Abstract
No conclusive information is available about the relation between the consumption of whole grains and the risk of mortality. We aimed to conduct a meta-analysis of prospective cohort studies to summarize the relation between whole-grain intake and risk of mortality from all causes, cardiovascular disease, and total and specific cancers. A systematic search of the literature published earlier than March 2015 was conducted in Medline and PubMed, SCOPUS, EMBASE, and Cochrane Library to identify relevant articles. Prospective cohort studies that examined the association of total whole-grain intake or specific whole-grain foods with risk of mortality from all causes, cardiovascular disease, and total and specific cancers were considered. Twenty prospective cohort studies were included in the systematic review: 9 studies reported total whole-grain intake and 11 others reported specific whole-grain food intake. In a follow-up period of 5.5 to 26 y, there were 191,979 deaths (25,595 from cardiovascular disease, 32,746 from total cancers, and 2671 from specific cancers) in 2,282,603 participants. A greater intake of both total whole grains and specific whole-grain foods was significantly associated with a lower risk of all-cause mortality in the meta-analysis. The pooled RR for all-cause mortality for an increase of 3 servings total whole grains/d (90 g/d) was 0.83 (95% CI: 0.79, 0.88). Total whole-grain intake (0.84; 95% CI: 0.76, 0.93) and specific whole-grain foods (0.82; 95% CI: 0.75, 0.90) were also associated with a reduced risk of mortality from cardiovascular disease. Each additional 3 servings total whole grains/d was associated with a 25% lower risk of mortality from cardiovascular disease. An inverse association was observed between whole-grain intake and risk of mortality from total cancers (0.94; 95% CI: 0.91, 0.98). We found an inverse association between whole-grain intake and mortality from all causes, cardiovascular disease, and total cancers.
Keywords: whole grain, mortality, meta-analysis, dose-response, cancer, cardiovascular
Introduction
High intake of whole-grain foods has been suggested as a key component of healthy eating for longevity (1). Whole grains—including dark bread, whole-grain breakfast cereal, popcorn, cooked oatmeal, wheat germ, brown rice, and bran—contain endosperm, germ, and bran, in contrast to refined grains, which contain only the endosperm and lose the germ and bran during the milling process (2). Compared with refined grains, whole grains contain higher amounts of dietary fiber, magnesium, phytochemicals, and other functional compounds (2, 3). In both developed and developing countries, cardiovascular events and cancer are the main causes of mortality (4). Therefore, dietary factors that are inversely associated with the incidence of these chronic conditions might also be associated with reduced mortality and help people live longer. Whole-grain intake has been linked inversely with the incidence of cardiovascular disease (CVD) and several cancers (5, 6).
Two meta-analyses of cohort studies (7, 8) reported the inverse associations of total dietary fiber intake with all-cause mortality. In addition, another recent meta-analysis of cohort studies (9) reported that cereal fiber intake was associated with lower risk of all-cause, CVD, and cancer mortality. Some, but not all (10–12), earlier investigations showed an inverse association between whole-grain intake and mortality. Several studies documented that consumption of whole-grain foods was associated with a lower risk of mortality (13–16), including death from cardiovascular events (15) and cancer (16). However, some others reported no significant association with all-cause mortality (10), or suggested an inverse association with cardiovascular mortality but not with cancer mortality (15). In addition, there has been a sex difference in the association of whole-grain intake and mortality. Whereas some studies reported a protective association in women (17), others reported such a relation in men, but not in women (18). However, most studies addressing the possible contribution of whole-grain consumption to mortality included relatively few cases of death, which limits the statistical power of any individual study to detect the associations. Furthermore, no information is available about the dose-response relation between consumption of whole grains and risk of mortality. Assessing the link between whole-grain intake and mortality is important for guiding consumer choices and setting and prioritizing dietary guidelines to reduce the risk. In the current study, we conducted a systematic review and meta-analysis of prospective cohort studies to summarize the relation between whole-grain intake and risk of mortality from all causes, CVD, and total and specific cancers. We hypothesized that whole-grain intake was associated with reduced risk of death from all causes, CVD, and cancer.
Methods
Search strategy.
We followed the Meta-Analysis of Observational Studies in Epidemiology for performing and reporting the current meta-analysis. Prospective cohort studies that examined the association of total whole-grain intake or specific whole-grain foods with risk of mortality from all causes, CVD, total cancers, and specific cancers were considered in this meta-analysis. A systematic search of the literature published earlier than March 2015 was conducted in Medline and PubMed, SCOPUS, EMBASE, and Cochrane Library by 2 independent investigators (SB-K and PS) to identify relevant articles. The following keywords were used in our search strategy: (“whole grain” or “whole-grain” or “whole-grains” or “oat” or “grains” or “cereals” or “whole wheat” or “brown rice” or “barley”) and (“mortality" or “fatal" or “death" or “survive" or “survival"). All keywords were selected from the MeSH database. No restrictions in terms of the language of publications and time were imposed. In addition, a manual search of references of the published papers was performed to find other relevant articles. Duplicate citations were then removed. The full text of related articles was obtained, in some cases through contacting the corresponding author.
Eligibility criteria.
Studies were included in the current meta-analysis if they met the following criteria: 1) they were cohort studies that had considered intake of total whole-grain or specific whole-grain foods as an exposure and mortality from all causes, CVD, total cancers, or specific cancers as the outcomes of interest; and 2) they had provided estimates of RRs, HRs, or rate ratios with corresponding 95% CIs. To identify eligible articles, we used a 2-step selection process. Two independent investigators (SB-K and PS) conducted an initial screening of all titles or abstracts and then assessed all potentially relevant papers on the basis of full text reviews. Studies that met our inclusion criteria were included in the analysis. In case of disagreements, the principal investigator (AE) was consulted.
Excluded studies.
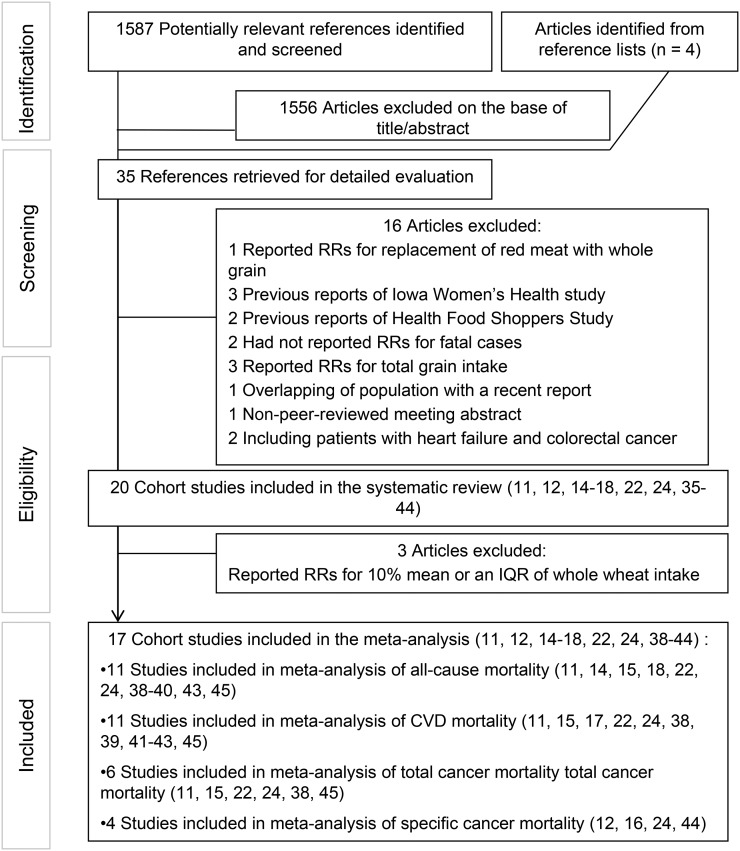
We excluded letters, comments, reviews, meta-analyses, ecological studies, and animal studies from the analysis. In total, 1587 articles were found in our initial search and 4 others were retrieved from hand-searching of reference lists. We excluded 1556 articles after reading the title and abstract. The other 16 papers were excluded for the following reasons: a study by Pan et al. (19) was not included in the current analysis because the risk of mortality was given for the replacement of 1 serving of meat with whole-grain consumption. From the Iowa Women’s Health study, 4 different reports (13, 20–22) were published that assessed whole-grain intake in relation to mortality; we included the last report of this study (22) for all-cause, CVD, and cancer mortality because it had the greatest number of deaths. Therefore, 3 previous reports from that study (13, 20, 21) were excluded from the analysis to avoid double counting data. In addition, of the 3 different reports from the Health Food Shoppers Study (10, 23, 24), we included the most recent one by Appleby et al. (24) and excluded 2 previous publications (10, 23). Two studies (25, 26) that did not separate risk ratios for fatal myocardial infarction (MI) from nonfatal MI also were excluded from this study. The study by Lo et al. (27) was not included because these researchers considered grain consumption (including whole grains, refined grains, and legumes) rather than whole grains individually. Three studies (28–30) were excluded because of the inclusion of both refined and whole grains as “cereals” in their analyses. The study by He et al. (31), which was performed on diabetic patients from the Nurses’ Health Study, was excluded because the included population in that study overlapped with that of the most recent study by Wu et al. (15). The study by Wengreen et al. (32) was also excluded because it was a non-peer-reviewed meeting abstract. We also did not include 2 studies (33, 34) conducted on patients with heart failure (34) and colorectal cancer (33) in the analysis, because subjects in these studies were not representative of the general population and they might have changed their diets after diagnosis of the disease. Three studies (35–37) that reported RRs for whole-grain intake as a continuous variable were excluded from the meta-analysis; however, these studies were included in the systematic review. Required information (RRs for highest compared with lowest intake of whole grain or RRs for fatal MI) for these studies could not be obtained even by contacting the authors. Finally, 20 cohort studies (11, 12, 14–18, 22, 24, 35–45) were included in the systematic review and 17 prospective cohort studies (11, 12, 14–18, 22, 24, 38–45) were included in this meta-analysis: 11 for all-cause mortality (11, 14, 15, 18, 22, 24, 38–40, 43, 45), 11 for CVD mortality (11, 15, 17, 22, 24, 38, 39, 41–43, 45), 6 for total cancer mortality (11, 15, 22, 24, 38, 45), and 4 for specific cancers mortality (12, 16, 24, 44) (Figure 1). Of the 20 studies included in the systematic review, 9 reported intake of total whole grain (11, 12, 14, 15, 22, 35, 37, 43, 45), and 11 others reported the intake of specific whole-grain foods (16–18, 24, 36, 38–42, 44).
FIGURE 1.
Flow diagram of search strategy and study selection process. CVD, cardiovascular disease; IQR, interquartile range.
In our dose-response analysis for all-cause mortality, 4 studies were not included because of the use of whole-grain intake as a dichotomous variable (e.g., greater than or equal to median compared with less than median) (18, 28, 40) or because they did not report the number of deaths in each category of whole-grain intake (43). Two other studies (38, 39) were also excluded because they considered consumption of specific whole-grain foods instead of total whole-grain intake. After these exclusions, 5 studies (11, 14, 15, 22, 45) remained in this dose-response analysis. For CVD mortality as an outcome, we did not include 5 studies in the dose-response meta-analysis because of the use of whole-grain intake as a dichotomous variable (24, 42) or lack of information about the number of deaths in each category (11, 17, 43). Therefore, 6 studies [3 for total whole grains (15, 22, 45) and 3 for specific whole-grain foods (38, 39, 41)] remained for this dose-response analysis. Regarding cancer mortality, 5 studies were not included in the dose-response meta-analysis because they considered specific whole-grain foods rather than total whole-grain intake (38, 44), considered specific cancer rather than total cancer (44), treated whole-grain intake as a dichotomous variable (24), did not report the number of deaths in each category of whole-grain intake (11, 16), or considered whole-grain intake in a days-per-week unit without reporting the quantity of intake in each category (12). Finally, 3 studies (15, 22, 45) were included to examine dose-response association between total whole-grain intake and mortality from total cancers. For mortality from specific cancers, dose-response analysis was not performed because of lack of adequate publications.
Data extraction.
Data extraction was conducted with a standardized data collection form. The primary exposure variable was consumption of total whole grains; however, we also examined intake of specific whole-grain foods when these foods were reported as the main source of whole-grain intake by investigators. According to published studies, specific whole-grain food intake included the consumption of whole-wheat bread, whole-meal bread, whole-grain bread, whole-grain cereals, whole-grain breakfast cereals, breakfast cereals, rye bread, and rye product. All the analyses were stratified based on exposure (total whole-grain intake or consumption of specific whole-grain food) to obtain the relations with total whole-grain intake and specific whole-grain foods separately. Outcomes of interest in the current study were mortality from all causes, CVD, and total and specific cancers.
The following information was extracted by 2 independent reviewers (SB-K and PS): the first author’s last name, date of publication, country, participants’ age range, sex, sample size, number of cases, duration of follow-up, method of assessment of whole-grain intake, comparisons, ascertainment of outcomes, HRs or RRs for all-cause mortality and for cause-specific mortality, and variables that entered into the multivariable model as potential confounders. In case of disagreements, the principal investigator (AE) was consulted. Discrepancies were resolved by consensus.
Assessment of methodologic quality.
The quality of included studies was examined by using the Newcastle–Ottawa Scale specific methods for cohort studies (46). The Newcastle–Ottawa Scale assigns a maximum of 9 points to each cohort study: 4 for selection and assessment of exposure, 2 for comparability, and 3 for assessment of outcomes. When a study got more than median stars, it was considered to be relatively high quality (or low risk of bias); otherwise, it was deemed to be low quality (or high risk of bias). Any discrepancies were resolved by discussion. Results from a quality assessment of studies included in the meta-analysis are presented in Supplemental Table 1.
Statistical methods.
RRs and HRs (and 95% CIs) for comparison of the highest and lowest categories of intake of total whole grains or specific whole-grain foods were used to calculate log RR ± SE. The analyses were performed with the use of a random-effects model in which we calculated both Q-statistic and I2 as indicators of heterogeneity. In case of significant between-study heterogeneity, we performed subgroup analysis to find possible sources of heterogeneity. Between-subgroup heterogeneity was examined through fixed-effects modeling. Publication bias was examined by visual inspection of funnel plots. Formal statistical assessment of funnel plot asymmetry was also done with the use of Egger’s regression asymmetry test and Begg’s test. A trim-and-fill method was used to detect the effect of missing studies in the overall effect of meta-analysis. We also conducted a sensitivity analysis in which each prospective cohort study was excluded to examine the influence of that study on the overall estimate.
We used a previously described method by Greenland and Orsini (47) for the dose-response analysis. The natural logs of the RRs and CIs across categories of total whole-grain intake or specific whole-grain foods were used to compute study-specific slopes (linear trends) and 95% CIs. In this method, the distribution of cases or person-years and the RRs with the variance estimates for ≥3 quantitative categories of exposure were required. We assigned the median or mean amount of total whole-grain intake or specific whole-grain foods in each category to the corresponding RR for each study. For studies that reported the intake as ranges, we estimated the midpoint in each category by calculating the mean of the lower and upper bound. When the highest category was open-ended, the length of the open-ended interval was assumed to be the same as that of the adjacent interval. When the lowest category was open-ended, the lower boundary was set to zero. For 2 studies (11, 15) that reported the total whole-grain intake as grams per day, we used 30 g as a serving size for recalculation of the intake to a common scale (servings daily) (48). We used restricted cubic splines (3 knots at fixed percentiles of 10%, 50%, and 90% of the distribution) to examine potential nonlinear dose-response associations between whole-grain consumption and risk of mortality (49, 50). Statistical analyses were conducted with the use of STATA version 12.0. P values < 0.05 were considered to be statistically significant for all tests, including Cochran’s Q test.
Results
Findings from the systematic review.
Characteristics of the 20 cohort studies included in the systematic review are shown in Table 1. Of the 20 cohort studies published between 1982 and 2015, 11 were conducted in the United States (12, 14–16, 22, 39, 40, 42–45), 2 in 7 different countries (35, 36), 1 in Norway (38), 1 in the United Kingdom (24), and the remaining 5 in Denmark (18), Netherlands (37), Spain (11), Finland (41), and China (17). The number of participants in these studies ranged from 535 to 970,045, with an age range from 16 to 98 y. In total, 2,282,603 participants were included in the 20 studies we considered. Of the 20 studies, 11 papers reported RRs for all-cause mortality, 11 publications for CVD mortality, 6 papers for total cancer mortality, and 4 reports for specific cancer mortality. All publications used FFQs for dietary assessment except for the studies by Appleby et al. (24), which used a short questionnaire, and the studies by Sahyoun et al. (43) and Jansen et al. (35, 36), which used dietary records. During the follow-up periods ranging from 5.5 to 26 y, the total number of deaths from all causes was 101,979; total numbers for CVD and cancer were 25,595 and 35,417, respectively. In total, 14 studies (11, 12, 15–18, 24, 37, 38, 40, 42–45) reported the estimates for both sexes combined; of those, 5 studies (12, 15, 17, 18, 37) also reported HRs for men and women separately. Five reports (16, 35, 36, 39, 41) studied only men, and 1 study (22) considered only women. Of 11 studies (11, 14, 15, 18, 22, 24, 37–40, 43, 45) with all-cause mortality as the main outcome, 6 studies (14, 22, 24, 38, 39, 45) found a protective association with whole-grain intake, and 4 others (11, 37, 40, 43) did not find a significant association. Another study found an inverse association in men, but not in women (18). One study reached an inverse association in women, but not in men (15). Of 11 publications (11, 15, 17, 22, 24, 38, 39, 41–43, 45) that reported RRs for CVD mortality, 6 studies (15, 22, 38, 39, 41, 43) reached an inverse association and 4 papers did not find any association (11, 24, 42, 45). One study found an inverse association in women, but not in men (17). Of 11 studies that reported RRs for cancer mortality (11, 12, 15, 16, 22, 24, 35, 36, 38, 44, 45), 3 studies reported a protective association (16, 44, 45), and 8 others did not find any significant association (11, 12, 15, 22, 24, 35, 36, 38).
TABLE 1.
Characteristics of cohort studies included in the systematic review1
| Study authors, year (ref) | Cohort name | Country | Age,2 y | Sex | Sample size, n | Cases, n | Duration of follow-up, y | Person-years | Exposure assessment | Outcome | Exposure/ comparison | HR or RR (95% CI) | Adjustments3 |
| Huang et al., 2015 (45) | NIH-AARP Diet and Health Study | United States | 50–71 | M/F | 367,442 | 46,067 | 14 | 5,148,760 | 124-item FFQ | All-cause mortality | Whole grains/ Q5 vs. Q1 (1.20 vs. 0.13 oz/d) | 0.94 (0.90, 0.97) | 1–7 |
| 11,283 | CVD mortality | 0.95 (0.88, 1.03) | |||||||||||
| 19,043 | Cancer mortality | 0.93 (0.88, 0.99) | |||||||||||
| Wu et al., 2015 (15) | Health Professionals Follow-Up Study | United States | 40–75 | M | 43,744 | 11,814 | 24 | 1,798,063 | 61-item FFQ | All-cause mortality | Whole grains/ Q5 vs. Q1 (47.8 vs. 5.9 g/d) | 0.95 (0.89, 1.00) | 1–7 |
| 3621 | CVD mortality | 0.84 (0.75, 0.93) | |||||||||||
| 3921 | Cancer mortality | 0.95 (0.86, 1.05) | |||||||||||
| Nurses′ Health Study | United States | 30–55 | F | 74,341 | 15,106 | 26 | 2,727,006 | 131-item FFQ | All-cause mortality | Whole grains/Q5 vs. Q1 (33.0 vs. 4.2 g/d) | 0.88 (0.84, 0.93) | ||
| 2989 | CVD mortality | 0.86 (0.76, 0.96) | |||||||||||
| 5964 | Cancer mortality | 0.99 (0.91, 1.07) | |||||||||||
| Buil-Cosiales et al., 2014 (11) | PREDIMED study | Spain | 55–75 | M/F | 7216 | 425 | 5.9 | 42,465 | 137-item FFQ | All-cause mortality | Whole grains/ quartile 4 vs. quartile 1 (84 vs. 0 g/d) | 0.92 (0.64, 1.33) | 1–7 |
| 103 | CVD mortality | 0.73 (0.34, 1.58) | |||||||||||
| 169 | Cancer mortality | 0.75 (0.40, 1.41) | |||||||||||
| Rebello et al., 2014 (17) | Singapore Chinese Health Study | Singapore | 45–74 | M | 23,501 | 1022 | 15 | 804,433 | 165-item FFQ | IHD mortality | Whole-wheat bread/T3 vs. T1 | 0.94 (0.66, 1.33) | 1–7 |
| F | 29,968 | 638 | (1.0 vs. 0.0 slice/d) | 0.51 (0.30, 0.89) | |||||||||
| van den Brandt, 2011 (37) | Netherlands Cohort Study | Netherlands | 55–69 | M | 58,279 | 6329 | 10 | NR | 150-item FFQ | All-cause mortality | Whole grains/ IQR (10.6 g/d) | 1.01 (0.99, 1.02) | 1–3, 5, 7 |
| F | 62,573 | 3362 | (13.5 g/d) | 1.00 (0.98, 1.03) | |||||||||
| Olsen et al., 2011 (18) | The Diet, Cancer and Health study | Denmark | 50–64 | M | 23,274 | 2383 | 12 | NR | 192-item FFQ | All-cause mortality | Rye bread ≥63 vs. <63 g/d | 0.84 (0.75, 0.94) | 1–7 |
| F | 27,016 | 1743 | 0.90 (0.80, 1.01) | ||||||||||
| Tognon et al., 2011 (40) | Gerontological and Geriatric Population Studies | Sweden | 60–74 | M/F | 1037 | 630 | 8.5 | NR | FFQ | All-cause mortality | Whole-grain cereals/ ≥median vs. <median (F: 74.2 g/d; M: 92.8 g/d) | 0.85 (0.73, 1.00) | 2, 5, 3 |
| Jacobs et al., 2007 (22) | The Iowa Women’s Health Study | United States | 55–69 | F | 27,312 | 5552 | 17 | 454,942 | 127-item FFQ | All-cause mortality | Whole grains/ Q5 vs. Q1 (≥19 vs. <3.5 servings/wk) | 0.79 (0.72, 0.87) | 1–7 |
| 1900 | CVD mortality | 0.73 (0.62, 0.86) | |||||||||||
| 1034 | CHD mortality | 0.72 (0.57, 0.90) | |||||||||||
| 414 | Stroke mortality | 0.85 (0.60, 1.21) | |||||||||||
| 2099 | Cancer mortality | 0.89 (0.77, 1.04) | |||||||||||
| Sahyoun et al., 2006 (43) | — | United States | 60–98 | M/F | 535 | NR | 12–15 | NR | Food record | All-cause mortality | Whole grains/ quartile 4 vs.quartile 1 (2.90 vs. 0.31 serving/d) | 0.82 (0.52, 1.28) | 1–7 |
| 89 | CVD mortality | 0.48 (0.25, 0.96) | |||||||||||
| Liu et al., 2003 (39) | The Physicians’ Health Study | United States | 40–84 | M | 86,190 | 3114 | 5.5 | NR | FFQ | All-cause mortality | Whole-grain breakfast cereals/ ≥1 serving/d vs. rarely | 0.83 (0.73, 0.94) | 1–6 |
| 1381 | CVD mortality | 0.80 (0.66, 0.97) | |||||||||||
| 488 | MI mortality | 0.71 (0.51, 0.98) | |||||||||||
| 146 | Stroke-mortality | 1.41 (0.85, 2.34) | |||||||||||
| Steffen et al., 2003 (14) | Atherosclerosis Risk in Communities Study | Washington | 45–64 | M/F | 11,940 | 867 | 11 | NR | 66-item FFQ | All-cause mortality | Whole grains/ Q5 vs. Q1 (3.0 vs. 0.1 serving/d) | 0.77 (0.61, 0.97) | 1, 2, 3, 4, 5, 7 |
| Appleby et al., 2002 (24) | Health Food Shoppers Study | United Kingdom | 16–79 | M/F | 10,741 | 2529 | 18–24 | 213,000 | Questionnaire | All-cause mortality | Whole-meal bread/ daily vs. <daily | 0.89 (0.82, 0.98) | 1, 3, 6 |
| 605 | IHD mortality | 0.86 (0.72, 1.03) | |||||||||||
| 356 | Cerebrovascular mortality | 0.89 (0.70, 1.13) | |||||||||||
| 680 | Cancer mortality | 1.01 (0.85, 1.20) | |||||||||||
| 81 | Lung cancer mortality | 1.08 (0.67, 1.76) | |||||||||||
| 100 | Colorectal cancer mortality | 1.21 (0.76, 1.93) | |||||||||||
| 40 | Stomach cancer mortality | 0.84 (0.42, 1.67) | |||||||||||
| 41 | Prostate cancer mortality | 1.24 (0.59, 2.57) | |||||||||||
| 39 | Pancreas cancer mortality | 0.86 (0.43, 1.73) | |||||||||||
| 90 | Breast cancer mortality | 1.22 (0.75, 1.97) | |||||||||||
| Jacobs et al., 2001 (38) | Norwegian County Study | Norway | 35–56 | M/F | 33,848 | 2058 | 6 | 488,500 | 66-item FFQ | All-cause mortality | Whole grain bread/ Q5 vs. Q1 (2.25–5.40 vs. 0.05–0.60 slice/d) | 0.75 (0.65, 0.88) | 1, 2, 3, 5, 6, 7 |
| 758 | CVD mortality | 0.77 (0.60, 0.98) | |||||||||||
| 553 | CHD mortality | 0.76 (0.56, 1.02) | |||||||||||
| 870 | Cancer mortality | 0.79 (0.62, 1.02) | |||||||||||
| McCullough et al., 2001 (12) | Cancer Prevention Study II cohort | United States | 56 | M | 436,654 | 910 | 14 | NR | 32-item FFQ | Stomach cancer mortality | Whole grains/ T3 vs. T1 (>4 vs. <1 d/wk) | 0.90 (0.77, 1.06) | 1, 2, 3, 6 |
| 55 | F | 533,391 | 439 | (>4.5 vs. <1 d/wk) | 0.97 (0.77, 1.24) | ||||||||
| Breslow et al., 2000 (44) | The National Health Interview Survey | United States | 18–87 | M/F | 20,195 | 158 | 8.5 | 162,558 | 59-item FFQ | Lung cancer mortality | Breakfast cereals/ quartile 4 vs. quartile 1 (>6 vs. <0.5 serving/wk) | 0.50 (0.30, 0.90) | 1, 3 |
| Jansen et al., 1999 (35) | Seven Countries Study | 7 countries | 40–59 | M | 12,763 | NR | 25 | NR | Food record | Stomach cancer mortality | Whole grains/ 10% of the mean intake (18.6 g) | 0.99 (0.95, 1.03) | 3, 7 |
| Jansen et al., 1999 (36) | Seven Countries Study | 7 countries | 40–59 | M | 12,763 | 162 | 25 | NR | Food record | Colorectal cancer mortality | Whole-grain bread/10% of the mean intake (17.8 g) | 0.98 (0.95–1.01) | 7 |
| Pietinen et al., 1996 (41) | Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | Finland | 50–69 | M | 21,930 | 581 | 6.1 | 126,970 | 279-item FFQ | CHD mortality | Rye product/ Q5 vs. Q1 (172.2 vs. 16.0 g/d) | 0.75 (0.58, 0.88) | 1–7 |
| Fraser et al., 1992 (42) | The Adventist Health Study | California | NR | M/F | 31,208 | 260 | 6 | NR | FFQ | Fatal CHD | Whole wheat bread vs. white bread (NR) | 0.89 (0.60, 1.33) | 1, 3, 5, 6 |
| Thun et al., 1992 (16) | Cancer Prevention Study II | United States | 57 | M | 337,505 | 611 | 6 | NR | 32-item FFQ | Colorectal cancer mortality | High-fiber grains/ Q5 vs. Q1 (NR) | 0.72 (0.52, 0.99) | 2, 5, 6, 7 |
AARP, American Association of Retired Persons; CHD, coronary heart disease; CVD, cardiovascular disease; IHD, ischemic heart disease; MI, myocardial infarction; NR, not reported; PREDIMED, Prevención con Dieta Mediterránea; Q, quintile; ref, reference; T, tertile.
Values are means or ranges.
For adjustments, 1 = age, 2 = BMI, 3 = smoking, 4 = alcohol consumption, 5 = physical activity, 6 = other dietary variables or nutrients, and 7 = total energy intake.
All studies except 4 (18, 35, 36, 40) adjusted for age (n = 15). Most cohorts controlled for some conventional risk factors, including BMI (n = 15), smoking (n = 18), and alcohol consumption (n = 10). Some studies also adjusted for physical activity (n = 15) and energy intake (n = 13) or other dietary variables or nutrients (n = 14). An assessment of study quality of 17 studies, which were included in the meta-analysis, yielded a median score of 6; 8 studies had a score of ≥7.
Findings from the meta-analysis on whole-grain consumption and all-cause mortality.
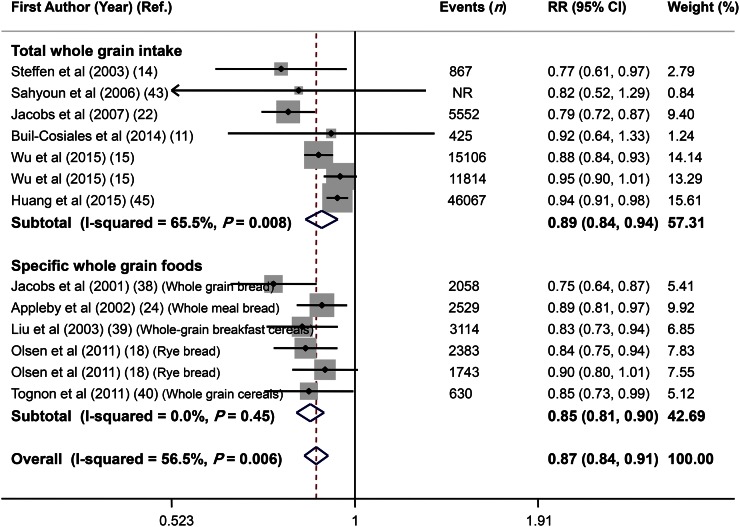
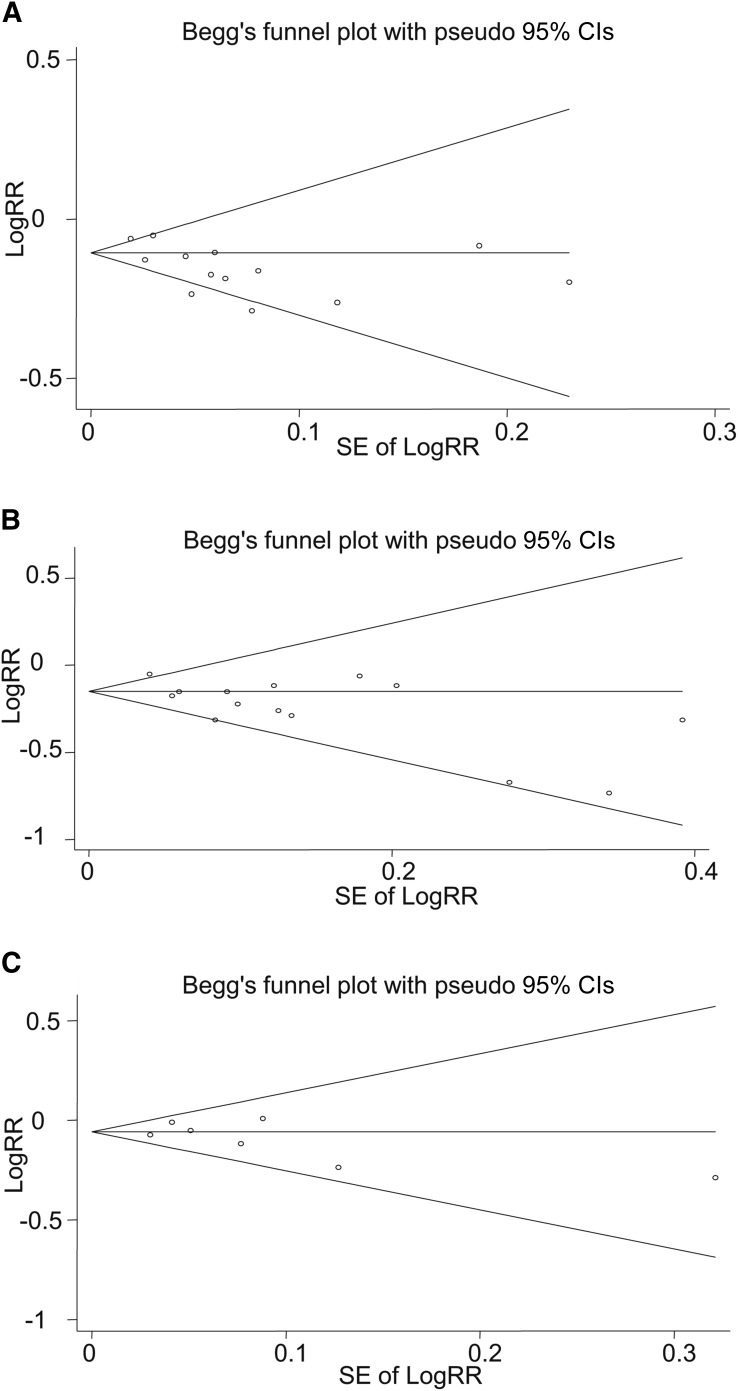
Overall, combining 13 effect sizes from 11 studies (11, 14, 15, 18, 22, 24, 38–40, 43, 45) that included 714,636 participants and 92,288 cases of death revealed that high consumption of whole grains (a combination of total whole grains and specific whole-grain foods) was inversely associated with all-cause mortality (RR: 0.87; 95% CI: 0.84, 0.91) (Figure 2), with a moderate between-study heterogeneity (I2 = 56.5%, P = 0.006). In subgroup analysis based on exposure (total whole-grain intake compared with specific whole-grain foods), we found significant inverse associations between both total whole-grain intake (RR: 0.89; 95% CI: 0.84, 0.94) and specific whole-grain food consumption (RR: 0.85; 95% CI: 0.81, 0.90) and all-cause mortality; the heterogeneity was significant for total whole-grain intake (I2 = 65.5%, P = 0.008), but not for specific whole-grain food consumption (I2 = 0.0%, P = 0.45) (Figure 2). Further subgroup analyses were done separately for total whole-grain intake and specific whole-grain foods according to study quality, duration of follow-up, sex, dietary assessment tools, and location (Table 2). Subgroup analysis based on study quality revealed a significant inverse association for both high-quality and low-quality studies for total whole-grain intake (high-quality studies: RR: 0.85; 95% CI: 0.73, 0.98; I2 = 85.0%, P = 0.001; low-quality studies: RR: 0.91; 95% CI: 0.86, 0.96; I2 = 24.4%, P = 0.27) and for specific whole-grain foods (high quality studies: RR: 0.87; 95% CI: 0.80, 0.94; I2 = 0%, P = 0.41; low-quality studies: RR: 0.84; 95% CI: 0.78, 0.90; I2 = 0%, P = 0.29). Subgroup analysis by other variables revealed no alteration in the findings across subgroups. Findings from sensitivity analysis revealed that the exclusion of any single study from the analysis did not alter the overall association (range of summary estimates: 0.84–0.91). Although no asymmetry was seen in Begg’s funnel plot (P = 0.39), findings from the Egger’s test (P = 0.02) rejected our null hypothesis about publication bias. Trim and fill did not change the overall effect. (Figure 3A).
FIGURE 2.
Forest plot of the association between whole-grain intake and all-cause mortality, stratified by exposure (total whole-grain intake compared with specific whole-grain foods). Combining 13 multivariable-adjusted RRs from 11 prospective cohort studies that included 714,636 participants and 92,288 cases of death, with the use of a random-effects model, revealed that high consumption of whole grains (combination of total whole grains and specific whole-grain foods) was inversely associated with all-cause mortality (RR: 0.87; 95% CI: 0.84, 0.91). Ref., reference.
TABLE 2.
Subgroup analysis for whole-grain intake and all causes mortality in prospective cohort studies
| Reference | Effect Size,1 n | I2, % | Q-test2 | RR (95%CI) | Pbetween3 | |
| Total whole-grain intake | ||||||
| Overall | 11, 14, 15, 22, 43, 45 | 7 | 65.5 | 0.01 | 0.89 (0.84, 0.94) | |
| Quality score4 | 0.82 | |||||
| High score (>median: 7) | 14, 22, 45 | 3 | 85.0 | 0.001 | 0.85 (0.73, 0.98) | |
| Low score (≤median: 7) | 11, 15, 43 | 4 | 24.4 | 0.27 | 0.91 (0.86, 0.96) | |
| Duration of follow-up | 0.96 | |||||
| ≥10 y | 14, 15, 22, 43, 45 | 6 | 71.3 | 0.004 | 0.89 (0.84, 0.94) | |
| <10 y | 11 | 1 | — | — | 0.92 (0.64, 1.33) | |
| Sex | 0.005 | |||||
| Women | 15, 22 | 2 | 74.2 | 0.05 | 0.84 (0.76, 0.93) | |
| Men | 15 | 1 | — | — | 0.95 (0.90, 1.01) | |
| Both | 11, 14, 43, 45 | 4 | 3.3 | 0.38 | 0.93 (0.88, 0.98) | |
| Dietary assessment tool | 0.64 | |||||
| FFQ | 11, 14, 15, 22, 45 | 6 | 70.9 | 0.004 | 0.89 (0.84, 0.94) | |
| Other tools | 43 | 1 | — | — | 0.82 (0.52, 1.29) | |
| Location | 0.96 | |||||
| United States | 14, 15, 22, 43, 45 | 6 | 71.3 | 0.004 | 0.89 (0.84, 0.94) | |
| Not United States | 11 | 1 | — | — | 0.92 (0.64, 1.33) | |
| Specific whole-grain foods | ||||||
| Overall | 18, 24, 38, 39, 40 | 6 | 0 | 0.45 | 0.85 (0.81, 0.90) | |
| Quality score4 | 0.62 | |||||
| High score (>median: 7) | 18 | 2 | 0 | 0.41 | 0.87 (0.80, 0.94) | |
| Low score (≤median: 7) | 24, 38, 39, 40 | 4 | 0 | 0.29 | 0.84 (0.78, 0.90) | |
| Duration of follow-up | 0.12 | |||||
| ≥10 y | 18, 24 | 3 | 0 | 0.65 | 0.88 (0.83, 0.93) | |
| <10 y | 38, 39, 40 | 3 | 0 | 0.47 | 0.81 (0.75, 0.88) | |
| Sex | 0.59 | |||||
| Women | 18 | 1 | — | — | 0.90 (0.80, 1.01) | |
| Men | 18, 39 | 2 | 0 | 0.89 | 0.84 (0.77, 0.91) | |
| Both | 24, 38, 40 | 3 | 45.1 | 0.16 | 0.84 (0.76, 0.93) | |
| Dietary assessment tool | 0.28 | |||||
| FFQ | 18, 38, 39, 40 | 5 | 0 | 0.47 | 0.84 (0.79, 0.89) | |
| Other tools | 24 | 1 | — | — | 0.89 (0.81, 0.97) | |
| Location | 0.42 | |||||
| United States | 39, 40 | 2 | 0 | 0.82 | 0.84 (0.76, 0.92) | |
| Not United States | 18, 24, 38 | 4 | 32.7 | 0.22 | 0.85 (0.80, 0.92) |
RRs or HRs for comparison of the highest and lowest categories of intake of total whole grains or specific whole grain.
Q-test or P-heterogeneity within subgroups with the use of a random-effects model.
P-heterogeneity between subgroups with the use of a fixed-effects model.
Based on the Newcastle–Ottawa Scale criteria (46).
FIGURE 3.
Begg’s funnel plots (with pseudo 95% CIs) in RRs compared with SEs of RR. All-cause mortality (A), cardiovascular disease mortality (B), and total cancer mortality (C).
Five studies (11, 14, 15, 22, 45) were included in the dose-response analysis of total whole-grain intake and risk of all-cause mortality, with 79,831 cases of death in 531,995 participants. The summary RR of all-cause mortality for an increase of 3 servings total whole grains/d (90 g/d) was 0.83 (95% CI: 0.79, 0.88) (I2 = 56%, P < 0.001). We did not find a nonlinear relation between consumption of total whole grains and risk of all-cause mortality (Pnonlinearity = 0.09), but a steeper reduction in risk was seen when increasing intake from low levels to ≤1 serving/d. Although the risk was reduced when increasing intake from >1 serving/d, the slope was slightly flattening (Supplemental Figure 1).
Findings from the meta-analysis on whole-grain intake and CVD mortality.
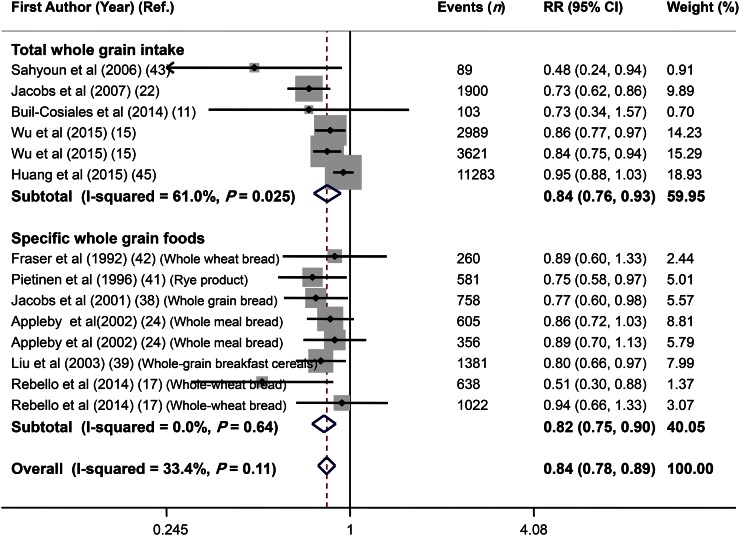
Overall, 14 effect sizes from 11 studies (11, 15, 17, 22, 24, 38, 39, 41–43, 45) were extracted for this association. These studies included a total of 757,966 participants; among them, 25,595 incident death cases were reported. Combining the estimates reported, we found that high whole-grain intake was associated with a 16% lower risk of cardiovascular mortality (RR: 0.84; 95% CI: 0.78, 0.89) (Figure 4). There was no evidence of between-study heterogeneity (I2 = 33.4%, P = 0.11). Subgroup analysis by exposure revealed that intake of both total whole grains (RR: 0.84; 95% CI: 0.76, 0.93) and specific whole-grain foods (RR: 0.82; 95% CI: 0.75, 0.90) was inversely associated with CVD mortality (Figure 4). No single study influenced the final association (range of summary estimates: 0.78–0.89). Findings from Begg’s test did not reject the null hypothesis of publication bias (P = 0.35); however, findings from Egger’s test (P = 0.01) rejected this hypothesis (Figure 3B). Trim and fill was applied, and filling added no study to the funnel plot, indicating a low degree of asymmetry and no change in the overall effect.
FIGURE 4.
Forest plot of the association between whole-grain intake and cardiovascular disease mortality, stratified by exposure (total whole-grain intake compared with specific whole-grain foods). Combining 14 multivariable-adjusted RRs from 11 prospective cohort studies that included 757,966 participants and 25,595 cases of death revealed that high whole-grain intake was inversely associated with risk of cardiovascular disease mortality (RR: 0.84; 95% CI: 0.78, 0.89). Ref., reference.
Findings from subgroup analyses based on CVD type, study quality, duration of follow-up, sex, dietary assessment tools, and study location are provided in Table 3. In these analyses, we found no significant association between total whole-grain intake and specific whole-grain foods and risk of mortality from stroke; however, significant inverse associations were detected for mortality from CVD, coronary heart disease, and ischemic heart disease. Subgroup analyses by other variables revealed no alteration in the findings.
TABLE 3.
Subgroup analysis for whole-grain intake and mortality from CVD in prospective cohort studies1
| Reference | Effectsize,2 n | I2, % | Q-test3 | RR (95%CI) | Pbetween4 | |
| Total whole-grain intake | ||||||
| Overall | 11, 15, 22, 43, 45 | 6 | 61.0 | 0.03 | 0.84 (0.76, 0.93) | |
| CVD type5 | 0.26 | |||||
| CVD | 11, 15, 22, 43, 45 | 6 | 61.0 | 0.03 | 0.84 (0.76, 0.93) | |
| CHD | 22 | 1 | — | — | 0.72 (0.57, 0.90) | |
| IHD | — | — | — | — | — | |
| Stroke | 22 | 1 | — | — | 0.85 (0.60, 1.21) | |
| Quality score6 | 0.64 | |||||
| High score (>median: 6) | 15, 22, 43, 45 | 5 | 68.3 | 0.01 | 0.84 (0.75, 0.94) | |
| Low score (≤median: 6) | 11 | 1 | — | — | 0.73 (0.34, 1.57) | |
| Duration of follow-up | 0.64 | |||||
| ≥10 y | 15, 22, 43, 45 | 5 | 68.3 | 0.01 | 0.84 (0.75, 0.94) | |
| <10 y | 11 | 1 | — | — | 0.73 (0.34, 1.57) | |
| Sex | 0.05 | |||||
| Women | 15, 22 | 2 | 60.8 | 0.11 | 0.80 (0.68, 0.94) | |
| Men | 15 | 1 | — | — | 0.84 (0.75, 0.94) | |
| Both | 11, 43, 45 | 3 | 53.7 | 0.12 | 0.77 (0.51, 1.17) | |
| Dietary assessment tools | 0.08 | |||||
| FFQ | 11, 15, 22, 45 | 5 | 58.9 | 0.05 | 0.85 (0.78, 0.94) | |
| Other tools | 43 | 1 | — | — | 0.48 (0.24, 0.94) | |
| Location | 0.64 | |||||
| United States | 15, 22, 43, 45 | 5 | 68.3 | 0.01 | 0.84 (0.75, 0.94) | |
| Not United States | 11 | 1 | — | — | 0.73 (0.34, 1.57) | |
| Specific whole-grain foods | ||||||
| Overall | 17, 24, 38, 39, 41, 42 | 8 | 0 | 0.64 | 0.82 (0.75, 0.90) | |
| CVD type5 | 0.36 | |||||
| CVD | 38, 39 | 2 | 0 | 0.81 | 0.79 (0.68, 0.98) | |
| CHD | 38, 41 | 2 | 0 | 0.95 | 0.75 (0.62, 0.92) | |
| IHD | 17, 24, 39, 42 | 5 | 14.8 | 0.32 | 0.81 (0.70, 0.95) | |
| Stroke | 24, 39 | 2 | 61.4 | 0.11 | 1.06 (0.68, 1.64) | |
| Quality score6 | 0.52 | |||||
| High score (>median: 6) | 17, 38 | 3 | 41.9 | 0.18 | 0.76 (0.58, 1.00) | |
| Low score (≤median: 6) | 24, 39, 41, 42 | 5 | 0 | 0.86 | 0.83 (0.75, 0.92) | |
| Duration of follow-up | 0.39 | |||||
| ≥10 y | 17, 24 | 4 | 22.3 | 0.28 | 0.85 (0.72, 0.99) | |
| <10 y | 38, 39, 41, 42 | 4 | 0 | 0.91 | 0.79 (0.70, 0.89) | |
| Sex | 0.19 | |||||
| Women | 17 | 1 | — | — | 0.51 (0.30, 0.88) | |
| Men | 17, 39, 41 | 3 | 0 | 0.60 | 0.81 (0.70, 0.93) | |
| Both | 24, 38, 42 | 4 | 0 | 0.84 | 0.85 (0.75, 0.95) | |
| Dietary assessment tools | 0.29 | |||||
| FFQ | 17, 38, 39, 41, 42 | 6 | 0 | 0.55 | 0.79 (0.70, 0.88) | |
| Other tools | 24 | 2 | 0 | 0.82 | 0.87 (0.75, 1.00) | |
| Location | 0.96 | |||||
| United States | 39, 42 | 2 | 0 | 0.64 | 0.82 (0.69, 0.97) | |
| Not United States | 17, 24, 38, 41 | 6 | 0 | 0.42 | 0.82 (0.74, 0.91) |
CHD, coronary heart disease; CVD, cardiovascular disease; IHD, ischemic heart disease.
RRs or HRs for comparison of the highest and lowest categories of intake of total whole grains or specific whole grain.
Q-test or P-heterogeneity within subgroups with the use of a random-effects model.
P-heterogeneity between subgroups with the use of a fixed-effects model.
This analysis includes reported effect sizes for both CVD and CVD subtypes from Rautiainen et al. (26), Jacobs et al. (38), and Liu et al. (39).
Based on the Newcastle–Ottawa Scale criteria (46).
Six studies (15, 22, 38, 39, 41, 45) were included in the dose-response analysis: 3 studies for total whole-grain intake (15, 22, 45), with 54,577 deaths in 512,839 participants, and 3 reports for specific whole-grain foods consumption (38, 39, 41), with 2720 cases of death in 141,968 participants. The summary RR for CVD mortality with an increase of 3 servings total whole grains/d (90 g/d) was 0.75 (95% CI: 0.68, 0.83). We did not find a nonlinear relation between consumption of total whole grains and CVD mortality (Pnonlinearity = 0.24). The overall RR for CVD mortality with an increase of 3 servings specific whole-grain foods/d was 0.83 (95% CI: 0.76, 0.91) (I2 = 0%, P = 0.86), with a significant nonlinear relation (Pnonlinearity = 0.04) (Supplemental Figure 2).
Findings from the meta-analysis on whole-grain consumption and total cancer mortality.
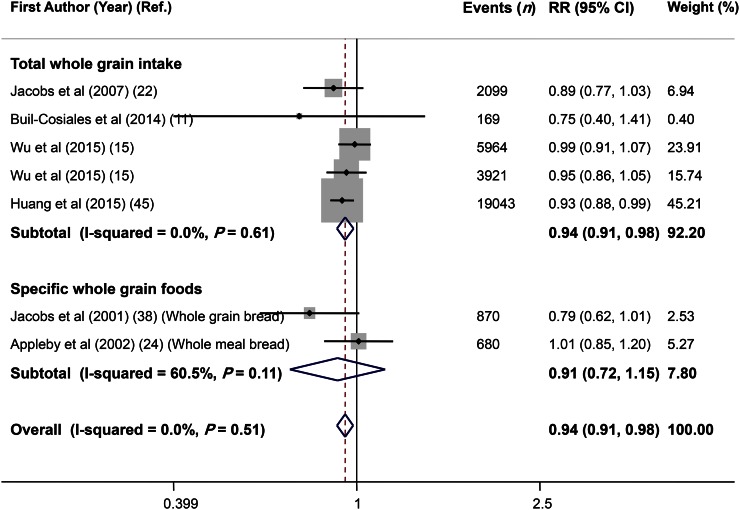
Combining 7 effect sizes from 6 studies (11, 15, 22, 24, 38, 45) that included 564,644 participants and 32,746 deaths revealed no significant association with total cancer mortality (RR: 0.94; 95% CI: 0.91, 0.98) and no evidence of heterogeneity (I2 = 0.0%, P = 0.51) (Figure 5). Subgroup analysis by exposure (total whole-grain intake compared with specific whole-grain foods) indicated a significant association between total cancer mortality and total whole-grain intake (RR: 0.94; 95% CI: 0.91, 0.98), but no significant association with specific whole-grain foods (RR: 0.91; 95% CI: 0.72, 1.15) (Figure 5). Excluding any single study did not affect this finding (range of summary estimates: 0.91–0.98). No asymmetry was seen in funnel plot and findings from the Begg’s test, and the Egger’s test did not reject our null hypothesis about publication bias (Begg’s test: P = 0.29; Egger’s test: P = 0.35) (Figure 3C).
FIGURE 5.
Forest plot of the association between whole-grain intake and total cancer mortality, stratified by exposure (total whole-grain intake compared with specific whole-grain foods). Combining 7 multivariable-adjusted RRs from 6 prospective cohort studies that included 564,644 participants and 32,746 cases of death revealed a significant association between whole-grain intake and total cancer mortality (RR: 0.94; 95% CI: 0.91, 0.98). Ref., reference.
In our dose-response analysis for total whole-grain intake, the summary RR for total cancer mortality in 3 included studies (15, 22, 45) with 30,991 cases of death in 512,839 participants was 0.90 (95% CI: 0.83, 0.98), meaning that an increase of 3 servings total whole grains/d was associated with a lower risk of cancer mortality. A nonlinear association between whole-grain intake and risk of cancer mortality was not found (Pnonlinearity = 0.22) (Supplemental Figure 3).
Findings from the meta-analysis on whole-grain intake and mortality from specific cancers.
Combining 3 effect sizes for stomach cancer, 2 effect sizes for lung cancer, and 2 others for colorectal cancer provided from 4 studies (12, 16, 28, 44) that included 1,338,486 participants and 2339 deaths, we found an overall summary RR of 0.92 (95% CI: 0.81, 1.05) for mortality from stomach cancer, 0.74 (95% CI: 0.35, 1.58) for mortality from lung cancer, and 0.91 (95% CI: 0.55, 1.50) for mortality from colorectal cancer. There was moderate evidence of between-study heterogeneity in the case of lung and colorectal cancer (for stomach cancer, I2 = 0.0%, P = 0.85; for lung cancer, I2 = 76.5%, P = 0.04; and for colorectal cancer, I2 = 69.0%, P = 0.07). Further analysis based on exposure (total whole-grain intake compared with specific whole-grain foods) was not possible because of the limited number of studies.
Discussion
Findings from the current meta-analysis supported the hypothesis that high consumption of whole grains was associated with a lower risk of mortality from all causes, CVD, and total cancers. However, no significant association was found between total whole-grain intake and mortality from specific cancers. An increase of 3 servings total whole grains/d was associated with a 17% lower risk of mortality from all causes, a 25% lower risk of mortality from CVD, and a 10% reduced risk of total-cancer mortality.
Similar to our findings, accumulating evidence from observational studies has shown significant inverse associations between whole-grain intake and risk of incident CVD (51–53). A meta-analysis of 7 cohort studies also revealed that greater whole-grain intake (2.5 servings/d compared with 0.2 servings/d) was associated with a 21% lower risk of CVD events (5). However, the Diet and Reinfarction Trial, the only clinical trial of dietary fiber intake in 2033 male survivors of MI, did not show a lower rate of coronary and total deaths with increased fiber intake during the 2 y of follow-up after MI (54). It is worth noting that this study was limited by the short duration of follow-up, poor compliance with the dietary intervention, and the generalizability of the patient population.
We found a significant inverse association between total whole-grain intake and risk of mortality from total cancers. This finding is consistent with a meta-analysis of prospective cohort and nested case-control studies that documented that 10 g cereal fiber/d was associated with a 10% reduction in risk of developing colorectal cancer and an increase of 3 servings whole grains/d was related to a 17% reduced risk of colorectal cancer (48). Some other previous observational studies also showed significant inverse associations between whole-grain intake and risk of incident cancer (55, 56). Studies that used plasma alkylresorcinol concentrations as a biomarker of whole-grain intake further demonstrated the beneficial effects of a high intake of whole grains on the risk of colon and rectal cancer (57).
The phrase “total whole grain” in earlier studies has been defined in different ways. Some studies defined whole grains as a grain product with a higher-than-X proportion from whole grains, whereas others, such as a study by Wu et al. (15), measured whole-grain intake as grams of whole grains, thus avoiding the need for an arbitrary cutoff to define whole-grain foods. In the NIH–American Association of Retired Persons Diet and Health Study (45), whole-grain foods were defined as those sources containing ≥25% whole grains and/or bran (including ready-to-eat cereals, high-fiber cereals, other fiber cereals, whole-grain breads or dinner rolls, cooked cereal, popcorn, pancakes, waffles, French toast or crepes, rice or other cooked grains, bagels, English muffins, tortillas, pasta, crackers, chips, cookies or brownies, sweet pastries, and pies); whereas in the Cancer Prevention Study II (12), whole-grain intake included brown rice, whole wheat or barley, bran or corn muffins, and oatmeal, shredded wheat, or bran cereals. Although whole-grain intake was considered to be the main exposure in the present meta-analysis, we did not limit the included studies to those with a specific definition of whole-grain intake, because the number of studies were few. A serving of whole grain also may mean something different in different studies. In addition, some studies assessed specific whole-grain foods rather than total whole-grain intake. Although some of these studies considered these specific foods as the main source of whole grain, it was impossible for us to make sure that the vast majority of whole grain was from these sources in some cases. These points should be considered carefully when interpreting our results. Applying a unified definition and serving size for whole-grain intake in future studies might be helpful in comparing their findings.
We included observational studies in this meta-analysis that could lead to considerable debate over the validity of our findings, because there was necessarily a concern that the observational studies were likely to be subject to unidentified sources of confounding and risk modification. Although we critically appraised the quality of studies with the use of an accurate instrument, we could not clearly assess the risk of bias because it was incompletely reported. The assessable risks were mostly low and did not considerably influence the results in the sensitivity analysis. In addition, we used systematic methods to minimize bias and provide more reliable findings. Furthermore, it is possible that published studies are systemically different from unpublished studies that are not found by searching the literature. Therefore, publication bias was inevitable in the present meta-analysis, as with other meta-analysis based on a literature search. We also tried to identify the sources of variation in responses or heterogeneity between studies; however, heterogeneity was not completely removed in some cases. This point also should be considered while interpreting the findings.
Whole-grain intake has been linked to lower long-term weight gain. Earlier epidemiologic studies consistently demonstrated that a higher intake of whole grains was associated with lower body weight, BMI, waist circumference, abdominal adiposity, and weight gain (58). However, a meta-analysis of 26 randomized controlled trials revealed that whole-grain consumption did not result in decreased body weight, but, rather a small beneficial effect on total body fat (59). In the present meta-analysis, most included studies (n = 13) adjusted for BMI, which is overadjustment. Because these studies did not provide multivariable models not adjusted for BMI, we could not assess the relation between whole-grain intake and mortality without controlling for BMI.
In a recent meta-analysis on fiber intake and mortality, fiber from cereal foods was found to be inversely associated with mortality (7). Therefore, fiber from whole-grain foods might provide an explanation for possible inverse association. In addition, high magnesium content and polyphenols in whole grains might also provide some other reasons (60, 61). Results from randomized controlled trials have shown that increased consumption of whole grains can contribute to a decrease in blood pressure (62, 63) and lipid profiles (64, 65), and an increase in insulin sensitivity (66). Plasma concentrations of antioxidants, including vitamin E, selenium, and phenolic compounds, increase in parallel with increased consumption of whole grains (60), and this could reduce the risk of cancer and CVD.
Our present meta-analysis had some strengths. To the best of our knowledge, this was the first comprehensive meta-analysis that explored the relation between total whole-grain intake and specific whole-grain foods and mortality. We included prospective cohort studies in this analysis. A prospective study design can minimize the possibility that the results are affected by recall or selection bias, which could be of concern in case-control studies. In addition, our analysis included a large number of cases that provided good statistical power for examining the association between whole-grain intake and mortality. We assessed the associations separately for mortality from all causes, CVD, total cancer, and specific cancers. We also evaluated the relation with total whole-grain intake and specific whole-grain foods separately. The quality assessment indicated that all the studies included in this meta-analysis were of either high or relatively good quality, and the majority of studies had adjusted for important confounders. Despite these strengths, several limitations also need to be acknowledged. First, some nondifferential misclassification of subjects in terms of whole-grain intake may have occurred in each study and, thus, in the meta-analysis, which may have attenuated any true association between whole-grain intake and mortality. Such possible misclassifications may be especially high for studies with long follow-up periods that assessed whole-grain intake at study baseline only. Although it was better to separately analyze each food item in a specific whole-grain category, because of the limited number of studies for each food item, we had to pool all specific whole-grain foods together. Because our quantitative assessment was based on observational studies, we cannot rule out the possibility that unknown and/or residual confounding still may have affected the results in each study and, thus, the pooled estimates in the meta-analyses. The meta-analysis of dose-response relations for total whole-grain intake included a rather limited number of studies: 5 for all-cause mortality, 6 for CVD mortality, and 3 for cancer mortality. Therefore, caution in the interpretation of these findings is required. The potential for bias or residual confounding because of the high correlation of whole-grain intake and healthy lifestyle such as physical activity and other dietary habits should be carefully considered when interpreting our results. Finally, in a meta-analysis of published studies, publication bias could be of concern.
In conclusion, in this meta-analysis of prospective cohort studies, we found an inverse association between whole-grain intake and mortality from all causes, CVD, and total cancers; however, the association with mortality from specific cancers needs further investigation. Our findings support the current recommendations on increased whole gain intake for longevity.
Acknowledgments
SB-K, PS, BL, and AE contributed to the conception, design, statistical analyses, data interpretation, and manuscript drafting; and MS-M contributed to the data analysis, data interpretation, and manuscript drafting. All authors read and approved the final manuscript.
References
- 1.Jones JM, Engleson J. Whole grains: benefits and challenges. Annu Rev Food Sci Technol 2010;1:19–40. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian RS, Lee RM, Kennedy MA, Ludwig DS, Mozaffarian D, Gortmaker SL. Identifying whole grain foods: a comparison of different approaches for selecting more healthful whole grain products. Public Health Nutr 2013;16:2255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu RH. Whole grain phytochemicals and health. J Cereal Sci 2007;46:207–19. [Google Scholar]
- 4.World Health Organization. The top 10 causes of death [Internet]. [cited 2016 Sep 15]. Available from: www.who.int/mediacentre/factsheets/fs310/en.
- 5.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis 2008;18:283–90. [DOI] [PubMed] [Google Scholar]
- 6.Lang R, Jebb SA. Who consumes whole grains, and how much? Proc Nutr Soc 2003;62:123–7. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Je Y. Dietary fiber intake and total mortality: a meta-analysis of prospective cohort studies. Am J Epidemiol 2014;180:565–73. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Zhao LG, Wu QJ, Ma X, Xiang YB. Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol 2015;181:83–91. [DOI] [PubMed] [Google Scholar]
- 9.Hajishafiee M, Saneei P, Benisi-Kohansal S, Esmaillzadeh A. Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 2016;116:343–52. [DOI] [PubMed] [Google Scholar]
- 10.Key TJ, Thorogood M, Appleby PN, Burr ML. Dietary habits and mortality in 11,000 vegetarians and health conscious people: results of a 17 year follow up. BMJ 1996;313:775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buil-Cosiales P, Zazpe I, Toledo E, Corella D, Salas-Salvado J, Diez-Espino J, Ros E, Fernandez-Creuet Navajas J, Santos-Lozano JM, Arós F, et al. Fiber intake and all-cause mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr 2014;100:1498–507. [DOI] [PubMed] [Google Scholar]
- 12.McCullough ML, Robertson AS, Jacobs EJ, Chao A, Calle EE, Thun MJ. A prospective study of diet and stomach cancer mortality in United States men and women. Cancer Epidemiol Biomarkers Prev 2001;10:1201–5. [PubMed] [Google Scholar]
- 13.Jacobs DR Jr, Meyer KA, Kushi LH, Folsom AR. Is whole grain intake associated with reduced total and cause-specific death rates in older women? The Iowa Women’s Health Study. Am J Public Health 1999;89:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steffen LM, Jacobs DR Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:383–90. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med 2015;175:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thun MJ, Calle EE, Namboodiri MM, Flanders WD, Coates RJ, Byers T, Boffetta P, Garfinkel L, Heath CW Jr. Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst 1992;84:1491–500. [DOI] [PubMed] [Google Scholar]
- 17.Rebello SA, Koh H, Chen C, Naidoo N, Odegaard AO, Koh WP, Butler LM, Yuan JM, van Dam RM. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: a prospective cohort study. Am J Clin Nutr 2014;100:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen A, Egeberg R, Halkjaer J, Christensen J, Overvad K, Tjonneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr 2011;141:639–44. [DOI] [PubMed] [Google Scholar]
- 19.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs DR Jr, Meyer KA, Kushi LH, Folsom AR. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women’s Health Study. Am J Clin Nutr 1998;68:248–57. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs DR, Pereira MA, Meyer KA, Kushi LH. Fiber from whole grains, but not refined grains, is inversely associated with all-cause mortality in older women: the Iowa women’s health study. J Am Coll Nutr 2000;19(3, Suppl)326S–30S. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs DR Jr, Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am J Clin Nutr 2007;85:1606–14. [DOI] [PubMed] [Google Scholar]
- 23.Burr ML, Sweetnam PM. Vegetarianism, dietary fiber, and mortality. Am J Clin Nutr 1982;36:873–7. [DOI] [PubMed] [Google Scholar]
- 24.Appleby PN, Key TJ, Burr ML, Thorogood M. Mortality and fresh fruit consumption. IARC Sci Publ 2002;156:131–3. [PubMed] [Google Scholar]
- 25.Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996;275:447–51. [DOI] [PubMed] [Google Scholar]
- 26.Rautiainen S, Levitan EB, Orsini N, Akesson A, Morgenstern R, Mittleman MA, Wolk A. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med 2012;125:974–80. [DOI] [PubMed] [Google Scholar]
- 27.Lo YT, Chang YH, Wahlqvist ML, Huang HB, Lee MS. Spending on vegetable and fruit consumption could reduce all-cause mortality among older adults. Nutr J 2012;11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasheras C, Fernandez S, Patterson AM. Mediterranean diet and age with respect to overall survival in institutionalized, nonsmoking elderly people. Am J Clin Nutr 2000;71:987–92. [DOI] [PubMed] [Google Scholar]
- 29.Gardener H, Wright CB, Gu Y, Demmer RT, Boden-Albala B, Elkind MS, Sacco RL, Scarmeas N. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr 2011;94:1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009;338:b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He M, van Dam RM, Rimm E, Hu FB, Qi L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010;121:2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wengreen H, Quach A, Cutler A, Munger R, Corcoran C. Whole-grain intake and risk of all-cause mortality among elderly men and women: the Cache, County Study on Memory, Health and Aging. FASEB J 2012; 26: 119.2. [Google Scholar]
- 33.Skeie G, Braaten T, Olsen A, Kyro C, Tjonneland A, Nilsson LM, Landberg R, Lund E. Whole grain intake and survival among Scandinavian colorectal cancer patients. Nutr Cancer 2014;66:6–13. [DOI] [PubMed] [Google Scholar]
- 34.Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, Snetselaar LG, Martin LW, Trevisan M, Howard BV, et al. Mediterranean and DASH diet scores and mortality in women with heart failure: The Women’s Health Initiative. Circ Heart Fail 2013;6:1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen MC, Bueno-de-Mesquita HB, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Kok FJ, Kromhout D. Consumption of plant foods and stomach cancer mortality in the seven countries study. Is grain consumption a risk factor? Seven Countries Study Research Group. Nutr Cancer 1999;34:49–55. [DOI] [PubMed] [Google Scholar]
- 36.Jansen MC, Bueno-de-Mesquita HB, Buzina R, Fidanza F, Menotti A, Blackburn H, Nissinen AM, Kok FJ, Kromhout D. Dietary fiber and plant foods in relation to colorectal cancer mortality: the Seven Countries Study. Int J Cancer 1999;81:174–9. [DOI] [PubMed] [Google Scholar]
- 37.van den Brandt PA. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am J Clin Nutr 2011;94:913–20. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs DR Jr, Meyer HE, Solvoll K. Reduced mortality among whole grain bread eaters in men and women in the Norwegian County Study. Eur J Clin Nutr 2001;55:137–43. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Sesso HD, Manson JE, Willett WC, Buring JE. Is intake of breakfast cereals related to total and cause-specific mortality in men? Am J Clin Nutr 2003;77:594–9. [DOI] [PubMed] [Google Scholar]
- 40.Tognon G, Rothenberg E, Eiben G, Sundh V, Winkvist A, Lissner L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. Age (Dordr) 2011;33:439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietinen P, Rimm EB, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Circulation 1996;94:2720–7. [DOI] [PubMed] [Google Scholar]
- 42.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med 1992;152:1416–24. [PubMed] [Google Scholar]
- 43.Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr 2006;83:124–31. [DOI] [PubMed] [Google Scholar]
- 44.Breslow RA, Graubard BI, Sinha R, Subar AF. Diet and lung cancer mortality: a 1987 National Health Interview Survey cohort study. Cancer Causes Control 2000;11:419–31. [DOI] [PubMed] [Google Scholar]
- 45.Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med 2015;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells GA, O’Connell D, Peterson J, Welch V, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [cited 2016 Sep 15]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 47.Orsini NBR, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6:40–57. [Google Scholar]
- 48.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsson SCON, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA 2010;303:1077–83. [DOI] [PubMed] [Google Scholar]
- 50.Larsson SCON, Wolk A. Dietary potassium intake and risk of stroke: a dose-response meta-analysis of prospective studies. Stroke 2011;42:2746–50. [DOI] [PubMed] [Google Scholar]
- 51.Anderson JW. Whole grains and coronary heart disease: the whole kernel of truth. Am J Clin Nutr 2004;80:1459–60. [DOI] [PubMed] [Google Scholar]
- 52.Erkkilä AT, Herrington DM, Mozaffarian D, Lichtenstein AH. Cereal fiber and whole-grain intake are associated with reduced progression of coronary-artery atherosclerosis in postmenopausal women with coronary artery disease. Am Heart J 2005;150:94–101. [DOI] [PubMed] [Google Scholar]
- 53.Djoussé L, Gaziano JM. Breakfast cereals and risk of heart failure in the Physicians’ Health Study I. Arch Intern Med 2007;167:2080–5. [DOI] [PubMed] [Google Scholar]
- 54.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fiber intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2:757–61. [DOI] [PubMed] [Google Scholar]
- 55.Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, Leitzmann MF, Thompson FE. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2007;85:1353–60. [DOI] [PubMed] [Google Scholar]
- 56.Mitrou PN, Kipnis V, Thiébaut AC, Reedy J, Subar AF, Wirfält E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461–8. [DOI] [PubMed] [Google Scholar]
- 57.Knudsen MD, Kyrø C, Olsen A, Dragsted LO, Skeie G, Lund E, Aman P, Nilsson LM, Bueno-de-Mesquita HB, Tjønneland A, et al. Self-reported whole-grain intake and plasma alkylresorcinol concentrations in combination in relation to the incidence of colorectal cancer. Am J Epidemiol 2014;179:1188–96. [DOI] [PubMed] [Google Scholar]
- 58.Thielecke F, Jonnalagadda SS. Can whole grain help in weight management? J Clin Gastroenterol 2014;48 Suppl 1:S70–7. [DOI] [PubMed] [Google Scholar]
- 59.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2013;98:872–84. [DOI] [PubMed] [Google Scholar]
- 60.Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc 2003;62:129–34. [DOI] [PubMed] [Google Scholar]
- 61.Shechter M, Merz CN, Paul-Labrador M, Meisel SR, Rude RK, Molloy MD, Dwyer JH, Shah PK, Kaul S. Oral magnesium supplementation inhibits platelet-dependent thrombosis in patients with coronary artery disease. Am J Cardiol 1999;84:152–6. [DOI] [PubMed] [Google Scholar]
- 62.Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr 2010;92:733–40. [DOI] [PubMed] [Google Scholar]
- 63.Saltzman E, Das SK, Lichtenstein AH, Dallal GE, Corrales A, Schaefer EJ, Greenberg AS, Roberts SB. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. J Nutr 2001;131:1465–70. [DOI] [PubMed] [Google Scholar]
- 64.Leinonen KS, Poutanen KS, Mykkanen HM. Rye bread decreases serum total and LDL cholesterol in men with moderately elevated serum cholesterol. J Nutr 2000;130:164–70. [DOI] [PubMed] [Google Scholar]
- 65.Maki KC, Beiseigel JM, Jonnalagadda SS, Gugger CK, Reeves MS, Farmer MV, Kaden VN, Rains TM. Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. J Am Diet Assoc 2010;110:205–14. [DOI] [PubMed] [Google Scholar]
- 66.Magnusdottir OK, Landberg R, Gunnarsdottir I, Cloetens L, Akesson B, Landin-Olsson M, Rosqvist F, Iggman D, Schwab U, Herzig KH, et al. Plasma alkylresorcinols C17:0/C21:0 ratio, a biomarker of relative whole-grain rye intake, is associated to insulin sensitivity: a randomized study. Eur J Clin Nutr 2014;68:453–8. [DOI] [PubMed] [Google Scholar]