Abstract
The raccoon (Procyon lotor) carnivore native to North America is a fast spreading, invasive species in the Europe now. At the moment, the highest population occupies areas near the German-Polish border. The data on the occurrence of Cryptosporidium spp. and microsporidia in raccoons is limited to North America’s territory and is totally lacking in the case of their introduction to Europe. Therefore, the objective of this study was to investigate the occurrence of microparasites, i.e., Cryptosporidium spp. and microsporidia in the introduced raccoons obtained from localities in Poland and Germany. A PCR-based approach that permitted genetic characterization via sequence analysis was applied to raccoon fecal samples (n = 49), collected during 2012–2014. All fecal samples were simultaneously tested with the use of genetic markers, and DNA of microsporidia and Cryptosporidium spp. was detected among the examined raccoons. The results of our research confirmed the presence of Cryptosporidium skunk genotype and Enterocytozoon bieneusi NCF2 genotype. The results suggest a possible role of raccoons in the contamination of the environment, including urban areas, with pathogens of zoonotic significance as well as their role in the transmission and introduction of new genotypes of microparasites in the areas where P. lotor has not been observed yet. To our knowledge, there has been no literature data on the above genotypes detected previously in humans or animals from the examined study sites so far.
Keywords: Raccoon, Cryptosporidium spp., Enterocytozoon bieneusi, Genotyping
Introduction
The raccoon is a North American carnivore which was introduced to Japan and Europe in the 20th century. In Europe, as a result of escaped pets, releases, and escapes from fur farms, raccoons are distributed almost across the whole mainland (Beltrán-Beck et al. 2012). A rapid expansion of this species has been observed in wild environment since the 1980s mainly on the German territory (Hohmann et al. 2001; Stubbe 1999). At present, the largest European stable population occurs in Germany (over one million individuals) (Hohmann et al. 2000; Michler and Michler 2012), but smaller populations inhabit also other European countries (Beltrán-Beck et al. 2012; Schley et al. 2001; Stubbe 1999). In Poland, the first individuals in wildlife were observed in the 1940s (Bogdanowicz and Ruprecht 1987). In the 1980s and the 1990s, a wild population was reported in Western Poland, and since that time, the abundance of the raccoons on the Polish territory has grown rapidly (Bartoszewicz et al. 2008; Bartoszewicz and Okarma 2007; Biedrzycka et al. 2014; Popiołek et al. 2011).
Raccoons, which become one of the fastest spreading wild living population, are often found in forested areas as well in urban space near human settlements, where they can find alternative sources of food but also contribute to the transmission of many zoonotic groups of parasites to other wildlife and humans (Kresta et al. 2009). Some studies have shown that species introduced into a novel environment often lose their own parasites during the course of a new population establishment (Torchin et al. 2003; Torchin and Mitchell 2004) but also encounter and accumulate parasites that occur in newly colonized areas. In addition, there may be a significant probability of raccoons introducing some new parasite species, recorded previously in individuals from North America, into European ones. The raccoon as an alien and invasive species, both wild living and potentially synanthropic, may serve as a susceptible host for opportunistic intestinal parasites. The current epidemiological data on Cryptosporidium spp. and microsporidia has raised public health concerns about the zoonotic nature of transmission of these microparasites. The knowledge on raccoon as reservoir hosts of the abovementioned group of parasites is rather limited and concerns Central and North Americas’ territories (Feng et al. 2007; Guo et al. 2014; Perz and Le Blancq 2001; Snyder 1988; Sulaiman et al. 2003; Zhou et al. 2004). On the other hand, there is no data on these microparasites in the case of invasive European raccoons.
Enterocytozoon bieneusi and Encephalitozoon spp. are the major microsporidians infecting humans and animals worldwide (Santin and Fayer 2011). At present, over 240 E. bieneusi genotypes have been identified (Matos et al. 2012; Zhao et al. 2015). By internal transcribed spacer (ITS) sequence analysis of E. bieneusi genotypes, eight different groups of all genotypes were established (Karim et al. 2014). A large cluster named as group 1 contains more than 94 % published genotypes of E. bieneusi (Henriques-Gil et al. 2010). The genotypes within this group are found both in humans and animals. Even though some genotypes are genetically similar to human pathogenic ones, they have been found only in animals so far, suggesting their zoonotic potential (Henriques-Gil et al. 2010). The remaining genogroups (2–8) are found mostly in specific hosts and wastewater (Guo et al. 2014). Encephalitozoon spp., another microsporidia group, has been generally studied among humans and domestic animals; there is still insufficient information on the role of wild living animals, including raccoons, which may be a potential source of zoonotic contamination with this microsporidia species.
So far, 30 species and over 100 genotypes of Cryptosporidium have been described in various vertebrate hosts and environmental sources (Kváč et al. 2014). Among them, Cryptosporidium hominis and Cryptosporidium parvum are responsible for over 90 % of human cryptosporidiosis cases (Rossle and Latif 2013). Wild living mammals, including carnivores, have been described as reservoirs of several Cryptosporidium species, especially C. parvum and Cryptosporidium muris (Fayer et al. 2010; Ryan and Hijjawi 2015) but also Cryptosporidium meleagridis, Cryptosporidium ubiquitum, Cryptosporidium felis, Cryptosporidium canis, Cryptosporidium cuniculus, Cryptosporidium skunk genotype, chipmunk genotype, and other novel genotypes (Chalmers et al. 2009, 2011; Elwin et al. 2012; Li et al. 2014; Plutzer and Karanis 2009; Robinson et al. 2008; Xiao 2010; Xiao et al. 1999).
Therefore, the aim of this preliminary study was to investigate the presence of intestinal microparasites occurring in the raccoon population in newly colonized areas of Western Poland and Germany. Molecular analyses were conducted to identify and genotype Cryptosporidium spp. and microsporidia species emphasizing their zoonotic potential in European raccoons.
Materials and methods
Study areas and collection of material
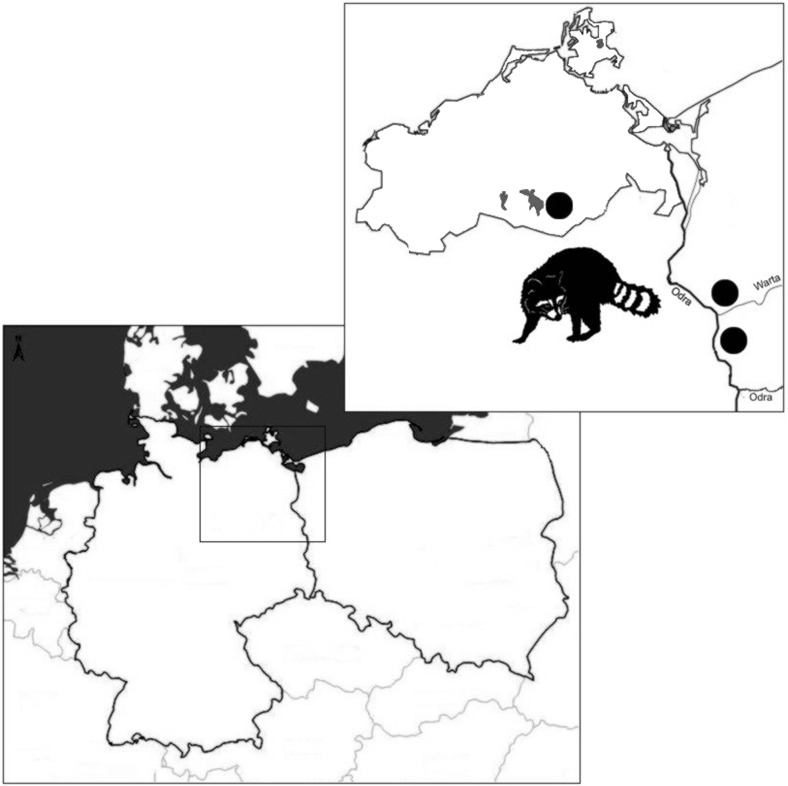
This study was carried out on 49 raccoons, comprising 31 males and 18 females, collected from hunters and road-kills from the area of Kostrzyn on the Oder and Warta Mouth National Park, Poland (n = 32), and from localities near the Müritz National Park, Mecklemburg-Vorpommern, Germany (n = 17) (Fig. 1). A suburban environment of the city of Kostrzyn is located in the surroundings of Warta Mouth National Park (WMNP) (52 °34′ N, 14° 43′ E) which covers about 80 km2 of the Warta River. Here, raccoons occupy relatively small territories and live in high density (0.7–2.5 individuals per 1 km2)—their home ranges overlap with an average of 80 %. This German-Polish border area is a zone of high human activity associated with traffic at petrol stations, restaurants, and hotels. WMNP is a part of Natura 2000 project, which makes it also attractive for tourists. Müritz National Park (MNP) (53° 27′ N, 12° 49′ E) located in Mecklenburg-Vorpommern contains a large amount of wetland habitats, especially bog and swamp districts. This area demonstrates a very opportune habitat for raccoon—its population density in the area is twice as much as that in the middle part of Germany (6–8 individuals per 1 km2) (Fischer et al. 2016; Hohmann 1998; Köhnemann and Michler 2008; Muschik et al. 2011).
Fig. 1.
The map of Poland and Germany showing geographical origin (black dots) of wild raccoons obtained for this study
Frozen carcasses were delivered to the laboratory of Department of Parasitology UWr and dissected. Collected fecal samples were kept at −20 °C for further analysis. Each animal was used for only one fecal specimen.
DNA extraction and PCR amplification
DNA was isolated from all 49 fecal samples using GeneMATRIX Stool DNA Purification Kit (EURx, Gdańsk, Poland) according to the manufacturer’s instructions. Obtained DNA was stored at −20 °C until further use.
PCR amplification was performed on a sets of nested primers amplifying the ITS region of the ribosomal ribonucleic acid (rRNA) gene, i.e., EBITS3, EBITS4 and EBITS1, EBITS2.4 for E. bieneusi (Buckholt et al. 2002) and INT580F, INT580R and Msp3, Msp4a for Encephalitozoon spp. (Katzwinkel-Wladarsch et al. 1996). A fragment of Cryptosporidium 18S rRNA and Cryptosporidium oocyst wall protein (COWP) genes were amplified (Pedraza-Diaz et al. 2001; Spano et al. 1997; Xiao et al. 1999). For the amplification of actin genes, we used cycling parameters elaborated by Sulaiman et al. (2002). For all PCR reactions, negative and positive controls were performed with sterile water and reference DNA, respectively. Secondary PCR products were subjected to electrophoresis on a 1.0 % agarose gel and stained with Midori Green (Nippon Genetics Europe GmbH). Products of expected size were purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and stored at 4 °C until sequencing.
Nucleotide sequencing
Products were sequenced in both directions on Applied Biosystems ABI PRISM 3100-Avant Sequencer (SEQme, the Czech Republic). The nucleotide sequences obtained in this study were edited using DNA Baser Sequence Assembly software (Heracle BioSoft SRL Romania) then aligned with reference sequences of Cryptosporidium spp. and E. bieneusi available in GenBank. Phylogenetic analyses were performed using MEGA6 software (Tamura et al. 2013). Trees were inferred by neighbor joining (NJ) method based on the Kimura 2-parameter distance model; bootstrapping was performed using 1000 replicates. Sequences from this study have been deposited in GenBank database under the accession numbers KX639723 and KX621279.
Statistical analysis
Prevalence was expressed as a ratio of a number of PCR positive samples for Cryptosporidium spp. 18S rRNA or/and COWP genes and the total number of examined samples. Contingency tables were used to compare prevalence between the sex of the raccoons and the different sampling areas using the chi-square test; p < 0.05 was considered statistically significant (STATISTICA®12).
Results
Nested PCR detected E. bieneusi in 2 of 49 (4.1 %) examined fecal samples of raccoons. Both positive samples, one obtained for a female and the other one for a male raccoon, were recorded from the area of Poland. The overall prevalence of Cryptosporidium spp. was estimated in 34.7 % (17/49) with infection rates of 38.9 % (7/18) and 32.3 % (10/31) observed in female and male raccoons, respectively. The prevalence of parasites according to the study sites was determined in 43.8 % (14/32) and 17.6 % (3/17) for Poland and Germany, respectively. No statistically significant differences were found in the occurrence of Cryptosporidium spp. and E. bieneusi between the sampling areas and the sex of the examined raccoons. In our survey, we did not detect any DNA of Encephalitozoon spp. in raccoons.
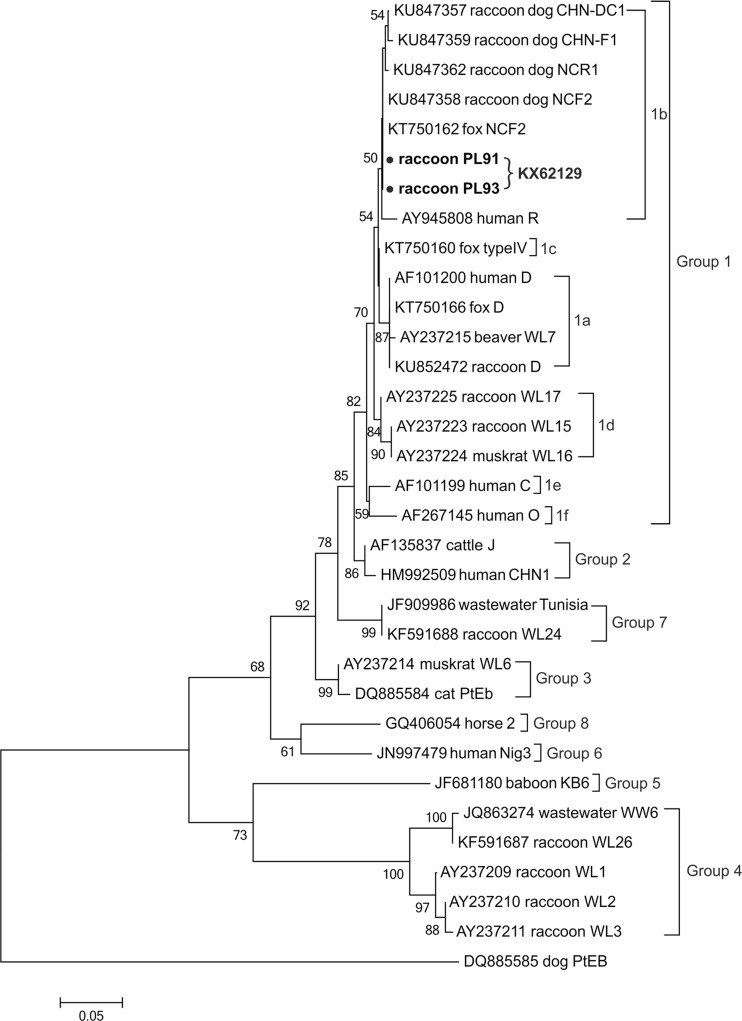
The analysis of the ITS region of E. bieneusi revealed the existence of one known genotype in both positive samples, namely NCF2. The phylogenetic analysis showed that the genotype was identical to the ones previously reported in fox (KT750163) and raccoon dog (KU847358) in China and clustered into group 1 (Fig. 2).
Fig. 2.
The phylogenetic relationship of Enterocytozoon bieneusi genotypes identified in the present study (indicated by solid circles) and others as inferred by a neighbor-joining analysis of ITS sequences. Bootstrapping was performed using 1000 replicates, and the values below 50 % are not shown. The E. bieneusi group terminology is based on the works of Guo et al. (2014), Zhao et al. (2015), and Xu et al. (2016)
The isolates from Cryptosporidium positive samples obtained from the amplification of 18S rRNA and/or COWP genes were genotyped by the sequence analysis of the actin gene. The only Cryptosporidium genotype, namely skunk genotype (identified from sequences of the actin gene), was detected in 9 out of 14 actin positive raccoons. Although the obtained sequences were not of the same length, they were identical to the isolate obtained from Eastern fox squirrel (KT027546) (Fig. 3). Isolates from the remaining actin positive raccoons yielded sequences of insufficient quality to include in the analyses.
Fig. 3.
The phylogenetic relationship of Cryptosporidium sp. skunk genotype identified in present study (indicated by solid circles) and others as inferred by a neighbor-joining analysis of the actin gene sequences. Bootstrapping was performed using 1000 replicates, and the values below 50 % are not shown
Discussion
In this study, we have molecularly identified the presence of E. bieneusi NCF2 genotype and Cryptosporidium skunk genotype in the Polish-German population of introduced raccoons. To our knowledge, this has been the first report on these groups of parasites in raccoons colonizing Europe. By now, literature data concerning this issue is based on the reports from the area of North America, where raccoon represents the native fauna. Also there are no available studies on the parasites in the population of raccoons introduced to Japan, but there are surveys on the raccoons introduced to Europe and Japan that concern some other bacterial and viral pathogens as well as parasites of protozoan and helminth species (Bauer 2013; Beltrán-Beck et al. 2012; Popiołek et al. 2011).
Our survey has shown that 2 out of 49 fecal samples of raccoons were E. bieneusi positive. During the phylogeny analysis, the detected genotype NCF2, clustered with other genotypes of group 1, suggesting its zoonotic potential. This newly identified E. bieneusi NCF2 genotype in European raccoons has so far been present only in farmed foxes and raccoon dogs from China (Xu et al. 2016; Zhang et al. 2016). The studies undertaken by Guo et al. (2014) showed that the infection rate of E. bieneusi in raccoons from a New York watershed area was as high as 82 % (18/22). The molecular studies conducted by Sulaiman et al. (2003) and Guo et al. (2014) revealed the presence of human pathogenic genotypes i.e., Peru 11, EbpC, WL15, and D genotypes. Additionally, the following raccoon-adapted genotypes were identified WL1-3, WL13, WL15-17, WL24, WL26, and WW6 (Guo et al. 2014; Sulaiman et al. 2003).
Cryptosporidium infection has been reported in raccoons (Carlson and Nielsen 1982; Snyder 1988; Zhou et al. 2004; Ziegler et al. 2007). Using indirect immunofluorescent assay, Snyder (1988) found that 13 % of wild raccoons were infected. By using molecular tools, C. parvum infection was found in 1 of 5 (20 %) raccoons from wildlife parks in New York State (Perz and Le Blancq 2001), and 2 of 51 (3.9 %) raccoons were Cryptosporidium skunk genotype positive in wetlands adjacent to the Chesapeake Bay (Zhou et al. 2004). Cryptosporidium skunk genotype and C. ubiquitum were identified in the raccoons and storm water from a New York watershed area (Feng et al. 2007). The Cryptosporidium skunk genotype was detected also in Eastern gray, American red and fox squirrels, river otters, and striped skunks from the area of the USA (Feng et al. 2007; Stenger et al. 2015; Ziegler et al. 2007). The phylogenetic analysis showed that the isolates obtained in the present study were organized in skunk genotype clade containing isolates previously identified among squirrels and skunk. Although uncommon, human infections with skunk genotype have also been reported (Davies et al. 2009; Elwin et al. 2012; Robinson et al. 2008). The wildlife origin of genotypes is increasingly recognized as an important environmental source of Cryptosporidium infection in humans, and shifting boundaries between wildlife and humans could result in the emergence of novel pathogens (Stenger et al. 2015). Considering that more than 75 % of human cases of cryptosporidiosis are determined to have the zoonotic origin and are related to wildlife and domestic animals, studies concerning a wide range of wild living hosts seem to be reasonable.
Despite the fact that the raccoon as an alien species has been present in Europe for 80 years, the knowledge about its parasitofauna is still insufficient, especially concerning microparasites. As our results have shown, raccoon as an introduced species lost many of its originally detected Cryptosporidium spp. and E. bieneusi genotypes. On the other hand, it encountered the E. bieneusi genotype which has not been identified either in Europe or in the North America. We suppose that E. bieneusi NCF2 genotype might be of the raccoon dog origin which is another species introduced from the area of East Asia. Cryptosporidium skunk genotype has been detected for the first time in the examined areas suggesting a North American origin. Thus, our results have shown that introduced raccoons could be considered as a potential source of human pathogenic Cryptosporidium skunk genotype and potentially pathogenic E. bieneusi NCF2 genotype.
Acknowledgments
We are grateful to Dr. M. Krappe (Kratzeburg, Germany) and the staff of “Warta Mouth” National Park for their help in collecting raccoon samples. Fecal samples of raccoons used in the study have been collected within the National Science Centre, Poland, project no. 2014/15/B/NZ8/00261.
References
- Bartoszewicz M, Okarma H. Szopy nad Wartą. Łowiec Polski. 2007;3:26–29. [Google Scholar]
- Bartoszewicz M, Okarma H, Zalewski A, Szczęsna J. Ecology of the raccoon (Procyon lotor) from western Poland. Ann Zool Fennici. 2008;45:291–298. doi: 10.5735/086.045.0409. [DOI] [Google Scholar]
- Bauer C. Baylisascariosis—infections of animals and humans with ‘unusual’ roundworms. Vet Parasitol. 2013;193:404–412. doi: 10.1016/j.vetpar.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Beltrán-Beck B, Garcia FJ, Gortázar C. Raccoons in Europe: disease hazards due to the establishment of an invasive species. Eur J Wildl Res. 2012;58:5–15. doi: 10.1007/s10344-011-0600-4. [DOI] [Google Scholar]
- Biedrzycka A, Zalewski A, Bartoszewicz M, Okarma H, Jędrzejewska E. The genetic structure of raccoon introduced in Central Europe reflects multiple invasion pathways. Biol Invasions. 2014;16:1611–1625. doi: 10.1007/s10530-013-0595-8. [DOI] [Google Scholar]
- Bogdanowicz W, Ruprecht AL. Przypadki stwierdzeń szopa pracza, Procyon lotor (Linnaeus, 1758), w Polsce. Przegląd Zoologiczny. 1987;31:375–383. [Google Scholar]
- Buckholt MA, Lee H, Tzipori S. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol. 2002;68:2595–2599. doi: 10.1128/AEM.68.5.2595-2599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BL, Nielsen SW. Cryptosporidiosis in a raccoon. J Am Vet Med Assoc. 1982;181:1405–1406. [PubMed] [Google Scholar]
- Chalmers RM, Elwin K, Thomas AL, Guy EC, Mason B. Long-term Cryptosporidium typing reveals the etiology and species-specific epidemiology of human cryptosporidiosis in England and Wales, 2000 to 2003. Euro Surveill. 2009;14:19086. doi: 10.2807/ese.14.02.19086-en. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Smith R, Elwin K, Clifton-Hadley FA, Giles M. Epidemiology of anthroponotic and zoonotic human cryptosporidiosis in England and Wales, 2004-2006. Epidemiol Infect. 2011;139:700–712. doi: 10.1017/S0950268810001688. [DOI] [PubMed] [Google Scholar]
- Davies AP, Campbell B, Evans MR, Bone A, Roche A, Chalmers RM. Asymptomatic carriage of protozoan parasites in children in day care centers in the United Kingdom. Pediatr Infect Dis J. 2009;28:838–840. doi: 10.1097/INF.0b013e31819d646d. [DOI] [PubMed] [Google Scholar]
- Elwin K, Hadfield SJ, Robinson G, Chalmers RM. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000-2008. Epidemiol Infect. 2012;140:673–683. doi: 10.1017/S0950268811000860. [DOI] [PubMed] [Google Scholar]
- Fayer R, Santin M, Macarisin D. Cryptosporidium ubiquitum n.sp. in animals and humans. Vet Parasitol. 2010;172:23–32. doi: 10.1016/j.vetpar.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Feng Y, Alderisio KA, Yang W, Blancero LA, Kuhne WG, Nadareski CA, Reid M, Xiao L. Cryptosporidium genotypes in wildlife from a New York watershed. Appl Environ Microbiol. 2007;73:6475–6483. doi: 10.1128/AEM.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ML, Sullivan MJP, Greiser G, Guerrero-Casado J, Heddergott M, Hohmann U, Keuling O, Lang J, Martin I, Michler FU, Winter A, Klein R. Assessing and predicting the spread of non-native raccoons in Germany using hunting bag data and dispersal weighted models. Biol Invasions. 2016;18:57–71. doi: 10.1007/s10530-015-0989-x. [DOI] [Google Scholar]
- Guo Y, Alderisio KA, Yang W, Cama V, Feng Y, Xiao L. Host specificity and source of Enterocytozoon bieneusi genotypes in a drinking source watershed. Appl Environ Microbiol. 2014;80:218–225. doi: 10.1128/AEM.02997-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques-Gil N, Haro M, Izquierdo F, Fenoy S, del Águila C. Phylogenetic approach to the variability of the microsporidian Enterocytozoon bieneusi and its implications for inter- and intrahost transmission. Appl Environ Microbiol. 2010;76:3333–3342. doi: 10.1128/AEM.03026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann U (1998) Untersuchungen zur Raumnutzung des Waschbären (Procyon lotor L. 1758) im Solling, Süd-Niedersachsen, unter besonderer Berücksichtigung des Sozialverhaltens. Dissertation, University of Götingen
- Hohmann U, Gerhard R, Kasper M. Home range size of adult raccoons (Procyon lotor) in Germany. Z Säugetierkunde. 2000;65:124–127. [Google Scholar]
- Hohmann U, Voigt S, Andreas U. Quo Vadis raccoon? New visitors in our backyards—on the urbanization of an allochthone carnivore in Germany. In: Gottschalk E, Barkow A, Mühlenberg M, Settele J, editors. Naturschutz und Verhalten. Lepzig: UFZ-Berichte; 2001. pp. 143–148. [Google Scholar]
- Karim MR, Wang R, Dong H, Zhang L, Lib J, Zhanga S, Rumec FI, Qia M, Jiana F, Sund M, Yange G, Zouf F, Ninga C, Xiao L. Genetic polymorphism and zoonotic potential of Enterocytozoon bieneusi from nonhuman primates in China. Appl Environ Microbiol. 2014;80:1893–1898. doi: 10.1128/AEM.03845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzwinkel-Wladarsch S, Lieb M, Heise W, Löscher T, Rinder H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop Med Int Health. 1996;3:373–378. doi: 10.1046/j.1365-3156.1996.d01-51.x. [DOI] [PubMed] [Google Scholar]
- Köhnemann BA, Michler FU. Der Waschbär in Mecklenburg-Strelitz. Labus. 2008;27:50–58. [Google Scholar]
- Kresta AE, Henke SE, Pence DB. Gastrointestinal helminths in raccoons in Texas. J Wildl Dis. 2009;45:1–13. doi: 10.7589/0090-3558-45.1.1. [DOI] [PubMed] [Google Scholar]
- Kváč M, McEvoy J, Stenger B, Clark M. Cryptosporidiosis in other vertebrates. In: Cacciò SM, Widmer G, editors. Cryptosporidium: parasite and disease. Vienna: Springer Vienna; 2014. pp. 237–323. [Google Scholar]
- Li N, Xiao L, Alderisio K, Elwin K, Cebelinski E, Chalmers R, Santin M, Fayer R, Kvac M, Ryan U, Sak B, Stanko M, Guo Y, Wang L, Zhang L, Cai J, Roellig D, Feng Y. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg Infect Dis. 2014;2:217–224. doi: 10.3201/eid2002.121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos O, Lobo ML, Xiao L. Epidemiology of Enterocytozoon bieneusi infection in humans. J Parasitol Res. 2012;2012:981424. doi: 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler FU, Michler BA. Ökologische, ökonomische und epidemiologische Bedeutung des Waschbären (Procyon lotor) in Deutschland- eine aktuelle Übersicht. Beiträge zur Jagd- und Wildforschung. 2012;37:389–397. [Google Scholar]
- Muschik I, Köhnemann B, Michler FU. Untersuchungen zur Entwicklung des Raum- und Sozialverhaltens von Waschbär- Mutterfamilien (Procyon lotor L.) und dessen jagdrechtliche Relevanz. Beiträge zur Jagd- und Wildtierforschung. 2011;36:573–585. [Google Scholar]
- Pedraza-Diaz S, Amar C, Nichols GL, McLauchlin J. Nested polymerase chain reaction for amplification of the Cryptosporidium oocyst wall protein gene. Emerg Infect Dis. 2001;7:49–56. doi: 10.3201/eid0701.010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perz JF, Le Blancq SM. Cryptosporidium parvum infection involving novel genotypes in wildlife from lower New York State. Appl Environ Microbiol. 2001;67:1154–1162. doi: 10.1128/AEM.67.3.1154-1162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzer J, Karanis P. Genetic polymorphism in Cryptosporidium species: an update. Vet Parasitol. 2009;165:187–199. doi: 10.1016/j.vetpar.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Popiołek M, Szczęsna-Staśkiewicz J, Bartoszewicz M, Okarma H, Smalec B, Zalewski A. Helminth parasites of an introduced invasive carnivore species, the raccoon (Procyon lotor L.), from the Warta Mouth National Park (Poland) J Parasitol. 2011;97:357–360. doi: 10.1645/GE-2525.1. [DOI] [PubMed] [Google Scholar]
- Robinson G, Elwin K, Chalmers RM. Unusual Cryptosporidium genotypes in human cases of diarrhoea. Emerg Infect Dis. 2008;14:1800–1802. doi: 10.3201/eid1411.080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossle NF, Latif B. Cryptosporidiosis as threatening health problem: a review. Asian Pac J Trop Biomed. 2013;3:916–924. doi: 10.1016/S2221-1691(13)60179-3. [DOI] [Google Scholar]
- Ryan U, Hijjawi N. New developments in Cryptosporidium research. Int J Parasitol. 2015;45:367–373. doi: 10.1016/j.ijpara.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Santin M, Fayer R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci. 2011;90:363–371. doi: 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Schley L, Schanck C, Schaul M, Sinner C. Neubürger und Heimkehrer unter den Wildtieren Luxemburgs. Beiträge zur Jagd- und Wildforschung. 2001;26:141–154. [Google Scholar]
- Snyder DE. Indirect immunofluorescent detection of oocysts of Cryptosporidium parvum in the feces of naturally infected raccoons (Procyon lotor) J Parasitol. 1988;74:1050–1052. doi: 10.2307/3282233. [DOI] [PubMed] [Google Scholar]
- Spano F, Putignani L, McLauchlin J, Casemore DP, Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:207–217. doi: 10.1016/S0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- Stenger BL, Clark ME, Kváč M, Khan E, Giddings CW, Prediger J, McEvoy JM. North American tree squirrels with overlapping ranges host different Cryptosporidium species and genotypes. Infect Genet Evol. 2015;36:287–293. doi: 10.1016/j.meegid.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Stubbe M. Procyon lotor (Linnaeus, 1758) In: Jones AJ, Amori G, Bogdanowicz W, Krystufek B, Reijnders P, Spitzenberger F, Stubbe M, Thissen JBM, Vohohralik V, Zima J, editors. The atlas of European mammals. London: Mitchell Academic Press; 1999. pp. 326–327. [Google Scholar]
- Sulaiman IM, Lal AA, Xiao L. Molecular phylogeny and evolutionary relationships of Cryptosporidium parasites at the actin locus. J Parasitol. 2002;88:388–394. doi: 10.1645/0022-3395(2002)088[0388:MPAERO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW, Xiao L. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol. 2003;69:4495–4501. doi: 10.1128/AEM.69.8.4495-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchin ME, Mitchell CE. Parasites, pathogens and invasions by plants and animals. Front Ecol Environ. 2004;2:183–190. doi: 10.1890/1540-9295(2004)002[0183:PPAIBP]2.0.CO;2. [DOI] [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Ma X, Zhang H, Zhang XX, Zhao JP, Ba HX, Rui-Du XXM, Wang QK, Zhao Q. Prevalence, risk factors and molecular characterization of Enterocytozoon bieneusi in raccoon dogs (Nyctereutes procyonoides) in five provinces of Northern China. Acta Trop. 2016;161:68–72. doi: 10.1016/j.actatropica.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Zhang XX, Cong W, Lou ZL, Ma JG, Zheng WB, Yao QX, Zhao Q, Zhu XQ. Prevalence, risk factors and multilocus genotyping of Enterocytozoon bieneusi in farmed foxes (Vulpes lagopus), Northern China. Parasit Vectors. 2016;9:72. doi: 10.1186/s13071-016-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang W, Yang F, Zhang L, Wang R, Cao J, Shen J, Liu A. Enterocytozoon bieneusi in dairy cattle in the Northeast of China: genetic diversity of ITS gene and evaluation of zoonotic transmission potential. J Eukaryot Microbiol. 2015;62:553–560. doi: 10.1111/jeu.12210. [DOI] [PubMed] [Google Scholar]
- Zhou L, Fayer R, Trout JM, Ryan UM, Schaefer FW, Xiao L. Genotypes of Cryptosporidium species infecting fur-bearing mammals differ from those of species infecting humans. Appl Environ Microbiol. 2004;70:7574–7577. doi: 10.1128/AEM.70.12.7574-7577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler PE, Wade SE, Schaaf SL, Stern DA, Nadareski CA, Mohammed HO. Prevalence of Cryptosporidium species in wildlife populations within a watershed landscape in southeastern New York State. Vet Parasitol. 2007;43:586–596. doi: 10.1016/j.vetpar.2007.03.024. [DOI] [PubMed] [Google Scholar]