Abstract
Granulocyte-macrophage colony stimulating factor (GM-CSF) has a role in inducing emergency hematopoiesis upon exposure to inflammatory stimuli. Although GM-CSF generated murine bone marrow derived cells have been widely used as macrophages or dendritic cells in research, the exact characteristics of each cell population have not yet been defined. Here we discriminated GM-CSF grown bone marrow derived macrophages (GM-BMMs) from dendritic cells (GM-BMDCs) in several criteria. After C57BL/6J mice bone marrow cell culture for 7 days with GM-CSF supplementation, two main populations were observed in the attached cells based on MHCII and F4/80 marker expressions. GM-BMMs had MHCIIlowF4/80high as well as CD11c+CD11bhighCD80−CD64+MerTK+ phenotypes. In contrast, GM-BMDCs had MHCIIhighF4/80low and CD11chighCD8α− CD11b+CD80+CD64−MerTKlow phenotypes. Interestingly, the GM-BMM population increased but GM-BMDCs decreased in a GM-CSF dose-dependent manner. Functionally, GM-BMMs showed extremely high phagocytic abilities and produced higher IL-10 upon LPS stimulation. GM-BMDCs, however, could not phagocytose as well, but were efficient at producing TNFα, IL-1β, IL-12p70 and IL-6 as well as inducing T cell proliferation. Finally, whole transcriptome analysis revealed that GM-BMMs and GM-BMDCs are overlap with in vivo resident macrophages and dendritic cells, respectively. Taken together, our study shows the heterogeneicity of GM-CSF derived cell populations, and specifically characterizes GM-CSF derived macrophages compared to dendritic cells.
Keywords: dendritic cell, GM-CSF, macrophage, phenotype
INTRODUCTION
M Granulocyte-macrophage colony stimulating factor (GM-CSF) mediates various signals for cellular proliferation and survival, especially in hematopoietic cells (Hercus et al., 2009). As one can easily suppose from its name, GM-CSF helps form hematopoietic progenitor colonies, which can differentiate into granulocytes or macrophages. Although serum GM-CSF levels are distinctively absent in the steady state, (Cebon et al., 1994; Cheers et al., 1988) production rapidly increases upon exposure to inflammatory stimuli. Therefore GM-CSF has a role in inducing emergency hematopoiesis and not in the steady state. In this way, it is not surprising that mice with GM-CSF or GMCSF receptor deficiency showed almost normal hematopoietic cell composition. Only minor cell populations were affected by GM-CSF deficiency. Alveolar macrophages showed reduced TNF-α and leukotrienes, indicating that GM-CSF has tissue specific activity in alveolar macrophages (Paine et al., 2001). In addition, elicited peritoneal macrophages (Becker et al., 2012) as well as inflammatory dendritic cells (DCs) were also diminished in GM-CSF deficient mice suggesting that GM-CSF has a role in inflammation.
For mice research, the relative numbers of macrophages to be obtained from mice are low so isolating sufficient numbers for comprehensive studies can be logistically burdensome. Therefore the majority of applications currently rely on the in vitro generation of macrophages from murine bone marrow (BM) cells with the appropriate haematopoietic growth factor M-CSF (Xu et al., 2007). M-CSF grown BM-derived macrophages (M-BMMs) are used commonly because of their resemblance to resident homeostatic macrophages, and M-CSF can produce relatively homogenous macrophage populations in vitro. In contrast, although GM-CSF grown BM-derived macrophages (GM-BMM) are also accepted as source of macrophages, (Murray et al., 2014) they are contaminated with other cell types, predominantly DCs. Nonetheless, GM-BMMs still have some value as a macrophage source (Fleetwood et al., 2007) because GM-BMMs have different functional and phenotypic characteristics from M-BMMs that are similar to inflammatory macrophages such as thioglycollate-induced peritoneal macrophages (Becker et al., 2012; Fleetwood et al., 2007). Thus there are some benefits to use GM-BMMs such as in screening anti-inflammatory drugs or investigating the biology of inflammatory macrophages in inflamed tissues, for example. However, general experimental protocols produce GM-CSF grown BM-derived dendritic cells (GM-GMDCs) as well as GM-BMMs. Thus, we need to clarify the exact characteristics and composition of each population.
In this study, we compared GM-BMMs and GM-BMDCs produced in the same culture environment and found several differences. We found differences in cell surface markers, profiles of cytokine secretion, cell morphology, ability to phagocytize latex beads and ability to proliferate T cells. We also found that microarray profiling of GM-BMMs overlaps with resident macrophages, and GM-BMDCs overlaps with resident DCs in vivo. Thus, we present here the heterogeneity of GM-CSF grown bone marrow cells, and the functional and phenotypic characteristics of GM-BMMs and GM-BMDCs.
MATERIALS AND METHODS
Cell preparation
C57BL/6J mice were obtained from Jackson Laboratory. Male mice of five to ten weeks of age were used to isolate bone marrow cells. The mice were housed under specific pathogen-free (SPF) conditions and cared according to the Guide for the Care and Use of Laboratory Animals prepared by the Institution of Animal Care and Use Committee (IACUC) of Seoul National University. All of the experiments were approved by the IACUC of the Seoul National University (accession number SNU-130311-2-2). Isolated bone marrow cells from femurs and tibiae were cultured for 7 days at a density of 106/ml in RPMI medium (Thermo, USA) supplemented with 10% FBS (Gibco, Carlsbad, California, USA), 10 units/ml penicillin, 10 μg/ml streptomycin, 2 mM L-glutamine (Gibco, USA) (hereafter termed complete medium) and 25 ng/ml murine GM-CSF (Miltenyi Biotech, Germany) at 37°C in a humidified atmosphere with 5% CO2. On day 3, floating cells were discarded and fresh medium containing 25 ng/ml GM-CSF was added. Cells were further differentiated for 4 days with GM-CSF containing complete medium. Floating and attached cells were separately examined for their surface marker expressions and we obtained attached cells in this study by scrapping after gently washing the culture plates with warm PBS twice.
Flow cytometry
Floating cells and attached cells were separately prepared for FACS analysis. Cells were then incubated for 20 min with antibodies diluted at the optimal concentrations in FACS buffer (PBS, 5% FBS, 5 mM EDTA, and 1% NaN3). LSRII (BD) was used for multiparameter analysis of stained cell suspensions, followed by analysis with FlowJo software (Tree Star) as described previously (Seok et al., 2013). Monoclonal antibodies to mouse F4/80 (BM8), CD11b (M1/70), IA/IE (M5/114.15.2), CD8α (53-6.7), CD115 (AFS98), FcεRI (MAR-1), Siglec F (E50-2440, BD Biosciences), Ly6C (HK1.4), MerTK (clone 125518, R&D systems), CD64 (X54-5/7.1, BD Biosciences), CD11c (HL3), CD80 (16-10A1, BD Biosciences), CD86 (GL1), and CD103 (2E7), were all from eBioscience, unless indicated. MHCIIhighF4/80low and MHCIIlowF4/80high populations were sorted with a FACSAria II (BD). Sorting purity was always checked and confirmed that was up to 95~98%.
Latex bead phagocytosis
Bone marrow cells were differentiated in a 12-well plate at a density of 106/ml for 7 days. After media change, cells were incubated with 1 μm diameter Alexa 350-tagged latex beads (Molecular Probe) for 2 h at 107 concentration per each well. Phagocytozed beads counts were analyzed on a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software. For imaging of beads phagocytozed by DCs and macrophages, differentiated cells were sorted into DCs (MHCIIhighF4/80low population) and macrophages (MHCIIlowF4/80high population) and seeded in a 4-chamber well slide. After beads incubation for 2 h, attached cells were washed three times, fixed with 4% paraformaldehyde for 10 min, mounted and photographed using a Leica AF6000 fluorescence microscope.
Cytokine production
Sorted DCs and macrophages were seeded in 96-well plates at 5 × 104/200 μl with complete media and rested for 6 h. Cells were stimulated with LPS (100 ng/ml) or PolyI:C (50 μg/ml) for 24 h. Supernatants were collected and stored at −80°C until TNFα, IL-12p70, IL-6 and IL-10 quantification. For IL-1β quantitation, cells at 24 h time point were lysed in RIPA buffer and stored at −80°C. Cells in each well were analyzed for their protein concentration using the BCA assay kit (Thermo, USA) as a normalization factor for cytokine quantitation. Cytokine concentrations were determined using the duoset ELISA kit (R&D systems, USA).
Mixed leukocyte reaction
DCs and macrophages were sorted according to the above description. For M-CSF grown macrophages, C57BL/6J bone marrow cells were differentiated with 20% L929 culture supernatant for 7 days and sorted for the F4/80+CD11b+ population. To prepare T cells, Balb/c splenocytes were isolated and incubated in a 100π dish for 2 h to remove attached cells. Floating splenocytes were collected and labeled with 5 μM CFSE (Molecular Probe) for 5 min at 37°C. Cells were washed with complete medium twice and seeded in a 96-well U bottom plate for mixed leukocyte reaction. Total 2 × 104 of antigen presenting cells and 105 of splenocytes were mixed and incubated for 5 days. CD4+ T cell proliferation was examined using FACS analysis.
Microarray analysis, normalization and data analysis
RNA was prepared from sorted DCs and macrophages with Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. Global gene expression analysis was performed using Affymetrix GeneChip® Mouse Gene 1.0 ST oligonucleotide arrays. Sample preparation was performed according to the instructions and recommendations provided by the manufacturer. Expression data were generated by Affymetrix Expression Console software version1.1. For normalization, the RMA (Robust Multi-Average) algorithm was implemented in Affymetrix Expression Console software. Obtained microarray data of GM-BMDCs, GM-BMMs and M-BMMs in this study was assigned a GEO accession number (GSE65425). Microarray gene expression data for lung DC, mesenteric lymph node CD8+ DC, splenic CD11b+ DC, thioglycollate induced F4/80high macrophage, lung CD11b+ macrophage and microglia were collected from the Immunological Genome Project (GSE15907) (Gautier et al., 2012; Heng et al., 2008). Expression data are pooled from 2 replicate microarray experiments.
For real-time PCR, cDNA was synthesized from 1-μg total RNA using M-MLT reverse transcriptase (Enzynomics, Korea). Reverse-transcribed RNA (20 ng) was used as template for quantitative real-time PCR and set up in 96-well plates using Taqman Universal Mastermix II (Applied Biosystems, USA). The primers for RelB detection were: 5d-GTTCCAGTGA CCTCTCTTCCC-3′ (Forward), 5′-CCAAAGCCGTTCTCCTT AATGTA-3′ (Reverse); for MafB detection were: 5′-GTTATA GGGGAGGTCTAGGTGT-3TAT (Forward), 5′-AAGCTCGTTT CCGATGCAG-3′ (Reverse), for Maf detection were: 5′-CTGC CGCTTCAAGAGGGTGCAG C-3′ (Forward), 5′-GATCTCC TGCTTGAGGTGGTC-3′ (Reverse). Gene expression levels were quantified using an ABI Prism 7900 sequence detection system (Applied Biosystems). The relative expression of each sample was normalized to 18Sr-RNA (Applied Biosystems) and compared with the controls according to the relative Ct method.
Statistical analysis
All data unless otherwise indicated are shown as mean ± SEM and were tested using two-tailed Student’s t test or two-way ANOVA using GraphPad Prism 4.
RESULTS
GM-CSF generates various cell populations from bone marrow cells
As GM-CSF is known to induce the expansion of various myeloid lineages, we cultured bone marrow cells in the presence of GM-CSF for 7 days to examine the exact cell populations derived from GM-CSF in vitro culture. We found that attached cells were mainly composed of two populations, based on the MHCII and F4/80 expressions. We assumed that MHCIIhigh F4/80low and MHCIIlowF4/80high populations correspond to DCs and macrophages, respectively. These GM-CSF grown, bone marrow cell derived DCs (GM-BMDCs) comprised up to 18% of total attached cells. In contrast, GM-CSF grown bone marrow cell derived macrophages (GM-BMMs) were the main cells (57% of the attaching cells) of the mixed populations. Floating cells consisted of monocytes (CD115+CD11b+, 56.5 ± 1.6%), basophils (CD115−FcεRIα+, 4.6 ± 3%) and eosinophils (CD115− SiglecF+, 3.6 ± 1.1%) and we did not observe neutrophils (Gr1+CD11b+F4/80−) (Fig. 1B). All of these cell populations were CD11b+ (data not shown). Much of the floating cells expressed F4/80 and they seemed to be transitioning from monocytes to macrophages or DCs. Monocyte expression of Ly6C was heterogenous and up to 54% of monocytes were Ly6C+ (Fig. 1A). From these results, we revealed that attached cells isolated from GM-CSF grown bone marrow cell cultures were heterogenous and were relatively favored into differentiation of MHCIIlowF4/80high macrophages.
Fig. 1.
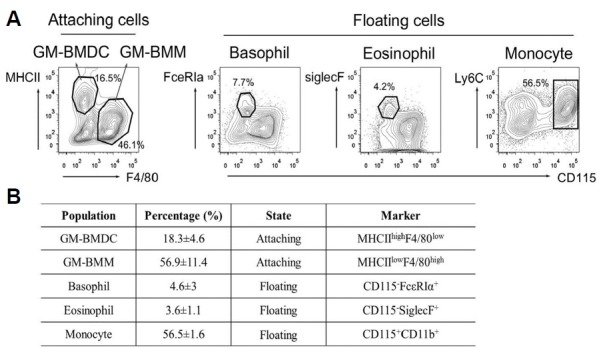
Population composition of GMCSF derived bone marrow cells. (A) Bone marrow cells were isolated from C57BL/6J mice and cultured with 25 ng/ml for 7 days. On day 3, fresh medium containing GM-CSF was added. Attached and floating cells were examined for the indicated marker expressions using flow cytometry. Contour plots are representative of five independent experiments. (B) A table describing the population percentages and phenotypes of GM-BMDCs, GM-BMMs, basophils, eosinophils and monocytes in GM-CSF derived bone marrow cells. Percentage indicates mean ± SEM. n = 3
GM-CSF derived DCs and macrophages have distinct surface marker expressions
DCs and macrophages themselves show heterogenous phenotypes in vivo (Hashimoto et al., 2011). To characterize GM-BMDCs and GM-BMMs more clearly, we next investigated the surface marker expressions of these cells in detail (Fig. 2). As expected, MHCIIhighF4/80low GM-BMDCs expressed high levels of CD11c and CD11b. GM-BMMs expressed relatively higher levels of CD11b but lower levels of CD11c compared to GM-BMDCs (Fig. 2A). GM-BMMs also expressed more CD64 and MerTK, which are known resident macrophage markers (Gautier et al., 2012). CD80 expression was only observed on GM-BMDCs. Taken together, we show that GM-CSF differentiates mixed DC and macrophage populations with distinct marker expressions in that GM-BMDC has MHCIIhighF4/80lowCD11chighCD8α−CD11b+CD80+CD64−MerTKlow phenotype and GM-BMM has MHCIIlowF4/80highCD11c+CD11blowCD80− CD64+MerTK+ phenotype.
Fig. 2.
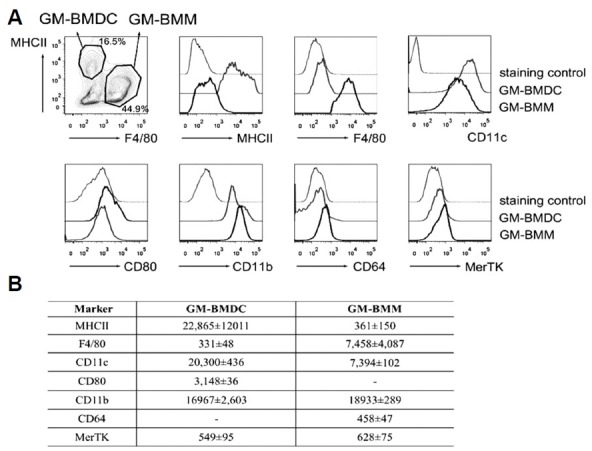
Surface marker expressions of GM-BMDCs and GM-BMMs. (A) GM-BMDCs and GM-BMMs were gated as MHCIIhighF4/80low and MHCIIlowF4/80high populations respectively, and further analyzed for the indicated marker expressions using flow cytometry. Histograms are representative of three independent experiments. (B) A table describing mean fluorescence intensity (mean ± SEM, n = 3) of examined surface markers analyzed by flow cytometry.
Macrophage population is increased dependent of GM-CSF concentration
GM-CSF concentration varies depending on inflammatory conditions in vivo. Because GM-CSF can induce different signaling pathways depending on the extracellular concentration (Hercus et al., 2009), we investigated dose dependent effects of GM-CSF on the ratio of DCs and macrophages differentiation in in vitro culture conditions. We treated bone marrow cells with 5, 10, 25 and 100 ng/ml of GM-CSF and cultured them for 7 days. At 5 ng/ml, we could not distinguish DC and macrophage populations clearly in a MHCII and F4/80 scatter plot (Fig. 3A). Two populations were distinguished with treatment of 10 ng/ml of GM-CSF. At the usual concentration (25 ng/ml) of GM-CSF, the DC:macrophage ratio increased to 1:3~1:4 as already shown in Figs. 1 and 2. Interestingly, GM-BMDCs decreased in a GM-CSF dose-dependent manner. At 100 ng/ml, 67.3% of attached cells were GM-BMMs but only 7.6% was comprised of GM-BMDCs. A quantitative population graph of GM-BMDCs and GM-BMMs with various doses of GM-CSF is depicted in Fig. 3B. From this results, we can see that high dose of GM-CSF favors macrophage differentiation.
Fig. 3.
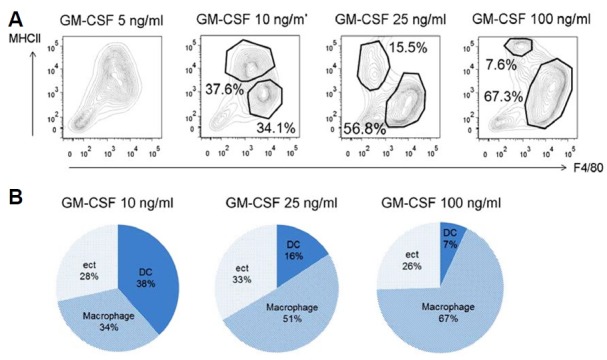
Increasing GM-CSF concentration favors GM-BMMs development. (A) Dot plots of MHCII and F4/80 expressions on cultured bone marrow cells with varying concentrations of GM-CSF. Bone marrow cells were differentiated with 5, 10, 25 or 100 ng/ml of GM-CSF for 7 days. Attached cells were examined for their population compositions of GM-BMDCs and GM-BMMs. Data are representative of three independent experiments. (B) Quantitative graph showing relative population compositions of GM-BMDCs and GM-BMMs examined in (A).
GM-BMMs have enhanced phagocytic ability compared to GM-BMDCs
Macrophages are efficient at phagocytosis and scavenging cellular debris in vivo. To compare the functional differences between GM-BMDCs and GM-BMMs, we first examined their phagocytic abilities. As expected, only 7.7% of MHCIIhighF4/80low GM-BMDCs contained beads. In contrast, most MHCIIlowF4/80high GM-BMMs (76.8%) had beads, illustrating their superior phagocytic ability (Fig. 4A). Bead count per cell was also significantly different between GM-BMDCs and GM-BMMs (Fig. 4B). The 7.3% of DCs had an uptake of 1 to 2 beads per cell, whereas 27.2% of macrophages had an uptake of more than 5 beads per cell. In accordance with this data, we obtained enriched phagocytosis gene ontology biological processes (GO: BP) in GM-BMMs by microarray analysis (Table 1). To observe any cellular morphology differences between the two populations, we seeded sorted GM-BMDCs and GM-BMMs in a chamber well slide, incubated them with latex beads and imaged them using fluorescence microscope (Fig. 4C). GM-BMDCs were relatively round and had many dendrites. On the other hand, GM-BMMs seemed to be flatter, had no evident dendrites and had many beads in their cytoplasm. Collectively, these results demonstrated that GM-CSF derived macrophages have sufficient phagocytic ability but DCs do not.
Fig. 4.
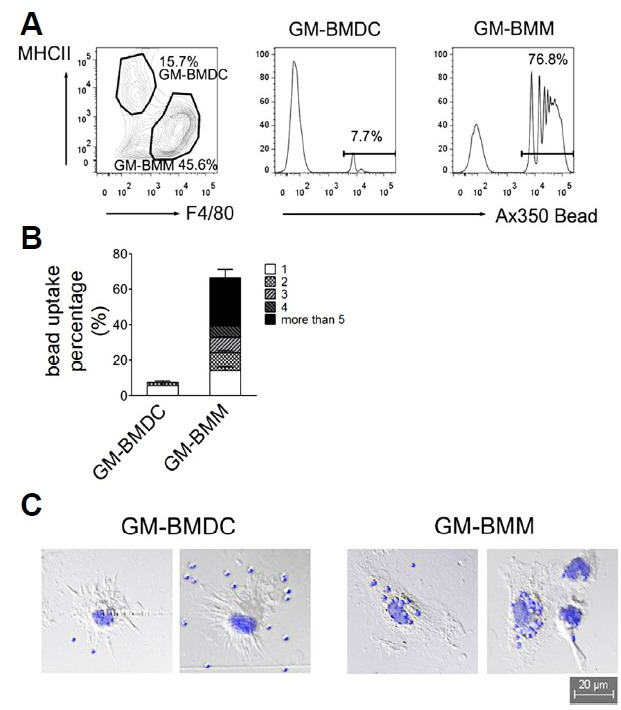
Bead phagocytosis by GM-BMDCs and GM-BMMs. (A) Gated GM-BMDCs and GM-BMMs by MHCII and F4/80 expressions (left dot plot) were further analyzed for bead uptake. Alexa 350-tagged latex beads were incubated with differentiated GM-CSF derived bone marrow cells for 2 h, washed twice and labeled with IA/IE and F4/80 antibodies. Internalized beads were analyzed using flow cytometry (Middle and right histograms). Data are representative of three independent experiments. (B) Quantitative bar graph indicating the cell percentages with internalized bead counts of GM-BMDCs and GM-BMMs. Error bars, SEM. n = 3 (C) Cell morphologies of sorted GM-BMDCs and GM-BMMs after bead internalization were photographed using fluorescence microscope. Beads are shown as blue with 1 μm diameter inside or around the cells.
GM-BMDCs produce much more pro-inflammatory cytokines compared to GM-BMMs
Both DCs and macrophages are known to produce inflammatory cytokines and stimulate innate immune responses upon Toll-like receptor ligation. To study the cytokine responses of GM-BMDCs and GM-BMMs, we sorted the two populations and stimulated them with Myd88/TRIF dependent TLR4 agonist LPS or TRIF dependent TLR3 agonist PolyI:C for 24 h (Fig. 5). Interestingly, GM-BMDCs synthesized much more TNFα, IL-12p70 and IL-6 compared to GM-BMMs upon LPS stimulation. Pro-IL-1β synthesis was not significantly different between DCs and macrophages. In contrast, anti-inflammatory cytokine IL-10 was produced two times higher in GM-BMMs than GM-BMDCs. LPS induced comparable effects on cytokine production, however PolyI:C did not, demonstrating that the effects of the Myd88 dependent pathway might be different in GM-CSF derived DCs and macrophages. IL-10 synthesis depends on endogenous ERK contents and it is known that macrophages are the main producers of IL-10 in an ERK-dependent manner, (Saraiva and O’Garra, 2010) further supporting our results. Taken together, we confirmed that GM-BMDCs have the ability to produce pro-inflammatory cytokines TNFα, IL-12p70 and IL-6 than GM-BMMs upon LPS stimulation. Conversely, GM-BMMs produce much more IL-10 than DCs.
Fig. 5.
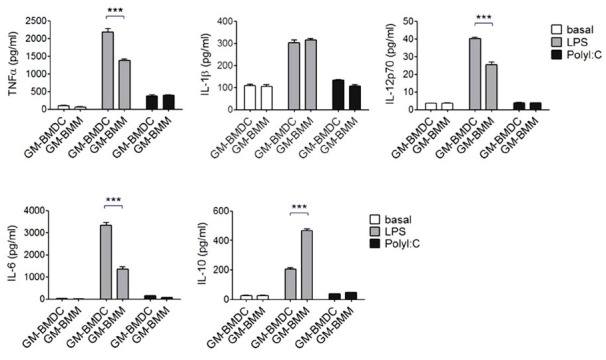
Cytokine productions of GM-BMDCs and GM-BMMs. Sorted GM-BMDCs and GM-BMMs were seeded in 96-well plates at 5 × 105 cells per well. Cells were stimulated with LPS (100 ng/ml) or polyI:C (50 μg/ml) for 24 h. TNFα, IL-12p70, IL-6 and IL-10 in culture supernatants were quantified using ELISA. IL-1β was analyzed in the cell lysates using ELISA. Data are representatives of three independent experiments. Error bars, SEM, ***, p < 0.001 analyzed by one-way ANOVA with Bonferroni correction, n = 3.
GM-BMDCs have enhanced T-cell proliferation ability than GM-BMMs
DCs are professional antigen presenting cells with an ability to stimulate T cells (Banchereau and Steinman, 1998). To ascertain T cell proliferating ability of GM-BMDCs, we performed mixed leukocyte reaction (Fig. 6). CD11b+F4/80+ M-CSF grown bone marrow derived macrophages (M-BMMs) were also examined to compare T cell proliferation ability. Sorted GM-BMDCs or GM-BMMs were mixed with allogeneic MHCII-carrying Balb/c slpenocytes for 5 days and CFSE-labeled CD4+ T cells were examined for their proliferation by flow cytometry. As expected, GM-BMDCs expanded T cells most extensively, resulting in the proliferation of 90.1% of CD4+ T cells (Fig. 6A). GM-BMMs and M-BMMs resulted in 65.3% and 40.9% of proliferated T cells, respectively. In support of this, we also obtained enriched GO: BP of antigen processing and peptide presentation or polysaccharide antigen presentation via MHC class II in DC population by microarray analysis (Table 2). A quantitative graph showing proliferated CD4+ T cells reveals sequential T cell stimulation abilities of GM-BMDCs, GM-BMMs and M-BMMs (Fig. 6B).
Fig. 6.
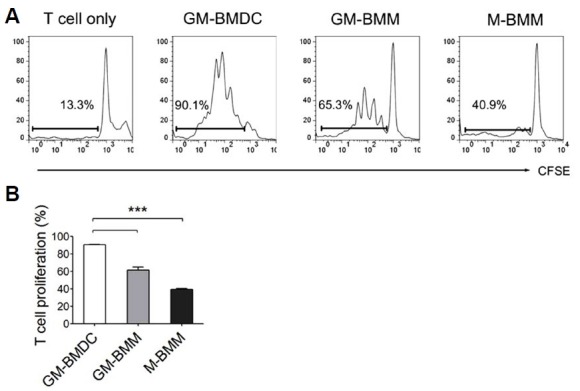
Mixed leukocyte reactions (MLR) of GM-BMDCs, GM-BMMs and M-BMMs. (A) Histograms showing CFSE-labeled CD4+ T cells after MLR mixed with C57BL/6J bone marrow derived cells. Gates indicate proliferated T cell percentages. GM-BMDCs and GM-BMMs were sorted into MHCIIhighF4/80low and MHCIIlowF4/80high populations after 7 days of differentiation of bone marrow cells with GM-CSF. M-BMMs were sorted into F4/80+CD11b+ population after differentiation for 7 days in 20% L-cell culture media. These cells were used as antigen presenting cells and mixed with Balb/c originated CFSE-labeled splenocytes at a 1:1 ratio (105 cells per well each). After 5 days of co-culture, floating cells were examined for their CFSE dye intensities after CD4 positive cell gating using flow cytometry. Data are representatives of three independent experi-Quantitative graph indicating proliferated CD4+ T cell percentages mixed with GM-BMDCs, GM-BMMs and M-BMMs. Error bars, SEM, ***, p < 0.001 analyzed by one-way ANOVA with Bonferroni correction, n = 3.
Whole transcriptome analysis shows that GM-BMDCs and GM-BMMs are clustered with tissue resident DCs and macrophages, respectively
Due to the ambiguity of characterizing cell populations using surface markers or specific functions, we sought to define GM-BMDCs and GM-BMMs based on their profiles of gene transcripts (Satpathy et al., 2012). Using gene hierarchical clustering mapping and principal component analysis (PCA) of tissue resident DC and macrophage populations from the ImmGen Project (Gautier et al., 2012), we confirmed that in vitro-generated GM-BMDCs and GM-BMMs resemble organ DCs and macrophages, respectively. Surprisingly, GM-BMMs overlap with M-BMMs as well as CD11b+ lung macrophages, microglia and thioglycollate induced peritoneal macrophages, but not with GM-BMDCs (Fig. 7A). As shown in the PCA result of all expressed genes (Fig. 7B), although resident macrophages from three specific organs have diverse gene expression patterns compared to DC populations from mesenteric lymph node, lung and spleen, GM-BMDCs most resemble tissue resident DC populations, and GM-BMMs as well as M-BMMs most resemble resident macrophage populations when comparing 2 principle component criteria. Finally, we compared lineage specific transcription factor gene expressions by real-time PCR. Expression of RelB, a transcription factor of DC (Egawa et al., 2013), was significantly higher in sorted GM-BMDC than GM-BMMs (Fig. 7C). In contrast, gene expression levels of MafB and Maf, both of which are used as macrophage lineage specific markers, were higher in sorted GM-BMMs than GM-BMDCs (Fig. 7D). Collectively, our results indicated that GM-CSF generates mixed DC and macrophage populations in in vitro culture conditions, and these share some degree of similarities to their corresponding in vivo populations.
Fig. 7.
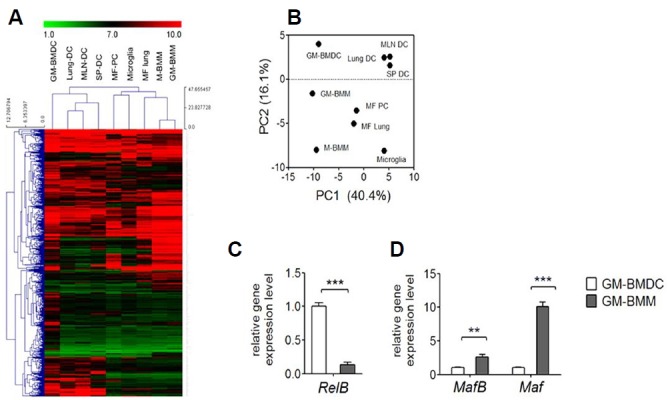
Whole transcriptome analysis of GM-BMDCs and GM-BMMs. (A) Hierarchical clustering of GM-BMDCs, GM-BMMs and M-BMMs based on the 15% of genes with the greatest variability compared to populations collected by the Immunological Genome Project (GSE15907). Lung DC; CD103+ lung DCs, MLN DC; mesenteric lymph node CD8+ DCs, SP DC; spleen CD11b+ DCs, MF PC; thioglycollate induced F4/80high macrophages, MF lung; CD11b+ lung macrophages. (B) Principal component analysis of GM-BMDCs, GM-BMMs and M-BMMs total gene expressions with tissue resident DCs and macrophages used in (A). Expression data are pooled from 2 replicate microarray experiments. (C) Gene expression of RelB by GM-GMDCs and GM-BMMs was examined by real-time PCR. Error bars, SEM, ***p < 0.001 analyzed by student t-test, n = 3. (D) Gene expressions of MafB and Maf by GM-GMDCs and GM-BMMs were examined by real-time PCR. Error bars, SEM, **p < 0.005, ***p < 0.001 analyzed by student t-test, n = 3.
DISCUSSION
GM-CSF grown bone marrow cells are widely used as a model system for DC development and function (Nikolic et al., 2003; Zhang et al., 1998), although bioinformatics analysis of their transcriptome indicates that they are closer to macrophages than DCs (Crozat et al., 2010; Robbins et al., 2008). It was recently suggested that GM-CSF grown bone marrow derived macrophages are still acceptable as macrophage sources (Murray et al., 2014), however the authors worried about the contamination with DC populations. M-CSF grown macrophages, the preferred source for macrophages, show much similarity with tissue macrophages, but as we stated, GM-CSF grown macrophages have unique utility as an experimental platform to investigate inflammation specific exacerbated signaling pathways because they resemble inflammatory macrophages (Fleetwood et al., 2007). In fact, these GM-CSF grown macrophages could produce even more extensive proinflammatory cytokines compared to M-CSF grown, INFγ/LPS polarized macrophages (preliminary data). Despite the remarkable inflammatory phenotype of GM-CSF grown macrophages, there is much confusion about the use of these cells because they have been used as both macrophages and DCs without any guiding criteria (Bhattacharya et al., 2011; Chung et al., 2015; Ganesh et al., 2009). Consequently, we sought to distinguish amongst the heterogenous populations of GM-CSF derived cells and especially focused on characteristics of GM-BMMs compared to GM-BMDCs in this study.
It is not surprising that we found two populations in the attached cells based on F4/80 and MHCII expression (Fig. 1A), as GM-CSF is known to produce granulocytes, macrophages and DCs from common progenitor cells (Inaba et al., 1993). We consider the MHCIIhighF4/80low population as GM-BMDCs in several aspects. They expressed high CD11c, showed a low phagocytic ability and had a standard DC-like morphology with small round shapes and many dendrites (Xu et al., 2007).
In addition, they could expand T cells efficiently, with similar gene ontology enrichment of antigen presentation. Recently reported classical DC-specific genes including Zbtb46, Flt3, kit and ccr7 were all upregulated in GM-BMDCs compared to GM-BMMs and M-BMMs, further indicating their resemblance with DCs (Supplementary Fig. S1), rather than macrophages. As Xu et al. (2007) had reported that DCs derived in vitro with GM-CSF/IL-4 are the equivalents of induced inflammatory Tip-DCs in vivo, we also found that GM-BMDCs extensively produced TNFα upon LPS stimulation in this study, indicating their inflammatory DC phenotype.
Standard 25 ng/ml of GM-CSF concentration resulted in a domination of MHCIIlowF4/80high GM-BMMs among attached cells. We could omit the possibility of these cells as immature DCs,(Mellman and Steinman, 2001) because they highly expressed F4/80 on their surface and had low gene transcripts of CD80 and CD86 compared to GM-BMDCs (Supplementary Fig. S1). Macrophage specific marker CD64 (Gautier et al., 2012) was only detected on the surface of GM-BMMs, albeit was not highly expressed. A substantial level of CD11c was also detected on GM-BMMs, indicating that DCs are not the sole CD11c-positive cells. Actually the expression of F4/80 and CD11c often overlap in macrophages and DCs in nonlympoid tissues, and our results show that GM-BMMs express F4/80, MHCII as well as CD11c like with colonic macrophages and lung macrophages (Miller et al., 2012). MafB and Maf, lineage specific transcription factors for macrophages, were also expressed higher in GM-BMMs compared to GM-BMDCs (Fig. 7C). Most of all, they demonstrated remarkable phagocytic ability, comparable to M-BMMs (data not shown), and showed a morphology typical of macrophages (Fig. 4). Enriched gene ontology biological process pathways in GM-BMMs includes lysosome organization, phagocytosis, tissue remodeling, tissue homeostasis and response to wounding, all indicating macrophage specific functions of these cells (Table 1).
Interestingly, the macrophage population increased in a GM-CSF concentration dependent manner, suggesting a preference toward macrophage development by newly infiltrated haematopoietic cells in a highly inflammatory environment.
In an effort to ensure the heterogeneity of GM-CSF derived cells, Helft et al. (2015) has been recently published an article in accord of most of our data. They found that CD11c+CD11bhigh MHCIIInt cell population, previously considered as immature BMDCs, actually corresponds to macrophage expressing CD64, CD115 and CD14 whereas CD11c+CD11bInt MHCIIhi cell population is primarily consisted with DC. Although they showed sufficient data supporting the existence of two populations in GM-CSF mouse bone marrow cultures, they originally focused on the methods of in vitro expansion of DCs thus examined on non-adherent as well as loosely adherent cells harvested by gentle washing with PBS. In this study, we aimed to focus on the characteristics of macrophages and showed contaminated DC population in attached GM-CSF derived bone marrow cells. We also revealed GM-CSF dose-dependent formation of macrophages as well as their phagocytic abilities and pronounced IL-10 productions compared to GM-BMDCs. Our data will give relevant information to readers having interests in using GM-CSF derived macrophages.
Collectively, our results clarify the characteristics of GM-CSF grown, bone marrow derived murine macrophages. They have a MHCIIlowF4/80highCD11c+CD64+ phenotype and are efficient at phagocytosis. GM-CSF also simultaneously produces a MHCIIhighF4/80low attached DC population, but this rate of formation diminishes with increasing GM-CSF concentration. Using mixed populations in experimental settings is common; however, it may be desirable to enhance macrophage purity as much as possible. This study might give useful information for researchers using bone marrow derived macrophages.
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by Bumsuk Academic Research Fund in 2014.
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Becker L., Liu N.C., Averill M.M., Yuan W., Pamir N., Peng Y., Irwin A.D., Fu X., Bornfeldt K.E., Heinecke J.W. Unique proteomic signatures distinguish macrophages and dendritic cells. PLoS One. 2012;7:e33297. doi: 10.1371/journal.pone.0033297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Gopisetty A., Ganesh B.B., Sheng J.R., Prabhakar B.S. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J. Leukocyte Biol. 2011;89:235–249. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebon J., Layton J.E., Maher D., Morstyn G. Endogenous haemopoietic growth factors in neutropenia and infection. Br. J. Haematol. 1994;86:265–274. doi: 10.1111/j.1365-2141.1994.tb04725.x. [DOI] [PubMed] [Google Scholar]
- Cheers C., Haigh A.M., Kelso A., Metcalf D., Stanley E.R., Young A.M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect. Immun. 1988;56:247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Ranjan R., Lee Y.G., Park G.Y., Karpurapu M., Deng J., Xiao L., Kim J.Y., Unterman T.G., Christman J.W. Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL-10: implication in hyperglycemia. J. Leukocyte Biol. 2015;97:327–339. doi: 10.1189/jlb.3A0514-251R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat K., Guiton R., Guilliams M., Henri S., Baranek T., Schwartz-Cornil I., Malissen B., Dalod M. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol. Rev. 2010;234:177–198. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- Egawa M., Mukai K., Yoshikawa S., Iki M., Mukaida N., Kawano Y., Minegishi Y., Karasuyama H. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Fleetwood A.J., Lawrence T., Hamilton J.A., Cook A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- Ganesh B.B., Cheatem D.M., Sheng J.R., Vasu C., Prabhakar B.S. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int. Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier E.L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K.G., Gordonov S., et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D., Miller J., Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J., Böttcher J., Chakravarty P., Zelenay S., Huotari J., Schraml B.U., Goubau D., Reis e Sousa C. GMCSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015;42:1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Heng T.S., Painter M.W. Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- Hercus T.R., Thomas D., Guthridge M.A., Ekert P.G., King-Scott J., Parker M.W., Lopez A.F. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Deguchi M., Hagi K., Yasumizu R., Ikehara S., Muramatsu S., Steinman R.M. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc. Natl. Acad. Sci USA. 1993;90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Steinman R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Miller J.C., Brown B.D., Shay T., Gautier E.L., Jojic V., Cohain A., Pandey G., Leboeuf M., Elpek K.G., Helft J., et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic T., de Bruijn M.F., Lutz M.B., Leenen P.J. Developmental stages of myeloid dendritic cells in mouse bone marrow. Int. Immunol. 2003;15:515–524. doi: 10.1093/intimm/dxg050. [DOI] [PubMed] [Google Scholar]
- Paine R., 3rd, Morris S.B., Jin H., Wilcoxen S.E., Phare S.M., Moore B.B., Coffey M.J., Toews G.B. Impaired functional activity of alveolar macrophages from GM-CSF-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1210–1218. doi: 10.1152/ajplung.2001.281.5.L1210. [DOI] [PubMed] [Google Scholar]
- Robbins S.H., Walzer T., Dembele D., Thibault C., Defays A., Bessou G., Xu H., Vivier E., Sellars M., Pierre P., et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M., O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Satpathy A.T., Wu X., Albring J.C., Murphy K.M. Re(de)fining the dendritic cell lineage. Nat. Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S.H., Heo J.I., Hwang J.H., Na Y.R., Yun J.H., Lee E.H., Park J.W., Cho C.H. Angiopoietin-1 elicits proinflammatory responses in monocytes and differentiating macrophages. Mol Cells. 2013;35:550–556. doi: 10.1007/s10059-013-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhan Y., Lew A.M., Naik S.H., Kershaw M.H. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J. Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Harada A., Wang J.B., Zhang Y.Y., Hashimoto S., Naito M., Matsushima K. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood. 1998;92:118–128. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


