Abstract
Purpose
In this work a spreadsheet based program is presented that to a large extent independently verifies the calculations of individual plans of brachytherapy treatment planning systems for low dose rate, high dose rate and pulsed dose rate techniques.
Material and methods
The verification program has been developed based on workbooks/spreadsheets. The treatment planning system output text files are automatically loaded into the new program, allowing the use of the source coordinates, the desired calculation point coordinates, and the dwell times of a patient plan. The source strength and the reference dates are entered by the user and then dose points calculations are independently performed. The program shows its results in a comparison of its calculated point dose data with the corresponding TPS outcome.
Results
Results of 250 clinical cases show agreement with the TPS outcome within a 2% level.
Conclusions
The program allows the implementation of the recommendations to verify the clinical brachytherapy dosimetry in a simple and accurate way, in only few minutes and with a minimum of user interactions.
Keywords: brachytherapy, physics dosimetry, planning system, verification
Purpose
Current European recommendations [1] state that, similarly to external planning, an independent verification of the calculations performed by a treatment planning system (TPS) should be performed for each patient plan in low dose rate (LDR), high dose rate (HDR) and pulsed dose rate (PDR) brachytherapy. As described in the ESTRO Booklet No. 8, many factors affect the outcome of such calculations [1]. For example, in the case of HDR and PDR, sources are used for several months (about one half-life of the radionuclide), even in standard applications the number and length of catheters may vary, and the optimization procedure of the plan can lead to large differences in individual dwell times. Therefore it is not easy to intuitively see whether or not the resulting plan has been correctly calculated. For these reasons, at the very least an inspection on the ‘reasonableness’ of a plan should be performed to identify serious errors [1]. An actionable threshold of 20% deviation as a minimum goal was suggested for this purpose in the NRC (Nuclear Regulatory Commission) misadministration rule for brachytherapy [2], though more precise checks are preferable.
The purpose of this work is to present a computer program for the independent verification of absorbed dose point calculations in individual brachytherapy treatment planning. The outcome of the program allows detection of a number of possible errors, thus seeking a compromise for efficiency both in the time required for the extra calculation and in the level of reliability. The program is applicable for a wide variety of treatment techniques and is available in several versions to serve with different treatment planning systems.
Material and methods
Material
At our departments different types of brachytherapy applications with LDR, HDR, and PDR modalities are performed. The sources currently used for these various types of implants are the Ir-192 mHDR-v2 source (MicroSelectron units, Nucletron®), and the IsoSeed I-125 (IBT Bebig Group®) seeds, with either loose or stranded (IsoCord®) seeds. Dosimetric data for these sources in TG-43 format can be found on the ESTRO website [3] which is maintained by the GEC-ESTRO BRAPHYQS Physics Network. The data for the Ir-192 mHDR-v2 source are those recommended by the ESTRO BRAPHYQS network [3], while for the IsoSeed I-125 source the consensus data recommended by the AAPM [4] are used in clinical dosimetry.
The following TPSs are used to perform the clinical brachytherapy planning calculations: PLATO (version 14.2.5); Oncentra MasterPlan Brachy (version 3.2); Oncentra Prostate (version 3.1.11); and SPOT-PRO (version 3.1). These products are all distributed by Nucletron. Furthermore the TPS Plaque Simulator X (version 5.3.6) is used, distributed by the IBt Bebig Group. The use of each system in various applications is shown in Table 1.
Table 1.
Treatment planning systems and sources/seeds used at our departments for which the verification spreadsheets are used
| Treatment planning system | Application | Source/Seed |
|---|---|---|
| PLATO | HDR and PDR intracavitary and interstitial implants | mHDR-v2 |
| Oncentra MasterPlan Brachy | HDR and PDR intracavitary and interstitial implants | mHDR-v2 |
| Oncentra Prostate | HDR, Prostate intraoperative implants | mHDR-v2 |
| SPOT-PRO | LDR seeds, Prostate intraoperative implants | IsoCord |
| TPS Plaque Simulator X | LDR seeds, Ophtalmic implants | IsoSeed I-125 |
The TG-43 updated formalism (TG-43 U1) [4] includes for low energy (I-125 and Pd-103) a point-source (1D) model and a line-source (2D) model to describe the dosimetric behaviour of the seed sources. In the latter case the orientation of the source is taken into account using the anisotropy function along with the linear geometric factor, the radial dose function and the dose rate constant. In the point source approximation the anisotropy factor and the point geometry factor are used. All TPSs included in this study consider the linear nature of the geometry of the sources, i.e. they use the 2D general formalism proposed by the TG-43 U1 [4] to perform the calculations.
The verification program has been made on Microsoft Excel 2002 workbooks/spreadsheets and is available in different versions which have been adapted to the particularities of each TPS and to the type of implants.
Methods
In our procedure for clinical dosimetry in brachytherapy, it is organised in such a way that the verification step is performed at the end of the treatment planning procedure, and always prior to treatment. To speed up the process the verification has been automated to the greatest possible extent. All the required information which is included in the text file of the TPS plan is imported directly into the program through a simple interface step. For each treatment planning system, the process is slightly different. The common ground is that the coordinates of the source dwell positions and their accompanying dwell times, the coordinates of the dose calculation points, and the other parameters needed for the verification are automatically extracted from this text file.
In the PLATO and Brachy OMP TPS, the generation of dose points is an explicit requirement for a dose prescription. These dose points can be points associated with a structure, with the applicator, etcetera. These points are typically used to perform the prescription and subsequently the optimization step. In contrast, in the TPS used in prostate implants (Prostate Oncentra and SPOT-PRO), as the prescription is made on volumes, a set of points must be added. The chosen points are representative for the plan, both in total absorbed dose and in position within the prostate and distributed inside the regions of interest. The coordinates of the calculation points are derived from the ultrasound transverse planes of the prostate.
The number of verification points per patient is different depending on the type of plan. For prostate LDR applications 5 points are verified. For HDR and PDR the range is strongly variable, depending on the type of implant. If we deal with standard applications, the dose prescription points are related to the applicator (e.g., with the dome type vaginal applicators, the Valencia surface applicator, etc.). In more complex implants the prescription is usually associated with a structure. Thus, depending on the case, any number from 5 up to several hundreds of dose points are included and verified in the program.
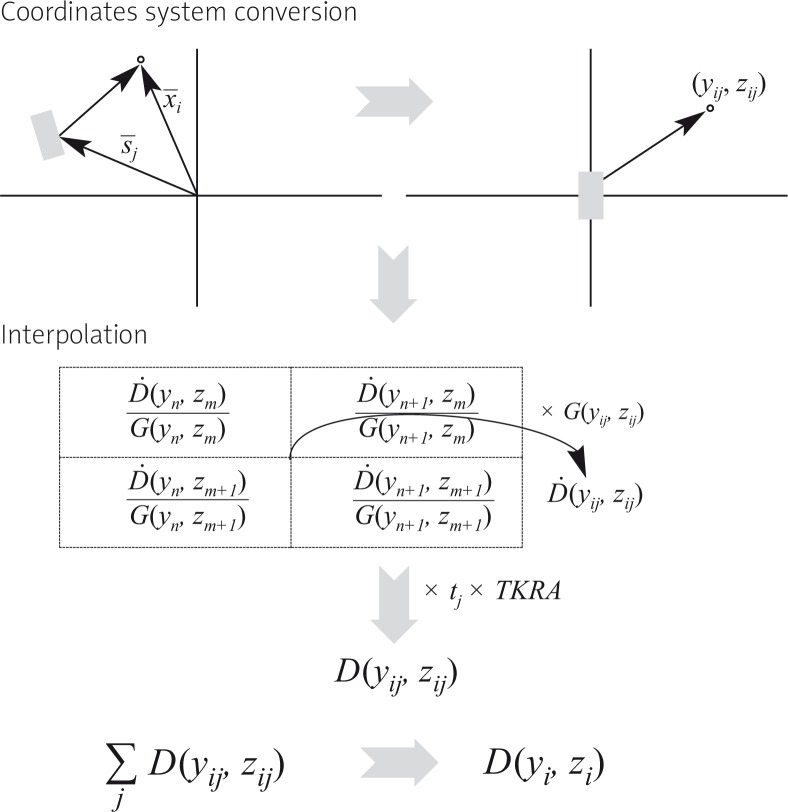
The spreadsheets implemented in the program to perform the verification make use of tables of the absolute absorbed dose rate per unit air-kerma strength at grid points in rectangular coordinates. For the Ir-192 mHDR-v2 source these were taken from the publication of Daskalov et al. [5] and can be found with the recommended data sets of the GEC-ESTRO BRAPHYQS group [1]. For each calculation point the process by which the necessary data are taken from the text file and how the absorbed dose is obtained can be followed in the diagram of Fig. 1. From the text file the information obtained with respect to a dwell position, refers to the coordinate set of the centre of the source. In order to take into account the orientation of the source at a given dwell position, it is assumed that it is well defined by the vector joining one dwell position with the next one.
Fig. 1.
Diagram showing the verification process for each point, in HDR and PDR calculations. Ḋ is the dose rate per unit air-kerma strength, G is the geometry function (linesource model) and D is the absorbed dose at point i
For the IsoSeed I-125 source the verification is based on the constants and line source functions of the AAPM TG-43 U1 [4] (dose rate constant, radial dose function, geometric factor, and anisotropy function). In this case, the coordinate system is changed to polar coordinates referring to the source, and an interpolation is made in each of the tables of the various functions. In this case the orientation of the sources, is supposed to be aligned with the longitudinal direction of the implant, following the ultrasound probe longitudinal axis direction. It is noted that in our case we use the stranded source type.
Based on these algorithms and the pre-entered source data sets, the workbook programs adds up all contributions from the different dwell or source positions to the absorbed dose at each of the dose points. The air-kerma strength as well as the required reference dates/times for the calculation are all independently entered by the user who carries out the verification.
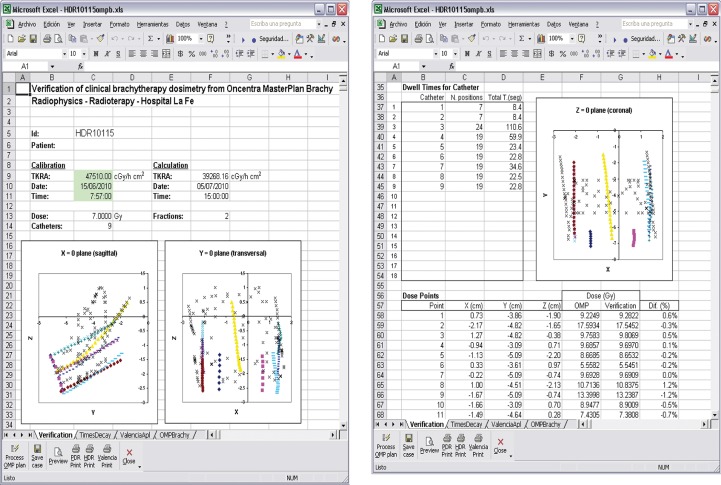
The values of the absorbed dose calculated by the TPS are also read from the text file. Finally the results of the verification along with the other parameters of the treatment are displayed in tabular form by the spreadsheet. Graphics are generated that correspond to the main views of the implant (axial, sagittal and coronal) in order to show: (i) the reconstructed geometry of the sources, and (ii) the dose points in the implant. See the screenshots in Fig. 2.
Fig. 2.
Screen shots of the result of a verification. On the left, the data for the implant are shown along with two of the three main views. On the right, the third main view as well as the comparison for the first eleven points
Results
To illustrate the results typically obtained with the independent dose calculation spreadsheets, we have summarized the outcome of a total of 250 clinical plans (90 HDR cases, 5 PDR cases, and LDR 155 cases) of patients treated along 2010. Table 2 shows, for each modality and in percent differences, the mean difference, the standard deviation of the differences and the maximum deviation obtained for all plans.
Table 2.
Mean deviation, standard deviation (1sd), and maximum difference observed between the TPS calculations and the verification program
| Application | N° cases | Difference (%) | ||
|---|---|---|---|---|
| Mean | Standard deviation | Maximum difference | ||
| HDR-PDR | 95 | –0.22 | 0.48 | –1.93 |
| LDR | 155 | 0.17 | 0.07 | 0.42 |
Since the algorithms and data base in the compiled program are essentially the same as that utilized in the TPS, there is a very good agreement between the two. The explanation of some possible small deviations is found in small differences between the recommended data sets used in the program and the vendor supplied data used by the TPS. These may result from certain differences in the management of the TG-43 functions such as the use of polynomial functions, and in the resolution of the calculation window of the planning system. The major differences occur at points very close to the sources (within 2 mm) which are not among the points of clinical interest. In all our plans the deviations have been less than 2%.
Discussion
Errors in the use of a brachytherapy treatment planning can have a very different origin. A carefully designed QA programme in a department is needed to prevent the occurrence of serious errors. This includes acceptance tests, commissioning, documentation, training, and development of a series of quality control procedures specific for the types of clinical applications in the department. Still, in clinical routine several situations can be identified that can lead to misadministration of dose to one or more patients. Examples of real accidents are published in booklets as the IAEA Safety Report Series No 17, “Lessons learned from accidental exposures in radiotherapy” [6] and in the more recent ICRP Publication 97 “Prevention of High-dose-rate Brachytherapy Accidents” [7]. Common factors in the described accidents are the lack of training of involved personnel, lack of double (and independent) check procedures, and moments of inattention. Typical errors which may occur, which can affect several patients, and which are often not so trivial to detect are, for example, unintended changes in or corruption of source data bases, and (typing) errors made in the setting of dates such as the calibration date after a source exchange.
Various methods have been proposed in the literature meant to verify in a feasible way the ‘reasonableness’ of the resulting treatment plan. Sometimes these methods consider the implant globally by calculating the absorbed dose at one or more dose points at a larger distance. For example, this can be done by the addition of some extra calculation points located at 5 cm or 10 cm from the centre of the implant in the plan and comparing the result with the absorbed dose obtained using a manual calculation [1]. Others may wish to use the TRAK, i.e. the Total Reference Air Kerma of a calculated plan to be compared with a standard value (note that in the radium-226 era the ‘milligram-hours’ or the ‘milligram-hours-equivalent’ concept was used, even for dose prescription; a very similar concept).
This type of manual check calculation can however sometimes still be quite complex and therefore different other approaches have been proposed, in which a resulting index is compared with pre-calculated values. Some of these approaches [8–14] are discussed in more detail in ESTRO Booklet No. 8 [1]. In the LDR seed techniques prostate planners may roughly correlate total absorbed dose administered, the volume and number of seeds by using nomograms [15], but these methods do not account for the quality of the absorbed dose distribution, for example at the level of the urethra.
All these verifications that are based on approximations and representative indices ignore information about the distribution of the absorbed dose inside the implant volume and have, of course, much higher tolerances than the TPS calculations. This largely depends on the approximations made in each specific case. They usually result well within the 20% limit stated by NRC [2].
The optimal check, a 2nd full recalculation of the plan in another TPS is usually not feasible, due to lack of time, manpower, or simply because it is not available. Furthermore, optimization steps may result in different solutions from 2 TPSs, so in different dwell times. Any check procedure has its limitations. In this respect it is important to realise that some techniques such as prostate implants (both LDR and HDR) are performed intraoperatively and the time efficiency of a verification step is crucial. The computer program described in this paper makes use of the same reconstructed geometric data of the dwell positions and dose points of the plan. It then calculates independently the dose at the dose points, using separate source data files and algorithms. User interaction is required only for entering source strength and calibration data. Comparison is made on the level of dose points, so detailed information is available of dose at relevant points and not only on a global level. The carefully performed initial validation of the system and the reported results of a sample of 250 cases shows that deviations between the TPS and the program are smaller than 2%. Although the program is not completely independent from the TPS, it has shown to work reliably, time efficient, and reassuring for the brachytherapy team.
Conclusions
An independent computer program capable of meeting the recommendations for independent verification of clinical dosimetry was developed and utilized routinely in all plans in our Hospitals. Time consumption is only a few minutes being compatible with intraoperative procedures. The utilisation of the program is possible in combination with a variety of brachytherapy treatment planning systems. It was demonstrated to be a valuable tool in the clinical practice. The developed calculus book is available to the interested medical physicists. The contact address of the authors, Mr. V. Carmona Meseguer, is provided to this paper.
References
- 1.Venselaar J, Pérez-Calatayud J, editors. European guidelines for quality assurance in radiotherapy. Brussels: 2004. ESTRO Booklet No. 8. A practical guide to quality control of brachytherapy equipment. [Google Scholar]
- 2.Nag S, Bice W, DeWyngaert, et al. The American Brachytherapy Society recommendations for permanent prostate brachytherapy postimplant dosimetric analysis. Int J Radiat Oncol Biol Phys. 2000;46:221–230. doi: 10.1016/s0360-3016(99)00351-x. [DOI] [PubMed] [Google Scholar]
- 3. http://www.estro.org/estroactivities/Pages/TG43BTDOSMETRICPARAMETERS.aspx last accessed 28 August 2010.
- 4.Rivard MJ, Coursey BM, DeWerd LA, et al. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31:633–674. doi: 10.1118/1.1646040. [DOI] [PubMed] [Google Scholar]
- 5.Daskalov G, Loffler E, Williamson J. Monte Carlo-aided dosimetry of a new high dose-rate brachytherapy source. Med Phys. 1998;25:2200–2208. doi: 10.1118/1.598418. Erratum. Med Phys 2000; 27: 1999. [DOI] [PubMed] [Google Scholar]
- 6.International Atomic Energy Agency. IAEA Safety Report Series No 17, Lessons learned from accidental exposures in radiotherapy. Vienna: IAEA; 2000. [Google Scholar]
- 7.International Commission on Radiological Protection. ICRP Publication 97. Prevention of High-dose-rate Brachytherapy Accidents. Annals of the ICRP. 2005;35:2. doi: 10.1016/j.icrp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Venselaar JLM, Bierhuizen HWJ, Klop R. A method to check treatment time calculations in Ir-192 high dose rate volume implants. Med Phys. 1995;22:1499–1500. doi: 10.1118/1.597417. [DOI] [PubMed] [Google Scholar]
- 9.Ezzell GA. Quality assurance of treatment plans for optimized high dose rate brachytherapy – planar implants. Med Phys. 1994;21:659–661. doi: 10.1118/1.597246. [DOI] [PubMed] [Google Scholar]
- 10.Rogus RD, Smith MJ, Kubo HD. An equation to QA check the total treatment time for single-catheter HDR brachytherapy. Int J Radiat Oncol Biol Phys. 1998;40:245–248. doi: 10.1016/s0360-3016(97)00487-2. [DOI] [PubMed] [Google Scholar]
- 11.Kubo HD. Verification of treatment plans by mathematical formulas for single catheter HDR brachytherapy. Med Dosimetry. 1992;17:151–155. doi: 10.1016/0958-3947(92)90033-c. [DOI] [PubMed] [Google Scholar]
- 12.Kubo HD, Chin RB. Simple mathematical formulas for quick-checking of single-catheter high dose rate brachytherapy treatment plans. Endocurie Hypertherm Oncol. 1992;8:165–169. [Google Scholar]
- 13.Williamson JF, Ezzell GA, Olch A, et al. Quality assurance for high dose rate brachytherapy. In: Nag S, editor. High Dose Rate Brachytherapy: A Textbook. Armonk, New York: Futura Publishing Company Inc; 1994. Library of Congress Cataloging-in-Publication Data. [Google Scholar]
- 14.Thomadsen BR. Institute of Physics Publishing. Bristol and Philadelphia: IOP Press; 2000. Achieving quality in brachytherapy. Medical Science Series. [Google Scholar]
- 15.Yu Y, Schell MC, Anderson LL, et al. Permanent prostate seed implant brachytherapy: report of the American Association of Physicist in Medicine Task Group No. 64. Med Phys. 1999;26:2054–2076. doi: 10.1118/1.598721. [DOI] [PubMed] [Google Scholar]