Abstract
Background:
Blood-based parameters are gaining increasing interest as potential prognostic biomarkers in patients with diffuse large B-cell lymphoma (DLBCL). The aim of this study was to comprehensively evaluate the prognostic significance of pretreatment plasma uric acid levels in patients with newly diagnosed DLBCL.
Methods:
The clinical course of 539 DLBCL patients, diagnosed and treated between 2004 and 2013 at two Austrian high-volume centres with rituximab-based immunochemotherapy was evaluated retrospectively. The prognostic influence of uric acid on overall survival (OS) and progression-free survival (PFS) were studied including multi-state modelling, and analysis of conditional survival.
Results:
Five-year OS and PFS were 50.4% (95% CI: 39.2–60.6) and 44.0% (33.4–54.0) in patients with uric acid levels above the 75th percentile of the uric acid distribution (Q3, cut-off: 6.8 mg dl−1), and 66.2% (60.4–71.5) and 59.6% (53.7–65.0%) in patients with lower levels (log-rank P=0.002 and P=0.0045, respectively). In univariable time-to-event analysis, elevated uric acid levels were associated with a worse PFS (hazard ratio (HR) per 1 log increase in uric acid 1.47, 95% CI: 1.10–1.97, P=0.009) and a worse OS (HR=1.60, 95% CI: 1.16–2.19, P=0.004). These associations prevailed upon multivariable adjustment for the NCCN-IPI score. Uric acid levels significantly improved the predictive performance of the R-IPI and NCCN-IPI scores, and in multi-state analysis, it emerged as a highly significant predictor of an increased risk of death without developing recurrence (transition-HR=4.47, 95% CI: 2.17–9.23, P<0.0001).
Conclusions:
We demonstrate that elevated uric acid levels predict poor long-term outcomes in DLBCL patients beyond the NCCN-IPI risk index.
Keywords: prognosis, DLBCL, uric acid
Diffuse large B-cell lymphoma (DLBCL) is histologically characterised by a diffuse growth pattern and diffuse nodal architectural destruction or extranodal infiltration by large B lymphoid cells. The incidence averages out 3–4 cases per 100 000 per year, increasing significantly with age from 0.3/100 000 per year in people 35–39 years old to 26.6/100 000 per year in people aged over 80 years (Tilly et al, 2012).
DLBCL is remarkably diverse in both clinical presentation and outcome, reflecting its molecular and genetic heterogeneity. This makes it challenging to stratify patients according to their risk of disease progression and death, and thus to target the right treatment modality to the right patient. Historically, clinicians and investigators have relied on prognostic schemes that imply clinical risk factors to predict the risk for disease progression, relapse and death of patients with aggressive non Hodgkin lymphoma. One of the most commonly used risk stratification models, the International Prognostic Index (IPI) for lymphomas, represented a robust clinical prognostic index for aggressive lymphomas in the pre-rituximab era. The IPI distinguishes four risk groups with different 5-year overall survival (OS), ranging from 26 to 73% (Shipp et al, 1993). In the era of rituximab treatment, a revised IPI (R-IPI) has been introduced, showing superior prediction in outcome of DLBCL patients treated with standard immunochemotherapy. The R-IPI identifies three distinct prognostic groups, with a very good (4-year OS 94%), good (OS 79%) and poor (OS 55%) outcome, respectively (Sehn et al, 2007). Recently, an advanced IPI (National Comprehensive cancer Network NCCN-IPI) was published by Zhou et al, established in patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) at 7 NCCN-cancer centres in the United States and in the British Columbia Cancer Agency (Zhou et al, 2014). This score emphasises the impact of older age and highly increased LDH levels, and also underscores the relevance of extranodal disease for prognosis. Nevertheless, a large group of patients with an unfavourable outcome remains insufficiently characterised. Sub-classifying tumours using molecular markers is associated with high monetary and man-power costs, and the techniques are not routinely performed in most routine laboratories. Therefore, the search for cheap and easily applicable markers that might help improve the prognostic accuracy of existing prognostic scores is warranted.
Uric acid is the final end product of purine metabolism, indicating a high turnover of nucleic acids in cellular elements. Therefore patients with solid cancers or haematological malignancies often present with high levels of uric acid in the blood. Importantly, a number of studies have demonstrated that elevated uric acid levels both increases the risk of cancer incidence and mortality, and correlate with a poor prognosis in several types of solid cancer, including pancreatic cancer, oesophageal cancer and colon cancer (Stotz et al, 2014; Yan et al, 2015; Chen et al, 2016). To our knowledge the exact role of uric acid level for prognostic risk stratification in DLBCL has not been established. In the present study we aimed to explore the prognostic significance of the pretreatment uric acid levels in a large middle European bi-centre study of DLBCL patients. Particular attention was paid to dissecting the complex epidemiological relationships between uric acid, disease recurrence and long-term survival, as well as the conditional OS experience of our patients according to pretreatment uric acid levels.
Patients and methods
This retrospective bi-center analysis included data from 539 consecutive patients who were histologically diagnosed with DLBCL according to the 2008 World Health Organization (Campo et al, 2011) criteria between January 2004 and April 2013. Patients were diagnosed and treated either at the Division of Hematology at the Medical University of Graz (‘Graz cohort') or at the third Medical Department at the Paracelsus Medical University Salzburg (‘Salzburg cohort'), both located in Austria. All clinico-pathological data were retrieved from medical records as well as of pathology reports from the respective institutions. The clinico-pathological parameters included in the study were histological confirmation of DLBCL; patient gender and age, the R-IPI and NCCN-IPI, and Ann Arbor tumour stage. Laboratory data, including whole blood count and uric acid levels, were obtained by pre-diagnosis exploration 1–7 days before histological diagnosis was obtained. A pretreatment plasma uric acid level is routinely measured at our institutions for all patients who undergo a consecutive chemotherapeutic treatment regimen. Patients were treated by standard R-CHOP, CHOP-like or third-generation anthracycline-containing regimens but all received the monoclonal antibody rituximab additionally to the chemotherapeutic regimen. Post-treatment surveillance included routine clinical- and laboratory examination every 3 months within the first year, 6 months for 3 years and annually thereafter. Patients were excluded in case of seropositivity for human immunodeficiency virus or missing laboratory parameters at diagnosis. Dates of death were obtained from clinical records or the central registry of the Austrian Bureau of Statistics. The study was approved by the local ethics committee of the Medical University of Graz (No. 25–434 ex 12/13) and by the Ethics Committee of the provincial government of Salzburg, Austria (415-EP/73/127–2012) (Troppan et al, 2014; Melchardt et al, 2014).
Statistical analyses
All statistical analyses were performed using Stata (Windows version 13.0, Stata Corp., Houston, TX, USA) and R (Windows version 3.2.0, The R Core Development Team, Vienna, Austria). Continuous variables were reported as medians (25th—75th percentile), and categorical variables by absolute frequencies and percentages. Correlations between two continuous variables, such as uric acid levels, were explored with Spearman's rank correlation coefficient. Rank-sum tests, Kruskal–Wallis tests, as well as multiple linear regression was applied to compare means of continuous variables between two or more groups. The median follow-up time was estimated with the reverse Kaplan–Meier method according to Schemper and Smith (Schemper and Smith, 1996).
The co-primary endpoints of the study were OS, defined as the time between DLBCL diagnosis and death-from-any-cause or censoring, and progression-free survival (PFS), defined as the time between DLBCL diagnosis and the first of the three events disease recurrence, death-from-any-cause, or censoring. OS and PFS were estimated with the Kaplan–Meier product limit estimator, and OS and PFS functions were compared between groups using log-rank tests. Time-to-death and time-to-progression-or-death were modelled with uni- and multivariable Cox models. Because the distribution of uric acid levels was highly skewed to the right (Supplementary Figure 1A), we included uric acid as a log-transformed variable in these models. Log-transformation appropriately stabilized the distribution of uric acid (Supplementary Figure 1B). Two final multivariable model for PFS and OS were derived by including all individual NCCN-IPI items and uric acid all variables. The proportional hazards assumption was assessed by a test of the slope of scaled Schoenfeld residuals. Uric acid was dichotomized into a binary variable at the 75th percentile (Q3) of its distribution. In a sensitivity analysis, a further binary uric acid variable was developed by deriving a cut-off that maximised the number of correctly classified patients under the receiver operating characteristic (ROC) curve for discriminating patients that survived and died according to uric acid levels. The predictive performance of different multivariable Cox models for predicting 5-year OS and PFS was compared using Harrell's C statistic. In the Graz cohort, we estimated the impact of progression on OS by fitting a three-state, unidirectional, semi-Markov, illness-death model (Supplementary Figure 2). In this multi-state model, we examined the differential impact of uric acid on (1) risk of progression, (2) risk of death-from-any-cause without progression, and (3) risk of death-from-any-cause after progression. The cumulative incidence of progression, accounting for competing all-cause-mortality, was estimated using competing risk cumulative incidence estimators according to Marubini & Valsecchi (Marubini et al, 1983). Landmark analysis with a landmark date 2 years after DLBCL was performed to visualise the impact of progression on OS. Finally, we analysed the relationship between uric acid levels and 3-year conditional overall (3-year COS) and conditional PFS (3-year CPFS). The 3-year CPFS and COS were defined as the probability of surviving and remaining progression-free, or surviving, respectively, for an additional 3 years at a given time point after DLBCL diagnosis.
Results
Baseline analysis
Overall, 275 (51.0%) male and 264 (49.0%) female patients with DLBCL were included in the study (Table 1). One hundred and forty-six (27.1%) patients were younger than 60 years. At diagnosis, an Ann Arbor tumour stage of stages III or IV disease was present in 261 (48.4%) patients. According to the R-IPI classifier, 46 patients (8.5%) had a very good R-IPI; 296 (54.9%) a good R-IPI; and 197 (36.5%) a poor R-IPI. Applying the NCCN-IPI classifier, 42 (7.8%) patients had low risk NCCN-IPI; 198 (36.7%) a low-intermediate risk; 218 (40.5%) a high-intermediate; and 81 (15.0%) a high risk NCCN-IPI (Table 1).
Table 1. Clinico-pathological characteristics of patients with DLBCL (n=539).
| Overall (n=539) | Uric acid<Q3 (n=404) | Uric acid⩾Q3 (n=135) | P-value | |
|---|---|---|---|---|
|
Variable | ||||
| Gender | ||||
| Male | 275 (51.0%) | 192 (47.5%) | 83 (61.5%) | – |
| Female | 264 (49.0%) | 212 (52.5%) | 52 (38.5%) | 0.005 |
| BMI (kg m-2)a | 25.4 (23.2–28.1) | 24.9 (22.6–27.2) | 27.7 (24.8–30.8) | <0.0001 |
| Age at diagnosis (years) | 68 (59–77) | 67 (56–77) | 71 (63–79) | 0.001 |
| Serum creatinine (mg dl−1) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 1.1 (0.9–1.3) | <0.0001 |
| LDH (units) | 264 (197–431) | 259 (192–411) | 303 (208–507) | 0.027 |
| Location | ||||
| Graz | 335 (62.2%) | 242 (59.9%) | 93 (68.9%) | – |
| Salzburg | 203 (37.9%) | 162 (40.1%) | 42 (31.1%) | 0.062 |
|
Individual (R-)IPI and NCCN-IPI items> | ||||
| Age category | ||||
| ⩽40 years | 43 (8.0%) | 40 (9.9%) | 3 (2.2%) | – |
| 41–60 years | 109 (20.2%) | 90 (22.3%) | 19 (14.1%) | – |
| 61–75 years | 211 (39.2%) | 152 (37.6%) | 59 (43.7%) | – |
| >75 years | 176 (32.7%) | 122 (30.2%) | 54 (40.0%) | 0.002 |
| Site | ||||
| No extranodal disease | 298 (55.3%) | 229 (56.7%) | 69 (51.1%) | – |
| Extranodal disease | 241 (44.7%) | 175 (43.3%) | 66 (48.9%) | 0.260 |
| LDH ratio | ||||
| <ULN | 219 (40.6%) | 170 (42.1%) | 49 (36.3%) | – |
| 1–3 × ULN | 269 (49.9%) | 204 (50.5%) | 65 (48.2%) | – |
| >3 × ULN | 51 (9.5%) | 30 (7.4%) | 21 (15.6%) | 0.018 |
| Clinical stage (Ann Arbor) | ||||
| I&II | 278 (51.6%) | 207 (51.2%) | 71 (52.6%) | – |
| III&IV | 261 (48.4%) | 197 (48.8%) | 64 (47.4%) | 0.785 |
| ECOG PS | ||||
| 0–2 | 455 (84.4%) | 340 (84.2%) | 115 (85.2%) | – |
| ⩾2 | 84 (15.6%) | 64 (15.8%) | 20 (14.8%) | 0.776 |
|
Prognostic indices | ||||
| R-IPI | ||||
| Very good | 46 (8.5%) | 36 (8.9%) | 54 (40.0%) | – |
| Good | 296 (54.9%) | 225 (55.7%) | 71 (52.6%) | – |
| Poor | 197 (36.6%) | 143 (35.4%) | 54 (40.0%) | 0.597 |
| NCCN-IPI | ||||
| Low | 42 (7.8%) | 33 (8.2%) | 9 (6.7%) | – |
| Low intermediate | 198 (36.7%) | 159 (39.4%) | 39 (28.9%) | – |
| High intermediate | 218 (40.5%) | 155 (38.4%) | 63 (46.7%) | – |
| High | 81 (15.0%) | 57 (14.1%) | 24 (17.8%) | 0.115 |
Abbreviations: BMI=body mass index; DLBCL=diffuse large B-cell lymphoma; ECOG PS=Eastern Cooperative Oncology Group (ECOG) performance status (PS); LDH=lactate dehydrogenase; mg/dl=milligram per decilitre; NCCN-IPI=National Comprehensive cancer network international prognostic Index; R-IPI=revised international prognostic index; ULN=upper limit of normal.
BMI was only observed in a subset of the Graz cohort (n=235).
Continuous variables such as BMI are reported as medians (25–75th percentile), whereas count data are reported as absolute frequencies (percentages). Distribution overall and by uric acid level dichotomized at its 75th percentile (Q3, cut-off: 6.8 mg dl−1).
Q3: 75th percentile of the uric acid distribution.
P: P-value from a Wilcoxon's rank-sum test. Bold indicates P<0.05.
The median uric acid level was 5.5 mg dl−1 (25–75th percentile: 4.3–6.8, Supplementary Figure 1A). Uric acid was dichotomized into a binary variable at the 75th percentile of its distribution (Q3, cut-off: 6.8 mg dl−1). On average, patients with uric acid at or above this cut-off were older (P=0.001), had a higher BMI (P<0.0001), a higher LDH (P=0.027), a higher creatinine level (P<0.0001) and had a higher probability of being male (P=0.005). However, clinical stage, R-IPI and NCCN-IPI did not significantly differ between these two groups, respectively (Table 1). Using ROC analysis, we determined a cut-off value of 9.1 mg dl−1 for uric acid levels as optimal to discriminate between patients' survival and death, as well as disease-free survival and death. This cut-off corresponded to the 93rd percentile (i.e., higher than the empirical cut-off at Q3), and correctly classified 70.7% of patients as deceased or alive, respectively.
Analysis of progression and survival outcomes
In the overall cohort, the 1-, 5- and 10-year PFS probabilities were 76.9% (95% CI: 72.9–80.4), 55.8% (50.6–60.6) and 33.4% (15.4–52.7), respectively. The corresponding 1-, 5- and 10-year OS probabilities were 83.8% (80.3–86.8), 62.4% (57.2–68.2) and 40.7% (18.1–62.4), respectively. Both the R-IPI (Harrell's C for 5-year DFS and OS: 0.63 and 0.65, respectively) and NCCN-IPI (Harrell's C for 5-year DFS and OS: 0.66 and 0.70, respectively) prognostic index excellently stratified patients according to their future risk of progression or death, although stratification was superior with the NCCN-IPI (Supplementary Figure 3A–D).
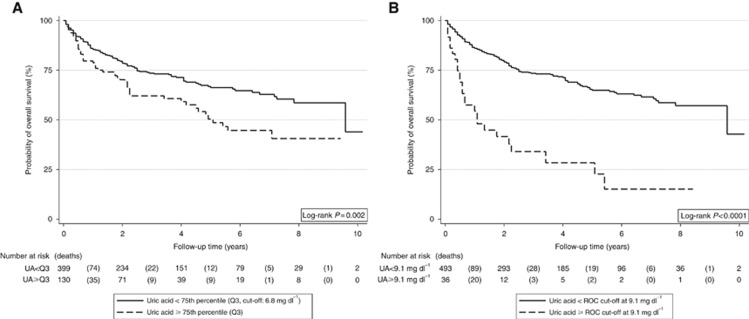
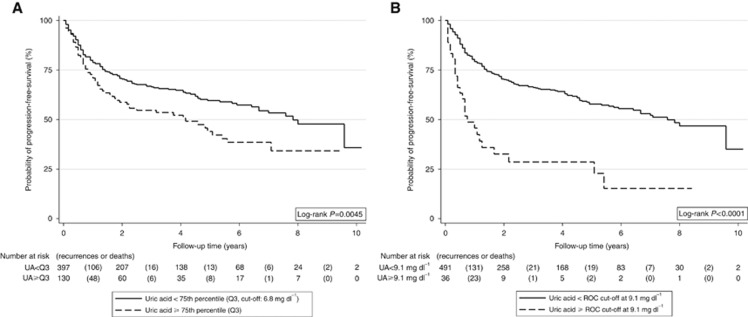
In Kaplan–Meier analysis, we detected a highly significant association between higher pretreatment uric acid levels and poor OS (Figure 1A) and PFS (Figure 2A). In detail, 5-year OS and PFS were 50.4% (95% CI: 39.2–60.6) and 44.0% (33.4–54.0) in patients with uric acid at or above the 75th percentile of its distribution, and 66.2% (60.4–71.5) and 59.6% (53.7–65.0%) in patients below this cut-off, respectively (log-rank P=0.002 and P=0.0045, respectively). Using the higher ROC-derived cut-off at the 93rd percentile, we observed even stronger differences (5-year OS: 28.4% vs 64.9%, log-rank P<0.0001, Figure 1B; 5-year PFS: 28.6% vs 57.8%, log-rank P<0.0001, Figure 2B), which suggests that the adverse impact of uric acid on PFS and OS may be primarily driven by the patients with very high pretreatment levels of uric acid.
Figure 1.
OS experience of the total cohort (n=539) according to pretreatment uric acid levels. Two different cut-offs were used for this analysis to investigate the sensitivity of our results to cut-off choice. In A, an empirical cut-off at the 75th percentile was employed, whereas in B we used a ROC-derived cut-off that maximised the number of patients that were correctly classified as alive or deceased. This cut-off corresponded to the 93rd percentile of the distribution. Abbreviation: UA=uric acid.
Figure 2.
PFS experience of the total cohort (n=539) according to pretreatment uric acid levels. Two different cut-offs were used for this analysis to investigate the sensitivity of our results to cut-off choice. In A, an empirical cut-off at the 75th percentile was employed, whereas in B we used a ROC-derived cut-off that maximised the number of patients that were correctly classified as alive or deceased. This cut-off corresponded to the 93rd percentile of the distribution. Interestingly, ROC-analysis yielded the same cut-off for overall and PFS (9.1 mg dl−1). Abbreviation: UA=uric acid.
In univariable time-to-death regression, higher pretreatment uric acid levels were associated with a worse PFS (hazard ratio (HR) for 1 log increase in uric acid=1.47, 95% CI: 1.10–1.97, P=0.009) and a worse OS (HR for 1 log increase=1.60, 95% CI: 1.16–2.19, P=0.004, Table 2). Further univariable analyses of OS identified older age (HR per 10 years increase=1.69, 95% CI: 1.47–1.94, P<0.0001)), a higher pretreatment LDH (HR per 100 units increase=1.04, 95% CI: 1.03–1.06, P<0.0001), an elevated serum creatinine (HR per 1 mg dl−1 increase=1.16, 95% CI: 1.02–1.31, P=0.024), advanced clinical stage (HR for I and II vs III and IV=1.83, 95% CI: 1.34–2.49, P<0.0001), a poor ECOG performance status (HR for PS0–2 vs PS>2=2.75, 95% CI: 1.96–3.85, P<0.0001) a high R-IPI (P<0.0001) and NCCN-IPI (P<0.0001) as poor prognostic factors for OS and PFS in this patient cohort. Similar results were observed for the univariable analysis of PFS, except that serum creatinine did not associate with PFS (Table 2). Male gender, a higher BMI and extranodal involvement did not emerge to be associated with OS or PFS.
Table 2. Univariable Cox proportional hazards models of uric acid and clinic-pathological parameters for the prediction overall and PFS in patients with DLBCL (n=539).
|
Univariable analysis of OS |
Univariable analysis of PFS |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Uric acid (per 1 log increase) | 1.60 (1.16–2.19) | 0.004 | 1.47 (1.10–1.97) | 0.009 |
| Uric acid (per 1 mg dl−1 increase) | 1.02 (1.00–1.05) | 0.031 | 1.02 (1.00–1.04) | 0.102 |
| Uric acid | ||||
| <75th percentile | 1 (ref.) | – | 1 (ref.) | – |
| ⩾75th percentile | 1.65 (1.20–2.29) | 0.002 | 1.53 (1.14–2.06) | 0.005 |
| Uric acid | ||||
| <ROC cut-off | 1 (ref.) | – | 1 (ref.) | – |
| ⩾ROC cut-off | 3.74 (2.44–5.73) | <0.0001 | 3.11 (2.05–4.70) | <0.0001 |
| BMI (per 5 kg m−2 increase) | 0.93 (0.68–1.27) | 0.647 | 0.91 (0.70–1.19) | 0.503 |
| Age at diagnosis (per 5 years increase) | 1.30 (1.21–1.39) | <0.0001 | 1.17 (1.11–1.24) | <0.0001 |
| LDH (per 100 units increase) | 1.04 (1.03–1.06) | <0.0001 | 1.04 (1.03–1.06) | <0.0001 |
| Serum creatinine (per 1 mg dl−1 increase) | 1.16 (1.02–1.31) | 0.024 | 1.10 (0.96–1.26) | 0.153 |
| Location | ||||
| Graz | 1 (ref.) | – | 1 (ref.) | – |
| Salzburg | 1.09 (0.80–1.48) | 0.586 | 0.83 (0.63–1.10) | 0.193 |
| Gender | ||||
| Female | 1 (ref.) | – | 1 (ref.) | – |
| Male | 0.86 (0.63–1.16) | 0.315 | 0.83 (0.63–1.10) | 0.193 |
| Age category | ||||
| ⩽40 years | 1 (ref.) | – | 1 (ref.) | – |
| 41–60 years | 1.91 (0.55–6.67) | 0.307 | 1.40 (0.63–3.10) | 0.408 |
| 61–75 years | 4.76 (1.49–15.15) | 0.008 | 2.40 (1.16–4.96) | 0.018 |
| >75 years | 10.86 (3.43–34.36) | <0.0001 | 4.22 (2.05–8.71) | <0.0001 |
| Site | ||||
| No extranodal disease | 1 (ref.) | – | 1 (ref.) | – |
| Extranodal disease | 1.26 (0.93–1.70) | 0.140 | 1.22 (0.93–1.60) | 0.159 |
| LDH ratio | ||||
| <ULN | 1 (ref.) | – | 1 (ref.) | – |
| 1–3 × ULN | 1.60 (1.13–2.26) | 0.008 | 1.52 (1.12–2.07) | 0.007 |
| >3 × ULN | 3.05 (1.92–4.84) | <0.0001 | 2.72 (1.77–4.17) | <0.0001 |
| Clinical stage (Ann Arbor) | ||||
| I&II | 1 (ref.) | – | 1 (ref.) | – |
| III&IV | 1.83 (1.34–2.49) | <0.0001 | 1.67 (1.27–2.20) | <0.0001 |
| ECOG PS | ||||
| 0–2 | 1 (ref.) | – | 1 (ref.) | – |
| ⩾2 | 2.75 (1.96–3.85) | <0.0001 | 2.20 (1.60–3.02) | <0.0001 |
|
Prognostic indices | ||||
| R-IPI | ||||
| Very good | 1 (ref.) | – | 1 (ref.) | – |
| Good | 7.07 (1.74–28.78) | 0.006 | 3.54 (1.44–8.69) | 0.006 |
| Poor | 14.54 (3.58–59.07) | <0.0001 | 6.54 (2.66–16.06) | <0.0001 |
| NCCN-IPI | ||||
| Low | 1 (ref.) | – | 1 (ref.) | – |
| Low intermediate | 3.65 (0.88–15.17) | 0.075 | 1.57 (0.71–3.45) | 0.263 |
| High intermediate | 9.38 (2.30–38.19) | 0.002 | 3.00 (1.39–6.47) | 0.005 |
| High | 21.32 (5.19–87.64) | <0.0001 | 6.34 (2.88–13.93) | <0.0001 |
Abbreviations: BMI=body mass index; CI=confidence interval; DLBCL=diffuse large B-cell lymphoma; ECOG PS=Eastern Cooperative Oncology Group (ECOG) performance status (PS); HR=hazard ratio; LDH=lactate dehydrogenase; mg/dl=milligram per decilitre; NCCN-IPI=National Comprehensive Cancer Network International Prognostic Index; OS=overall survival; PFS=progression-free survival; P=Wald-test P-value; ref=reference category; R-IPI=revised international prognostic index; ULN=upper limit of normal. Bold indicates P<0.05.
To determine the independent prognostic value of uric acid for OS and PFS, multivariable analyses including all individual NCCN-IPI items and creatinine levels were performed (Table 3). Here, the prognostic association between a high pretreatment uric acid and worse OS and PFS prevailed. Furthermore, high uric acid remained significantly associated with a worse OS upon adjusting for the full R-IPI and NCCN-IPI prognostic indices (Supplementary Table 1). In this analysis, we evaluated 5-year OS because this was the endpoint in the development originally leading to the R-IPI and NCCN-IPI, and observed that addition of pretreatment uric acid to these two scores slightly but statistically significantly improved the discriminative performance of these scores for 5-year OS (as indicated by Harrell's C statistic), and this was independent of whether a ROC-derived uric acid cut-off or a cut-off at the 75th percentile was chosen (Supplementary Table 1).
Table 3. Multivariable Cox proportional hazards models of uric acid, individual NCCN-IPI items, and serum creatinine for the prediction of overall and PFS in patients with DLBCL (n=539).
|
Multivariable analysis OS |
Multivariable analysis PFS |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Uric acid (per 1 log increase) | 1.54 (1.07–2.22) | 0.020 | 1.45 (1.05–2.01) | 0.025 |
| Age category | ||||
| ⩽40 years | 1 (ref.) | – | 1 (ref.) | – |
| 41–60 years | 1.97 (0.56–6.88) | 0.289 | 1.43 (0.64–3.18) | 0.385 |
| 61–75 years | 3.98 (1.24–12.74) | 0.020 | 2.10 (1.01–4.36) | 0.048 |
| >75 years | 9.31 (2.90–29.95) | <0.0001 | 3.64 (1.73–7.68) | 0.001 |
| Site | ||||
| No extranodal disease | 1 (ref.) | – | 1 (ref.) | – |
| Extranodal disease | 1.21 (0.88–1.65) | 0.242 | 1.16 (0.87–1.54) | 0.317 |
| LDH ratio | ||||
| <ULN | 1 (ref.) | – | 1 (ref.) | – |
| 1–3 × ULN | 1.22 (0.85–1.75) | 0.286 | 1.26 (0.91–1.75) | 0.157 |
| >3 × ULN | 1.87 (1.15–3.03) | 0.011 | 1.93 (1.23–3.02) | 0.004 |
| Clinical stage (Ann Arbor) | ||||
| I&II | 1 (ref.) | – | 1 (ref.) | – |
| III&IV | 1.78 (1.27–2.48) | 0.001 | 1.55 (1.15–2.09) | 0.004 |
| ECOG PS | ||||
| 0–2 | 1 (ref.) | – | 1 (ref.) | – |
| ⩾2 | 1.46 (1.00–2.12) | 0.049 | 1.40 (0.98–1.99) | 0.065 |
| Serum creatinine (per 1 mg dl−1 increase) | 1.04 (0.89–1.21) | 0.643 | 1.00 (0.85–1.17) | 0.951 |
Abbreviations: CI=confidence interval; DLBCL=diffuse large B-cell lymphoma; ECOG PS=Eastern Cooperative Oncology Group (ECOG) performance status (PS); HR=hazard ratio; LDH=lactate dehydrogenase; NCCN-IPI=National Comprehensive Cancer Network International Prognostic Index; OS=Overall survival; P=Wald-test P-value; PFS=progression-free survival. Bold indicates P<0.05.
LDH levels are indeed available for every single patient in our study, because this parameter was routinely measured within the routine pretreatment risk assessment (R-IPI, NCCN-IPI). As expected, we observed a weak correlation between LDH and uric acid (Spearman's rho=0.09, P=0.043), suggesting that cellular decay (as indicated by an elevated LDH) also contributes to an elevated uric acid level. However, what is particularly interesting is that uric acid and LDH appear to be independently associated with the outcomes under study. In detail, a multivariable Cox model for PFS predicts that after adjusting for elevated LDH (as indicated by the NCCN-IPI cut-off), 1 log increase in uric acid is associated with a 52% increase in the risk of progression or death (HR=1.52, 95% CI: 1.12–2.06, P=0.007). After adjusting for uric acid, LDH was as well associated with an increased risk of this endpoint (HR adjusted for uric acid=1.63, 95% CI: 1.32–2.01, P<0.0001). Highly similar results were observed for the OS endpoint (not shown).
Competing risk and multi-state models of progression and long-term mortality outcomes in patients with DLBCL
In the Graz cohort (n=308 with fully observed data relevant for this analysis; progressions: n=58, deaths: n=89), the cumulative 1-, 3-, 5- and 10-year incidence of progression (accounting for all-cause-mortality as a competing risk) was 8.4% (95% CI: 5.6–12.0), 17.9% (13.5–22.8), 22.4% (17.3–28.0) and 27.4% (20.0–35.4), respectively. Only three progressions occurred after 5 years of follow-up. In multi-state analysis (Supplementary Figure 2), the onset of these progression was associated with an almost 14-fold increase in the risk of death-from-any-cause (transition HR (THR)=13.66, 95% CI: 8.58–21.74, P<0.0001). In landmark analysis, 10-year OS was 14.0% in patients that developed progression within the first 2 years of follow-up, as compared to 54.9% in patients that did not develop progression within the first 2 years of follow-up (Mantel–Byar P<0.0001, Supplementary Figure 4).
Progressions occurring later during follow-up were not associated with a better prognosis than recurrences occurring earlier after diagnosis (change in THR for every year elapsed between diagnosis and onset of progression=0.92, 95% CI: 0.68–1.26, P=0.61). Including uric acid into the multi-state model, we observed that uric acid emerged as a highly significant predictor of an increased risk of death without developing progression (THR for ROC-derived variable=4.47, 95% CI: 2.17–9.23, P<0.0001), but was only weakly associated with progression (THR for ROC-derived variable=2.17, 95% CI: 0.86–5.43, P=0.099). Furthermore, uric acid did not predict survival after the onset of progression (THR for ROC-derived variable=1.39, 95% CI: 0.48–4.01, P=0.544). Interestingly, the NCCN-IPI emerged not only as a highly significant predictor of progression risk (THR per 1 category increase=1.73, 95% CI: 1.25–2.39, P=0.001) and risk of death without progression (THR per 1 category increase=2.86, 95% CI: 1.98–4.14, P<0.0001), but also as a weak predictor of post-progression survival (THR per 1 category increase=1.56, 95% CI: 1.02–2.38, P=0.042), which suggests that when progressions occur, they are more harmful in patients with a poor pretreatment NCCN-IPI.
Analysis of 3-year conditional overall (COS) and progression-free survival (CPFS)
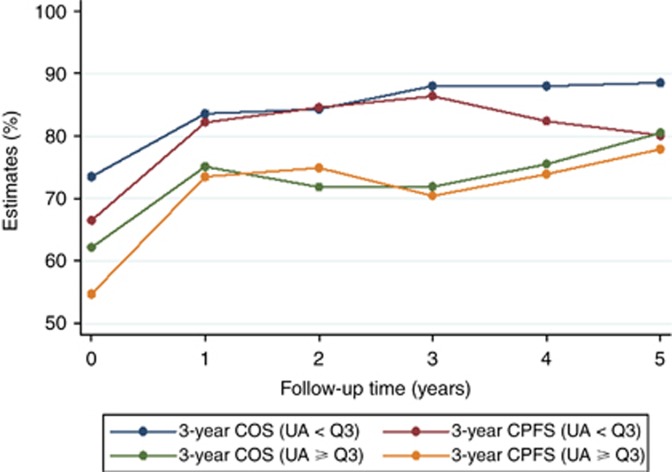
In the overall cohort, 3-year COS improved from 70.7% (95% CI: 66.2–74.2) at baseline, to 81.6% (76.9–85.4), 84.6% (78.2–89.3) and 87.0% (76.8–92.9) at 1, 3 and 5 years after diagnosis, respectively (Supplementary Figure 5). Three-year CPFS strongly improved very early on from 63.6% (59.0–67.9) at baseline to 80.2% (75.3–84.3) at 1 year after baseline, and then remained roughly constant at 82.8% (75.8–88.0), and 79.9% (0.67–88.2) at 3 and 5 years after diagnosis, respectively (Supplementary Figure 5). Improvements in 3-year COS and CPFS were also seen in patients with high (i.e., ⩾Q3) and lower (i.e., <Q3) pretreatment uric acid. However, both 3-year COS and CPFS were lower at baseline in patients with uric acid ⩾Q3, and COS consistently remained below the improvements in COS observed in patients with uric acid <Q3 (Figure 3). Although patients with uric acid ⩾Q3 never reached the COS of patients with uric acid <Q3, CPFS became similar between patients with higher and lower pretreatment uric acid that had remained free from progression and death for at least 5 years after diagnosis. This suggests a persistent adverse impact of elevated pretreatment uric acid on progression- and mortality-outcomes in long-term survivors of DLBCL, which slowly improves over time. The strongest improvements in 3-year COS and 3-year CPFS were seen in patients who had a poor-risk NCCN-IPI at baseline (Supplementary Figure 6).
Figure 3.
Three-year conditional overall and PFS experience of the total cohort (n=539) according to pretreatment uric acid levels. The empirical cut-off at the 75th percentile (Q3) was used for this analysis. Patients with elevated pretreatment uric acid levels (⩾Q3) initially had a worse 3-year conditional progression- and OS experience, which improved over time, and approached the 3-year COS and 3-year CPFS experience of patients with uric acid levels <Q3 after around 5 years of follow-up. Abbreviations: COS=conditional OS, CPFS=conditional PFS; UA=uric acid, Q3–75th percentile.
Discussion
In this large bi-center study on patients with DLBCL treated with first-line rituximab-based chemo-immunotherapy, we observed that uric acid plasma levels at the time of diagnosis present a significant and independent prognostic marker for PFS and OS, as well as impairment for conditional survival. Beyond this, uric acid improved the predictive ability of established prognosis scores including the R-IPI and the NCCN-IPI, as well as represented a significant factor in multi-stage analysis for recurrence-free survival.
Despite all recent efforts to elucidate the origins of DLBCL, using gene expression profiling, chromosomal aberration analysis and differential microRNA expression (Alizadeh et al, 2000; Shepshelovich et al, 2015), traditional prognostic indices like the R-IPI or the NCCN-IPI, which are constructed of routinely measured clinico-laboratory parameters, remain the standard tool to classify patients into distinct prognostic risk categories. However, there remain subgroups of patients, who fail to be correctly classified with these already existing risk scores. An addition of parameters, especially easy and cheap determinable parameters, may help to better classify those patient subgroups. Uric acid that is routinely measured since many years without any additional laborious effort has been proven to have prognostic utility in various solid cancers (Szkandera et al, 2014; Stotz et al, 2014; Yan et al, 2015; Chen et al, 2016). Regardless of the type of solid cancer, these studies showed that elevated pretreatment uric acid levels have a negative impact on PFS and OS of patients.
The predictive impact of uric acid levels after chemotherapy was shown by Selcukbiricik et al (2016). That study demonstrated a correlation of high uric acid levels with improved outcome after the application of bevacizumab in colorectal cancer, which supports the role of uric acid as an easily available marker for cell turnover. Nevertheless, the authors concluded that the predictive role of uric acid should be further investigated in other studies.
In cancer, high uric acid levels are caused by high cell turn over and/or tumour lysis syndrome demonstrating the rapid cell metabolism of purine-containing nucleic acids from tumour cells (Hochberg and Cairo, 2008). Especially in aggressive haematological malignancies, a high cell turnover in the pretreatment setting, as well as during the first courses of chemotherapy, characterises the disease. Therefore, one might see unsteadiness in the absolute levels while using this biomarker.
Establishing a routine under consideration of this drawback is key to making this easily and generally available, low-price biomarker, represent an advantage. A structured sequence of sample processing, as performed in the two centres routinely, adds confidence and is still inexpensive compared with the pitfalls with pre-analytics of molecular marker analysis.
By following a routinely used sample processing procedure, instability of laboratory parameters can be minimised (Redrup et al, 2016). Uric acid has been proven to be relatively stable at varying time (up to 72 h) and temperature (4 °C or 23 °C; Marjani, 2006) of sample storage. Another study showed a slight increase in uric acid levels, but only after storage longer than 30 h in tubes without gel barrier (Cuhadar et al, 2012). In the two centres, included in this study, a prompt sample processing on the same day with delays no longer than 4 h can be guaranteed and therefore a bias regarding specimen instability most likely excluded.
In 1982, uric acid was hypothesised to provide a primary defence against cancer because of its capacity to purify singlet oxygen and its efficiency to inhibit lipid peroxidation (Hille, 2005). Nonetheless, elevated values of serum urate, the dominant monosodium form of uric acid, were found to exhibit strong statistical association with increased premature cancer death in both men and women. This suggests a more complex role for uric acid in cancer biology than being a general antioxidant (Petersson and Trell, 1983; Terkeltaub, 2010; Fini et al, 2012). Normal uric acid levels are usually between 2.4 and 7.0 mg dl−1. Stark hyperuricemia (uric acid levels >7.0 mg dl−1) can reach levels of 14 mg dl−1 or higher and is associated with an increased risk for gout and acute kidney injury arising by the deposition of urate crystals in the kidney (Terkeltaub, 2010).
However, there are also indications out of old prospective studies, suggesting that uric acid elevation may precede detection or coincide with the development of cancer. After adjusting for various confounding factors, Levine et al observed that uric acid measured prospectively at the start of the study—before the development of cancer—was significantly associated with all site cancer mortality, for those patients developing cancer in the observation period of over more than 10 years of follow-up, in women aged 55–64. Therefore, the study authors concluded that hyperuricemia does not develop secondarily to the development of cancer (Levine et al, 1989).
To the best of our knowledge, this is the first study evaluating the impact of uric acid levels in DLBCL. Five hundred and thirty-nine patients with DLBCL of two independent Austrian centres were included in this retrospective analysis. In this large retrospective cohort with excellent data quality, we could clearly demonstrate a statistically significant and clinically important difference in OS and PFS for patients with increased uric acid levels prior to treatment. Comparing the empirical cut-off at the 75th percentile with the higher ROC-derived cut-off at the 93rd percentile of the uric acid distribution, we could observe that the magnitude of the deterioration in OS and PFS with higher pretreatment uric acid levels was mainly attributable to the 10% or so patients with the highest uric acid levels. Importantly, we also used linear specifications of the uric acid variable (continuous untransformed and loge-transformed), and the results were also robust with these parameterizations. Relevant for investigating how uric acid levels contribute to worse outcomes, we could not only evaluate the impact of uric acid on OS and PFS, but also dissect the complex relationships between uric acid, progression and death using a multi-state model. Multi-state models have recently been shown to be able to clarify complex prognostic relationships between biomarkers, intermediate events and death (Posch et al, 2016), and are therefore very appropriate for this study on the prognostic potential of uric acid. In our multi-state model, it appeared that uric acid was most relevant for predicting death. Although the relative risk of progression with elevated uric acid was high (as indicated by the HR), uric acid was only weakly associated with progression, which could be related to the smaller sample size we had available for this subtype of analysis (Graz cohort only). Further, uric acid at baseline did not appear to predict survival after the occurrence of progression in our multi-state analysis. This finding was in contrast to the NCCN-IPI, where patients with an unfavourable NCCN-IPI prognostic index at baseline did worse after the onset of progression than patients with a more favourable index. This analysis adds a novel feature to the epidemiology of the NCCN-IPI, namely the prognostic potential of this score towards not only OS but also post-progression survival. Clinically, these results can be interpreted in the sense that progressions occurring in patients that have a poor-risk NCCN-IPI at baseline are more ‘harmful' than progressions occurring in patients with better risk NCCN-IPI risk indices. Looking at conditional survival outcomes of our cohort, both COS and CPFS improved over time. This is important information for counselling DLBCL patients, because we could show that given DLBCL patients survive progression-free for 5 years, their long-term survival outcomes were excellent. Patients with poor-risk NCCN-IPI at baseline experienced the highest improvements in conditional progression and survival outcomes. These improvements were also seen in patients with an elevated uric acid level at baseline, whose initially adverse PFS and OS experience considerably improved over time. Nevertheless, these patients showed consistently worse conditional survival outcomes in comparison to patients with lower uric acid levels at baseline, with their conditional outcomes only approaching the lower uric acid patients after around 5 years after diagnosis. This suggests that an elevated pretreatment uric acid negatively affects long-term outcomes in DLBCL in a comprehensive manner.
A major limitation of this study is that we cannot provide a separate cohort to externally validate uric acid as a biomarker for risk stratification in DLBCL. We therefore refrain from premature conclusions that this biomarker should be incorporated into presently available risk stratification rules. Although our study population comprises two separate cohorts, external validation in more populations will also be necessary for proposing a generalisable cut-off that defines an elevated uric acid level in the DLBCL setting. Thus, the two cut-offs used in this study (empirical and ROC-derived) should be regarded as tentative and exploratory with respect to our primary study question. Finally, we want to highlight that although we showed statistically significant improvements in risk prediction when adding pretreatment uric acid to the NCCN-IPI, it is now well understood that definitive ‘added benefit' regarding prediction upon inclusion of a novel biomarker to an established risk score needs should only be declared when an integrative analysis using clinically relevant net reclassification statistics and/or decision curve analysis has been performed (Kerr et al, 2014).
However, these analyses would represent separate stand-alone projects beyond the scope of this manuscript.
In conclusion, this study indicates for the first time that a high uric acid level is a negative prognostic factor regarding OS, PFS, COS and CDFS in DLBCL patients. These data allow us to carefully hypothesise that uric acid may add prognostic information to the present armamentarium of clinical risk prediction tools in DLBCL. Further studies should focus on (1) externally validating pretreatment uric acid levels for risk stratification of patients with DLBCL, (2) defining a generalisable cut-off for elevated uric acid levels in this setting and (3) demonstrating improvements in risk prediction with uric acid according to a clinically meaningful reclassification of patients into higher or lower risk strata.
Acknowledgments
KTT and TM designed the study and collected the data, with the help of KS, AD and LW. FP, TG and MP performed the statistical analysis and the research. CBS, DN and EK contributed by performing the pathological investigations. KTT and TM wrote the manuscript with the help of PN, AE, RG, MP and FP.
None of the contributing authors has any conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tra,n T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511. [DOI] [PubMed] [Google Scholar]
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES (2011) WHO classification of tumours of hematopoietic and lymphoid tissues. Blood 117: 5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Li Q, Chen DT, Pan JH, Chen YH, Wen ZS, Zeng WA (2016) Prognostic value of pre-operative serum uric acid levels in esophageal squamous cell carcinoma patients who undergo R0 esophagectomy. Cancer Biomark 17: 89–96. [DOI] [PubMed] [Google Scholar]
- Cuhadar S, Atay1 A, Koseoglu M, Dirican A, Hur A (2012) Stability studies of common biochemical analytes in serum separator tubes with or without gel barrier subjected to various storage conditions. Biochem Med 22: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini MA, Elias A, Johnson RJ, Wright RM (2012) Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R (2005) Molybdenum-containing hydroxylases. Arch Biochem Biophys 433: 107–116. [DOI] [PubMed] [Google Scholar]
- Hochberg J, Cairo MS (2008) Tumor lysis syndrome: current perspective. Haematologica 93: 9–13. [DOI] [PubMed] [Google Scholar]
- Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS (2014) Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology 25: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Staudt LM (2010) Aggressive lymphomas. N Engl J Med 362: 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine W, Dyer AR, Shekelle RB, Schoenberger JA, Stamler J (1989) Serum uric acid and 11.5-year mortality of middle-aged women: findings of the Chicago Heart Association Detection Project in Industry. J Clin Epidemiol 42: 257–267. [DOI] [PubMed] [Google Scholar]
- Marjani A (2006) Effect of storage time and temperature on some serum analytes. Internet J Lab Med 2: 1–6. [Google Scholar]
- Marubini E, Morabito A, Valsecchi MG (1983) Prognostic factors and risk groups: some results given by using an algorithm suitable for censored survival data. Stat Med 2: 295–303. [DOI] [PubMed] [Google Scholar]
- Melchardt T, Troppan K, Weiss L, Hufnagl C, Neureiter D, Tränkenschuh W, Hopfinger G, Magnes T, Deutsch A, Neumeister P, Hackl H, Greil R, Pichler M, Egle A (2014) A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and β2 -microglobulin. Br J Haematol 168: 239–245. [DOI] [PubMed] [Google Scholar]
- Petersson B, Trell E (1983) Raised serum urate concentration as risk factor for premature mortality in middle aged men: relation to death from cancer. Br Med J 287: 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch F, Riedl J, Reitter EM, Kaider A, Zielinski C, Pabinger I, Ay C (2016) Hypercoagulabilty, venous thromboembolism, and death in patients with cancer. A Multi-State Model. Thromb Haemost 115: 817–826. [DOI] [PubMed] [Google Scholar]
- Redrup MJ, Igarashi H, Schaefgen J, Lin J, Geisler L, Ben M'Barek M, Ramachandran S, Cardoso T, Hillewaert V (2016) Sample management: recommendation for best practices and harmonization from the global bioanalysis consortium harmonization team. AAPS J 18: 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17: 343–346. [DOI] [PubMed] [Google Scholar]
- Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109: 1857–1861. [DOI] [PubMed] [Google Scholar]
- Selcukbiricik F, Kanbay M, Solak Y, Bilici A, Kanıtez M, Balık E, Mandel NM (2016) Serum uric acid as a surrogate marker of favorable response to bevacizumab treatment in patients with metastatic colon cancer. Clin Transl Oncol; e-pub ahead of print 19 January 2016; doi:10.1007/s12094-016-1485-1. [DOI] [PubMed]
- Shepshelovich D, Ram R, Uziel O, Kushnir M, Lithwick-Yanai G, Hoshen M, Feinmesser M, Bairey O, Lahav M (2015) MicroRNA signature is indicative of long term prognosis in diffuse large B-cell lymphoma. Leuk Res 39: 632–637. [DOI] [PubMed] [Google Scholar]
- Shipp MA et al on behalf of The International Non-Hodgkin's Lymphoma Prognostic Factors Project (1993) A predictive model for aggressive Non-Hodgkin's Lymphoma. N Engl J Med 329: 987–994. [DOI] [PubMed] [Google Scholar]
- Stotz M, Szkandera J, Seidel J, Stojakovic T, Samonigg H, Reitz D, Gary T, Kornprat P, Schaberl-Moser R, Hoefler G, Gerger A, Pichler M (2014) Evaluation of uric acid as a prognostic blood-based marker in a large cohort of pancreatic cancer patients. PLoS One 18: e104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkandera J, Gerger A, Liegl-Atzwanger B, Stotz M, Samonigg H, Ploner F, Stojakovic T, Gary T, Leithner A, Pichler M (2014) Uric acid levels in blood are associated with clinical outcome in soft-tissue sarcoma patients. Clin Chem Lab Med 53: 493–597. [DOI] [PubMed] [Google Scholar]
- Terkeltaub R (2010) Update on gout: new therapeutic strategies and options. Nat Revies Rheumatol 6: 30–38. [DOI] [PubMed] [Google Scholar]
- Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, André M, Pfreundschuh M, Dreyling M ESMO Guidelines Working Group (2012) Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23: 78–82. [DOI] [PubMed] [Google Scholar]
- Troppan K, Deutsch A, Gerger A, Stojakovic T, Beham-Schmid C, Wenzl K, Neumeister P, Pichler M (2014) The derived neutrophil to lymphocyte ratio is an independent prognostic factor in patients with diffuse large B-cell lymphoma. Br J Cancer 110: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Zhang P, Xu W, Liu Y, Wang B, Jiang T, Hua C, Wang X, Xu D, Sun B (2015) Serum uric acid increases risk of cancer incidence and mortality: a systematic review and meta-analysis. Mediators Inflamm 2015: 764250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN (2014) An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.