Abstract
The 2015 Dietary Guidelines Advisory Committee indicated that magnesium was a shortfall nutrient that was underconsumed relative to the Estimated Average Requirement (EAR) for many Americans. Approximately 50% of Americans consume less than the EAR for magnesium, and some age groups consume substantially less. A growing body of literature from animal, epidemiologic, and clinical studies has demonstrated a varied pathologic role for magnesium deficiency that includes electrolyte, neurologic, musculoskeletal, and inflammatory disorders; osteoporosis; hypertension; cardiovascular diseases; metabolic syndrome; and diabetes. Studies have also demonstrated that magnesium deficiency is associated with several chronic diseases and that a reduced risk of these diseases is observed with higher magnesium intake or supplementation. Subclinical magnesium deficiency can exist despite the presentation of a normal status as defined within the current serum magnesium reference interval of 0.75–0.95 mmol/L. This reference interval was derived from data from NHANES I (1974), which was based on the distribution of serum magnesium in a normal population rather than clinical outcomes. What is needed is an evidenced-based serum magnesium reference interval that reflects optimal health and the current food environment and population. We present herein data from an array of scientific studies to support the perspective that subclinical deficiencies in magnesium exist, that they contribute to several chronic diseases, and that adopting a revised serum magnesium reference interval would improve clinical care and public health.
Keywords: serum magnesium, plasma magnesium, magnesium deficiency, reference interval, chronic disease
Introduction
The perspectives gathered in this article stem from a workshop on addressing an evidence-based reference interval for serum total magnesium concentration (STMC)15 that was held in April 2015 in Lowell, Massachusetts. The objectives of this article are to 1) gather and categorize studies that have used serum and/or urinary magnesium, 2) evaluate whether these studies collectively support the need for an evidence-based re-evaluation of the clinical reference interval, 3) determine whether the magnesium biomarkers in serum and/or urine are consistent across population groups, and 4) identify data gaps for serum or urinary magnesium in terms of population groups (specific age, sex, and ethnicity) that are necessary to inform a re-evaluation of the clinical reference interval for serum magnesium.
Approximately half (48%) of the US population has been shown to consume less than the daily requirement of magnesium from foods (1), partly because of the processing of food, a lower consumption of whole grains and fruits and vegetables than recommended, and a greater consumption of fast food that has a low magnesium content. The 2015 Dietary Guidelines Advisory Committee found magnesium to be underconsumed relative to the Estimated Average Requirement (EAR) and characterized it as a shortfall nutrient of public health concern (2). The European Food Safety Authority recently published a scientific opinion on dietary reference values for magnesium and found that “although the role of Mg in bone structure and physiology is well established, there are few quantitative data for using this relation for setting dietary reference values for magnesium” (3). Nevertheless, the impact of chronically low magnesium intake on serum magnesium concentrations and long-term health remains poorly studied; most trials have been of short duration, and most observational studies have lacked repeated serum measures.
Overt signs of clinical magnesium deficiency have not been routinely recognized in the healthy population. However, relatively low magnesium intake and/or status has been associated with critical health issues, such as but not limited to hypertension (4–9), cardiovascular disease (CVD) (10–14), type 2 diabetes (T2D) (15–18), and osteoporosis (19, 20). In most cases, risk was elevated at serum magnesium concentrations higher than the present clinical cutoff for deficiency, raising the question of potential subclinical deficiency and chronic latent magnesium deficiency and justifying a review of the current research to evaluate contemporary ideas on “healthy” or “normal” serum total magnesium values. Therefore, this perspective is oriented toward supporting a need for identifying a clinical reference interval of STMC that is needed for optimal health.
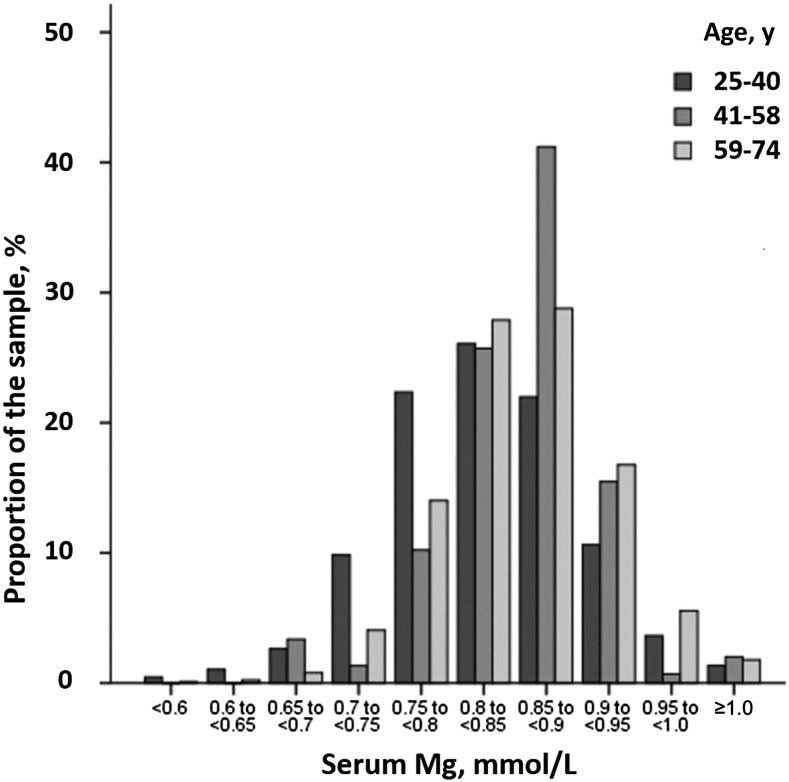
STMC is the predominant test used by healthcare providers to assess magnesium status. The current reference interval for STMC was determined by measuring serum magnesium in then-representative healthy normal individuals of NHANES I (1974) (21). The central 95th percentile of this measure in 15,820 apparently healthy individuals aged 18–74 y was defined as the normal range (Figure 1).
FIGURE 1.
Age-specific distributions of serum magnesium in US adults. Data were derived from NHANES I (1971–1975). Mg, magnesium.
This set the reference interval for serum magnesium at 0.75–0.95 mmol/L. It is important to highlight that this reference interval was based on the distribution in a normal population—not the relation between serum magnesium and clinical outcomes. Re-evaluating this reference interval while also taking into account health outcomes is therefore justified (22). Physicians may be led to assume that patients have normal magnesium status when they may have chronic latent magnesium deficiency (23, 24). Complicating the picture further, the last time serum magnesium was measured in NHANES was >40 y ago, and given changes in the food supply, changes in population distribution, prevalence of diseases such as obesity and T2D, and so on, the current distribution of serum magnesium in the United States is effectively an unknown.
A simple, rapid, and accurate test to assess total body magnesium status is lacking. Although STMC is most commonly used to assess the status of patients, >99% of total body magnesium (22–26 g in adults) is extravascular, mostly in the bones (>50%), with only 0.3% in the serum (25). Thus, this parameter does not necessarily reflect the true total body magnesium content. The serum concentration of ionized magnesium, the biologically active form, may be more reflective of true magnesium status; however, clinical reliance on this measure remains controversial (26). Another noninvasive method is to analyze urinary magnesium excretion. Because renal magnesium excretion decreases in response to dietary deficiency, this can be an important parameter during the assessment of magnesium status (27). The combined determination of the STMC and urinary magnesium excretion are currently the most practical tests to assess magnesium status (28), but their reliability as biomarkers needs rigorous evaluation.
Although normal serum magnesium concentrations can be seen in the presence of intracellular magnesium deficiency, once serum magnesium declines, it is unlikely that the intracellular magnesium concentration remains normal. In addition, taking into account that magnesium homeostasis depends on the relation between intake and loss, a rational approach for a reliable magnesium biomarker should take into account the relation between intake, serum concentrations, and urinary concentrations. Available data suggest that serum and plasma magnesium concentrations, RBC concentrations, and urinary magnesium excretion respond to dietary manipulation (29).
Discussions from the previously mentioned workshop on the critical evaluation of the clinical reference interval are summarized in the sections that follow along with their applicability in evaluating the STMC reference range. The perspectives presented in this review reflect a gathering of current literature that has measured serum total, urinary, and/or dietary magnesium in both healthy humans and in humans with various chronic diseases (osteoporosis, T2D, hypertension, CVD).
What Have We Learned from Balance Data with Respect to Dietary Requirements and Status Indicators of Magnesium?
Because dietary intake of magnesium is often used to support the judgment of status for determining reference values, adequate and deficient intakes need to be based on data obtained from well-controlled studies with humans. The EAR and RDA set for magnesium in 1997 in the United States and Canada were based on highly variable balance data from only 34 men and women on self-selected diets (30). Since then, improved balance data have been reported that can be used to determine the Dietary Reference Intakes for magnesium. These include data from 27 tightly controlled metabolic ward studies (including 243 healthy men and women aged 19–77 y whose weight ranged from 46 to 136 kg) that found neutral magnesium balance, without considering surface or phlebotomy losses, occurred at an intake of 165 mg/d with a 95% prediction interval of 113–237 mg/d (31). With the use of the upper 95% value of 237 mg/d and considering that 98% is the upper interval level used for setting RDAs, the metabolic unit balance data indicated an RDA of ∼245 mg/d. Considering surface and phlebotomy losses in the balance studies would increase the RDA to 250 mg/d for a healthy 70-kg adult.
Body weight and environmental and dietary factors can have a marked effect on the magnesium requirement. These factors need to be considered if or when dietary magnesium intake is used to indicate magnesium status in the determination of reference values (31–38).
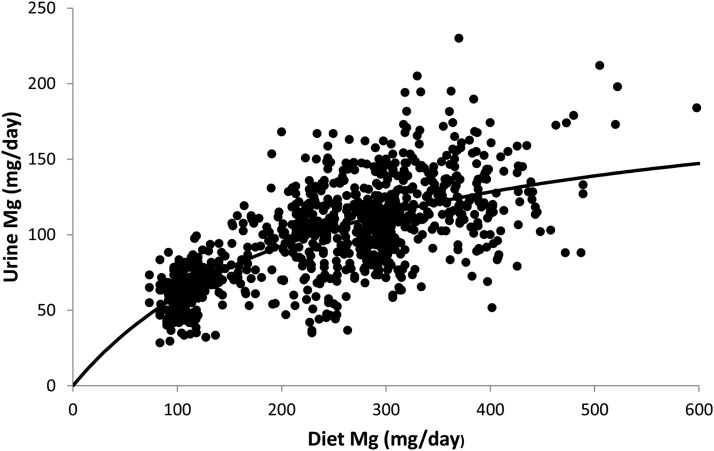
The balance data obtained from the metabolic unit studies of magnesium depletion and repletion also have provided additional information that may be useful for determining reference values. These studies determined changes in serum and urinary magnesium with changes from deficient to adequate magnesium intakes (39–42). These findings indicate that urinary magnesium is a relatively good indicator of magnesium intake, that urinary excretion <80 mg/d indicates a risk for a current dietary magnesium deficiency, and that urinary excretion 80–160 mg/d is associated with magnesium intakes >250 mg/d (Figure 2). However, the rapid change in urinary excretion with a change from deficient to adequate magnesium intake, or vice versa, indicates that urinary magnesium measurement alone is not a good indicator of status if dietary changes were recent or short term. In such cases, individuals excreting <80 mg/d could still be magnesium adequate, whereas individuals excreting >80 mg/d could be magnesium deficient.
FIGURE 2.
Data derived from urinary magnesium excretion in 93 men and 150 women participating in 27 different tightly controlled metabolic unit studies in which dietary magnesium ranged from 84 to 598 mg (3.46–246 mmol/d). Diet, dietary; Mg, magnesium.
Serum magnesium does not respond as quickly or consistently as does urinary magnesium when magnesium deficiency is present based on results from metabolic unit studies (39–42). This indicates that those individuals with serum magnesium concentrations indicating adequacy actually could be in, or approaching, a chronic latent magnesium-deficient state caused by a less-than-adequate magnesium intake.
In conclusion, metabolic unit balance and depletion and repletion experiments indicate that serum magnesium concentrations <0.82 mmol/L (2.0 mg/dL) (i.e., higher than the current cutoff) with a urinary magnesium excretion of 40–80 mg/d, indicating magnesium intake of <250 mg/d, strongly suggest that an individual is magnesium deficient, thus potentially increasing the risk for some chronic diseases. In addition, these individuals should respond well to an increase in magnesium intake.
Does Inflammation and Its Relation to Chronic Disease Help Inform a Reference Range for Serum Magnesium?
Many pathologic conditions involving low serum magnesium status have been associated with an increased inflammatory response and oxidative stress in both animals (43) and humans (34). This is well characterized by the activation of several leukocytes and macrophages as well as the release of numerous inflammatory cytokines and acute-phase proteins. Although low magnesium status may not be a direct cause of inflammatory diseases, insufficient magnesium has consistently been shown across multiple laboratories to increase chronic low-grade inflammation, which is thought to play a major role in chronic disease etiology. Animal studies limiting magnesium intake support data in humans confirming the biological plausibility behind low magnesium status and low-grade chronic inflammation (34), although the lack of dose-response randomized controlled trial (RCT) data makes it difficult to ascertain a direct effect. It is plausible that low magnesium status may be an indicator of other suboptimal dietary and lifestyle patterns that lead to low-grade chronic inflammation (e.g., inadequate intakes of fruits and vegetables).
However, most studies have indicated that increased dietary intakes of magnesium have been linked to a decrease in several markers of systemic inflammation and endothelial dysfunction such as, but not limited to, IL-6, TNF-α, soluble intracellular adhesion molecule 1, soluble vascular cell adhesion molecule 1, and C-reactive protein (CRP). A systematic review and meta-analysis of observational and experimental studies indicated an inverse association between magnesium intake and serum CRP (44). A recent RCT of 62 men and nonpregnant women (45) supported this finding and indicated that low serum magnesium status is correlated with higher serum CRP. Individuals who received magnesium chloride in this RCT had higher serum magnesium concentrations (0.86 ± 0.08 compared with 0.69 ± 0.16 mmol/L; P = 0.002) and lower serum CRP concentrations (4.8 ± 15.2 compared with 17.1 ± 21.0 nmol/L; P = 0.01) than participants in the control group. Two mechanisms have been proposed to explain the increase in inflammatory response caused by magnesium deficiency or insufficiency. First, reactive oxygen species are increased when an individual undergoes a state of magnesium deficiency, as measured by serum magnesium status. The increase in reactive oxygen species promotes membrane oxidation and NF-kB production. Second, the attenuation of the calcium channel-blocking effect of magnesium during magnesium deficit allows for increased calcium entry within immune-competent cells, stimulating an inflammatory response (46).
These studies support a role for magnesium in the inflammation-based etiology of many chronic diseases and suggest a causal role for magnesium in lowering certain markers of inflammation. However, more studies are needed if magnesium’s role in inflammation can or should be used to support a revision of reference intervals, especially because many markers of inflammation do not yet have clinically useful reference intervals. Nevertheless, the relations demonstrated to date on the role of magnesium and inflammation suggest that this and accruing evidence could support a disease and/or optimal health-based re-evaluation of the current serum magnesium reference interval.
Are Skeletal Studies Informative for Redefining the Reference Range for Serum Magnesium?
Magnesium consists of ∼0.5–1.0% bone ash and plays an important role in bone and mineral homeostasis. Several animal studies have demonstrated that magnesium deficiency results in bone loss (47, 48). The small body of clinical evidence suggesting that long-term low dietary intakes of magnesium influence bone mass, bone turnover, bone-related hormones, and cytokine concentrations has been previously reviewed (37). There is growing evidence that magnesium may not only affect bone cell function and hydroxyapatite crystal formation but may also be an important factor in quantitative changes of the bone matrix that predict fragility. Magnesium has been evaluated with respect to bone health in a meta-analysis of 7 studies (49) and 6 case-control studies (50–55). In these studies, the association between serum magnesium concentrations in postmenopausal women with osteoporosis compared with a healthy control were examined. The 6 case-control studies were all included in the meta-analysis (49), which suggested that serum magnesium concentrations have an inverse relation with bone mineral density (BMD). Taken together, these studies suggest low serum magnesium is a plausible risk factor for osteoporosis among postmenopausal women. BMD is currently the only risk biomarker of osteoporosis considered valid by the FDA; however, other markers of bone turnover have also been used to assess the effect of serum magnesium status in relation to bone health and fracture risk (56, 57). Men, but not women, enrolled in the European Prospective Investigation into Cancer and Nutrition-Norfolk cohort showed significant inverse trends in fracture risk across serum magnesium concentration groups for spine fractures (P = 0.02) and total hip, spine, and wrist fractures (P = 0.02). The mean serum magnesium for men was 0.81 ± 0.12 mmol/L (58).
Postmenopausal women with osteoporosis had consistently lower serum magnesium concentrations across studies than their healthy controls (49). In addition, RBC magnesium concentrations have been shown to be lower in postmenopausal women with osteoporosis than a healthy control (59). These data are consistent with other studies that have indicated that the dietary intake of magnesium is inversely associated with DXA bone status measurements (60). A recent analysis of the Women’s Health Initiative Observational Study, which did not assess serum or urine magnesium, suggested that lower dietary magnesium intake is associated with lower BMD of the hip and whole body but not with an increased risk of fractures (61). Magnesium intake has been shown to have a positive association with BMD in both cross-sectional studies that used dietary recalls (20, 62–65) and clinical studies of supplemental intake (66–68).
The limited evidence to date, primarily in the form of case-control studies, points to an inverse relation between serum magnesium and osteoporosis, a relation further supported by observational studies of dietary magnesium in bone health. The literature on magnesium in bone health is notably dwarfed by the vast literature on calcium, a mineral that often competes with magnesium; i.e., magnesium has been largely ignored. More data from trials and prospective observational studies will be needed to support an appropriate serum magnesium reference interval for older individuals, particularly for postmenopausal women, who represent the highest and largest risk group for osteoporosis and for whom serum magnesium is a plausible risk factor for osteoporosis.
How Do Data from Studies in Healthy Individuals Inform a Reference Range for Serum Magnesium?
Cross-sectional studies
Serum magnesium.
To our knowledge, 35 cross-sectional studies or surveys have evaluated serum magnesium in healthy individuals, 4 of which were national health surveys. Table 1 presents the means and ranges of serum magnesium in these studies in addition to the cutoff and prevalence of hypomagnesemia. As noted earlier, NHANES I, which included 15,820 white and black men and women aged 1–74 y, is the only national study in the United States to our knowledge to have collected serum magnesium concentrations (21). Several other countries have included serum magnesium in their measures, including the 2006 Mexican National Health and Nutrition Survey, which consisted of a population of representative men and women aged >20 y (70), adolescents (71), and children (72). In addition, the China Health and Nutrition Survey initiated in 1989 collected blood samples in 2009 from 8511 participants aged ≥18 y from 228 communities across 9 provinces in China (73), and the 1998 Comprehensive Survey of Living Conditions of the People on Health and Welfare examined a subset of 62 Japanese adults aged 20–90 y (74). Table 1 presents a summary of these results.
TABLE 1.
Reference intervals of serum magnesium from nationally representative studies and surveys1
| Reference (country) | Mean serum magnesium, mmol/L | Range of serum magnesium, mmol/L | Prevalence of hypomagnesemia | Additional study findings |
| Lowenstein and Stanton (21) (United States) | 0.85 | 0.75–0.96 | Women: 21% (<0.8 mmol/L); men: 1.5% (<0.7 mmol/L) | NHANES I (1971–1975) reported 12 age categories; small sex differences were observed between the ages of 18 and 45 y, with men having higher concentrations than women; both white men and women had higher serum concentrations than black men and women of the same age; these differences were statistically significant in many age groups, particularly in young and middle-aged adults; low serum magnesium (<0.80 mmol/L) was associated with all-cause mortality (69) |
| Mejía-Rodríguez et al. (70) (Mexico) | 0.81 (median) | 0.70–0.95 (median) | Women: 36.3% (<0.75 mmol/L); men: 31% (<0.75 mmol/L) | 5410 adults representing ∼59 million Mexican adults from the National Health and Nutrition Survey (2006) aged ≥20 y; 63.2% women; 70% overweight or obese) |
| De la Cruz-Góngora et al. (71) (Mexico) | 0.79 (no difference by sex) | NR | Overall: 37.6%; females: 40%; males: 35.4% (<0.75 mmol/L) | 2447 adolescents representing ∼17 million adolescents from the National Health and Nutrition Survey (2006); mean age: 15.1 y (range: 12–19 y); survey included 54% females, of which 35.4% were overweight or obese; 7.29 (7.7% females and 6.8% males) had a CRP >6 mg/dL; overall median daily magnesium intake was 235 mg/d; no significant associations were found for serum magnesium with sex, BMI (in kg/m2), CRP, or ethnicity |
| Morales-Ruán Mdel et al. (72) (Mexico) | 0.86 | 0.86–0.90 (<5 y); 0.82–0.87 (5–11 y) | Overall: 22.6%; aged 1–4 y: 12%; aged 5–11 y: 28.4% (<0.75 mmol/L) | 5060 children representing ∼24 million children from the National Health and Nutrition Survey (2006); age range: 1–11 y; 49% females |
| Zhan et al. (73) (China) | Men: 0.95; women: 0.93 | 0.84–1.05 | NR | Samples collected from 8511 participants; significant interaction found with serum magnesium and sex and low serum ferritin (P < 0.001); prevalence of anemia decreased with increasing concentrations of serum magnesium |
| Akizawa et al. (74) (Japan) | 0.85 | 0.54–1.19 | NR | Participants from the 1998 Comprehensive Survey of Living Conditions of the People on Health and Welfare; distribution of serum magnesium was normal; daily magnesium intake (322 ± 147 mg/d) correlated with serum magnesium (0.28; P = 0.05) |
CRP, C-reactive protein; NR, not reported.
Five studies were conducted exclusively in children aged <18 y (75–79). Two studies evaluated adults >65 y. The first of these studies compared residents living in nursing homes with those who were not (n = 345) to document serum magnesium and zinc and associated deficiency symptoms (80). In total, 33% of the seniors sampled were found to be hypomagnesemic. The second study, part of the Prospective Investigation of the Vasculature in Uppsala Seniors (81), evaluated 897 seniors to validate the Nordic Reference Interval Project for a battery of clinical chemistry tests. No individuals with T2D were studied in this population, but 80% of the seniors were on medications, predominantly for CVD. Serum magnesium for both men and women without CVD ranged from 0.70 to 0.96 mmol/L and was similar to those previously documented for the entire Nordic population.
Twelve studies evaluated participants with a BMI (in kg/m2) <25 (70, 74, 75, 77, 82–89), and 14 studies reported on participants with a BMI >25 (70, 81, 84–88, 90–96). In total, 9 of the 14 studies (64%) that enrolled participants with a BMI >25 demonstrated hypomagnesemia (<0.75 mmol/L) compared with 2 of the 12 studies (16%) that enrolled participants with a BMI <25. Sharifi et al. (94) found that the prevalence of magnesium deficiency (<0.70 mmol/L) in women with polycystic ovary disease was significantly higher than in women without the disease (13% compared with 0%; P = 0.02).
Dietary magnesium.
In addition to measuring serum magnesium, 5 studies also collected information on dietary magnesium (70, 77, 88, 96, 97). One of these studies (88) evaluated concentrations of magnesium in the water in 3 different geographical regions in Serbia. Individuals in 2 soft-water (low concentration of dissolved calcium and magnesium) regions had lower concentrations of serum magnesium (0.72 ± 0.05 mmol/L) than the 1 hard-water (high concentration of dissolved calcium and magnesium) region (0.87 ± 0.09 mmol/L).
Urinary magnesium.
Two large multinational population-based studies (98, 99) collected 24-h urinary magnesium along with other biomarkers of interest. Although neither collected serum magnesium concentrations, thereby limiting the inferences that can be made, these studies did supply useful dietary and associated risk-factor data. The INTERMAP Study (98) investigated the role of multiple dietary factors and urinary metabolites on blood pressure (BP) concentrations among 4680 middle-aged men and women in the United States, United Kingdom, China, and Japan. Mean dietary magnesium intake in 2194 US participants aged 49.1 ± 5.4 y was 148.2 ± 40 mg/1000 kcal, which is within the normal range (113–237 mg/d). Urinary magnesium was 4.25 ± 1.58 mmol/d (104 mg/d), above the deficiency cutoff cited earlier. In the WHO CARDIAC Study (99), which evaluated 24-h magnesium excretion and CVD factors in 4211 participants aged 48–56 y in 50 population samples from 22 countries, the reported magnesium:chromium ratio (mg:g) by quintile ranging from a low of 34.7 ± 12.1 to a high (fifth quintile) of 136.7 ± 44.9. magnesium:chromium ratios were found to be inversely associated with BMI, systolic and diastolic BP, and total cholesterol. The risk of hypertension was significantly higher only in the lowest magnesium:chromium ratio quintile (P < 0.001).
Collectively, these data provide serum magnesium and/or 24-h urinary concentrations across the spectrum of age groups for both men and women over a range of BMI measures and in multiple ethnic groups with diverse dietary patterns. Lacking are studies collecting both dietary data and serum and/or urinary magnesium data; such studies could help inform the biomarker use in assessing magnesium intake or its ability to improve a less-than-adequate magnesium status.
RCTs
Table 2 shows basal circulating magnesium concentrations from 28 RCTs (7 of which were cross-over) that enrolled a total of 2106 healthy participants or participants with CVD risk factors given either magnesium supplementation or a placebo in studies that lasted 28 d to 12 mo. These studies evaluated magnesium status via serum and/or urine and the impact of magnesium supplement or placebo upon biomarkers of insulin resistance, glycemic control, blood lipids, and/or inflammation.
TABLE 2.
Serum and plasma magnesium concentrations reported at baseline in trials of healthy participants and those with risk factors for cardiovascular disease1
| Blood pressure |
|||||||
| Healthy, no risk factors | Glucose-intolerant | Overweight or obese2 | Elevated cholesterol | Hypomagnesemic | Normotensivesubjects | Hypertensive subjects | |
| Numbers of studies (references) | 10 (100–109) | 9 (106, 110–117) | 15 (45, 100, 108, 110–114, 117–123) | 3 (103, 108, 109) | 10 (45, 104, 107, 110, 112, 113, 116–118, 122, 124) | 16 (45, 105, 106, 111, 112, 118, 121, 122, 124–129) | 14 (126, 130–143) |
| Range of serum and plasma magnesium, mmol/L | 0.61–0.87 | 0.56–0.89 | 0.79–0.94 | 0.82–0.88 | 0.56–0.74 | 0.53–1.17 | 0.62–1.01 |
Some studies enrolled participants that may have presented with >1 risk factor and are referenced accordingly.
BMI (in kg/m2) >25.
Very few studies enrolled exclusively men or exclusively women. Only one study—the Trial of Hypertension Prevention, which was the largest of the studies—reported serum values separately for men, women, blacks, and whites (100). Data from 13 different countries are represented in this healthy RCT data set. The 20–39- and 40–59-y age groups were the predominant groups studied; 2 studies were identified with participants aged <18 y, and 4 studies enrolled participants ≥75 y. Serum and plasma magnesium was collected in 23 studies. Dietary data were collected in 5 of these studies, urinary data were collected in 4, and 3 studies collected all 3 status markers. No studies collected urinary magnesium data alone, but 2 collected urinary data along with dietary data.
Together, these population-based cross-sectional studies and clinical trials indicate that some 10–30% of a given population, considered healthy, may have serum magnesium concentrations below typically used cutoffs (<0.80 mmol/L). This points to several potential realities and questions. If, in fact, serum magnesium concentrations are clinically low in as much as a third of any given population, such a reality would warrant considerable attention and potential intervention, not to mention additional research (notably trials) on the causes of such a high incidence of hypomagnesemia and the potential effects of magnesium intake in reducing or preventing chronic disease. Of course, as highlighted elsewhere, the possibility remains that existing clinical cutoffs are too low. If so, the proportion of the population with suboptimal serum magnesium concentrations, along with any associated adverse health consequences, may be even higher.
How Do Oral Magnesium Studies in Participants with Elevated BP Inform a Reference Range for Serum Magnesium?
BP is an extremely easy physiologic marker to measure, and hypertension is an established and reliable risk factor for CVD morbidity and mortality. Meta-analyses of clinical trials of oral magnesium therapy and BP have also shown varying results (4–8) depending on the meta-analysis inclusion and exclusion criteria. Overall, however, these meta-analyses have established a statistically significant effect of oral magnesium in lowering high BP. In a recent meta-analysis that investigated oral magnesium supplementation, Zhang et al. (9) showed a mean rise of 0.05 mmol/L in serum magnesium in 27 trials in a median time of 87 d and found significance (P < 0.001).
Clinical studies
Our search of magnesium in BP and hypertension resulted in 80 studies (53 clinical trials and 6 cohort, 3 case-control, and 18 cross-sectional studies) (Supplemental References) that included measurements of serum and/or urinary magnesium, 2 of which were in adolescents (1 RCT in those aged 14–18 y and 1 cohort study in those aged 12–14 y). The remaining 78 studies were conducted in adults, of which 5 included some elderly participants aged ≥75 y. Our search returned no studies on young children or infants. Thus, there are considerable gaps in the data for the understanding of magnesium and BP in children, infants, teens, and the elderly. In the vast majority of prospective and cross-sectional studies, serum, urinary, and dietary magnesium were inversely associated with hypertension and BP, except in one study in teens. In general, these studies show that when urinary, serum, and/or dietary magnesium goes up, both systolic and diastolic BP go down.
Intervention trials
Our search resulted in 53 publications that reported 65 human clinical trials of oral magnesium therapy for BP with measurements of serum and/or urinary magnesium (Supplemental References). Of these 65 trials, 44 were RCTs, 9 were non-RCTs, and 12 were noncontrolled trials. Of the 44 RCTs, 30 reported serum magnesium measurements. Table 2 shows the range of baseline serum and plasma magnesium reported in these 30 RCTs on normotensive and hypertensive participants.
This large number of clinical trials that used oral magnesium for BP in adults provides a rich source of data on serum and urinary magnesium with changes in BP during a stated supplemental oral magnesium dose. All of these studies, both those on normotensive as well as hypertensive participants, show increases in serum and urinary magnesium with oral magnesium supplementation along with variable results on BP (analyzed in the section that follows).
How Do Cohort Studies of CVD Outcomes Inform a Reference Range for Serum Magnesium?
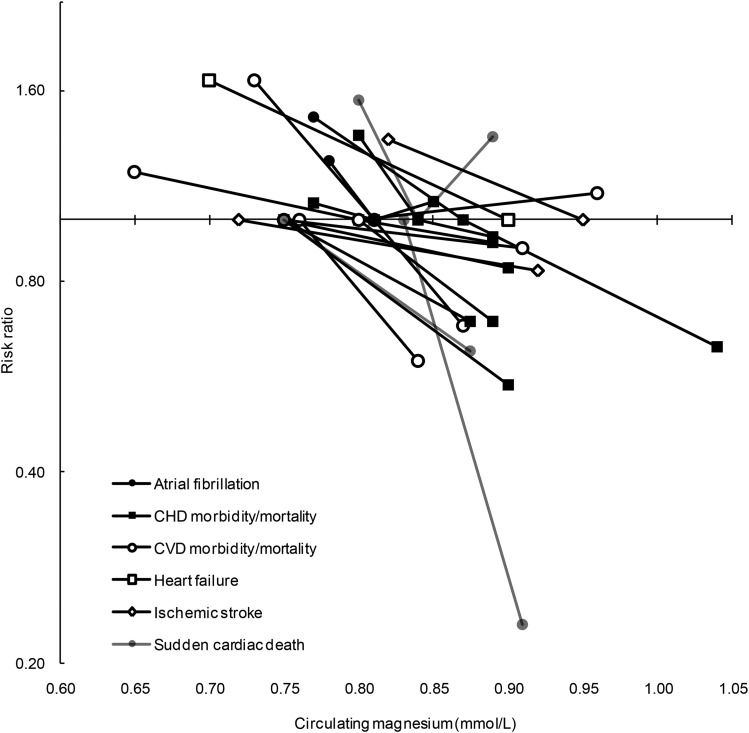
Magnesium is believed to be linked to CVD risk through a broad range of physiologic roles, several of which have been described in the previous sections; e.g., low circulating concentrations have been associated with impaired glucose homeostasis and insulin action, elevated BP, chronic inflammation, impaired vasomotor tone and peripheral blood flow, and electrocardiogram abnormalities (4, 15, 144, 145). To date, no RCT to our knowledge has explored whether magnesium supplementation is related to lower CVD risk. However, prospective observational studies in initially generally healthy populations have linked low circulating magnesium to a higher risk of CVD morbidity and mortality. Many of these observational studies were included in one or both of the 2 meta-analyses (10, 146) published in 2013. Del Gobbo et al. (10) estimated that per 0.2-mmol/L increment in circulating magnesium, the risk of total CVD was 30% lower (RR: 0.70; 95% CI: 0.56, 0.88), the risk of ischemic heart disease was 17% lower (RR: 0.83; 95% CI: 0.65, 1.05), and the risk of fatal ischemic heart disease (e.g., fatal myocardial infarction) was 39% lower (RR: 0.61; 95% CI: 0.37, 1.00). Results were similar, albeit slightly weaker, in the meta-analysis from Qu et al. (146) for total CVD events (RR: 0.91; 95% CI: 0.85, 0.97 per 0.05 mmol/L). The studies included in the meta-analyses and 4 additional studies [2 nested case-control (147, 148) and 2 cohort (11, 149)] resulting from a recent literature search are summarized in Figure 3 (Supplemental Table 1 displays the CIs for this data set), which shows that the risk of mortality and morbidity of several cardiovascular diseases goes down as magnesium status markers rise. Two studies that reported the prevalence of low magnesium status (magnesium <0.73–0.80 mmol/L), as opposed to quantile-based analyses, observed that ∼25% of the populations had low serum magnesium (149, 150). Across all of these prospective studies in initially healthy populations, a higher risk of CVD morbidity and mortality tended to begin to be observed at circulating magnesium circulations <0.75–0.85 mmol/L.
FIGURE 3.
Risk estimates and corresponding risk and reference circulating magnesium concentrations from prospective studies of incident cardiovascular diseases. Estimated risks [from published ORs, RRs, or HRs (11, 147–162)] were derived from comparing the cutoff of circulating magnesium in cases to controls or the risk quantile compared with the reference quantile. The risk estimate at a given circulating magnesium concentration is connected to its corresponding reference magnesium concentration by a line for the following outcomes: atrial fibrillation (closed circles), coronary heart disease morbidity or mortality (closed squares), cardiovascular disease morbidity or mortality (open circles), heart failure (open squares), ischemic stroke (open diamonds), and sudden cardiac death (shaded circles). By way of example, for sudden cardiac death (shaded circle), 1 study (157) observed an RR of 0.23 at a circulating magnesium concentration >0.86 mmol/L relative to <0.78 mmol/L, which in this case was the reference concentration (RR = 1). Published and/or derived risk estimates (along with CIs) used to create this figure are shown in Supplemental Table 2. CHD, coronary heart disease; CVD, cardiovascular disease.
Compared with ischemic heart disease and stroke, there are far fewer prospective studies of other cardiac conditions such as atrial fibrillation and heart failure. In 2 studies, ARIC (Atherosclerosis Risk in Communities) (151) and the Framingham Heart Study Offspring (152), each of which included 18–20 y of follow-up, lower circulating magnesium (<0.76 and 0.73 mmol/L, respectively) was associated with a 30% and 50% higher risk, respectively, of incident atrial fibrillation. In ARIC, low serum magnesium (≤0.70 mmol/L) was also associated with a 70% higher risk of incident heart failure (153).
Findings from ARIC are particularly informative when evaluating the serum magnesium interval optimal for CVD health because it includes a large (∼16,000) population-based sample of blacks and whites and because participants experience many clinical CVD events over >25 y of follow-up. Serum magnesium measured at the baseline visit (1987–1989) followed a normal distribution, with 98% of individuals having serum magnesium concentrations between 0.6 and 1.0 mmol/L, and 11.3% were deemed hypomagnesemic (<0.75 mmol/L). Within ARIC, low serum magnesium has been linked to a greater risk of incident CVD-related risk factors, such as hypertension (163), diabetes (164), and chronic kidney disease (165). Furthermore, it has been linked to numerous CVD outcomes, including ischemic heart disease (157), sudden cardiac death (155), heart failure (153), atrial fibrillation (151), and ischemic stroke (156). Unfortunately, the published ARIC studies did not present serum magnesium in a uniform way in their statistical models (i.e., serum magnesium was variously modeled as quintiles, quartiles, and according to prespecified categories). However, when reviewing the totality of the data, it appears that the risk of CVD outcomes typically increases around serum magnesium concentrations ≤0.75 mmol/L. Although these findings are observational and based on a single study population, they are consistent with the wider body of evidence in suggesting that concentrations of serum magnesium >0.75 mmol/L may be associated with lower CVD risk. Whether magnesium supplementation to concentrations ≥0.75 mmol/L leads to lower CVD is unknown and awaits testing in an appropriately powered RCT.
What Is the Clinical Evidence for Magnesium and T2D That Can Inform a Serum Reference Range for Magnesium?
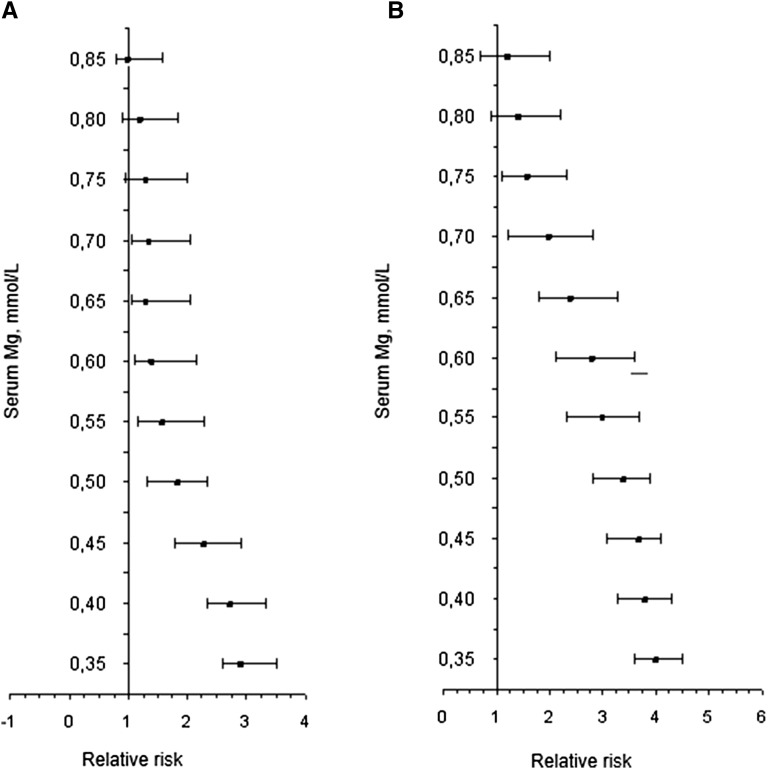
Magnesium deficiency is frequently observed in individuals with T2D because of increased diuresis, a feature of uncontrolled diabetes (166). Among healthy individuals, reports from a 10-y (8735 person-years) follow-up study highlighted that serum magnesium concentrations <0.74 mmol/L predicted incident-impaired glucose tolerance and T2D (90). A longer follow-up of 15 y (11,905 person-years) corroborates the hypothesis that serum magnesium concentrations ≤0.74 mmol/L are related to the risk of developing glucose metabolic disorders (90) (Figure 4). These results strongly suggest that hypomagnesemia is not only a feature of diabetes but also a risk factor for the development of glucose and insulin disorders, thus supporting the idea that in addition to dietary magnesium intake, individuals with diabetes, as well as those at risk of developing the disease, may experience health benefits by increasing their consumption of magnesium. However, multiple conditions can modify the response to dietary and supplemental magnesium, masking their efficacy.
FIGURE 4.
Associations between the risk of developing impaired glucose tolerance (A) and type 2 diabetes (B) in relation to serum magnesium concentrations in individuals followed for ≤15 y. Poisson regression models were adjusted for age, sex, family history of diabetes, waist circumference, and HOMA-IR index. Mg, magnesium.
To define which diabetes populations might benefit from oral magnesium supplementation and to recognize gaps that require resolution before public health advocacy for the expansion of the use of magnesium supplements for the prevention of T2D, we searched evidence derived from RCTs that evaluated serum, urinary, or intracellular magnesium concentrations in adult humans. A total of 10 RCTs were identified (125, 126, 130, 131, 167–172) that enrolled 315 participants with T2D (mean duration of diabetes: 10.1 ± 4.3 y) who received magnesium supplementation (mean dose: 20.5 ± 8.2 mmol elemental magnesium/d) over 11.5 ± 5.9 wk. Between baseline and final conditions, serum magnesium concentrations showed a significant increase (0.73 ± 0.2 to 0.83 ± 0.1 mmol/L; P < 0.005) and fasting plasma glucose (FPG) concentrations a mild but significant decrease (10.6 ± 2.5 to 9.5 ± 2.1 mmol/L; P < 0.01) (Supplemental Figure 1).
By focusing on improving FPG as the critical outcome of magnesium supplementation and stratifying the study populations by age, length of diabetes, serum magnesium at baseline, and baseline FPG, Supplemental Table 2 highlights that after magnesium supplementation, final FPG was significantly lower in participants with diabetes who had higher FPG and lower serum magnesium concentrations at baseline. This suggests that individuals with poorly controlled, untreated, or uncontrolled T2D and serum magnesium <0.74 mmol/L would likely benefit considerably from magnesium supplementation.
Among RCTs that have assessed urinary magnesium (125, 130, 162, 163, 165, 166, 171, 172), a total of 144 participants with T2D (mean duration of diabetes: 9.3 ± 5.1 y) were enrolled and received a mean dose of 24.4 ± 11.3 mmol elemental magnesium/d during a mean of 8.8 ± 4.4 wk. Between baseline and final conditions, serum (0.74 ± 0.3 to 0.80 ± 0.1 mmol/L; P < 0.005) and urinary (3.2 ± 1.5 to 4.3 ± 1.4 mmol/d; P < 0.05) magnesium significantly increased. It is important to highlight that serum magnesium concentrations of targeted populations at baseline were within normal reference values; however, supplementation was effective for decreasing FPG concentrations in those participants with T2D.
These data show that magnesium supplementation may benefit individuals with T2D, particularly among those with serum magnesium <0.74 mmol/L and FPG ≥7.4 mmol/L. Among the most important challenges in the field is the need for identifying a reliable biomarker of magnesium deficiency in the diabetic population. Evidence is currently insufficient for determining an appropriate cutoff of serum magnesium concentrations to establish hypomagnesemia in T2D; additional studies are required, and any future reference range should address disease states, including chronic diseases.
Do Dose and Time Responses of Serum and Plasma Magnesium Biomarkers to Oral Magnesium Supplementation Support a Redefinition of the Serum Reference Range?
Based on RCT data, changes in serum and plasma magnesium concentrations in response to magnesium supplementation in certain doses and durations may provide useful information on the utility of serum and plasma magnesium in reflecting magnesium status. A previous meta-analysis of 22 intervention studies (up to September 2008) showed a 0.03-mmol/L elevation (95% CI: 0.01, 0.06) of circulating magnesium concentrations in response to magnesium intake (29) and that serum and urinary magnesium responded to dietary magnesium manipulation.
From a recently published meta-analysis (173), we found that 41 RCTs examined serum magnesium (29 RCTs) and plasma magnesium (12 RCTs) among 1388 and 506 participants, respectively. The median serum and plasma magnesium concentrations at baseline were similar among the magnesium supplementation and placebo groups for all included trials (serum magnesium: 0.785 compared with 0.79 mmol/L; plasma magnesium: 0.75 compared with 0.75 mmol/L). After magnesium supplementation at a median dose of 365 mg/d for a median duration of 12 wk, serum magnesium concentrations were significantly elevated by 0.05 mmol/L (95% CI: 0.02, 0.07; P < 0.0001) compared with placebo groups. Similarly, plasma magnesium was higher in magnesium groups than placebo groups after a median duration of 2 mo (weighted mean difference: 0.03 mmol/L; 95% CI: 0.01, 0.05; P < 0.0001). Dose- and time-response analyses indicated that serum and plasma magnesium concentrations were elevated immediately after magnesium supplementation and gradually peaked at a dose of 500 mg/d (Supplemental Figure 2) over a duration of 25 wk (Supplemental Figure 3). In addition, serum and plasma magnesium changes did not significantly vary by age, sex, magnesium formulation (organic or inorganic magnesium supplements), cardiometabolic health status (participants free of or with diabetes, CVD, and/or hypertension), trial sample size, or trial quality (P-interaction > 0.05 for all).
This quantitative assessment of available RCT data shows similarly substantial dose and time responses of serum and plasma magnesium concentrations to oral magnesium supplementation. These results provide direct evidence that both serum and plasma magnesium are useful for their effectiveness in reflecting long-term magnesium status (i.e., 25 wk for serum and 15 wk for plasma magnesium measurements), although the sensitivity and specificity of serum and plasma magnesium concentrations in determining magnesium status need to be reliably calibrated in well-designed and rigorously conducted RCTs with the gold-standard measure of magnesium status, i.e., the magnesium loading test. In addition, further studies are needed to fully explore potential biological modifiers of serum and plasma magnesium concentrations.
The Case for Transitioning STMC to an Evidence-Based Reference Interval
Modern medicine has chosen STMC to evaluate magnesium status, but it represents only ∼0.3% of the total body magnesium content and is not in equilibrium with other body tissues except nominally with the bone. As noted earlier, the reference interval for STMC was determined in a US population as part of NHANES I with the use of atomic absorption spectrometry. The identified reference interval (central 95th percentile) was 0.75–0.95 mmol/L with a mean concentration of 0.85 mmol/L (21) and followed a normal Gaussian distribution curve.
Can the clinical laboratory accurately and precisely measure STMC?
A reference system for accurately determining STMC has been established (174). The definitive method for magnesium is isotope dilution/MS as indicated by the National Institute of Standards and Technology. The clinical laboratory reference method for magnesium is flame atomic absorption spectrometry. Reference materials for magnesium are available from the National Institute of Standards and Technology. Standard reference material (SRM) 929 is a preparation of magnesium gluconate dihydrate, and SRM 3131a is a stock solution of magnesium at a concentration of 10 g/L and 10% HNO3. Furthermore, SRM 909 is a human serum with certified values for many analytes, including magnesium.
Most clinical laboratories in the United States now use a colorimetric method (96.8%) for determining STMC. A few laboratories use an enzymatic method (3.2%), as indicated by the College of American Pathologists Proficiency Testing Survey. The 2015 survey set C-C (the first “C” indicating chemistry and the second “C” the third survey of the year) for magnesium report results from 4942 clinical laboratories with 5 separate challenges (175). The mean CV among all results was 4.94%, indicating excellent precision among methods. Furthermore, there was excellent agreement for the mean result among methods. Thus, we have in place today in clinical laboratories across the country an accurate and precise methodology for determining STMC.
Is STMC a Valid Clinical Indicator of Magnesium Status?
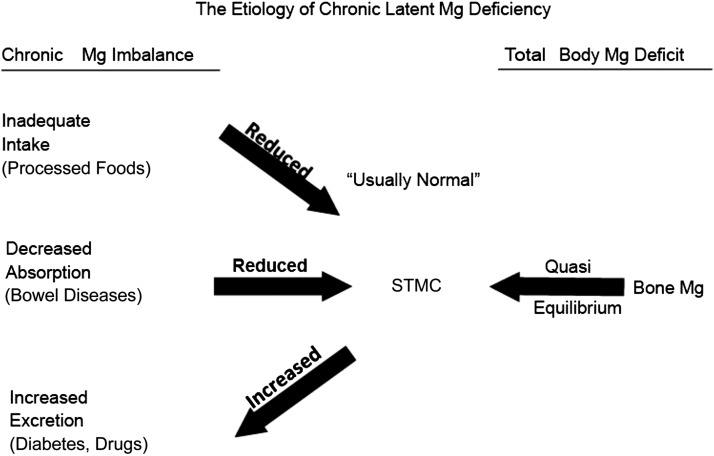
Several factors are needed for humans to achieve and maintain magnesium balance. Figure 5 depicts the factors needed for magnesium balance and lays the foundation for the etiology of chronic latent magnesium deficiency (23). It is chronic because it extends over years and often lasts a lifetime. It is latent because STMC is frequently within the reference interval, albeit at the lower end, and an individual is thus assessed as having normal magnesium status.
FIGURE 5.
The etiology of chronic latent magnesium deficiency. Mg, magnesium; STMC, serum total magnesium concentration. Adapted from reference 23 with permission.
The normal individual in magnesium balance has an adequate intake of magnesium and normal absorption of magnesium from the gastrointestinal tract and does not waste magnesium through overexcretion in the urine. However, a change in any of these 3 entities that is chronic may lead to chronic latent magnesium deficiency. The considerable decrease in magnesium in the food supply is likely a major cause of chronic latent magnesium deficiency. This has led to a subtle chronic magnesium imbalance that occurs over years or a lifetime. In the vast majority of individuals, this imbalance is not detected by measuring STMC because magnesium is slowly leeched from the bone to maintain STMC within the lower part of the reference interval. The best evidence that magnesium has been taken from the bone in a state of chronic latent magnesium deficiency is a study that shows a very significant inverse correlation (r = −0.992; P < 0.0001) between bone magnesium content and the magnesium retention test (176). It is this equilibrium between the bone and STMC that facilitates the development of chronic latent magnesium deficiency in normal individuals. As noted earlier, postmenopausal women with low serum magnesium are at increased risk for osteoporosis.
STMC has been determined to be a valid biomarker for magnesium status. In an extensive review of the literature, Witkowski et al. (29) determined that there were 3 effective biomarkers of magnesium status: plasma and serum magnesium, RBC magnesium, and urinary magnesium. Although STMC is supplemented by bone magnesium during periods of magnesium deficiency, it is still a valid biomarker of magnesium status and essentially the only test used to assess magnesium status by clinical medicine at this time. The availability of a valid and reliable biomarker is essential for determining an evidence-based reference interval. Furthermore, the capacity of labs nationwide to measure serum magnesium accurately and precisely at a relatively low cost is advantageous in thinking about targeted screening for low magnesium concentrations.
What is the impact of chronic latent magnesium deficiency on human health?
Studies have shown that humans need an STMC ≥0.85 mmol/L for health (177). Thus, based on the study by Lowenstein and Stanton (21) and data from Table 1, ≥25% of the people in the United States may have chronic latent magnesium deficiency. Decreased magnesium favors oxidation with an increase in free radicals and endothelial dysfunction (178, 179) as well as systemic inflammation, as noted earlier. Endothelial dysfunction with an increase in free radicals accelerates the atherosclerotic process and risk for CVD. As described previously, studies of CVD in humans (e.g., stroke, heart failure, atrial fibrillation, heart disease morbidity and mortality) support adverse risk at serum magnesium concentrations <0.80 mmol/L. Several animal studies have documented this relation (43, 180–183). Furthermore, based on this mechanism, there is an increased risk for T2D, which we also discussed previously. In addition, in clinical settings, serum magnesium concentrations of 0.75–1.0 mmol/L have been shown to prolong QTc intervals on electrocardiograms, increasing the risk of cardiac arrhythmias. A recent retrospective hospital chart review in 3200 participants (free of medications that would alter the electrocardiogram) demonstrated a mean QTc of 465.4 ± 1.1 ms with serum magnesium >1.0 mmol/L, whereas the mean serum magnesium of <1.0 mmol/L had a longer QTc that averaged 470.1 + 0.99 ms (P < 0.001) (184), suggesting an increased risk in vulnerable population groups even when serum STMC may be within or above the upper limit of the reference interval.
What is needed to recognize chronic latent magnesium deficiency?
The model for approaching an evidence-based reference interval for STMC is similar to that done over many years for blood lipids, particularly cholesterol (185). The reference interval for the serum total cholesterol concentration was established with the use of normal individuals and conventional statistics. This was in part because the reference interval from hospital samples varied greatly, with some having an upper reference cutoff >300 mg/dL. An additional component of establishing the evidence-based reference interval for cholesterol was that the medical literature documented a direct relation between the serum total cholesterol concentration and risk of heart disease. A consensus conference was held at the NIH that established the upper limit of the reference interval for serum total cholesterol of 200 mg/dL (185). We now recommend a similar consensus conference with subject experts to establish an evidence-based reference interval for STMC (Figure 5) that reflects the US population.
Based on the review of literature presented herein, we propose adopting an evidenced-based reference interval for STMC ≥0.85 mmol/L to reduce the risk of CVD, T2D, and other diseases (Figure 6). We further propose a program similar to what was done for blood lipids to establish this evidenced-based STMC reference interval (Figure 7).
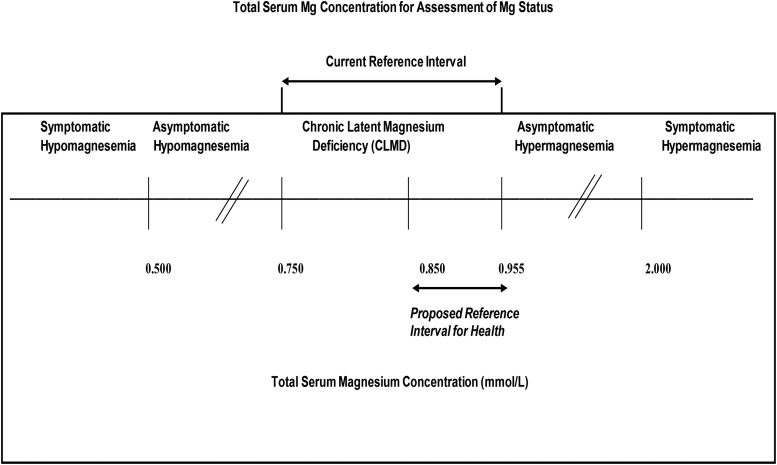
FIGURE 6.
Current and proposed clinical cut-offs of the serum total magnesium concentration for assessing magnesium status. Current reference range derived from reference 21.
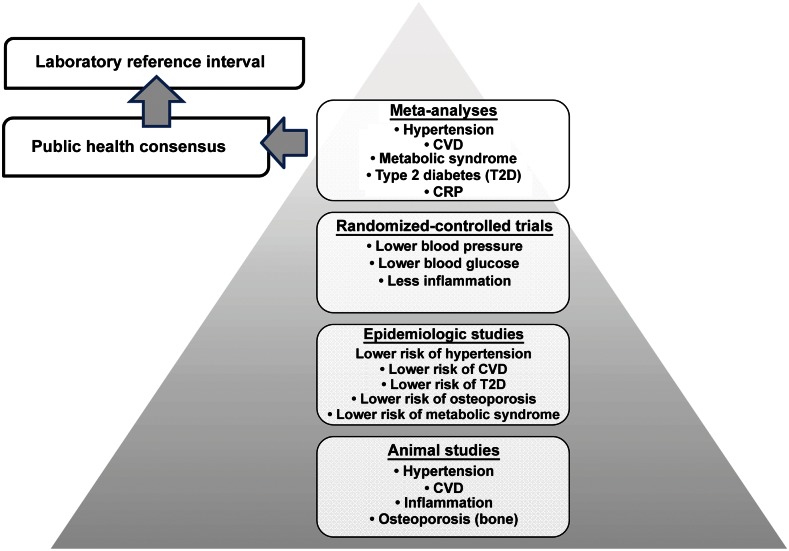
FIGURE 7.
Summary of the accumulated evidence base to inform a revised serum reference interval. CRP, C-reactive protein; CVD, cardiovascular disease; Mg, magnesium; T2D, type 2 diabetes.
Conclusions
Because magnesium has been deemed a shortfall nutrient for the US population, more research is urgently needed. The key to advancing the field of magnesium research is validating a biomarker that most reflects magnesium status whether based on dietary intake and urinary and/or serum magnesium concentrations in healthy individuals or individuals at risk for chronic diseases. Increased public health emphasis and educational messages on the importance of magnesium in the diet to foster optimal health are needed for all age groups. Systems are needed to monitor the impact of magnesium insufficiency and to address methods for improving the intake of magnesium in crops and packaged food products, especially for populations at high risk for magnesium deficiency. Furthermore, it has been >40 y since magnesium status was assessed in a nationally representative population-based sample. Contemporaneous measurement of serum magnesium in a nationally representative sample is urgently needed. The system used to determine the present magnesium reference interval was based on the distribution of magnesium in the population, not health outcomes. As detailed herein, a substantial body of evidence suggests that the current cutoff is too low.
We support the need for a timely re-evaluation of the conventional serum total magnesium reference interval based upon evidence from the literature linking magnesium to health outcomes. Implementing an evidence-based reference interval will allow institutions and health professionals to provide the necessary dietary and therapeutic interventions to increase magnesium concentrations, thereby stemming the tide of adverse health outcomes that may occur as a consequence of chronic latent magnesium deficiency.
Acknowledgments
We thank Joyce Merkel for her editorial assistance in preparing this manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ARIC, Atherosclerosis Risk in Communities; BMD, bone mineral density; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; EAR, Estimated Average Requirement; FPG, fasting plasma glucose; RCT, randomized control trial; SRM, standard reference measure; STMC, serum total magnesium concentration; T2D, type 2 diabetes.
References
- 1.Moshfegh A, Goldman JD, Ahuja J, Rhodes D, LaComb R. What we eat in America, NHANES 2005–2006: usual nutrient intakes from food and water compared to 1997 Dietary Reference Intakes for vitamin D, calcium, phosphorus, and magnesium. Washington (DC): USDA; 2009. [Google Scholar]
- 2.US Department of Health and Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee [Internet]. [cited 2015 Oct 1]. Available from: http://health.gov/dietaryguidelines/2015-scientific-report.
- 3.European Food Safety Authority. Scientific opinion on dietary reference values for magnesium. EFSA J 2015;13:4186. [Google Scholar]
- 4.Jee SH, Miller ER III, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens 2002;15:691–6. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson HO, Nicolson DJ, Campbell F, Cook JV, Beyer FR, Ford GA, Mason J. Magnesium supplementation for the management of essential hypertension in adults. Cochrane Database Syst Rev 2006;3:CD004640. [DOI] [PubMed] [Google Scholar]
- 6.Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr 2012;66:411–8. [DOI] [PubMed] [Google Scholar]
- 7.Rosanoff A. Magnesium supplements may enhance the effect of antihypertensive medications in stage 1 hypertensive subjects. Magnes Res 2010;23:27–40. [DOI] [PubMed] [Google Scholar]
- 8.Rosanoff A, Plesset MR. Oral magnesium supplements decrease high blood pressure (SBP>155 mmHg) in hypertensive subjects on anti-hypertensive medications: a targeted meta-analysis. Magnes Res 2013;26:93–9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016;68:324–33. [DOI] [PubMed] [Google Scholar]
- 10.Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2013;98:160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joosten MM, Gansevoort RT, Mukamal KJ, van der Harst P, Geleijnse JM, Feskens EJ, Navis G, Bakker SJ. Urinary and plasma magnesium and risk of ischemic heart disease. Am J Clin Nutr 2013;97:1299–306. [DOI] [PubMed] [Google Scholar]
- 12.Seelig MS, Elin RJ, Antman EM. Magnesium in acute myocardial infarction: still an open question. Can J Cardiol 1998;14:745–9. [PubMed] [Google Scholar]
- 13.Shlezinger M, Amitai Y, Goldenberg I, Shechter M. Desalinated seawater supply and all-cause mortality in hospitalized acute myocardial infarction patients from the Acute Coronary Syndrome Israeli Survey 2002–2013. Int J Cardiol 2016;220:544–50. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Manson JE, Cook NR, Albert CM, Buring JE, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol 2005;96:1135–41. [DOI] [PubMed] [Google Scholar]
- 15.He K, Song Y, Belin RJ, Chen Y. Magnesium intake and the metabolic syndrome: epidemiologic evidence to date. J Cardiometab Syndr 2006;1:351–5. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Li C, McGuire LC, Mokdad AH, Liu S. Intake of dietary magnesium and the prevalence of the metabolic syndrome among U.S. adults. Obesity (Silver Spring) 2007;15:1139–46. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Moran M, Simental Mendia LE, Zambrano Galvan G, Guerrero-Romero F. The role of magnesium in type 2 diabetes: a brief based-clinical review. Magnes Res 2011;24:156–62. [DOI] [PubMed] [Google Scholar]
- 18.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 2011;34:2116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr 2009;28:131–41. [DOI] [PubMed] [Google Scholar]
- 20.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 1999;69:727–36. [DOI] [PubMed] [Google Scholar]
- 21.Lowenstein FW, Stanton MF. Serum magnesium levels in the United States, 1971–1974. J Am Coll Nutr 1986;5:399–414. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg GD. Medscape editorial on magnesium [Internet]. [cited 2016 Jul 6]. Available from: http://www.medscape.com/viewarticle/844214.
- 23.Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res 2010;23:S194–8. [DOI] [PubMed] [Google Scholar]
- 24.Ismail Y, Ismail AA, Ismail AA. The underestimated problem of using serum magnesium measurements to exclude magnesium deficiency in adults; a health warning is needed for “normal” results. Clin Chem Lab Med 2010;48:323–7. [DOI] [PubMed] [Google Scholar]
- 25.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012;5(Suppl 1):i3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayuk J, Gittoes NJ. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem 2014;51:179–88. [DOI] [PubMed] [Google Scholar]
- 27.Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr 2008;99(Suppl 3):S24–36. [DOI] [PubMed] [Google Scholar]
- 28.Lameris AL, Monnens LA, Bindels RJ, Hoenderop JG. Drug-induced alterations in Mg2+ homoeostasis. Clin Sci (Lond) 2012;123:1–14. [DOI] [PubMed] [Google Scholar]
- 29.Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnes Res 2011;24:163–80. [DOI] [PubMed] [Google Scholar]
- 30.Lakshmanan FL, Rao RB, Kim WW, Kelsay JL. Magnesium intakes, balances and blood levels of adults consuming self-selected diets. Am J Clin Nutr 1984;40:1380–9. [DOI] [PubMed] [Google Scholar]
- 31.Hunt CD, Johnson LK. Magnesium requirements: new estimations for men and women by cross-sectional statistical analyses of metabolic magnesium balance data. Am J Clin Nutr 2006;84:843–52. [DOI] [PubMed] [Google Scholar]
- 32.Anke M, Glei M, Vormann J, Müller R, Hoppe C, Schäfer U. Magnesium in the nutrition of man. In: Porr PJ, Nechifor M, Durlach J, editors. Advances in magnesium research: new data. Montrouge (France): John Libbey Eurotext; 2006. p. 175–86. [Google Scholar]
- 33.Palacios C, Wigertz K, Braun M, Martin BR, McCabe GP, McCabe L, Pratt JH, Peacock M, Weaver CM. Magnesium retention from metabolic-balance studies in female adolescents: impact of race, dietary salt, and calcium. Am J Clin Nutr 2013;97:1014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev 2010;68:333–40. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen FH, Milne DB. A moderately high intake compared to a low intake of zinc depresses magnesium balance and alters indices of bone turnover in postmenopausal women. Eur J Clin Nutr 2004;58:703–10. [DOI] [PubMed] [Google Scholar]
- 36.Volpe SL. Magnesium In: Erdman JWJ, Macdonald EA, Zeisel SH, editors. Present knowledge in nutrition. 10th ed Oxford (United Kingdom): Wiley-Blackwell; 2012: p. 459–74. [Google Scholar]
- 37.Rude RK, Shils ME. Magnesium In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RI, editors. Modern nutrition in health and disease. 10th ed Philadelphia: Lippincott Williams & Wilkins; 2006. p. 223–47. [Google Scholar]
- 38.Coudray C, Demigne C, Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J Nutr 2003;133:1–4. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen FH, Milne DB, Gallagher S, Johnson L, Hoverson B. Moderate magnesium deprivation results in calcium retention and altered potassium and phosphorus excretion by postmenopausal women. Magnes Res 2007;20:19–31. [PubMed] [Google Scholar]
- 40.Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in post menopausal women. J Am Coll Nutr 2007;26:121–32. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen FH, Milne DB. Some magnesium status indicators and oxidative metabolism responses to low-dietary magnesium are affected by dietary copper in postmenopausal women. Nutrition 2003;19:617–26. [DOI] [PubMed] [Google Scholar]
- 42.Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr 2002;132:930–5. [DOI] [PubMed] [Google Scholar]
- 43.Malpuech-Brugère C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, Lebreton J, Mazur A, Rayssiguier Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta 2000;1501:91–8. [DOI] [PubMed] [Google Scholar]
- 44.Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, Wallace R, Liu S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010;33:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simental-Mendía LE, Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: a clinical randomized double-blind placebo-controlled trial. Arch Med Res 2014;45:325–30. [DOI] [PubMed] [Google Scholar]
- 46.Romani AM. Magnesium in health and disease. Met Ions Life Sci 2013;13:49–79. [DOI] [PubMed] [Google Scholar]
- 47.Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem 2004;15:710–6. [DOI] [PubMed] [Google Scholar]
- 48.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos Int 2006;17:1022–32. [DOI] [PubMed] [Google Scholar]
- 49.Zheng J, Mao X, Ling J, He Q, Quan J, Jiang H. Association between serum level of magnesium and postmenopausal osteoporosis: a meta-analysis. Biol Trace Elem Res 2014;159:8–14. [DOI] [PubMed] [Google Scholar]
- 50.Reginster JY, Strause L, Deroisy R, Lecart MP, Saltman P, Franchimont P. Preliminary report of decreased serum magnesium in postmenopausal osteoporosis. Magnesium 1989;8:106–9. [PubMed] [Google Scholar]
- 51.Gür A, Colpan L, Nas K, Cevik R, Sarac J, Erdogan F, Duz MZ. The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J Bone Miner Metab 2002;20:39–43. [DOI] [PubMed] [Google Scholar]
- 52.Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res 2007;35:692–5. [DOI] [PubMed] [Google Scholar]
- 53.Liu SZ, Yan H, Xu P, Li JP, Zhuang GH, Zhu BF, Lu SM. Correlation analysis between bone mineral density and serum element contents of postmenopausal women in Xi’an urban area. Biol Trace Elem Res 2009;131:205–14. [DOI] [PubMed] [Google Scholar]
- 54.Haliloglu B, Aksungar FB, Ilter E, Peker H, Akin FT, Mutlu N, Ozekici U. Relationship between bone mineral density, bone turnover markers and homocysteine, folate and vitamin B12 levels in postmenopausal women. Arch Gynecol Obstet 2010;281:663–8. [DOI] [PubMed] [Google Scholar]
- 55.Okyay E, Ertugrul C, Acar B, Sisman AR, Onvural B, Ozaksoy D. Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas 2013;76:320–5. [DOI] [PubMed] [Google Scholar]
- 56.Aydin H, Deyneli O, Yavuz D, Gozu H, Mutlu N, Kaygusuz I, Akalin S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res 2010;133:136–43. [DOI] [PubMed] [Google Scholar]
- 57.Dimai HP, Porta S, Wirnsberger G, Lindschinger M, Pamperl I, Dobnig H, Wilders-Truschnig M, Lau KH. Daily oral magnesium supplementation suppresses bone turnover in young adult males. J Clin Endocrinol Metab 1998;83:2742–8. [DOI] [PubMed] [Google Scholar]
- 58.Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr 2015;102:376–84. [DOI] [PubMed] [Google Scholar]
- 59.Odabasi E, Turan M, Aydin A, Akay C, Kutlu M. Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann Acad Med Singapore 2008;37:564–7. [PubMed] [Google Scholar]
- 60.Nielsen FH, Lukaski HC, Johnson LK, Roughead ZK. Reported zinc, but not copper, intakes influence whole-body bone density, mineral content and T score responses to zinc and copper supplementation in healthy postmenopausal women. Br J Nutr 2011;106:1872–9. [DOI] [PubMed] [Google Scholar]
- 61.Orchard TS, Larson JC, Alghothani N, Bout-Tabaku S, Cauley JA, Chen Z, LaCroix AZ, Wactawski-Wende J, Jackson RD. Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr 2014;99:926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tucker KL, Hannan MT, Kiel DP. The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. Eur J Nutr 2001;40:231–7. [DOI] [PubMed] [Google Scholar]
- 63.Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary intake and bone mineral density. Bone Miner 1988;4:265–77. [PubMed] [Google Scholar]
- 64.Yano K, Heilbrun LK, Wasnich RD, Hankin JH, Vogel JM. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr 1985;42:877–88. [DOI] [PubMed] [Google Scholar]
- 65.Ilich JZ, Brownbill RA, Tamborini L. Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. Eur J Clin Nutr 2003;57:554–65. [DOI] [PubMed] [Google Scholar]
- 66.Stendig-Lindberg G, Tepper R, Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes Res 1993;6:155–63. [PubMed] [Google Scholar]
- 67.Abraham GE, Grewal H. A total dietary program emphasizing magnesium instead of calcium. Effect on the mineral density of calcaneous bone in postmenopausal women on hormonal therapy. J Reprod Med 1990;35:503–7. [PubMed] [Google Scholar]
- 68.Ryder KM, Shorr RI, Bush AJ, Kritchevsky SB, Harris T, Stone K, Cauley J, Tylavsky FA. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J Am Geriatr Soc 2005;53:1875–80. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Xia J, Del Gobbo LC, Hruby A, He K, Dai Q, Song Y. Serum magnesium and mortality in the general US population: results from the NHANES I Epidemiologic Follow-Up Study. Circulation 2016;133(Suppl 1):AP146. [Google Scholar]
- 70.Mejía-Rodriguez F, Shamah-Levy T, Villalpando S, Garcia-Guerra A, Mendez-Gomez Humaran I. Iron, zinc, copper and magnesium deficiencies in Mexican adults from the National Health and Nutrition Survey 2006. Salud Publica Mex 2013;55:275–84. [DOI] [PubMed] [Google Scholar]
- 71.De la Cruz-Góngora V, Gaona B, Villalpando S, Shamah-Levy T, Robledo R. Anemia and iron, zinc, copper and magnesium deficiency in Mexican adolescents: National Health and Nutrition Survey 2006. Salud Publica Mex 2012;54:135–45. [DOI] [PubMed] [Google Scholar]
- 72.Morales-Ruán Mdel C, Villalpando S, Garcia-Guerra A, Shamah-Levy T, Robledo-Perez R, Avila-Arcos MA, Rivera JA. Iron, zinc, copper and magnesium nutritional status in Mexican children aged 1 to 11 years. Salud Publica Mex 2012;54:125–34. [DOI] [PubMed] [Google Scholar]
- 73.Zhan Y, Chen R, Zheng W, Guo C, Lu L, Ji X, Chi Z, Yu J. Association between serum magnesium and anemia: china health and nutrition survey. Biol Trace Elem Res 2014;159:39–45. [DOI] [PubMed] [Google Scholar]
- 74.Akizawa Y, Koizumi S, Itokawa Y, Ojima T, Nakamura Y, Tamura T, Kusaka Y. Daily magnesium intake and serum magnesium concentration among Japanese people. J Epidemiol 2008;18:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghasemi A, Syedmoradi L, Zahediasl S, Azizi F. Pediatric reference values for serum magnesium levels in Iranian subjects. Scand J Clin Lab Invest 2010;70:415–20. [DOI] [PubMed] [Google Scholar]
- 76.Jagarinec N, Flegar-Mestric Z, Surina B, Vrhovski-Hebrang D, Preden-Kerekovic V. Pediatric reference intervals for 34 biochemical analytes in urban school children and adolescents. Clin Chem Lab Med 1998;36:327–37. [DOI] [PubMed] [Google Scholar]
- 77.Jose B, Jain V, Vikram NK, Agarwala A, Saini S. Serum magnesium in overweight children. Indian Pediatr 2012;49:109–12. [DOI] [PubMed] [Google Scholar]
- 78.Chang X, Li J, Guo Y, Wei Z, Mentch FD, Hou C, Zhao Y, Qiu H, Kim C, Sleiman PM, et al. . Genome-wide association study of serum minerals levels in children of different ethnic background. PLoS One 2015;10:e0123499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shibutani Y, Sakamoto K, Katsuno S, Yoshimoto S, Matsuura T. Relation of serum and erythrocyte magnesium levels to blood pressure and a family history of hypertension. A follow-up study in Japanese children, 12–14 years old. Acta Paediatr Scand 1990;79:316–21. [DOI] [PubMed] [Google Scholar]
- 80.Wörwag M, Classen HG, Schumacher E. Prevalence of magnesium and zinc deficiencies in nursing home residents in Germany. Magnes Res 1999;12:181–9. [PubMed] [Google Scholar]
- 81.Carlsson L, Lind L, Larsson A. Reference values for 27 clinical chemistry tests in 70-year-old males and females. Gerontology 2010;56:259–65. [DOI] [PubMed] [Google Scholar]
- 82.Borghi L, Meschi T, Guerra A, Briganti A, Schianchi T, Allegri F, Novarini A. Essential arterial hypertension and stone disease. Kidney Int 1999;55:2397–406. [DOI] [PubMed] [Google Scholar]
- 83.Ghasemi A, Zahediasl S, Syedmoradi L, Azizi F. Low serum magnesium levels in elderly subjects with metabolic syndrome. Biol Trace Elem Res 2010;136:18–25. [DOI] [PubMed] [Google Scholar]
- 84.Guerrero-Romero F, Rodriguez-Moran M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol 2002;39:209–13. [DOI] [PubMed] [Google Scholar]
- 85.Guerrero-Romero F, Rodriguez-Moran M. Relationship between serum magnesium levels and C-reactive protein concentration, in non-diabetic, non-hypertensive obese subjects. Int J Obes Relat Metab Disord 2002;26:469–74. [DOI] [PubMed] [Google Scholar]
- 86.Guerrero-Romero F, Rodriguez-Moran M. Serum magnesium in the metabolically-obese normal-weight and healthy-obese subjects. Eur J Intern Med 2013;24:639–43. [DOI] [PubMed] [Google Scholar]
- 87.Panhwar AH, Kazi TG, Afridi HI, Talpur FN, Arain S, Kazi N. Distribution of potassium, calcium, magnesium, and sodium levels in biological samples of Pakistani hypertensive patients and control subjects. Clin Lab 2014;60:463–74. [DOI] [PubMed] [Google Scholar]
- 88.Rasic-Milutinovic Z, Perunicic-Pekovic G, Jovanovic D, Gluvic Z, Cankovic-Kadijevic M. Association of blood pressure and metabolic syndrome components with magnesium levels in drinking water in some Serbian municipalities. J Water Health 2012;10:161–9. [DOI] [PubMed] [Google Scholar]
- 89.Yu Y, Cai Z, Zheng J, Chen J, Zhang X, Huang XF, Li D. Serum levels of polyunsaturated fatty acids are low in Chinese men with metabolic syndrome, whereas serum levels of saturated fatty acids, zinc, and magnesium are high. Nutr Res 2012;32:71–7. [DOI] [PubMed] [Google Scholar]
- 90.Guerrero-Romero F, Rascon-Pacheco RA, Rodriguez-Moran M, de la Pena JE, Wacher N. Hypomagnesaemia and risk for metabolic glucose disorders: a 10-year follow-up study. Eur J Clin Invest 2008;38:389–96. [DOI] [PubMed] [Google Scholar]
- 91.Randell EW, Mathews M, Gadag V, Zhang H, Sun G. Relationship between serum magnesium values, lipids and anthropometric risk factors. Atherosclerosis 2008;196:413–9. [DOI] [PubMed] [Google Scholar]
- 92.Rodríguez-Moran M, Guerrero-Romero F. Insulin secretion is decreased in non-diabetic individuals with hypomagnesaemia. Diabetes Metab Res Rev 2011;27:590–6. [DOI] [PubMed] [Google Scholar]
- 93.Rotter I, Kosik-Bogacka D, Dolegowska B, Safranow K, Karakiewicz B, Laszczynska M. Relationship between serum magnesium concentration and metabolic and hormonal disorders in middle-aged and older men. Magnes Res 2015;28:99–107. [DOI] [PubMed] [Google Scholar]
- 94.Sharifi F, Mazloomi S, Hajihosseini R, Mazloomzadeh S. Serum magnesium concentrations in polycystic ovary syndrome and its association with insulin resistance. Gynecol Endocrinol 2012;28:7–11. [DOI] [PubMed] [Google Scholar]
- 95.Sharma A, Dabla S, Agrawal RP, Barjatya H, Kochar DK, Kothari RP. Serum magnesium: an early predictor of course and complications of diabetes mellitus. J Indian Med Assoc 2007;105:16. [PubMed] [Google Scholar]
- 96.Syedmoradi L, Ghasemi A, Zahediasl S, Azizi F. Prevalence of hypo- and hypermagnesemia in an Iranian urban population. Ann Hum Biol 2011;38:150–5. [DOI] [PubMed] [Google Scholar]
- 97.Johnson MA, Dooley SP, Caster WO, Ham CG. The relationship between dietary calcium and blood pressure in the elderly. J Clin Exp Gerontol 1987;9:89–102. [Google Scholar]
- 98.Kesteloot H, Tzoulaki I, Brown IJ, Chan Q, Wijeyesekera A, Ueshima H, Zhao L, Dyer AR, Unwin RJ, Stamler J, et al. . Relation of urinary calcium and magnesium excretion to blood pressure: the International Study Of Macro- And Micro-nutrients and Blood Pressure and the International Cooperative Study on Salt, Other Factors, and Blood Pressure. Am J Epidemiol 2011;174:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamori Y, Sagara M, Mizushima S, Liu L, Ikeda K, Nara Y. An inverse association between magnesium in 24-h urine and cardiovascular risk factors in middle-aged subjects in 50 CARDIAC Study populations. Hypertens Res 2015;38:219–25. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto ME, Applegate WB, Klag MJ, Borhani NO, Cohen JD, Kirchner KA, Lakatos E, Sacks FM, Taylor JO, Hennekens CH. Lack of blood pressure effect with calcium and magnesium supplementation in adults with high-normal blood pressure. Results from Phase I of the Trials of Hypertension Prevention (TOHP). Ann Epidemiol 1995;5:96–107. [DOI] [PubMed] [Google Scholar]
- 101.Chacko SA, Sul J, Song Y, Li X, LeBlanc J, You Y, Butch A, Liu S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: a randomized, double-blind, controlled, crossover trial in overweight individuals. Am J Clin Nutr 2011;93:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doyle L, Flynn A, Cashman K. The effect of magnesium supplementation on biochemical markers of bone metabolism or blood pressure in healthy young adult females. Eur J Clin Nutr 1999;53:255–61. [DOI] [PubMed] [Google Scholar]
- 103.Fu ZY, Yang FL, Hsu HW, Lu YF. Drinking deep seawater decreases serum total and low-density lipoprotein-cholesterol in hypercholesterolemic subjects. J Med Food 2012;15:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guerrero-Romero F, Simental-Mendía LE, Hernández-Ronquillo G, Rodriguez-Morán M. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: a double-blind placebo-controlled randomized trial. Diabetes Metab 2015;41:202–7. [DOI] [PubMed] [Google Scholar]
- 105.Itoh K, Kawasaka T, Nakamura M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br J Nutr 1997;78:737–50. [DOI] [PubMed] [Google Scholar]
- 106.Lee S, Park HK, Son SP, Lee CW, Kim IJ, Kim HJ. Effects of oral magnesium supplementation on insulin sensitivity and blood pressure in normo-magnesemic nondiabetic overweight Korean adults. Nutr Metab Cardiovasc Dis 2009;19:781–8. [DOI] [PubMed] [Google Scholar]
- 107.Lima de Souza E, Cruz T, Rodrigues LE, Ladeia AM, Bomfim O, Olivieri L, Melo J, Correia R, Porto M, Cedro A. Magnesium replacement does not improve insulin resistance in patients with metabolic syndrome: a 12-week randomized double-blind study. J Clin Med Res 2014;6:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Resnick LM, Oparil S, Chait A, Haynes RB, Kris-Etherton P, Stern JS, Clark S, Holcomb S, Hatton DC, Metz JA, et al. . Factors affecting blood pressure responses to diet: the Vanguard study. Am J Hypertens 2000;13:956–65. [DOI] [PubMed] [Google Scholar]
- 109.Shechter M, Saad T, Shechter A, Koren-Morag N, Silver BB, Matetzky S. Comparison of magnesium status using X-ray dispersion analysis following magnesium oxide and magnesium citrate treatment of healthy subjects. Magnes Res 2012;25:28–39. [DOI] [PubMed] [Google Scholar]
- 110.Carpenter TO, DeLucia MC, Zhang JH, Bejnerowicz G, Tartamella L, Dziura J, Petersen KF, Befroy D, Cohen D. A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J Clin Endocrinol Metab 2006;91:4866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, Rodríguez-Morán M. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab 2004;30:253–8. [DOI] [PubMed] [Google Scholar]
- 112.Guerrero-Romero F, Rodríguez-Morán M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: double-blind, randomized clinical trial. Eur J Clin Invest 2011;41:405–10. [DOI] [PubMed] [Google Scholar]
- 113.Held K, Antonijevic IA, Kunzel H, Uhr M, Wetter TC, Golly IC, Steiger A, Murck H. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry 2002;35:135–43. [DOI] [PubMed] [Google Scholar]
- 114.Mooren FC, Kruger K, Volker K, Golf SW, Wadepuhl M, Kraus A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects—a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab 2011;13:281–4. [DOI] [PubMed] [Google Scholar]
- 115.Nielsen FH, Johnson LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res 2010;23:158–68. [DOI] [PubMed] [Google Scholar]
- 116.Sacks FM, Willett WC, Smith A, Brown LE, Rosner B, Moore TJ. Effect on blood pressure of potassium, calcium, and magnesium in women with low habitual intake. Hypertension 1998;31:131–8. [DOI] [PubMed] [Google Scholar]
- 117.Sur G, Maftel O. Role of magnesium in essential hypertension in teenagers. In: Porr PJ, Nechifor M, Durlach J, editors. Advances in magnesium research: new data. Montrouge (France): John Libbey Eurotext; 2006. p. 55–9. [Google Scholar]
- 118.Cosaro E, Bonafini S, Montagnana M, Danese E, Trettene MS, Minuz P, Delva P, Fava C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr Metab Cardiovasc Dis 2014;24:1213–20. [DOI] [PubMed] [Google Scholar]
- 119.Lichodziejewska B, Klos J, Rezler J, Grudzka K, Dluzniewska M, Budaj A, Ceremuzynski L. Clinical symptoms of mitral valve prolapse are related to hypomagnesemia and attenuated by magnesium supplementation. Am J Cardiol 1997;79:768–72. [DOI] [PubMed] [Google Scholar]
- 120.Paolisso G, Sgambato S, Gambardella A, Pizza G, Tesauro P, Varricchio M, D’Onofrio F. Daily magnesium supplements improve glucose handling in elderly subjects. Am J Clin Nutr 1992;55:1161–7. [DOI] [PubMed] [Google Scholar]
- 121.Rodriguez-Hernandez H, Cervantes-Huerta M, Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation decreases alanine aminotransferase levels in obese women. Magnes Res 2010;23:90–6. [DOI] [PubMed] [Google Scholar]
- 122.Rodríguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation improves the metabolic profile of metabolically obese, normal-weight individuals: a randomized double-blind placebo-controlled trial. Arch Med Res 2014;45:388–93. [DOI] [PubMed] [Google Scholar]
- 123.Simental-Mendía LE, Rodríguez-Moran M, Reyes-Romero MA, Guerrero-Romero F. No positive effect of oral magnesium supplementation in the decreases of inflammation in subjects with prediabetes: a pilot study. Magnes Res 2012;25:140–6. [DOI] [PubMed] [Google Scholar]
- 124.Wary C, Brillault-Salvat C, Bloch G, Leroy-Willig A, Roumenov D, Grognet JM, Leclerc JH, Carlier PG. Effect of chronic magnesium supplementation on magnesium distribution in healthy volunteers evaluated by 31P-NMRS and ion selective electrodes. Br J Clin Pharmacol 1999;48:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eriksson J, Kohvakka A. Magnesium and ascorbic acid supplementation in diabetes mellitus. Ann Nutr Metab 1995;39:217–23. [DOI] [PubMed] [Google Scholar]
- 126.Barragán-Rodríguez L, Rodríguez-Morán M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res 2008;21:218–23. [PubMed] [Google Scholar]
- 127.Bashir Y, Sneddon JF, Staunton HA, Haywood GA, Simpson IA, McKenna WJ, Camm AJ. Effects of long-term oral magnesium chloride replacement in congestive heart failure secondary to coronary artery disease. Am J Cardiol 1993;72:1156–62. [DOI] [PubMed] [Google Scholar]
- 128.Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res 2010;23:131–7. [DOI] [PubMed] [Google Scholar]
- 129.Baker WL, Kluger J, White CM, Dale KM, Silver BB, Coleman CI. Effect of magnesium L-lactate on blood pressure in patients with an implantable cardioverter defibrillator. Ann Pharmacother 2009;43:569–76. [DOI] [PubMed] [Google Scholar]
- 130.de Valk HW, Verkaaik R, van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet Med 1998;15:503–7. [DOI] [PubMed] [Google Scholar]
- 131.Guerrero-Romero F, Rodríguez-Morán M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double-blind, placebo-controlled clinical trial. J Hum Hypertens 2009;23:245–51. [DOI] [PubMed] [Google Scholar]
- 132.Henderson DG, Schierup J, Schodt T. Effect of magnesium supplementation on blood pressure and electrolyte concentrations in hypertensive patients receiving long term diuretic treatment. Br Med J (Clin Res Ed) 1986;293:664–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borrello G, Mastroroberto P, Curcio F, Chello M, Zofrea S, Mazza M. The effects of magnesium oxide on mild essential hypertension and quality of life. Current therapeutic research. Curr Ther Res 1996;57:767–74. [Google Scholar]
- 134.Cohen L, Laor A, Kitzes R. Reversible retinal vasospasm in magnesium-treated hypertension despite no significant change in blood pressure. Magnesium 1984;3:159–63. [PubMed] [Google Scholar]
- 135.Plum-Wirell M, Stegmayr BG, Wester PO. Nutritional magnesium supplementation does not change blood pressure nor serum or muscle potassium and magnesium in untreated hypertension. A double-blind crossover study. Magnes Res 1994;7:277–83. [PubMed] [Google Scholar]
- 136.Ferrara LA, Iannuzzi R, Castaldo A, Iannuzzi A, Dello Russo A, Mancini M. Long-term magnesium supplementation in essential hypertension. Cardiology 1992;81:25–33. [DOI] [PubMed] [Google Scholar]
- 137.Witteman JC, Grobbee DE, Derkx FH, Bouillon R, de Bruijn AM, Hofman A. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr 1994;60:129–35. [DOI] [PubMed] [Google Scholar]
- 138.Olhaberry JV, Reyes AJ, Acosta-Barrios TN, Leary WP, Queiruga G. Pilot evaluation of the putative antihypertensive effect of magnesium. Mag-Bul 1987;9:181–4. [Google Scholar]
- 139.Wirell MM, Wester PO, Stegmayr B. Nutritional dose of magnesium given to short-term thiazide treated hypertensive patients does not alter the blood pressure or the magnesium and potassium in muscle -a double blind cross over study. Mag-Bul 1993;15:50–4. [Google Scholar]
- 140.Cappuccio FP, Markandu ND, Beynon GW, Shore AC, Sampson B, MacGregor GA. Lack of effect of oral magnesium on high blood pressure: a double blind study. Br Med J (Clin Res Ed) 1985;291:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dyckner T, Wester PO. Effect of magnesium on blood pressure. Br Med J (Clin Res Ed) 1983;286:1847–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Motoyama T, Sano H, Fukuzaki H. Oral magnesium supplementation in patients with essential hypertension. Hypertension 1989;13:227–32. [DOI] [PubMed] [Google Scholar]
- 143.Widman L, Wester PO, Stegmayr BK, Wirell M. The dose-dependent reduction in blood pressure through administration of magnesium. A double blind placebo controlled cross-over study. Am J Hypertens 1993;6:41–5. [DOI] [PubMed] [Google Scholar]
- 144.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 2006;23:1050–6. [DOI] [PubMed] [Google Scholar]
- 145.Rude RK. Magnesium In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, editors. Encyclopedia of dietary supplements. 2nd ed New York: Informa Healthcare; 2010. p. 527–37. [Google Scholar]
- 146.Qu X, Jin F, Hao Y, Li H, Tang T, Wang H, Yan W, Dai K. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One 2013;8:e57720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc 2013;2:e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Akarolo-Anthony SN, Jimenez MC, Chiuve SE, Spiegelman D, Willett WC, Rexrode KM. Plasma magnesium and risk of ischemic stroke among women. Stroke 2014;45:2881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kieboom BC, Niemeijer MN, Leening MJ, van den Berg ME, Franco OH, Deckers JW, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reffelmann T, Ittermann T, Dorr M, Volzke H, Reinthaler M, Petersmann A, Felix SB. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis 2011;219:280–4. [DOI] [PubMed] [Google Scholar]
- 151.Misialek JR, Lopez FL, Lutsey PL, Huxley RR, Peacock JM, Chen LY, Soliman EZ, Agarwal SK, Alonso A. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans–Atherosclerosis Risk in Communities (ARIC) study. Circ J 2013;77:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khan AM, Lubitz SA, Sullivan LM, Sun JX, Levy D, Vasan RS, Magnani JW, Ellinor PT, Benjamin EJ, Wang TJ. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation 2013;127:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lutsey PL, Alonso A, Michos ED, Loehr LR, Astor BC, Coresh J, Folsom AR. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2014;100:756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 1998;136:480–90. [DOI] [PubMed] [Google Scholar]
- 155.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2010;160:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ohira T, Peacock JM, Iso H, Chambless LE, Rosamond WD, Folsom AR. Serum and dietary magnesium and risk of ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2009;169:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiuve SE, Korngold EC, Januzzi JL Jr., Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr 2011;93:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ford ES. Serum magnesium and ischaemic heart disease: findings from a national sample of US adults. Int J Epidemiol 1999;28:645–51. [DOI] [PubMed] [Google Scholar]
- 159.Gartside PS, Glueck CJ. The important role of modifiable dietary and behavioral characteristics in the causation and prevention of coronary heart disease hospitalization and mortality: the prospective NHANES I follow-up study. J Am Coll Nutr 1995;14:71–9. [DOI] [PubMed] [Google Scholar]
- 160.Khan AM, Sullivan L, McCabe E, Levy D, Vasan RS, Wang TJ. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J 2010;160:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006;17:308–14. [DOI] [PubMed] [Google Scholar]
- 162.Marniemi J, Jarvisalo J, Toikka T, Raiha I, Ahotupa M, Sourander L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol 1998;27:799–807. [DOI] [PubMed] [Google Scholar]
- 163.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 1999;9:159–65. [DOI] [PubMed] [Google Scholar]
- 164.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med 1999;159:2151–9. [DOI] [PubMed] [Google Scholar]
- 165.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, Selvin E, Coresh J, Kao WH. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int 2015;87:820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White JR Jr, Campbell RK. Magnesium and diabetes: a review. Ann Pharmacother 1993;27:775–80. [DOI] [PubMed] [Google Scholar]
- 167.Paolisso G, Scheen A, Cozzolino D, Di Maro G, Varricchio M, D’Onofrio F, Lefebvre PJ. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994;78:1510–4. [DOI] [PubMed] [Google Scholar]
- 168.Eibl NL, Kopp HP, Nowak HR, Schnack CJ, Hopmeier PG, Schernthaner G. Hypomagnesemia in type II diabetes: effect of a 3-month replacement therapy. Diabetes Care 1995;18:188–92. [DOI] [PubMed] [Google Scholar]
- 169.de Lordes Lima M, Cruz T, Pousada JC, Rodrigues LE, Barbosa K, Cangucu V. The effect of magnesium supplementation in increasing doses on the control of type 2 diabetes. Diabetes Care 1998;21:682–6. [DOI] [PubMed] [Google Scholar]
- 170.Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care 2003;26:1147–52. [DOI] [PubMed] [Google Scholar]
- 171.Navarrete-Cortes A, Ble-Castillo JL, Guerrero-Romero F, Cordova-Uscanga R, Juarez-Rojop IE, Aguilar-Mariscal H, Tovilla-Zarate CA, Lopez-Guevara Mdel R. No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes Res 2014;27:48–56. [DOI] [PubMed] [Google Scholar]
- 172.Solati M, Ouspid E, Hosseini S, Soltani N, Keshavarz M, Dehghani M. Oral magnesium supplementation in type II diabetic patients. Med J Islam Repub Iran 2014;28:67. [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang X, Del Gobbo LC, Hruby A, Rosanoff A, He K, Dai Q, Costello RB, Zhang W, Song Y. The circulating concentration and 24-h urine excretion of magnesium dose- and time-dependently respond to oral magnesium supplementation in a meta-analysis of randomized controlled trials. J Nutr 2016;146:595–602. [DOI] [PubMed] [Google Scholar]
- 174.Elin RJ. Determination of serum magnesium concentration by clinical laboratories. Magnes Trace Elem 1991;10:60–6. [PubMed] [Google Scholar]
- 175.College of American Pathologists. Participant summary, set C-C clinical chemistry and therapeutic drug monitoring. 2015:44–5.
- 176.Cohen L, Laor A. Correlation between bone magnesium concentration and magnesium retention in the intravenous magnesium load test. Magnes Res 1990;3:271–4. [PubMed] [Google Scholar]
- 177.Von Ehrlich B. Magnesiummangelsyndrom in der internistischen praxis. [Magnesium deficiency syndrome in internal medicine practice.] Magnes Bulletin 1997;19:29–30. [Google Scholar]
- 178.Weglicki WB, Mak IT, Kramer JH, Dickens BF, Cassidy MM, Stafford RE, Philips TM. Role of free radicals and substance P in magnesium deficiency. Cardiovasc Res 1996;31:677–82. [PubMed] [Google Scholar]
- 179.Maier JA. Endothelial cells and magnesium: implications in atherosclerosis. Clin Sci (Lond) 2012;122:397–407. [DOI] [PubMed] [Google Scholar]
- 180.Resnick LM, Gupta RK, Sosa RE, Corbett ML, Sealey JE, Laragh JH. Effects of altered dietary calcium intake in experimental hypertension: role of intracellular free magnesium. J Hypertens Suppl 1986;4:S182–5. [PubMed] [Google Scholar]
- 181.Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J 2011;75:237–45. [DOI] [PubMed] [Google Scholar]
- 182.Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol 2008;294:H1103–18. [DOI] [PubMed] [Google Scholar]
- 183.Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol 1987;65:729–45. [DOI] [PubMed] [Google Scholar]
- 184.Bomb R, Flatt DM, Heckle MR. Effect of cation dysregulation on corrected QT interval. J Clin Invest 2016;64(2 Suppl 1):483–94. [Google Scholar]
- 185.Consensus conference. Lowering blood cholesterol to prevent heart disease. JAMA 1985;253:2080–6. [PubMed] [Google Scholar]