Gold MV (PerkinElmer) was added. Radioactivity was measured using a MicroBeta TriLux counter (PerkinElmer).
Association and Dissociation Assays
Association and dissociation rate kinetic assays were performed at room temperature using the same buffer conditions described for the equilibrium binding assays and 2.5 nM [3H]-8-OH-DPAT. Non-specific binding was defined by the addition of 10 μM serotonin. The amount of radioligand bound to the receptor was measured at different time intervals during a total incubation of 60 min in the absence or presence of 10 and 500 μM ZnCl2. For the dissociation assay, after incubating the membranes with radioligand for 60 min to achieve equilibrium, serotonin (10 μM), either alone or together with 10 or 500 μM of ZnCl2, was added, and the specifically bound radioligand was measured after incubations of different durations (from 0 to 60 min), which were terminated by rapid filtration.
Data Analysis
All experiments were performed in triplicate, and the results were obtained from at least three independent experiments. The data are expressed as the mean ± S.D. (standard deviation). The experimental data were analysed using GraphPad Prism 5.1 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). Analysis of the saturation binding data with respect to allosteric interactions was performed with the use of equation (1) [24]:
where K App is the apparent equilibrium dissociation constant of radioligand A ([3H]-8-OH-DPAT) observed in the presence of modulator B (ZnCl2); K A and K B are the equilibrium dissociation constants of the radioligand and allosteric modulator, respectively; logd is a constant representing the logarithm of the quotient of K A and α; and α defines the cooperativity factor, the magnitude by which the equilibrium dissociation constant of either ligand for its site on the receptor is modified by the concomitant presence of the other ligand. Values of α less than 1 (but greater than zero) denote negative cooperativity, values greater than 1 denote positive cooperativity and values not significantly different from 1 indicate neutral cooperativity.
Bell-shaped concentration-response curves were fit to a special model [16], based on equation (2):
where ρA denotes the fractional receptor occupancy by the orthosteric ligand; [A] and [B] are the concentrations of [3H]-8-OH-DPAT and ZnCl2, respectively; K A, K B1, and K B2 denote equilibrium dissociation constants; the subscript ‘1’ refers to the binding of B to the orthosteric site; the subscript ‘2’ refers to the binding of B to the allosteric site; the cooperativity factor α′ denotes the interaction between B and A; and β denotes the interaction between the two molecules of B.
The experimental data were fit to one-site and two-site models to determine the best fit. The statistical significance of differences between the means was evaluated by Student’s t test. The level of significance was set at p < 0.05.
Behavioural Studies
Animals and Housing
The experiments were performed on male Albino Swiss mice (23–25 g) and Sprague Dawley rats (200–250 g) from Charles River, Germany as well as TPH2-Cre-ERT2 x flx-5-HT1A-flx-YFP mice from Jackson Labs, Bangor ME and the Hen lab, respectively. The animals were kept under standard laboratory conditions with respect to lighting (light phase 7:00–19:00) and temperature (19–21 °C). Food and water were freely available. Each experimental group consisted of eight to ten animals. All of the animals were experimentally naive and were used only once in each test. The experiments were performed during the light period (9:00–14:00 h). All of the procedures were conducted according to the guidelines of the National Institutes of Health Animal Care and Use Committee and were approved by the Ethics Committees of the Institute of Pharmacology, Polish Academy of Sciences in Krakow and the Animal Care Committee, University of Ottawa.
Drug Administration
Zn (all doses refer to mg Zn/kg) was given as either Zn hydroaspartate (Farmapol, Poland) or Zn chloride (Sigma-Aldrich) and was dissolved in 0.9 % NaCl and administered intraperitoneally (i.p.). 8-OH-DPAT and WAY-100635 were dissolved in aqua pro-injection and were administered subcutaneously (s.c.). Controls were treated with the appropriate diluent, which was indicated as VEH on the graphs and in the tables.
Presynaptic 5-HT1AR Knockout Mice
A conditional knockout approach was used to eliminate the 5-HT1AR in the raphe nuclei in adult mice. To create the conditional 5-HT1AR knockout mice, a TPH2-Cre-ERT2 mouse line was crossed with a flx-5-HT1A-YFP mouse line. The TPH2-Cre-ERT2 confers specificity such that only serotonin neurons, which express tryptophan hydroxylase 2 (TPH2), can express Cre recombinase to knockout the 5-HT1AR gene and allow for GFP expression [25]. Once the mice reached adulthood (approximately 5–6 postnatal weeks), tamoxifen (180 mg/kg) was injected i.p. every day for five consecutive days (the tamoxifen binds to the ERT2 domain and enables Cre to enter the nucleus where it can excise the 5-HT1AR gene, allowing for YFP expression as a marker of recombination). The mice were then left undisturbed for at least 14 days to allow for recombination and the turnover of endogenous 5-HT1ARs.
Lower Lip Retraction
LLR was assessed according to the method described by Berendsen et al., [20]. The rats were individually placed in cages (30 × 25 × 25 cm) and were scored three times, 15, 30 and 45 min after the administration of Zn as follows: 0 = lower incisors not visible, 0.5 = partly visible and 1 = completely visible. Zn was administered at doses of 2 and 5 mg Zn/kg (given as Zn hydroaspartate, Farmapol, Poland). The maximum total score was 3/rat. In addition, the effects of Zn and WAY-100635 (0.1 mg/kg; Sigma) on the LLR induced by 8-OH-DPAT (1 mg/kg; Sigma) were tested. Zn and WAY-100635 were administered 45 min before 8-OH-DPAT, and the animals were scored 15, 30 and 45 min after the 8-OH-DPAT administration.
Behavioural Syndrome
The experiments were conducted according to previously published procedure [26], with a modification to the ranked intensity scale. Briefly, the rats were individually placed in cages (30 × 25 × 25 cm) 5 min before tested compounds were injected. Forepaw treading (FT) and flat body posture (FBP) were scored using a ranked intensity scale, where 0 = absent, 1 = equivocal and 2 = present. The Zn (2, 5, 7.5 and 11.5 mg Zn/kg, given as a Zn hydroaspartate)-induced behavioural syndrome was scored for each animal 3, 6, 9, 12 and 15 min after the Zn treatment. Each observation session lasted for 45 s. The maximum score, which was summed over 5 observation periods, was 10 for each symptom/rat. The effect of Zn on the behavioural syndrome induced by 8-OH-DPAT (5 mg/kg) was scored using the same scale. Zn was administered 60 min before 8-OH-DPAT, and the animals were scored 3, 6, 9, 12 and 15 min after the 8-OH-DPAT treatment.
Body Temperature in Mice and Rats
The effects of Zn (2, 5, 7.5 and 11.5 mg Zn/kg for rats and 2 and 5 mg/kg for mice, given as a Zn hydroaspartate), 8-OH-DPAT (5 mg/kg) and WAY-100635 (0.1 mg/kg) on rectal body temperature were recorded 30 and 60 min after their acute administration. The results were expressed as a change in body temperature with respect to the basal body temperature, which was measured at the beginning of the experiments.
Body Temperature in 5-HT1A Autoreceptor KO Mice
A separate experiment was performed to measure the effect of Zn on rectal body temperature in wild-type and 5-HT1A autoreceptor KO mice; however, in this experiment, only the higher dose of Zn (5 mg Zn/kg; Zn chloride, Sigma) was used.
Forced Swim Test (FST)
Rats
The test was carried out according to the method described previously [10]. On day one of the experiment, the animals were individually placed in plexiglass cylinders (40 cm in height, 18 cm in diameter), containing 25 cm of water maintained at 24–25 °C for a 15-min habituation period. After the rats were removed from the water, they were again placed in their home cages. On the second day, the rats were again placed in the cylinders, and the total duration of immobility was measured for a 5-min test period. Zn (1 or 2 mg Zn/kg, given as Zn hydroaspartate) and 8-OH-DPAT (0.1 or 0.3 mg/kg) were administered alone or jointly 30 min before the test. WAY-100635 (0.1 mg/kg) was administered 15 min before the Zn treatment.
5-HT1A Autoreceptor KO Mice
The behaviour of the mice in the water was recorded using a video camera. The test was performed under red lighting. The cylinder was filled with almost 4 l of water, to a depth that exceeded the distance to which the tail could extend, so the mouse could not balance on its tail at the bottom of the cylinder. The top of the cylinder was 9 cm above the surface of the water. The mice were placed in the individual glass cylinder (22 cm diameter × 37 cm high) for a standard 6-min test; however, the total duration of immobility from the last 4 min of the test was analysed. In this test, only one dose of Zn—5 mg Zn/kg (given as a Zn chloride)—was administered 30 min before the test.
Data Analysis
One-way ANOVA followed by Dunnett’s multiple comparison test (LLR, FBP, FT and FST) or Student’s t test (FST and body temperature in wild-type and 5-HT1A autoreceptor KO mice) was used, and p < 0.05 was considered significant.
Results
Receptor Binding Studies
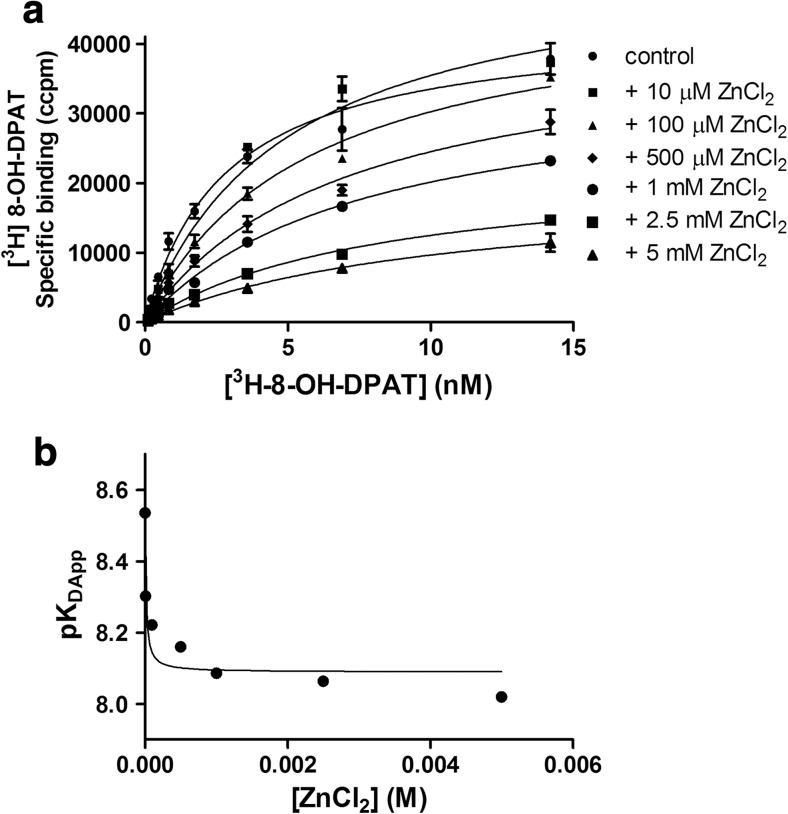
The binding of [3H]-8-OH-DPAT to 5-HT1ARs was saturable, yielding an equilibrium constant of K D = 2.9 ± 0.1 nM (n = 4) and a maximal receptor binding of B max = 4.5 ± 0.3 pM/mg prot. Saturation isotherms obtained for six increasing concentrations of Zn (10 μM–5 mM) revealed a decrease in radioligand binding (Fig. 1a). The K D values for [3H]-8-OH-DPAT increased from 5 nM at 10 μM of Zn to 9.6 nM at 5 mM of Zn relative to the K D obtained for [3H]-8-OH-DPAT without Zn (Table 1). At the same time, unusual changes in B max values were observed. Zn at 10 μM caused an increase in specific binding (B max = 5.8 ± 0.7 pM/mg prot.), Zn at 0.5 and 1 mM recovered the control values (4.7 ± 0.7 and 4.0 ± 0.6 pM/mg prot., respectively), and the two highest concentrations of Zn (2.5 and 5 mM) significantly decreased B max values (3.1 ± 1.0 and 2.2 ± 0.8 pM/mg prot., respectively).
Fig. 1.
Effect of increasing concentrations of Zn on the saturation binding of [3H]-8-OH-DPAT in 5-HT1A receptor-expressing HEK293 cells. a Representative set of radioligand saturation binding curves obtained in the absence and presence of Zn. b Nonlinear regression analysis of the saturation data according to equation 1
Table 1.
Effects of Zn2+ on K D and B max values of [3H]8-OH-DPAT obtained in saturation binding experiments in 5-HT1A receptors in HEK293 cells
| Zn2+ [μM] | 0 | 10 | 100 | 500 | 1000 | 2500 | 5000 |
| K D [nM] | 2.9 ± 0.1 | 5.0 ± 0.1 | 6.0 ± 0.4 | 6.9 ± 0.2 | 8.2 ± 0.9 | 8.6 ± 0.6 | 9.6 ± 0.6 |
| B max [pM/mg prot] | 4.5 ± 0.3 | 5.8 ± 0.7* | 6.2 ± 2.0 | 4.7 ± 0.7 | 4.0 ± 0.6 | 3.1 ± 1.2* | 2.5 ± 0.8* |
*p < 0.05
The data derived from the saturation experiments were fit using nonlinear regression according to equation 1 (Fig. 1b), and the calculated value of the cooperativity factor (α = 0.37) indicated negative modulation between the binding of Zn and the agonist radioligand. This is generally consistent with the results described by Barrondo and Salles for rat cortical membranes, except that increased B max values at lower Zn concentrations were not observed in their saturation studies [13].
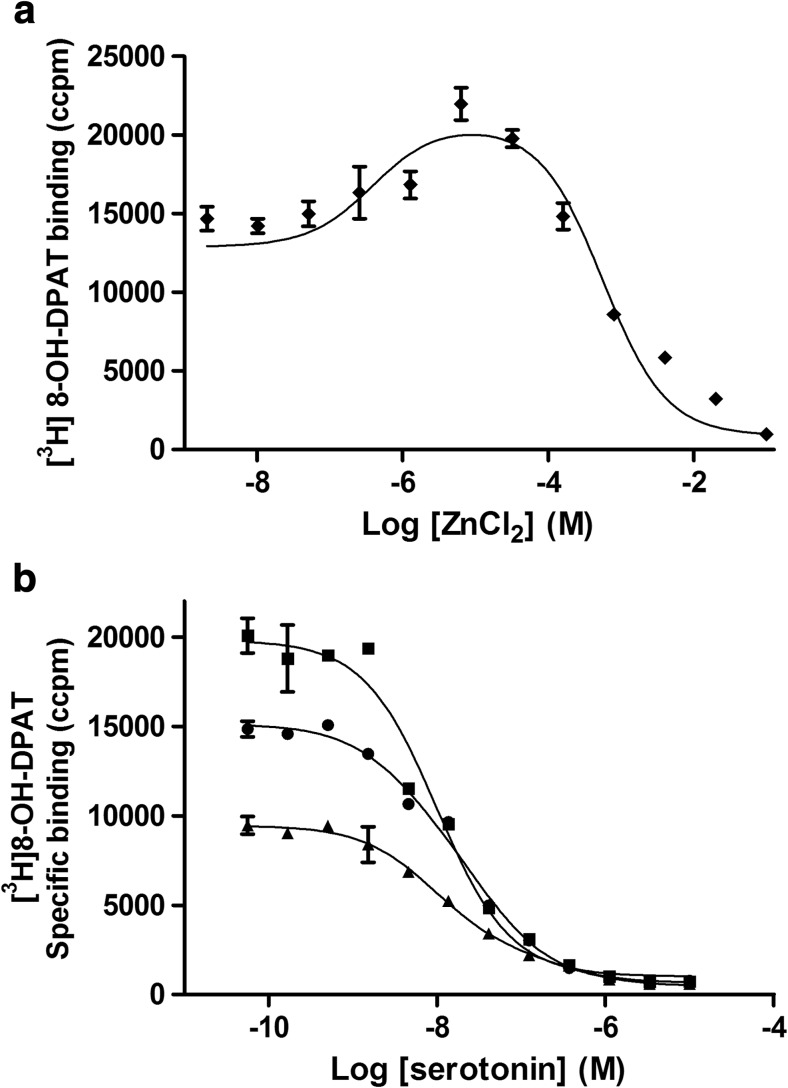
Next, a Zn titration curve against a single, fixed concentration of [3H]-OH-DPAT (2.5 nM) was evaluated in competition-like experiments. As observed in Fig. 2a, a bell-shaped binding curve was obtained, with an ~25 % increase of [3H]-8-OH-DPAT specific binding at 10 μM of Zn and subsequent inhibition at >100 μM. This type of binding is characteristic of ligands exhibiting some degree of allosteric enhancement; therefore, the classic model of negative cooperativity is not a complete description of the action of Zn ions on agonist binding at 5-HT1ARs. Thus, it was interesting to check the influence of Zn ions on serotonin (as an endogenous orthosteric agonist) in displacement experiments in the absence and presence of 10 and 500 μM of Zn. The 5-HT tested alone completely inhibited [3H]-8-OH-DPAT binding at 5-HT1ARs, with a K i of 8.7 ± 0.6 nM; addition of 10 μM of Zn ions caused a small but significant reduction of Ki value for 5-HT (K i = 5.4 ± 0.2 nM, p < 0.05, F test), while at higher Zn concentrations the affinity of 5-HT remained unchanged (K i = 8.1 ± 0.6 nM). Figure 2b shows a representative set of inhibition curves of [3H]8-OH-DPAT for 5-HT obtained in the absence and presence of Zn ions.
Fig. 2.
The ‘bell-shaped’ Zn titration curve obtained in the competition-like experiments, with the enhancement (~10 μM of Zn) and inhibition (above 100 μM of Zn) of [3H]-8-OH-DPAT binding at 5-HT1ARs. The curve was generated by fitting the data to equation 2 (a). Displacement of specific [3H]-8-OH-DPAT binding by 5-HT in the absence (black circle; K i = 8.7 ± 0.6 nM) or presence of 10 μM (black square; K i = 5.4 ± 0.2 nM; p < 0.05) and 500 μM (black triangle; K i = 8.1 ± 0.6 nM) of Zn (b)
Kinetic Studies
It is well known that allosteric modulators may increase or decrease the association and/or dissociation rates of an orthosteric ligand at its binding site in a way that enhancers increase the association rate and/or decrease the dissociation rate, whereas negative allosteric modulators act in the opposite way. On the other hand, competitive orthosteric ligands can influence the association rate by increasing the time needed for the radioligand to reach equilibrium, but do not change the dissociation rate [27].
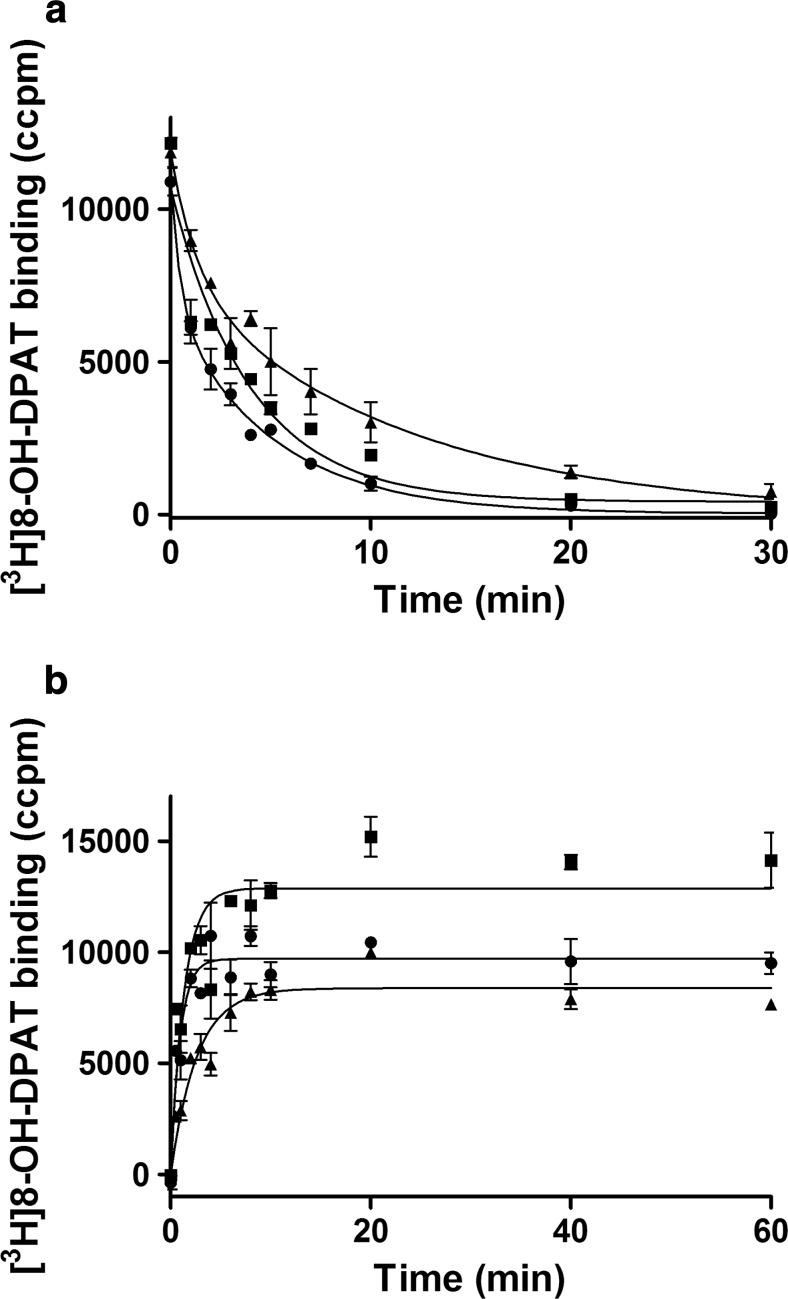
Taking into account the complex mechanism of Zn influence on agonist binding, dissociation and association (see Fig. 3) rates were measured in kinetic assays; the values of kinetic parameters are listed in Table 2.
Fig. 3.
Effect of Zn on the dissociation (a) and association (b) rates of [3H]-8-OH-DPAT in the absence (black circle) or presence of 10 μM (black square) and 500 μM (black triangle) of Zn
Table 2.
Association and dissociation rate constants of [3H]-8-OH-DPAT obtained in the kinetic experiments in the absence and presence of Zn
| k on [M−1 min−1] | k off fast [min−1] | k off slow [min−1] | |
|---|---|---|---|
| Control | 3.4 ± 0.4 × 10+8 | 2.33 ± 0.31 | 0.21 ± 0.02 |
| +10 μM Zn2+ | 6.4 ± 0.8 × 10+8* | 0.25 ± 0.04a | |
| +500 μM Zn2+ | 3.6 ± 0.3 × 10+8 | 0.58 ± 0.07** | 0.06 ± 0.02** |
*p < 0.07; **p < 0.05
aone phase
In the absence of Zn, the dissociation rate for [3H]-8-OH-DPAT at 5-HT1ARs was biphasic, and it remained biphasic at high Zn concentration, but both phases were significantly reduced without affecting the proportion of each state. However, the presence of a low concentration of Zn led to the disappearance of the fast radioligand dissociation rate, and the kinetics of [3H]-8-OH-DPAT became monophasic. These results suggest that Zn modifies receptor conformation, reducing agonist radioligand dissociation, which is in line with the enhancing effects exerted by Zn in the competition-like experiments.
As detailed in Table 2, [3H]-8-OH-DPAT (2.5 nM) binds at 5-HT1ARs with an association rate constant (kon) of 3.4 ± 0.4 × 108 M−1 min−1. In the presence of 10 μM of Zn ions, a small increase in the association rate was observed, while at a higher concentration (500 μM), Zn had no effect on the kon values.
Behavioural Studies
Effects of Zn Treatment on the LLR, FBP and FT in Rats
8-OH-DPAT (1 mg/kg), the 5-HT1A agonist, induced LLR (p < 0.001) in the rats. Zn, given at a dose of 2 and 5 mg/kg, and WAY-100635, a 5-HT1A antagonist, given at the dose of 0.1 mg/kg, did not evoke LLR; however, they blocked the LLR induced by 8-OH-DPAT (Zn: p < 0.05 for the dose of 2 mg/kg and p < 0.001 for the dose of 5 mg/kg; p < 0.01 for WAY-100635, vs. 8-OH-DPAT) (see Table 3). When given at the doses of 2, 5, 7.5 and 11.5 mg/kg, Zn did not induce FBP or FT in the rats (see Table 4); however, at the higher doses of 11.5 mg/kg (p < 0.001), 7.5 and 11.5 mg/kg (p < 0.001 and p < 0.01, respectively), Zn blocked the 8-OH-DPAT-induced FBP and FT.
Table 3.
Lower lip retraction score as a function of 8-OH-DPAT for the Zn, WAY-100635 and control groups
| Mean ± LLR score | ||
|---|---|---|
| Treatment (mg/kg) | (A) VEH | (B) 8-OH-DPAT 1 mg/kg |
| VEH | 0.0 ± 0.0 | 2.25 ± 0.46### |
| Zn 2 | 0.0 ± 0.0 | 1.17 ± 0.98* |
| Zn 5 | 0.0 ± 0.0 | 0.46 ± 0.75*** |
| WAY 100635 0.1 | 0.0 ± 0.0 | 0.67 ± 0.76** |
(A) Each animal was scored for LLR three times, 15, 30 and 45 min after the administration of Zn as follows: 0 = lower incisors not visible, 0.5 = partly visible and 1 = completely visible. The maximum total score was 3/rat. Zn was administered at doses of 2 and 5 mg Zn/kg. (B) The effect of Zn and WAY-100635 on the LLR induced by 8-OH-DPAT was scored using the same scale. Zn and WAY-100635 was administered 45 min before 8-OH-DPAT and the animals were scored 15, 30 and 45 min after 8-OH-DPAT treatment. The values represent the mean ± SEM (n = 8–10 rats per group)
### p < 0.01 vs. VEH; *p < 0.05, **p < 0.01 and ***p < 0.001 vs. 8-OH-DPAT. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post hoc test
Table 4.
Induction of behavioural syndrome by Zn (A) and the effect of Zn on the 8-OH-DPAT-induced behavioural syndrome (B)
| Treatment | Dose (mg/kg) | Mean ± SEM behavioural score | |||
|---|---|---|---|---|---|
| (A) VEH | (B) 8-OH-DPAT | ||||
| FBP | FT | FBP | FT | ||
| VEH | 0.0 ± 0.0 | 0.0 ± 0.0 | 7.83 ± 0.7 | 7.5 ± 0.6 | |
| Zn | 2 | 0.2 ± 0.2 | 0.3 ± 0.2 | 7.7 ± 0.3 | 7.1 ± 0.6 |
| 5 | 0.3 ± 0.3 | 0.2 ± 0.2 | 7.5 ± 0.8 | 7.5 ± 0.5 | |
| 7.5 | 0.5 ± 0.5 | 0.2 ± 0.2 | 7.0 ± 0.3 | 5.0 ± 0.6** | |
| 11.5 | 0.5 ± 0.2 | 0.2 ± 0.2 | 3.4 ± 0.6*** | 0.4 ± 0.2*** | |
(A) In each animal, the Zn-induced behavioural syndrome was scored at 3, 6, 9, 12 and 15 min after Zn treatment. FBP, flat body posture, and FT, forepaw treading, were scored using the following scale: 0 = absent, 1 = equivocal, and 2 = present. The maximum score, which was summed over 5 observation periods, was 10 for each symptom/rat. (B) The effect of Zn on the behavioural syndrome induced by 8-OH-DPAT was scored using the same scale. Zn was administered 60 min before 8-OH-DPAT (5 mg/kg), and the animals were scored at 3, 6, 9, 12 and 15 min after 8-OH-DPAT treatment. The data represent the mean ± SEM for n = 6 rats
***p < 0.001; **p < 0.01, compared to the VEH + 8-OH-DPAT group. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post hoc test
Effects of Zn Treatment on the Body Temperature of Rats and Mice
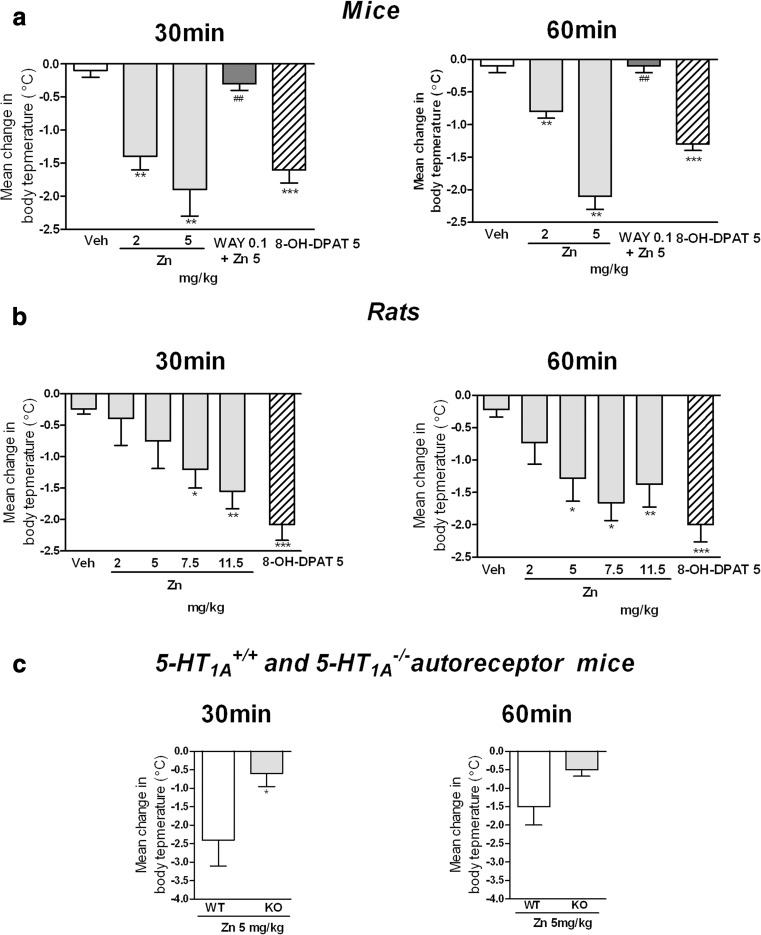
As shown in Fig. 4, 8-OH-DPAT (5 mg/kg) induced a significant decrease in body temperature of the mice (Fig. 4a) and in rats (Fig. 4b) compared to saline group (p < 0.001). Similarly to 8-OH-DPAT, Zn significantly and dose-dependently decreased body temperature of the mice at both 30 (p < 0.01) and 60 min (p < 0.01) after administration (Fig. 4a). Zn also significantly decreased the body temperature of the rats, at both 30 min (p < 0.01 for the dose of 11.5 mg/kg, and p < 0.05 for the dose of 7.5 mg/kg) and 60 min after the injection (p < 0.01 for the dose of 11.5 mg/kg; p < 0.01 for the dose of 7.5 mg/kg; and p < 0.05 for the dose of 5 mg/kg) (Fig. 4b).
Fig. 4.
Effect of Zn and 8-OH-DPAT on body temperature in mice (a), rats (b) and 5-HT1A +/+ (WT) and 5-HT1A autoreceptor−/− (KO) mice (c). The change in body temperature was calculated for each animal by comparing the baseline temperature to the temperature reached 30 and 60 min following Zn or 8-OH-DPAT administration. WAY-100635 at a dose of 0.1 mg/kg was administered (sc) 15 min before the Zn (a). The test was performed 30 and 60 min after injection of Zn (5 mg/kg) (ip). The data bars represent the mean ± SEM for n = 8 (mice); n = 5 (WT mice) and n = 8 (KO mice) and n = 6 (rats) animals. *p < 0.05; **p < 0.01; ***p < 0.001, compared to the VEH group. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post hoc test (a and b) and Student’s t test (c)
Effects of Zn Treatment on the Body Temperature of the 5-HT1A Autoreceptor KO Mice
The effect of Zn treatment on the body temperature of the 5-HT1A autoreceptor KO mice was shown in Fig. 4c. There was no difference in basal body temperature between the wild-type and 5-HT1A autoreceptor KO mice (37.7 ± 0.23 and 37.9 ± 0.19, respectively). Zn administered at a dose of 5 mg /kg, decreased the body temperature of the wild-type mice but not of the autoreceptor KO mice (p < 0.05 at 30 min and p = 0.06 at 60 min).
Effect of Zn in the FST
Rats
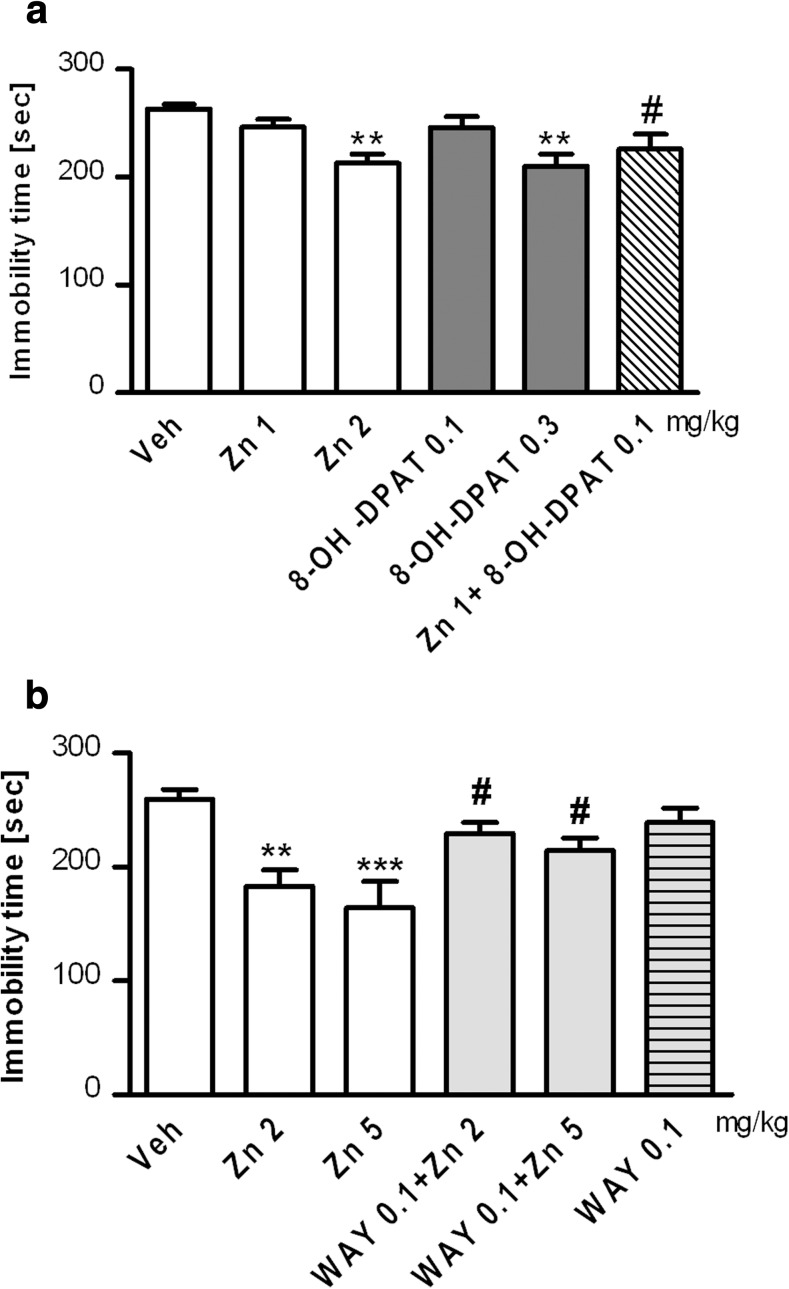
As shown in Fig. 5a, Zn administered at a dose of 2 mg/kg but not 1 mg/kg significantly decreased the immobility time of the rats in the FST (p < 0.01 and p > 0.05, respectively). 8-OH-DPAT administered at a dose of 0.3 mg/kg (p < 0.05) but not 0.1 mg/kg (p > 0.05) decreased the immobility time of the rats in the FST. Zn given jointly with 8-OH-DPAT at the doses that were ineffective in the FST significantly decreased the immobility time of the rats (p < 0.01) compared to the VEH-treated group. As shown in Fig. 5b, Zn at a dose of 2 and 5 mg/kg significantly decreased the immobility time of the rats in the FST (p < 0.05 and p < 0.01, respectively). When given alone, WAY-100635, an antagonist of 5-HT1ARs (0.1 mg/kg), did not change the behaviour of the animals in this test, but it antagonized the Zn-induced decrease in the immobility time of the rats (p < 0.05 vs. the Zn-treated groups).
Fig. 5.
Effect of Zn and 8-OH-DPAT given alone and jointly using the doses that did not affect immobility time in the FST in rats (a) and the effect of pretreatment with WAY-100635 on Zn-induced reduction in immobility time (b). Zn and 8-OH-DPAT were administered alone or jointly 30 min before the FST (a). WAY-100635 was administered 15 min before the Zn treatment, and the FST test was performed 30 min after Zn treatment (b). The values represent the mean ± SEM (n = 5–8 rats). *p < 0.05; **p < 0.01 vs. the VEH group; # p < 0.05 vs. the Zn treatment group. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post hoc test
5-HT1A Autoreceptor KO Mice
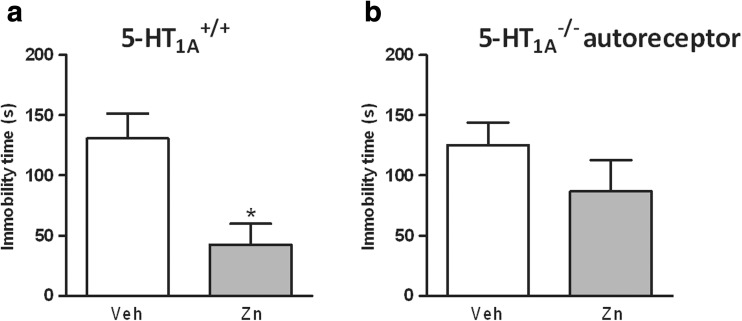
Zn administered at a dose of 5 mg/kg induced a slight (30 %) decrease in the immobility time of the 5-HT1A autoreceptor KO mice compared to the VEH-treated KO mice (see Fig. 6b), while in wild-type mice (see Fig. 6a), a significant decrease in immobility time after Zn treatment was observed (p < 0.05).
Fig. 6.
The effect of Zn treatment on the immobility time in the FST in the 5-HT1A +/+ (WT) (a) and 5-HT1A autoreceptor−/− (KO) (b) mice. Zn at the dose of 5 mg/kg was administered 30 min before the FST. The data bars represent the mean ± SEM for n = 3–5 mice. *p < 0.05 compared to the VEH group. Statistical analysis was performed using Student’s t test
Discussion
Receptor Binding Studies
As an endogenous trace element, Zn has been suggested to act as an allosteric modulator of a number of G protein-coupled receptors, such as dopamine D1, D2 and D4; melanocortin MC1 and MC4; α1A and β2 adrenergic; μ, κ and δ opioid; and serotonin 5-HT1A receptors [28]. Despite the previous identification of Zn ions as allosteric inhibitors of both agonists and antagonists of 5-HT1ARs [13], the exact mechanism of Zn’s action in relation to orthosteric agonists appears complex and is not fully understood. Thus, in the present study, an extended set of in vitro radioligand binding experiments (saturation, competition-like and kinetic tests) were undertaken using [3H]-8-OH-DPAT as an agonist probe to further characterize the effects of Zn on 5-HT1ARs.
Consistent with the data presented by Barrondo and Salles for native tissue [13], the results of the current saturation experiments using stable expression of 5-HT1ARs in HEK293 cells suggest negative allosteric modulation of Zn on [3H]-8-OH-DPAT binding (α = 0.37). Nevertheless, a model of negatively cooperative interactions does not account for all of the additional data obtained in the study.
At first, Zn tested alone in a competition-like assay with [3H]-8-OH-DPAT yielded a bell-shaped binding curve, with a marked increase in agonist radioligand binding at low modulator concentrations and a decrease in binding at high concentrations (Fig. 2a). This type of curve was also shown for several adenosine A 1 allosteric modulators investigated in agonist radioligand binding assays [27, 29, 30]. The results of those studies have invariably been interpreted as evidence that the investigated molecules recognize the allosteric site at low concentrations but also bind to the orthosteric site at higher concentrations, combining the two mechanisms of action: allosteric enhancement and competitive inhibition [27, 29, 30].
Interestingly, in the competition experiments, an enhancement of agonist binding to 5-HT1ARs was evident in the presence of 10 μM of Zn for 5-HT only, while a concentration of 500 μM of Zn did not change the K i values for this orthosteric agonist. Likewise, the association kinetics of [3H]-8-OH-DPAT showed small increase in association rate (k on) at a low (10 μM) concentration of Zn ions (Table 2), and the lack of statistically significant effects on k on values at a higher Zn concentration (500 μM) was observed (Table 2, Fig. 3). However, the results of the dissociation kinetic experiments showed that both concentrations of Zn used slowed the radioligand dissociation rates, which is a characteristic of positive allosteric modulation (Table 2, Fig. 3).
The complex pattern of presently obtained data, when the behaviour of Zn in the saturation experiments and competition and kinetic assays are compared, supports the conclusion that the previously assumed negatively cooperative model is not a complete description of Zn’s effects on agonist binding. Because Zn displayed negative cooperative interactions with [3H]-8-OH-DPAT in the saturation experiments but caused an increase in B max values at low concentrations and directly showed properties of positive allosteric modulation in both the competition-like and kinetic experiments, a dual mode of Zn action against agonist binding at 5-HT1ARs should be considered.
It is worth noting that in the case mentioned above, in which adenosine A 1 ligands showed positive cooperativity in interactions with agonists, when the same modulators were tested against antagonist probes, they were characterized only by inhibitory properties [29, 30]. Similar probe dependence can be observed by comparing the data we obtained for the effects of Zn on agonist binding at 5-HT1ARs with earlier results describing clear negative modulation of antagonist ([3H]-WAY-100635) binding [13].
It should be mentioned that a complex mechanism of Zn action has also been detected for several other proteins. In electrophysiological studies of 5-HT3 receptor, low concentrations of Zn (0.3–10 μM) enhanced and high concentrations of Zn (30–200 μM) depressed, the 5-HT-induced response [31]. Biphasic effects involving potentiation at sub-micromolar and inhibition at sub-millimolar Zn concentrations have been detected for glycine receptors [32]. Similar effects were also demonstrated for β 2 adrenergic receptors, in which the presence of 5 μM of Zn enhanced agonist affinity, whereas 500 μM of Zn inhibited antagonist binding [33].
Summing up, Zn released into synaptic space from neuronal vesicles (of mostly glutamatergic terminals) was found to act at various channels and membrane receptors and these modulatory effects of Zn can be positive or negative depending on its concentration. The exact amount of Zn release is controversial; however, many laboratories have indicated that Zn increases in the extracellular space may reach 1–100 μM [34–36]. Therefore, it seems that the effects observed at lower Zn concentrations should be physiologically more relevant, especially that the elevation of extracellular Zn level, over 300 μM, is reported as neurotoxic [37].
In Vivo Studies
The results of in vivo studies provide evidence that Zn is likely to have both an agonist and antagonist profile at 5-HT1A receptors that could be a consequence of dual Zn effects at 5-HT1A receptors suggested by in vitro studies.
Body Temperature
Induction of hypothermia in response to the administration of 8-OH-DPAT is one of the parameters which have been proposed as an index of 5-HT1AR autoreceptor mediated activity [23, 38]. In our study, Zn decreased the body temperature in mice and the intensity of this effect was similar to that observed for 8-OH-DPAT. Furthermore, pretreatment with WAY-100635, the 5-HT1AR antagonist, abolished the effect induced by Zn. On the other hand, our studies using 5-HT1A autoreceptor KO mice showed that lack of this receptor completely blocked the hypothermia induced by Zn, while in wild-type littermate mice, a consistent decrease in body temperature was observed. These results are consistent with those showing that hypothermic effect of 5-HT1AR agonists in mice is mediated by presynaptic 5-HT1AR [38, 39] and indicate an agonist-like profile of Zn at presynaptic 5-HT1ARs, perhaps enhancing the action of endogenous 5-HT.
In the present studies, we found that Zn can also induce hypothermia in rats; however, this effect was observed at higher doses than in mice. It is still controversial which 5-HT1ARs namely pre- or postsynaptic are responsible for the induction of decrease in the body temperature in rats. Some data [23, 40, 41] suggest that postsynaptic rather than presynaptic 5-HT1AR is implicated in this effect in rats. Despite that these data further suggest the agonist-like profile of Zn at 5-HT1AR as in mice.
Lower Lip Retraction and 5-HT Syndrome
Another behavioural approach used to characterize 5-HT1AR mediated activity is the induction of lower lip retraction (LLR). As shown by Berendsen et al. [20, 21], LLR was induced by the 8-OH-DPAT, buspirone, ipsapirone—agonists or partial agonists with a high affinity for 5-HT1A binding sites in rat brain but not by serotonergic agents such as 5-MeO-DMT, mCPP or DOI with weaker affinity for 5-HT1AR but with high affinity for 5-HT2AR, 5-HT2B/2CR or 5-HT2A/2CR, respectively [20, 21]. The effect induced by 8-OH-DPAT was attenuated by 5-HT1A antagonists such as WAY-100135 or WAY-100635 (which by themselves did not produce the LLR [42]), as well as by the above-mentioned nonselective serotonergic agents of moderate 5-HT1AR affinity which can act as antagonists of 5-HT1AR. In the case of location of receptors mediating this effect, it is suggested that LLR produced by the selective 5-HT1AR agonists has been attributed to autoreceptor activation [20, 21].
In our study, Zn did not induce LLR but significantly and dose-dependently blocked the LLR induced by 8-OH-DPAT. As was mentioned above, the LLR is specific only for compounds with high efficacy at 5-HT1ARs; thus, the lack of this effect after Zn treatment with simultaneous blockade of 8-OH-DPAT action might result from the fact that Zn, like 5-MeO-DMT, mCPP or DOI, can also modulate other types of serotonin receptors, especially from 5-HT2R family. It was, for instance, found that chronic Zn administration increased the density of 5-HT2A serotonin receptors in the frontal cortex [9]. Moreover, ritanserin, the 5-HT2A/C receptor antagonist, blocked the antidepressant-like effect of Zn in the FST [10].
Another behavioural effect induced by 5-HT1AR agonists is serotonin syndrome that is believed to reflect postsynaptic 5-HT1AR activation. It is observed after higher doses of 8-OH-DPAT and is associated with the enhanced 5-HT synthesis [43]. In the behavioural syndrome tests, Zn given alone did not evoke flat body posture or forepaw trading, however, in the higher doses strongly inhibited both FBP and FT showing a postsynaptic 5-HT1AR antagonist-like profile.
Taken together, our data suggest that Zn has concentration-dependent actions in the above-mentioned behavioural responses that are mediated by 5-HT1ARs. Thus, LLR and hypothermia are produced following administration of low doses of Zn or 8-OH-DPAT and are attributed selectively to autoreceptor activation. Interestingly, Zn induced hypothermia in mice at lower doses than in rats. Higher doses of Zn were needed to reverse 8-OH-DPAT induced LLR, and only higher doses of Zn blocked 8-OH-DPAT induced FBP/FT. These results are consistent with a potentiating effect of Zn on agonist actions at 5-HT1AR at low doses and an antagonist effect at higher doses, although the absolute dose depends on the animal model and behaviour studied.
Forced Swim Test
The 5-HT1AR agonist 8-OH-DPAT and several others serotonin agonists with varying degrees of selectivity for different subtypes of 5-HTRs produce antidepressant-like behaviour in the FST. Wieland and Lucki, [44] showed that p-chlorophenylalanine (pCPA), which is known to reduce the concentration of serotonin in the brain by inhibiting its biosynthesis, did not block the antidepressant effect of 8-OH-DPAT in the FST in rats. These and other studies suggest that the antidepressant-like effects of 5-HT1AR agonists are mediated by postsynaptic 5-HT1ARs [19, 44].
Zn also induces antidepressant-like effects in the FST both in rats and mice [10, 45–48]. Our earlier studies performed in mice [10] and the present studies in rats indicated that pretreatment with WAY-100635 blocked the effect of Zn. Furthermore, when Zn was given together with 8-OH-DPAT at the doses that were ineffective in the FST, it decreased immobility time, which suggests the additive effect of both compounds in this test and the agonist-like effect of Zn in the FST. Interestingly, the results of the 5-HT1A autoreceptor KO mice in the FST demonstrated that the lack of presynaptic 5-HT1ARs only partially blocked the anti-immobility effect induced by Zn in mice; however, pCPA pretreatment completely blocked the antidepressant-like effect of Zn in mice [10]. These data suggests that presynaptic receptors may be also implicated in the Zn-induced effects in the FST. These results also showed that Zn does not work without endogenous serotonin, which can be attributed to its allosteric mechanism of 5-HT1AR regulation because allosteric modulators may act only in conjunction with physiological receptor activation. It should be noted, however, that the allosteric nature of Zn described previously [13], which suggests only the inhibition of both agonist and antagonist interactions at 5-HT1ARs, cannot explain the agonistic-like effects of Zn observed in our in vivo studies. In contrast, the current in vitro binding data, which showed characteristics of positive allosteric modulation in the presence of 10 μM of Zn and the inhibition of agonist action at sub-millimolar Zn concentrations, are more coherent with the results observed in vivo.
Conclusions
In summary, our studies provide new data regarding the dual mechanism of Zn action at 5-HT1ARs, which may underlie its antidepressant-like effects observed in the behavioural tests. The in vitro radioligand results revealed biphasic effects, involving allosteric potentiation of agonist binding at sub-micromolar Zn concentrations and inhibition at sub-millimolar Zn concentrations. Given the therapeutic potential of Zn, the in vitro results obtained for lower Zn concentration (10 μM) are within the range of reported extracellular free Zn concentrations (1–100 μM) and thus potentiating effects may be physiologically more relevant than inhibition observed using higher Zn concentrations at which toxic effects may be expected.
In behavioural paradigms, which are commonly used to distinguish the pharmacological profile of new compounds at 5-HT1AR, both agonist and antagonist-like effects of Zn at 5-HT1ARs were found, and these data are consistent with results from the in vitro radioligand studies revealing biphasic Zn effects at 5-HT1AR, involving allosteric potentiation of agonist binding and inhibition. Taking into account results of in vivo studies with the use of wild-type and 5-HT1A autoreceptor knockout animals, it seems that Zn can affect both pre- and postsynaptic 5-HT1ARs. It should be, however, stressed that more studies are needed to explain this complex mechanism of antidepressant-like effect of Zn via modulation of serotonin system, especially that there are many controversies in the literature concerning the participation of the pre- and postsynaptic 5-HT1ARs in the behavioural tests in rodents.
Acknowledgments
This study was supported by the grants: No. 2012/05/N/NZ7/02110 (G.S.) and No. 2013/08/M/NZ7/00518 (B.S.) from the National Science Centre, Poland; grant No. 123426 from Canadian Institutes of Health Research (P.R.A) and partially by statutory funds of the Institute of Pharmacology, Polish Academy of Sciences in Krakow.
Authors’ Contribution
G.S. conducted in vitro experiments and participated in the in vitro data analysis; B.D. and A.J.B. conducted the in vitro study design and participated in the interpretation and description of the in vitro experimental data; T.L. developed and maintained the HEK293 cell line expressing 5-HT1ARs; K.S., A.R. and B.P. participated in the behavioural studies; M.D. and C.L. participated in the behavioural studies using the 5-HT1A autoreceptor KO mice; K.F.T. and R.H. generated the 5-HT1A autoreceptor KO mice; P.R.A. and B.S. conceived and designed the mouse experiments; P.R.A. and G.N. contributed to the revising of the last version of the manuscript; and B.S. initiated the present studies, designed and participated in all of the behavioural studies and created the final version of the manuscript.
Abbreviations
- DOI
(±)-2,5-Dimethoxy-4-iodoamphetamine
- FBP
Flat body posture
- FST
Forced swim test
- FT
Forepaw treading
- GalR1
Galanin 1 receptor
- HEK293
Human embryonic kidney 293
- 5-HT1AR
5-HT1A receptor
- 5-HT
5-Hydroxytryptamine (serotonin)
- 5-MeO-DMT
5-Methoxy-N,N-dimethyltryptamine
- 8-OH-DPAT
8-Hydroxy-2-(di-n-propylamino)tetralin
- LLR
Lower lip retraction
- mCPP
1-(3-Chlorophenyl)piperazine
- pCPA
p-Chlorophenylalanine
- PFC
Prefrontal cortex
- WAY-100135
(S)-N-tert-Butyl-3-(4-(2-methoxyphenyl)-piperazin-1-yl)-2-phenylpropanamide dihydrochloride
- WAY-100635
N-[2[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Brain Res Rev. 2000;34:137–148. doi: 10.1016/S0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 2.Haase H, Maret W. Fluctuations of cellular, available zinc modulate insulin signaling via inhibition of protein tyrosine phosphatases. J Trace Elem Med Biol. 2005;19:37–42. doi: 10.1016/j.jtemb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 4.Doboszewska U, Sowa-Kucma M, Mlyniec K, Pochwat B, Holuj M, Ostachowicz B, Pilc A, Nowak G, Szewczyk B. Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:254–263. doi: 10.1016/j.pnpbp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Mlyniec K, Davies CL, Budziszewska B, Opoka W, Reczynski W, Sowa-Kucma M, Doboszewska U, Pilc A, Nowak G. Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol Rep. 2012;64:567–575. doi: 10.1016/S1734-1140(12)70852-6. [DOI] [PubMed] [Google Scholar]
- 6.Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci. 2013;5:33. doi: 10.3389/fnagi.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siwek M, Dudek D, Paul IA, Sowa-Kucma M, Zieba A, Popik P, Pilc A, Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord. 2009;118:187–195. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Siwek M, Dudek D, Schlegel-Zawadzka M, Morawska A, Piekoszewski W, Opoka W, Zieba A, Pilc A, Popik P, Nowak G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord. 2010;126:447–452. doi: 10.1016/j.jad.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Cichy A, Sowa-Kucma M, Legutko B, Pomierny-Chamiolo L, Siwek A, Piotrowska A, Szewczyk B, Poleszak E, Pilc A, Nowak G. Zinc-induced adaptive changes in NMDA/glutamatergic and serotonergic receptors. Pharmacol Rep. 2009;61:1184–1191. doi: 10.1016/S1734-1140(09)70182-3. [DOI] [PubMed] [Google Scholar]
- 10.Szewczyk B, Poleszak E, Wlaz P, Wrobel A, Blicharska E, Cichy A, Dybala M, Siwek A, Pomierny-Chamiolo L, Piotrowska A, Branski P, Pilc A, Nowak G. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:323–329. doi: 10.1016/j.pnpbp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 12.Tena-Campos M, Ramon E, Lupala CS, Perez JJ, Koch KW, Garriga P (2015) Zinc is involved in depression by modulating G protein-coupled receptor heterodimerization. Mol Neurobiol. [DOI] [PubMed]
- 13.Barrondo S, Salles J. Allosteric modulation of 5-HT(1A) receptors by zinc: binding studies. Neuropharmacology. 2009;56:455–462. doi: 10.1016/j.neuropharm.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Heusler P, Rauly-Lestienne I, Tourette A, Tardif S, Ailhaud MC, Croville G, Cussac D. Actions of the prototypical 5-HT1A receptor agonist 8-OH-DPAT at human alpha2-adrenoceptors: (+)8-OH-DPAT, but not (−)8-OH-DPAT is an alpha2B subtype preferential agonist. Eur J Pharmacol. 2010;640:8–14. doi: 10.1016/j.ejphar.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Monsma FJ, Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- 16.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 17.Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 19.Assie MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortere R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A. F15599, a highly selective post-synaptic 5-HT(1A) receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol. 2010;13:1285–1298. doi: 10.1017/S1461145709991222. [DOI] [PubMed] [Google Scholar]
- 20.Berendsen HH, Jenck F, Broekkamp CL. Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- 21.Berendsen HH, Bourgondien FG, Broekkamp CL. Role of dorsal and median raphe nuclei in lower lip retraction in rats. Eur J Pharmacol. 1994;263:315–318. doi: 10.1016/0014-2999(94)90728-5. [DOI] [PubMed] [Google Scholar]
- 22.Cryan JF, Kelliher P, Kelly JP, Leonard BE. Comparative effects of serotonergic agonists with varying efficacy at the 5-HT(1A) receptor on core body temperature: modification by the selective 5-HT(1A) receptor antagonist WAY 100635. J Psychopharmacol. 1999;13:278–283. doi: 10.1177/026988119901300310. [DOI] [PubMed] [Google Scholar]
- 23.Millan MJ, Rivet JM, Canton H, Le Marouille-Girardon S, Gobert A. Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther. 1993;264:1364–1376. [PubMed] [Google Scholar]
- 24.Leach K, Sexton PM, Christopoulos A. Quantification of allosteric interactions at G protein-coupled receptors using radioligand binding assays. Curr Protoc Pharmacol. 2011;52:1.22.1–1.22.41. doi: 10.1002/0471141755.ph0122s52. [DOI] [PubMed] [Google Scholar]
- 25.Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, Madronal N, Donaldson ZR, Drew LJ, Dranovsky A, Gross CT, Tanaka KF, Hen R (2015) 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci 18:1606–1616 [DOI] [PMC free article] [PubMed]
- 26.Paluchowska MH, Mokrosz MJ, Bojarski A, Wesolowska A, Borycz J, Charakchieva-Minol S, Chojnacka-Wojcik E. On the bioactive conformation of NAN-190 (1) and MP3022 (2), 5-HT(1A) receptor antagonists. J Med Chem. 1999;42:4952–4960. doi: 10.1021/jm991045h. [DOI] [PubMed] [Google Scholar]
- 27.Gregory KJ, Sexton PM, Christopoulos A (2010) Overview of receptor allosterism. Curr Protoc Pharmacol Chapter 1:Unit 1.21. doi:10.1002/0471141755.ph0121s51 [DOI] [PubMed]
- 28.van der Westhuizen ET, Valant C, Sexton PM, Christopoulos A. Endogenous allosteric modulators of G protein-coupled receptors. J Pharmacol Exp Ther. 2015;353:246–260. doi: 10.1124/jpet.114.221606. [DOI] [PubMed] [Google Scholar]
- 29.Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol Pharmacol. 1990;38:939–949. [PubMed] [Google Scholar]
- 30.Valant C, Aurelio L, Urmaliya VB, White P, Scammells PJ, Sexton PM, Christopoulos A. Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol Pharmacol. 2010;78:444–455. doi: 10.1124/mol.110.064568. [DOI] [PubMed] [Google Scholar]
- 31.Gill CH, Peters JA, Lambert JJ. An electrophysiological investigation of the properties of a murine recombinant 5-HT3 receptor stably expressed in HEK 293 cells. Br J Pharmacol. 1995;114:1211–1221. doi: 10.1111/j.1476-5381.1995.tb13335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maksay G, Biro T. Dual cooperative allosteric modulation of binding to ionotropic glycine receptors. Neuropharmacology. 2002;43:1087–1098. doi: 10.1016/S0028-3908(02)00213-7. [DOI] [PubMed] [Google Scholar]
- 33.Swaminath G, Steenhuis J, Kobilka B, Lee TW. Allosteric modulation of beta2-adrenergic receptor by Zn(2+) Mol Pharmacol. 2002;61:65–72. doi: 10.1124/mol.61.1.65. [DOI] [PubMed] [Google Scholar]
- 34.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. J Neurosci. 2011;31:16076–16085. doi: 10.1523/JNEUROSCI.3454-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol. 1991;103:1857–1864. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagdy G, To CT. Comparison of relative potencies of i.v. and i.c.v. administered 8-OH-DPAT gives evidence of different sites of action for hypothermia, lower lip retraction and tail flicks. Eur J Pharmacol. 1997;323:53–58. doi: 10.1016/S0014-2999(97)00021-6. [DOI] [PubMed] [Google Scholar]
- 41.Schenk S, Abraham B, Aronsen D, Colussi-Mas J, Do J. Effects of repeated exposure to MDMA on 5HT1a autoreceptor function: behavioral and neurochemical responses to 8-OHDPAT. Psychopharmacology (Berl) 2013;227:355–361. doi: 10.1007/s00213-013-2980-5. [DOI] [PubMed] [Google Scholar]
- 42.Przegalinski E, Filip M, Budziszewska B, Chojnacka-Wojcik E. Antagonism of (+)WAY 100135 to behavioral, hypothermic and corticosterone effects induced by 8-OH-DPAT. Pol J Pharmacol. 1994;46:21–27. [PubMed] [Google Scholar]
- 43.Kleven M, Ybema C, Carilla E, Hamon M, Koek W. Modification of behavioral effects of 8-hydroxy-2-(di-n-propylamino)tetralin following chronic ethanol consumption in the rat: evidence for the involvement of 5-HT1A receptors in ethanol dependence. Eur J Pharmacol. 1995;281:219–228. doi: 10.1016/0014-2999(95)00324-E. [DOI] [PubMed] [Google Scholar]
- 44.Wieland S, Lucki I. Antidepressant-like activity of 5-HT1A agonists measured with the forced swim test. Psychopharmacology (Berl) 1990;101:497–504. doi: 10.1007/BF02244228. [DOI] [PubMed] [Google Scholar]
- 45.Kroczka B, Zieba A, Dudek D, Pilc A, Nowak G. Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Pol J Pharmacol. 2000;52:403–406. doi: 10.1211/0022357001774156. [DOI] [PubMed] [Google Scholar]
- 46.Kroczka B, Branski P, Palucha A, Pilc A, Nowak G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res Bull. 2001;55:297–300. doi: 10.1016/S0361-9230(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 47.Szewczyk B, Poleszak E, Sowa-Kucma M, Wrobel A, Slotwinski S, Listos J, Wlaz P, Cichy A, Siwek A, Dybala M, Golembiowska K, Pilc A, Nowak G. The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids. 2010;39:205–217. doi: 10.1007/s00726-009-0412-y. [DOI] [PubMed] [Google Scholar]
- 48.Nowak G. Zinc, future mono/adjunctive therapy for depression: mechanisms of antidepressant action. Pharmacol Rep. 2015;67:659–662. doi: 10.1016/j.pharep.2015.01.015. [DOI] [PubMed] [Google Scholar]