Abstract
Objectives
Many studies have examined histopathological factors and various prognostic scores related to inflammation to predict outcomes. Here, we examined the prognostic value of the C-reactive protein/albumin (CRP/alb) ratio in oral squamous cell carcinoma (OSCC).
Materials and Methods
This retrospective study included 40 patients with OSCC. Using univariate and multivariate analyses, we focused on the correlation of the CRP/alb ratio with clinicopathological characteristics and with overall survival. We then compared five inflammation-based prognostic scores, CRP/alb ratio, modified Glasgow Prognostic Score (mGPS), neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and prognostic nutritional index (PNI), based on receiver operating characteristic (ROC) curves.
Results
The optimal cut-off value for the CRP/alb ratio was 0.085. The group with a high CRP/alb ratio had a high TNM clinical stage (P=0.002) and larger primary tumors (P=0.029), with statistically significant differences in lymph node metastasis and distant metastasis. In addition, when the CRP/alb ratio was high, multivariate analysis showed a lower survival rate (P=0.002; hazard ratio=6.078), and the ROC curve showed more outstanding discriminatory ability regarding overall survival compared to other inflammation-based prognostic scores.
Conclusion
The CRP/alb ratio can be an independent prognostic factor when predicting prognosis in OSCC and has good prognostic ability.
Keywords: Squamous cell carcinoma, C-reactive protein, Albumin
I. Introduction
Oral cancers represent approximately 1.6% of all cancers in Korea. The major type of oral cancer is squamous cell carcinoma, which reportedly comprises 80% to 90% of all oral carcinoma1,2. Treatment can include surgery, radiation therapy, and/or chemotherapy, but it is important to maintain functions of the head and neck and to consider aesthetic aspects. Additional radiation therapy and chemotherapy are used along with broad resection in advanced cancer3,4,5. In order to determine relevant treatment options and to predict patient prognosis, tumor-node-metastasis (TNM) staging systems and lymph node metastasis are widely used6.
It has been reported that, in various kinds of cancer, systemic inflammation is related to a poor prognosis7. Many studies are under way to assess the histopathological factors and various prognostic scores related to inflammation as a determinant of outcomes. In this context, a number of inflammation-based prognostic scores have been introduced, including the neutrophil-lymphocyte ratio (NLR), the platelet-lymphocyte ratio (PLR), the modified Glasgow Prognostic Score (mGPS), and the prognostic nutritional index (PNI)8,9,10,11,12.
Serological tests related to inflammation include neutrophil count, lymphocyte count, C-reactive protein (CRP), and albumin (alb). CRP is an acute phase response protein, and an increase in CRP is related to a worsening inflammatory status and progression of the cancer13, whereas a decrease in alb level is related to a chronic inflammatory state and poor nutritional status14. Recently, a new inflammation-based prognostic score, the CRP/alb ratio, was introduced and is reportedly comparable or superior to other inflammation-based prognostic scores in predicting prognosis in malignant tumors such as those of the liver and lung15,16,17,18. However, whether or not the CRP/alb ratio correlates with prognosis in patients with oral squamous cell carcinoma (OSCC) has yet to be determined.
We aimed to investigate the possibility of using the CRP/alb ratio preoperatively as an independent prognostic factor in OSCC and to compare the prognostic predictability of the CRP/alb ratio with that of four other inflammation-based prognostic scores.
II. Materials and Methods
1. Study population
We reviewed the medical records of patients with a diagnosis of primary OSCC as a result of histopathological tests carried out at the Department of Oral and Maxillofacial Surgery of Dankook University Dental Hospital from May 2004 through December 2011. Patients were excluded from the sample if they had no preoperative serological indicators, had died from causes other than cancer, had insufficient information regarding follow-up, had disorders such as rheumatic diseases or acute inflammation that might have affected the inflammation-related indicators, had a history of oral cancer treatment, or had primary malignant tumors located in other than the oral or maxillofacial area. Our final study cohort included 40 patients.
2. Clinical information
Along with basic information such as the age, sex, height, weight, and medical history, postoperative histopathological specimens were used to classify the clinical stage based on location of the primary tumor, the degree of differentiation, and the TNM staging system (American Joint Committee on Cancer, 7th edition)19. Tumor size was defined as the longest part of the resected specimen, and lymph node metastasis was confirmed by the presence or absence of the affected lymph node within the specimen, and distant metastases overall were diagnosed through histopathological tests on areas suspected to be involved.
3. Serological indicators and inflammation-based prognostic scores
White blood cell, neutrophil, lymphocyte, platelet count, and levels of CRP and alb constituted the serological indicators of inflammation, and the inflammation-based prognostic scores were defined as follows; 1) mGPS, based on the levels of CRP and alb: 0 for CRP <1 mg/dL, 1 for CRP >1 mg/dL and alb >3.5 g/dL, and 2 for CRP >1 mg/dL and alb <3.5 g/dL; 2) NLR, obtained by dividing the neutrophils (count/µL) by the lymphocytes (count/µL); 3) PLR, obtained by dividing the platelets (count/µL) by the lymphocytes (count/µL); 4) PNI, derived using the following equation: 10×alb (g/dL)+0.005×lymphocyte (count/µL); and 5) CRP/alb ratio, obtained by dividing the CRP level (mg/dL) by the alb level (mg/dL).
In each case, the preoperative inflammation-based prognostic score was used to avoid the possible effects of surgery, radiation therapy, and chemotherapy.
4. Treatment and follow-up
When radical excision was necessary, depending on the TNM clinical stage, reconstruction was undertaken at the same time as the surgery. When the clinical stage was advanced (T3 or T4) and the histologically determined degree of differentiation was low and when metastasis was confirmed, postoperative radiation therapy with or without concurrent chemotherapy was carried out. Follow-up lasted from the date of the diagnosis of OSCC to the date of death or the date when follow-up was terminated.
5. Statistical analysis
In order to identify risk factors that affected overall survival, we used the Kaplan-Meier method to calculate survival curves, taking into account characteristics such as sex, age, TNM clinical stage, size of the primary tumor, degree of differentiation, and inflammation-based prognostic scores. Univariate analysis was carried out using log-rank test to determine statistically significant differences in survival rates. Continuous variables such as age and size of the tumor were converted to a dichotomous variable, with the average providing a basis for analysis.
Inflammation-based prognostic scores for the CRP/alb ratio, PNI, NLR, and PLR were divided into two groups based on optimal cut-off values obtained from receiver operating characteristic (ROC) curves, and the results were compared.
The effects of all risk factors were considered using the Cox proportional hazards model. For factors that showed statistically significant results based on the log-rank test, we used multivariate analysis to identify variables that affected overall survival. The optimal cut-off value for the CRP/alb ratio regarding overall survival was set according to the web-based program designed by Budczies et al.20 (http://molpath.charite.de/cutoff/), and this cut-off value was used to classify the patients into two groups—one with a high CRP/alb ratio (>0.085) and one with a low CRP/alb ratio (≤0.085). Clinicopathological characteristics were compared between the two groups by means of the Mann-Whitney U test or Fisher's exact test, depending on the type of variable.
In order to compare the discriminatory ability of the CRP/alb ratio and the other inflammation-based prognostic scores regarding overall survival, we determined the area under the curve (AUC) using the ROC curve and evaluated the significance.
All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Co., Armonk, NY, USA), and P-values lower than 0.05 were considered statistically significant.
III. Results
The mean age at the time of diagnosis was 66 years (range, 31-83 years), and the sample included 27 men (67.5%) and 13 women (32.5%). In terms of TNM clinical stage, 10 tumors (25.0%) were classified as stage I, 7 (17.5%) as stage II, 6 (15.0%) as stage III, and 17 (42.5%) as stage IV. Primary tumors were located on the lips in 2 patients (5.0%), on the buccal mucosa in 3 patients (7.5%), on the floor of the mouth in 3 patients (7.5%), on the palate in 6 patients (15.0%), on the tongue in 8 patients (20.0%), on the gum in 12 patients (30.0%), in the maxillary sinus in 2 patients (5.0%), and on the retromolar pad in 4 patients (10.0%). Six of the patients (15.0%) underwent radical excision only; the other 34 patients (85.0%) were treated with both radical excision and reconstruction. Based on histological findings in the resected specimens, the tumors averaged 2.8 cm in size and consisted of carcinoma in situ in 1 patient (2.5%), well-differentiated tumors in 24 patients (60.0%), moderately differentiated tumors in 14 patients (35.0%), and poorly differentiated tumors in 1 patient (2.5%). Lymph node metastasis was observed in 10 patients (25.0%), and distant metastases (all to the lung) were present in 3 patients (7.5%).
The mean follow-up period was 35.58 months, and 17 patients (42.5%) died during the follow-up period. Table 1 shows the results of both the univariate analysis, comparing overall survival using the Kaplan-Meier method and log-rank test, and the multivariate analysis, using Cox proportional hazards model.
Table 1. Prognostic factors of overall survival, as identified by univariate and multivariate analyses (n=40).
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Value | P-value | Hazard ratio (95% CI) | P-value | |
| Time of diagnosis | 0.371 | |||
| Mean age (yr) | 66 (31–83) | - | ||
| ≤66 yr | 21 (52.5) | - | ||
| > 66 yr | 19 (47.5) | - | ||
| Sex | 0.059 | |||
| Male | 27 (67.5) | - | ||
| Female | 13 (32.5) | - | ||
| TNM | 0.011* | |||
| I | 10 (25.0) | - | ||
| II | 7 (17.5) | - | ||
| III | 6 (15.0) | - | ||
| IV | 17 (42.5) | - | ||
| CRP/alb ratio | <0.001* | 6.078 (1.971-18.743) | 0.002* | |
| ≤0.085 | 27 (67.5) | - | ||
| > 0.085 | 13 (32.5) | - | ||
| mGPS | <0.001* | |||
| 0 | 37 (92.5) | - | 0.044* | |
| 1 | 2 (5.0) | 4.949 (0.835-29.329) | 0.078 | |
| 2 | 1 (2.5) | 36.324 (2.33-566.372) | 0.01* | |
| NLR | 0.417 | |||
| ≤1.888 | 23 (57.5) | - | ||
| > 1.888 | 17 (42.5) | - | ||
| PLR | 0.025* | 4.827 (1.457-45.984) | 0.01* | |
| ≤124.8 | 20 (50.0) | - | ||
| > 124.8 | 20 (50.0) | - | ||
| PNI | 0.002* | |||
| ≤50.55 | 16 (40.0) | - | ||
| > 50.55 | 24 (60.0) | - | ||
| Size of primary tumor (cm) | 0.037* | |||
| ≤2.8 | 19 (47.5) | - | ||
| > 2.8 | 21 (52.5) | - | ||
| Lymph node metastasis | 0.03* | |||
| No | 30 (75.0) | - | ||
| Yes | 10 (25.0) | - | ||
| Distant metastasis | 0.079 | |||
| No | 37 (92.5) | - | ||
| Yes | 3 (7.5) | - | ||
(CRP/alb: C-reactive protein/albumin, mGPS: modified Glasgow Prognostic Score, NLR: neutrophil-lymphocyte ratio, PLR: platelet-lymphocyte ratio, PNI: prognostic nutritional index, CI: confidence interval)
*P<0.05.
Values are presented as mean (range) or number (%).
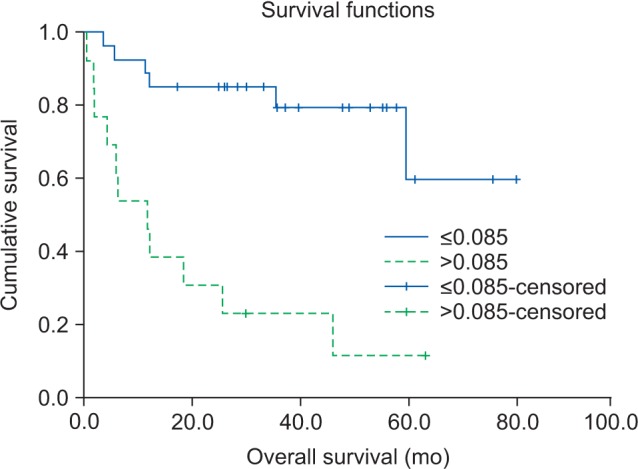
The group with high CRP/alb ratio showed a significantly higher overall survival (P<0.001).(Fig. 1) In addition, factors that showed statistically significant results were TNM clinical stage (I, II, III, or IV), mGPS (scores 0, 1, or 2), PLR (≤124.8 or >124.8), PNI (≤50.55 or >50.55), size of primary tumor (≤2.8 or >2.8 cm), and lymph node metastasis (yes or no). Based on the Cox proportional hazards model, CRP/alb ratio (hazard ratio=6.078; P=0.002), mGPS, and PLR were independent prognostic factors of overall survival.
Fig. 1. The overall survival curves according to the pretreatment C-reactive protein/albumin (CRP/alb) ratio. Compared with a lower CRP/alb ratio, a ratio of >0.085 was associated with significantly worse overall survival (P<0.001).
The mean CRP/alb ratio was 0.137 (range, 0.005-1.124). Using the cut-off finder, we determined that the optimal cut-off value of the CRP/alb ratio should be 0.085, with a sensitivity of 64.7% and a specificity of 91.3%. The cut-off values of the other inflammation-based prognostic scores were also found; NLR was 1.888, PLR was 124.8, and PNI was 50.55. Based on the cut-off value of CRP/alb ratio, the patients were classified into two groups: those with a high CRP/alb ratio (>0.085) and those with a low CRP/alb ratio (≤0.085); 27 patients (67.5%) were included in the high-CRP/alb ratio group and 13 patients (32.5%) in the low-CRP/alb ratio group. Table 2 compares the clinicopathological characteristics of these two groups using the Mann-Whitney U test or Fisher's exact test.
Table 2. Interrelation of CRP/alb ratio with clinicopathological characteristics (n=40).
| Characteristic | CRP/alb ≤0.085 | CRP/alb >0.085 | P-value |
|---|---|---|---|
| Sex | 0.072 | ||
| Male | 21 | 6 | |
| Female | 6 | 7 | |
| Mean age (yr) | 64.3 | 63.5 | 0.932 |
| TNM stage | 0.002* | ||
| I | 9 | 1 | |
| II | 7 | 0 | |
| III | 5 | 1 | |
| IV | 6 | 11 | |
| T stage | |||
| T1 | 10 | 1 | 0.002* |
| T2 | 8 | 0 | |
| T3 | 3 | 2 | |
| T4 | 6 | 10 | |
| N stage | 0.006* | ||
| N0 | 24 | 6 | |
| N1 | 3 | 7 | |
| N2 | 0 | 0 | |
| N3 | 0 | 0 | |
| M stage | 0.029* | ||
| M0 | 27 | 10 | |
| M1 | 0 | 3 | |
| Mean tumor size (cm) | 2.4 | 3.4 | 0.029* |
| Degree of differentiation | 0.386 | ||
| In situ | 0 | 1 | |
| Well | 15 | 9 | |
| Moderately | 11 | 3 | |
| Poorly | 1 | 0 | |
| Mean PNI | 53.41 | 47.96 | 0.002* |
| Mean NLR | 1.93 | 1.94 | 0.711 |
| Mean PLR | 0125.14 | 0130.87 | 0.568 |
| Mean WBC | 6.32 | 6.70 | 0.407 |
| mGPS | 0.029* | ||
| 0 | 27 | 10 | |
| 1 | 0 | 2 | |
| 2 | 0 | 1 |
(CRP/alb: C-reactive protein/albumin, PNI: prognostic nutritional index, NLR: neutrophil-lymphocyte ratio, PLR: platelet-lymphocyte ratio, WBC: white blood cell count, mGPS: modified Glasgow Prognostic Score)
*P<0.05.
Values are presented as number of patients or mean.
There were no significant differences in sex or degree of tumor differentiation (P>0.05). However, in the group with the high CRP/alb ratio, TNM clinical stage was advanced (P=0.002), the primary tumor was larger (P=0.029), and the probabilities of lymph node metastasis (P=0.006) and distant metastasis (P=0.029) were high.
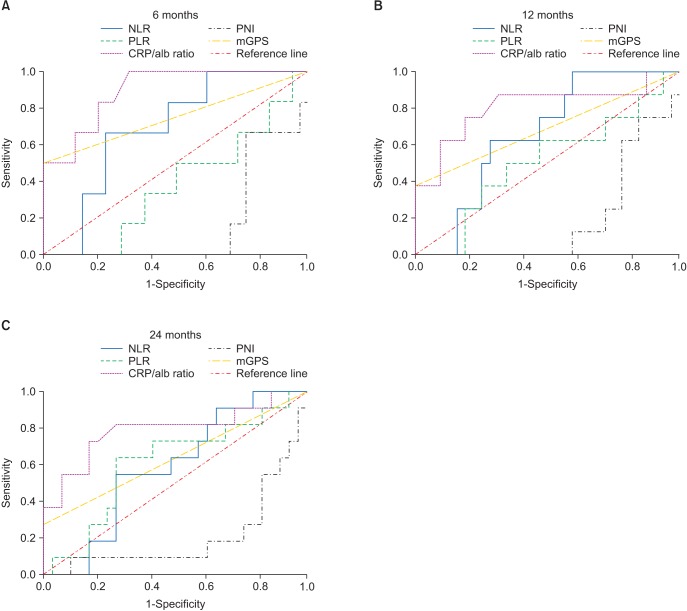
Regarding overall survival, we evaluated the discriminatory ability of the inflammation-based prognostic scores other than the CRP/alb ratio by comparing the values of the AUC to determine statistically significant differences using the receiver operation characteristic (ROC) curves corresponding to follow-up observations at 6, 12, and 24 months.(Table 3, Fig. 2) The longer was the period of observation, the greater was the decrease in AUC values for the CRP/alb ratio. At all follow-up examinations, the values of the CRP/alb ratio were higher than those of the other inflammation-based prognostic scores, and the CRP/alb ratio was the only variable with a P-value less than 0.05.
Table 3. Comparison of the AUC of the five inflammation-based prognostic scores.
| Prognostic score | AUC | 95% Cl | P-value |
|---|---|---|---|
| 6 mo | |||
| CRP/alb ratio | 0.897* | 0.784–1.000 | 0.002* |
| mGPS | 0.750 | 0.485–1.000 | 0.053 |
| PLR | 0.382 | 0.159–0.606 | 0.363 |
| NLR | 0.691 | 0.505–0.877 | 0.140 |
| PNI | 0.172 | 0.035–0.308 | 0.011 |
| 12 mo | |||
| CRP/alb ratio | 0.809* | 0.607–1.000 | 0.008* |
| mGPS | 0.688 | 0.446–0.929 | 0.105 |
| PLR | 0.508 | 0.283–0.733 | 0.946 |
| NLR | 0.660 | 0.487–0.834 | 0.166 |
| PNI | 0.191 | 0.008–0.055 | 0.008 |
| 24 mo | |||
| CRP/alb ratio | 0.790* | 0.608–0.972 | 0.005* |
| mGPS | 0.636 | 0.424–0.849 | 0.188 |
| PLR | 0.608 | 0.408–0.808 | 0.296 |
| NLR | 0.583 | 0.402–0.765 | 0.422 |
| PNI | 0.207 | 0.040–0.374 | 0.005 |
(AUC: area under the curve, CI: confidence interval, CRP/alb: C-reactive protein/albumin, mGPS: modified Glasgow Prognostic Score, PLR: platelet-lymphocyte ratio, NLR: neutrophil-lymphocyte ratio, PNI: prognostic nutritional index)
*P<0.05.
Fig. 2. Comparison of area under the curve (AUC) for outcome prediction among the five inflammation-based prognostic scores at 6 months (A), 12 months (B), and 24 months (C) of follow-up. The overall survival curves according to the pretreatment CRP/alb ratio. Compared with a lower CRP/alb ratio, a ratio of >0.085 was associated with significantly worse overall survival (P<0.001). (NLR: neutrophil-lymphocyte ratio, PLR: platelet-lymphocyte ratio, CRP/alb: C-reactive protein/albumin, PNI: prognostic nutritional index, mGPS: modified Glasgow Prognostic Score).
IV. Discussion
Inflammation represents a systematic reaction to malignancy when tumor cells secrete certain substances. When immune cells infiltrate the tumor tissue, the inflammatory response affects tumor progression. As these cells interact with each other, they cause tumor development to either accelerate or regress21,22.
Various mechanisms are involved in the relationship between inflammation and malignancy. First, growth of or invasion by a tumor can cause inflammation. Second, necrosis, tumor hypoxia, or local tissue damage can induce an inflammatory response. Third, tumor cells or lymphocytes related to the tumor can generate inflammatory cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-8, and vascular endothelial growth factor, and these inflammatory mediators induce growth, invasion, and metastasis; inhibit the immune reactions; and increase resistance to cytotoxic medicine7,23,24.
Among the different indicators related to inflammation, CRP is an acute phase protein. In response to an inflammatory trigger such as injury, cardiac disorder, or malignancy, CRP level increases. Of the pro-inflammatory cytokines that mediate this increase (e.g., IL-1, IL-6, and TNF), IL-6 is the most important25,26,27. In a malignant tumor, inflammatory cells act as early intrinsic defense mechanisms to resist the tumor; however, as the inflammation becomes chronic, it has a negative effect, resulting in tumor angiogenesis and DNA damage—both of which increase CRP level28. Hence, a preoperative increase in CRP can be used to predict prognosis in various kinds of cancers29,30. According to Khandavilli et al.13, patients with OSCC who demonstrated increased CRP level prior to surgery had reduced overall survival, larger tumors, and more advanced stages, which, when associated with CRP level, increased the prognostic power.
The relationship between inflammatory response and nutritional status has been confirmed in many studies. Albumin, as a chronic phase protein, is an indicator of a patient' s nutritional status and inflammatory status31,32,33. Systemic inflammation, followed by a decrease in alb level, results in poor performance, weight loss, and nutritional deficiency, all of which negatively affect the prognosis of patients with cancer34. Metgud and Patel35 have proposed that serum alb decreases in premalignant oral lesions and malignant tumors and may have a role in early diagnosis and prognosis.
To evaluate the relationship between inflammatory response and tumor development, researchers have recently introduced the use of inflammation-based prognostic scores, as determined by means of general serological indicators such as neutrophils, lymphocytes, CRP, and alb36,37,38. The initial study related to the CRP/alb ratio involved a patient with a severe illness. In assessing the prognosis of patients admitted to an acute medical unit, Fairclough et al.39 noted that the CRP/alb ratio is a better indicator upon exacerbation of chronic diseases among older patients. Ranzani et al.40 proposed that the CRP/alb ratio can be an independent risk factor in predicting prognosis in septic patients and showed that the results were more consistent than when CRP alone was used.
Recent studies have found that the CRP/alb ratio has good prognostic ability in various kinds of cancer. Kinoshita et al.18 demonstrated that the CRP/alb ratio could be an independent prognostic factor and had comparable prognostic value compared to other inflammation-based prognostic scores in patients with hepatocellular carcinoma. Analogous results were reported by Zhou et al.16 in patients with small-cell lung cancer and by Xu et al.41 in esophageal cancer. The optimal cut-off value for CRP/alb ratio of hepatocellular carcinoma, small-cell lung cancer, esophageal squamous cell carcinoma and OSCC is 0.037, 0.441, 0.09515,16,17,18,41, and 0.085, respectively. There are various cut-off values according to the kind of cancer. Although these other studies showed that the CRP/alb ratio had similar prognostic ability to other prognostic scores, the CRP/alb ratio had a better prognostic value in this study.
In the hopes of predicting prognosis in patients with OSCC, we conducted the first study designed to examine the clinicopathological and prognostic value of the CRP/alb ratio. In our study of 40 patients with OSCC, we found statistically significant relationships between the CRP/alb ratio and some important clinicopathological characteristics, indicating that the CRP/alb ratio is valuable in predicting prognosis.
On univariate analysis, all inflammation-based prognostic scores except NLR showed statistical significance; however, in the multivariate analysis, which considered confounding variables, the CRP/alb ratio, mGPS, and PLR were determined to be independent prognosis factors. In the group with a high CRP/alb ratio, the risk of death was 6.078 times higher than in the group with a low CRP/alb ratio.
According to the ROC curve, the CRP/alb ratio had far better discriminatory ability than did the other prognostic scores with regard to overall survival. The AUCs for the CRP/alb ratio at 6, 12, and 24 months were all larger than the AUCs of the other inflammation-based prognostic scores, and those scores that excluded the CRP/alb ratio were not statistically significant. A notable point is that the AUCs of the CRP/alb ratio and of the mGPS, which is determined using CRP and Alb values, are larger than those of the NLR and PLR, which is a white blood cell-based prognostic score. This indicates that the CRP-based prognostic score is better than the white blood cell-based prognostic score. Proctor et al.42 proposed that using neutrophils or platelets along with the mGPS could strengthen predictability; regarding the CRP/alb ratio, this conjecture should be tested in additional studies.
The inflammation-based prognostic scores that use CRP and alb include the mGPS and the CRP/alb ratio, but the results of these tests differ. Many researchers have shown that the mGPS had good prognostic ability in various kinds of cancers43,44,45,46. However, in our study, 37 of the 40 patients (92.5%) had an mGPS score of 0, and patients with a CRP level above 1 mg/dL were very rare. Unlike the CRP/alb ratio, the mGPS separates the values of CRP and alb and evaluates them independently by categorization, so it is possible to underestimate or overestimate a patient's prognosis. In our study, therefore, use of the mGPS to predict the prognosis of the patients with OSCC might not have been appropriate because this test indicated worse outcomes than did the CRP/alb ratio.
Our study had many limitations. First, the findings were retrospective and involved a sample that was relatively small and derived from a single institution. In addition, the cut-off value we chose for the CRP/alb ratio is not generalizable to all patients with OSCC. Because the optimal cut-off values for this ratio differ depending on the type of cancer and the institution, a meta-analysis is necessary to evaluate the prognostic value of the CRP/alb ratio in OSCC.
The results of this study confirm that the CRP/alb ratio is an independent factor for predicting prognosis in patients with OSCC, and it produces better results than other inflammation-based prognostic scores we examined. Prospective studies with larger sample sizes are needed to further explain the role of the CRP/alb ratio as a prognostic indicator in oral and other cancers.
V. Conclusion
In terms of clinicopathological characteristics, the group with a high CRP/alb ratio showed an advanced TNM clinical stage (P=0.002), larger primary tumors (P=0.029), and a statistically significant difference in lymph node metastasis and distant metastasis. In addition, on multivariate analysis, a high CRP/alb ratio was related to a lower survival rate (P=0.002; hazard ratio=6.078), and the associated ROC curve showed that the CRP/alb ratio had much greater discriminatory ability regarding overall survival than did the other inflammation-based prognostic scores. Consequently, the CRP/alb ratio could more effectively predict the prognosis of patients with OSCC and is simple and inexpensive to measure; hence, it should be considered a useful prognostic indicator.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Sun JR, Kim SM, Seo MH, Kim MJ, Lee JH, Myoung H. Oral cancer incidence based on annual cancer statistics in Korea. J Korean Assoc Oral Maxillofac Surg. 2012;38:20–28. [Google Scholar]
- 2.Lo Muzio L, Santarelli A, Panzarella V, Campisi G, Carella M, Ciavarella D, et al. Oral squamous cell carcinoma and biological markers: an update on the molecules mainly involved in oral carcinogenesis. Minerva Stomatol. 2007;56:341–347. [PubMed] [Google Scholar]
- 3.Spencer KR, Ferguson JW, Wiesenfeld D. Current concepts in the management of oral squamous cell carcinoma. Aust Dent J. 2002;47:284–289. doi: 10.1111/j.1834-7819.2002.tb00539.x. quiz 351. [DOI] [PubMed] [Google Scholar]
- 4.Klug C, Berzaczy D, Voracek M, Millesi W. Preoperative chemoradiotherapy in the management of oral cancer: a review. J Craniomaxillofac Surg. 2008;36:75–88. doi: 10.1016/j.jcms.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Genden EM, Ferlito A, Silver CE, Takes RP, Suárez C, Owen RP, et al. Contemporary management of cancer of the oral cavity. Eur Arch Otorhinolaryngol. 2010;267:1001–1017. doi: 10.1007/s00405-010-1206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geum DH, Roh YC, Yoon SY, Kim HG, Lee JH, Song JM, et al. The impact factors on 5-year survival rate in patients operated with oral cancer. J Korean Assoc Oral Maxillofac Surg. 2013;39:207–216. doi: 10.5125/jkaoms.2013.39.5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537–1549. doi: 10.1007/s00432-014-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31–39. doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 11.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI) Br J Cancer. 2012;106:1439–1445. doi: 10.1038/bjc.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Wu Y, Wang Z, Yao Y, Chen F, Zhang H, et al. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer. J Thorac Dis. 2013;5:783–789. doi: 10.3978/j.issn.2072-1439.2013.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khandavilli SD, Ceallaigh PO, Lloyd CJ, Whitaker R. Serum C-reactive protein as a prognostic indicator in patients with oral squamous cell carcinoma. Oral Oncol. 2009;45:912–914. doi: 10.1016/j.oraloncology.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Zhao L, Peng F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics (Sao Paulo) 2013;68:686–693. doi: 10.6061/clinics/2013(05)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8:339–345. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Zhan J, Hong S, Hu Z, Fang W, Qin T, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep. 2015;5:10481. doi: 10.1038/srep10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C, Jin Y, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 24.Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res. 2011;317:664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 26.Gallo O, Gori AM, Attanasio M, Martini F, Giusti B, Brunelli T, et al. Interleukin-6 and acute-phase proteins in head and neck cancer. Eur Arch Otorhinolaryngol. 1995;252:159–162. doi: 10.1007/BF00178104. [DOI] [PubMed] [Google Scholar]
- 27.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 30.Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006;12:3746–3750. doi: 10.3748/wjg.v12.i23.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs. 2005;9(Suppl 2):S35–S38. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberici Pastore C, Paiva Orlandi S, González MC. Association between an inflammatory-nutritional index and nutritional status in cancer patients. Nutr Hosp. 2013;28:188–193. doi: 10.3305/nh.2013.28.1.6167. [DOI] [PubMed] [Google Scholar]
- 34.Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110:1409–1412. doi: 10.1038/bjc.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metgud R, Patel S. Serum and salivary levels of albumin as diagnostic tools for oral pre-malignancy and oral malignancy. Biotech Histochem. 2014;89:8–13. doi: 10.3109/10520295.2013.793394. [DOI] [PubMed] [Google Scholar]
- 36.Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1068–E1074. doi: 10.1002/hed.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farhan-Alanie OM, McMahon J, McMillan DC. Systemic inflammatory response and survival in patients undergoing curative resection of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2015;53:126–131. doi: 10.1016/j.bjoms.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Fang HY, Huang XY, Chien HT, Chang JT, Liao CT, Huang JJ, et al. Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope. 2013;123:2690–2699. doi: 10.1002/lary.24105. [DOI] [PubMed] [Google Scholar]
- 39.Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009;9:30–33. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8:e59321. doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10:e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer. 2013;119:2325–2332. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 43.Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafique K, Proctor MJ, McMillan DC, Leung H, Smith K, Sloan B, et al. The modified Glasgow prognostic score in prostate cancer: results from a retrospective clinical series of 744 patients. BMC Cancer. 2013;13:292. doi: 10.1186/1471-2407-13-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou T, Hong S, Hu Z, Hou X, Huang Y, Zhao H, et al. A systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancer. Tumour Biol. 2015;36:337–343. doi: 10.1007/s13277-014-2623-4. [DOI] [PubMed] [Google Scholar]
- 46.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]