Abstract
Interest in the application of phenolic compounds from the diet or supplements for the prevention of chronic diseases has grown substantially, but the efficacy of such approaches in humans is largely dependent on the bioavailability and metabolism of these compounds. Although food and dietary factors have been the focus of intense investigation, the impact of disease states such as obesity or diabetes on their absorption, metabolism, and eventual efficacy is important to consider. These factors must be understood in order to develop effective strategies that leverage bioactive phenolic compounds for the prevention of chronic disease. The goal of this review is to discuss the inducible metabolic systems that may be influenced by disease states and how these effects impact the bioavailability and metabolism of dietary phenolic compounds. Because current studies generally report that obesity and/or diabetes alter the absorption and excretion of these compounds, this review includes a description of the absorption, conjugation, and excretion pathways for phenolic compounds and how they are potentially altered in disease states. A possible mechanism that will be discussed related to the modulation of phenolic bioavailability and metabolism may be linked to increased inflammatory status from increased amounts of adipose tissue or elevated plasma glucose concentrations. Although more studies are needed, the translation of benefits derived from dietary phenolic compounds to individuals with obesity or diabetes may require the consideration of dosing strategies or be accompanied by adjunct therapies to improve the bioavailability of these compounds.
Keywords: phenolic compounds, flavonoids, phase II metabolism, diabetes, obesity, inflammation
Introduction
Plant-derived phenolic compounds are a subclass of phytochemicals characterized by the presence of ≥1 phenol moieties, and their consumption is associated with a reduced risk of certain chronic diseases. Although evidence has primarily been drawn from the epidemiologic associations between foods rich in phenolic compounds and disease-risk outcomes (1), in vitro studies (2), animal models (3), and more crucially, clinical trials have begun to substantiate these epidemiologic associations (4). Although the results are not fully conclusive (5), these data generally support the notion that plant-derived phenolic compounds likely impart health benefits to humans. As a result, the desire to expand the application of these compounds as a disease-preventative agent is gaining momentum, in part due to the cost-effective nature of dietary prevention strategies relative to therapeutic approaches. This concept has been translated into the formulation of phenolic compounds in functional foods and dietary supplements for the broader public.
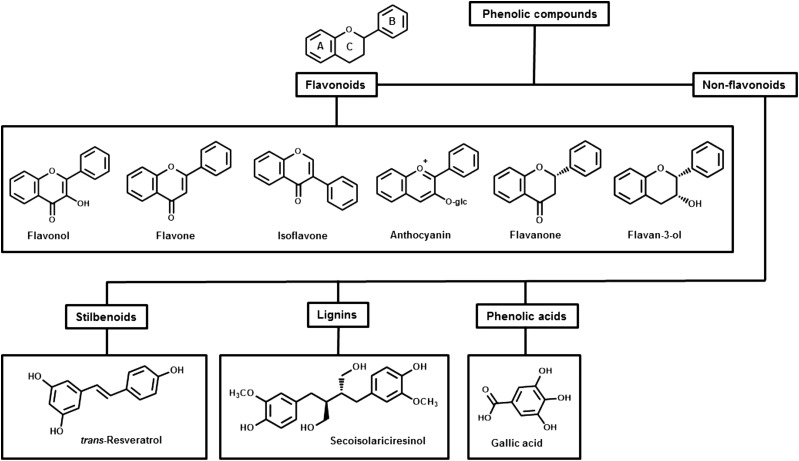
Phenolic compounds can be broadly divided into flavonoid and nonflavonoid derivatives (6) (see Figure 1). The amount of phenolic compounds in common foods and beverages has been systematically reviewed and documented in several publicly available databases, including the USDA’s Nutrient Data Laboratory Flavonoid Database and the Institut National de la Recherche Agronomique’s Phenol-Explorer (7–11). The predominant dietary sources of phenolic compounds in the Western diet are generally accepted to be fruit, vegetables, tea, wine, and cocoa products, with mean dietary intakes for flavonoids ranging from ∼250 mg/d for US adults (12), 250–300 mg/d for Greek men and women (13), and 320 mg/d for Korean adults (14) to 1000 mg/d for British adult populations (13). The flavan-3-ol epicatechin, which is found in high amounts in cocoa and grape seed and to a lesser extent in apples and tea, has received particular attention due to its presence in high quantities in certain chocolates combined with chocolate’s general popularity. Nonflavonoid phenolic compounds include phenolic acids, with one of the most common dietary sources being gallic acid, which is present in grapes and other fruit.
FIGURE 1.
Flowchart of the main classes of phenolics with key examples of their chemical structure. A, B, and C designations are nomenclature used for assignment of the phenol rings.
The diverse mechanisms proposed for the biological activities of phenolic compounds are dependent on their structure and cellular target. Regardless of the structure, dietary source of the phenolic compound, or specific mechanism by which it may impart its bioactivity, the ultimate efficacy of dietary phenolic compounds is dependent on the ability of the compounds to be absorbed intact or as a biologically relevant metabolite and transported to target tissues where they can exert biological activity (15). The bioavailability of phenolic compounds is therefore a critical factor to consider when evaluating the efficacy of a food or supplement or designing products for at-risk populations. In addition to these factors, disease states such as obesity or diabetes that are common in industrialized countries (16) can affect the bioavailability of nutrients and potentially phytochemicals (17), which may be related to the ability of these diseases to modulate gut function (18, 19), alter regulation of xenobiotic metabolizing systems (17), or perturb the gut microbiota critical to the metabolism of phenolic compounds (20).
This review will specifically focus on the effect of obesity and diabetes on the bioavailability and bioactivity of dietary phenolic compounds. Some outstanding questions on the absorption and metabolism of phenolic compounds include the following:
Why are phenolic compounds potentially more effective in improving physiologic conditions in lean or nondiabetic individuals?
Are the absorption, metabolism, and transport of phenolic compounds altered in obese or diabetic individuals?
If so, what mechanism may be effective to target in order to increase biological activity of phenolic compounds in these populations?
Current Status of Knowledge
Overview of the absorption, metabolism, and transport of phenolic compounds.
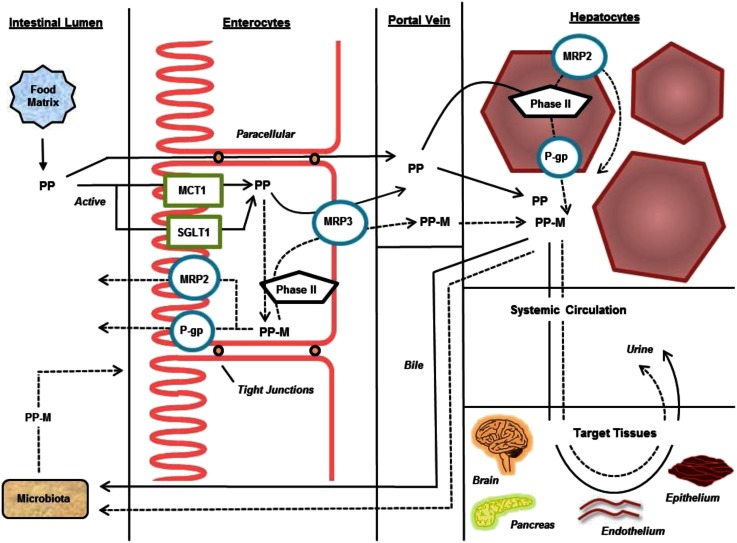
The preventative or therapeutic potential of phenolic compounds is highly dependent on their ability to reach target tissues in a form that can exert biological function. Multiple steps in this process include absorption, metabolism, and transport in both the upper and lower gastrointestinal tract (21) (summarized in Figure 2). Because the broader aspects of absorption, metabolism, and target tissue profiles have been the subject of several reviews (6, 22–25), only key aspects of this process will be covered, focusing on upper intestinal absorption and metabolism because they rely on both inducible and noninducible systems (26) that may be affected by both health status and previous exposure to phenolic compounds.
FIGURE 2.
Schematic of the key molecular processes involved in the transport and metabolism of (poly)phenolic compounds. Solid arrows indicate the pathway of native phenolic compounds; dashed arrows indicate the pathway of phenolic compound metabolites. MCT1, monocarboxylate transporter 1; MRP, multidrug resistance–associated protein; P-gp, P-glycoprotein; Phase II, phase II metabolizing enzymes; PP, (poly)phenolic compounds; PP-M, (poly)phenolic metabolites; SGLT1, sodium-dependent glucose transporter 1.
The first key step in the absorptive process is the release of phenolic compounds from the plant and food matrix into the soluble and bioaccessible fraction in the gut lumen. Soluble forms of phenolic compounds are then actively and passively transported from the lumen into the enterocyte but can be effluxed back out into the lumen by the action of ATP-binding cassette (ABC)6 transporters. Because of the nature of the described absorptive pathways, many phenolic compounds may directly interact and inhibit or decrease the activity of these transport proteins (27). These interactions can be highly specific or broad depending on the phenolic compound preparation and co-consumed food. Ongoing research in this area will continue to dissect the interactions between phenolic compounds and intestinal transport systems that may both alter the absorption of phenolic compounds but also modify the absorption of other bioactive compounds, including drugs. Within the enterocyte, phenolic compounds may also be metabolized before transport across the serosal side and into the portal vein for circulation to the liver, where further metabolism may occur before systemic circulation, excretion into bile or urine, or distribution to other tissues. Unabsorbed phenolic compounds are then carried to the lower gut and made available to the microbiota for catabolism into low-molecular-weight compounds, including several previously characterized metabolites of phenolic compounds (28).
Clinical trials comparing efficacy of phenolic compounds in healthy and obese or diabetic populations.
Because xenobiotic transport and metabolism are dependent on normal metabolic regulation, it can be expected that diseases such as obesity or diabetes may alter these systems and, by extension, affect the bioavailability and effectiveness of phenolic compounds. Banini et al. (29) found that the consumption of dietary grape phenolic compounds produced a differential response between diabetic and nondiabetic individuals on markers of cardiovascular health and glucose control. Diabetic and healthy individuals were given either 150 mL juice, wine, or a dealcoholized wine beverage made from muscadine grapes for 28 d. The BMI range (in kg/m2) for nondiabetic controls was from ∼27 to 30 (overweight), and the diabetic BMI range was from ∼35–40 (obese). All participants were advised to not alter their normal diet and physical activity. When comparing the changes in lipid profiles, grape juice consumption decreased plasma TG concentrations in only the nondiabetic participants, although insulin concentrations decreased in diabetics who consumed dealcoholized wine compared with baseline. One strength of this study is that it tested standard wine along with dealcoholized wine, which is important because alcohol can be a potential confounding factor by affecting health outcomes and/or bioavailability (30, 31). Although the authors did not report the phenolic compounds in the test beverages, an analysis elsewhere suggests that 150 mL of the wine could contain ∼443 mg total phenolic compounds, including 6 mg gallic acid (32).
Davison et al. (33) found that supplemental doses of cocoa phenolic compounds are needed to produce a response in markers of cardiovascular disease and glucose control in overweight and obese individuals. The mean BMI of participants was 33.5, and they consumed a cocoa beverage daily that provided low (36 mg) or high (902 mg) amounts of flavan-3-ols. All participants were advised to not modify their normal diet other than limiting their caffeinated beverages to 2/d and to avoid red wine and dark chocolate. In addition, participants were randomly assigned to either follow a regimen of regular modest exercise or to remain sedentary. This treatment occurred over 12 wk, with measurement tests conducted at baseline and at 6 and 12 wk. Measurements at 12 wk for the high-dose groups (data were pooled for the exercise and nonexercise groups) revealed increased flow-mediated dilatation, a marker of cardiovascular function, along with improved markers of insulin resistance. A strength of this study is the prolonged treatment time period (12 wk) in conjunction with the low and high doses to determine at which dose a physiologic response takes effect. A limitation of this study is that there was no normal-weight group to directly compare the results. However, this shows that higher supplemental doses were required to elicit a response compared with other studies such as Banini et al. (29), which used dietary sources of phenolic compounds.
Combined, these studies suggest that phenolic compounds may have differential effects on cardiovascular markers in lean compared with obese or diabetic populations. However, the underlying reasons for this difference are not clear, although alterations in the absorption and metabolism of phenolic compounds between healthy and obese or diabetic individuals may be responsible, in part, for these observations. Although the studies described did not directly measure the absorption of phenolic compounds, evidence from in vitro, animal, and other clinical studies provide more insight on this potential effect.
Metabolism of phenolic compounds.
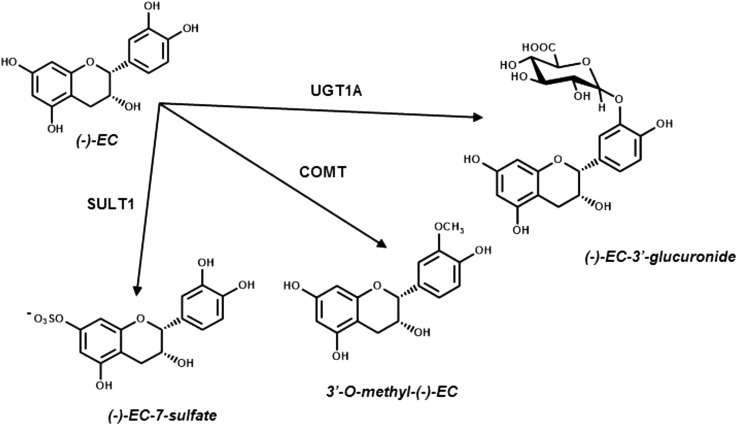
Phenolic compounds are largely transformed and transported via xenobiotic metabolizing systems expressed extensively in intestinal and hepatic tissues (34) but also in other tissues and compartments, including the blood-brain barrier (35, 36). These compounds undergo phase II conjugation to produce metabolites that are more hydrophilic or hydrophobic than the parent compound, thereby facilitating transport processes leading to excretion through bile or feces or urine (see Figure 3). Because the extent to which individual phenolic compounds are biotransformed may result in the alteration of specific biological activity (37–39), factors that ultimately affect the metabolism of these compounds are believed to have a substantial impact on their potential to exert protective therapeutic effects at target tissues.
FIGURE 3.
Phase II metabolism of the flavan-3-ol (–)-epicatechin can produce metabolites that differ in bioactivity due to a change in chemical reactivity and hydrophobicity compared with the parent compound. Conjugation can occur at other and additional hydroyl positions than those shown in the figure. COMT, catechol-O-methyltransferase; EC, epicatechin; SULT, sulfotransferase; UGT, UDP-glucuronyltransferase.
The methylation of phenolic compounds is driven primarily by catechol-O-methyltransferase (COMT) and results in a more hydrophobic and less chemically reactive species (26). This increased hydrophobicity may alter their transport across the enterocyte or other cell types (40), leading to the potential for an accumulation of methylated derivatives in certain tissues such as the brain (41–43). Although methylation generally decreases phenolic antioxidant capacity in vitro (44, 45), methylated epicatechin glucuronide derivatives were observed to have greater ability to enhance long-term potentiation in hippocampal slices relative to simple glucuronides (42). The effect of the methylation transformation on bioactivity is a subject of much debate and appears to be specific to the class of phenolic compound being metabolized.
Sulfotransferases (SULTs) conjugate anionic sulfate groups to phenolic moieties to produce a more hydrophilic species (46), resulting in either a more or less bioactive species depending on the cellular target (47). For example, sulfate metabolites of the grape phenolic resveratrol, in contrast to the resveratrol aglycone and its glucuronide, are not effective in decreasing the lipid content of differentiated adipocytes, whereas sulfate metabolites have been reported to be effective in decreasing the lipid content of maturing adipocytes (48).
Glucuronidation conjugates glucuronic acid to phenolic compounds through the action of UDP-glucuronyl transferase (UGT) to yield a more hydrophilic species that can be efficiently eliminated (49). However, glucuronidation does not appear to affect resveratrol’s in vitro antioxidant potential (50) or quercetin’s estrogenic activity (51). Although this metabolite in certain cases does not seem to have diminished bioactivity compared with the parent compound, it may have increased potential for excretion and thus may affect bioactivity by limiting the half-life and concentrations in target tissues.
Clinical trials suggest obesity and diabetes affect xenobiotic transport and metabolism absorption of phenolic compounds.
Because the bioactivity of phenolic compounds is largely dependent on their metabolism by key phase II enzymes (52), it is necessary to consider the systems that may be altered in disease conditions. Early research documented fundamental differences in the xenobiotic transport and metabolizing systems in obese or diabetic individuals (53). Although many studies in this area have focused on pharmaceuticals, interestingly, the effect of disease states on these systems has also been investigated by using compounds with phenolic structures. Because both phenolic compounds and many pharmaceuticals target the phase II and III metabolizing systems, research with the use of pharmaceuticals is also included in this discussion.
Although doses for many pharmaceutical treatments reflect changes in dose on the basis of weight, increased clearance of compounds metabolized by the phase II systems has been observed in obese populations, possibly indicating altered activity of this system (54). Considering that these observations are inferential, additional insights are needed to better define the extent to which obesity may directly alter the expression and/or activity of specific transporters. For example, Abernethy et al. (55) showed the potential influence of obesity on xenobiotic metabolizing systems. In this study, normal-weight and obese subjects were given a 30-mg oral dose of the benzodiazepine oxazepam, excreted after glucuronidation, and it was found that plasma clearance of oxazepam increased significantly in the obese group (157 mL/min) compared with the normal-weight group (50 mL/min).
In addition, clinical studies showed that pharmacokinetic parameters of the stilbenoid trans-resveratrol differ depending on the health status of the population (see Table 1). Boocock et al. (56) administered 1000 mg trans-resveratrol to healthy, nonobese participants and observed an AUC of 545 ng ⋅ h ⋅ mL−1 and a maximum concentration (Cmax) of 117 ng/mL, but when 1500 mg of trans-resveratrol was administered to an overweight and obese (mean BMI = 31.8) population with hepatic steatosis, it resulted in an AUC of 705 ng ⋅ h ⋅ mL−1 and a Cmax of 65.7 ng/mL (57). These studies can be more easily compared when normalizing these parameters by dose, which shows that healthy participants in Boocock et al. (56) had an ∼15% greater AUC/dose and a more striking 167% greater trans-resveratrol Cmax/dose than the overweight and obese population in Chachay et al. (57), which shows a potentially reduced ability to achieve a threshold for therapeutic concentrations. Because nonalcoholic hepatic steatosis has been reported to be present in ∼66% of obese (58) and even 18% of lean (59) populations, early stages of this condition may be involved in liver function. Thus, normal liver function may potentially be affecting phenolic compound metabolism in certain studies, given the prevalence of hepatic steatosis in the population.
TABLE 1.
Comparison of clinical pharmacokinetic studies that used resveratrol in overweight/obese and normal-weight populations1
| Study authors, year (ref) | Health status | Source | Time, h | Compound | Dose,2mg | AUC, ng ⋅ h ⋅ mL–1 | Cmax, ng ⋅ mL–1 | Tmax, h | AUC/dose, ng ⋅ h ⋅ mL–1 ⋅ mg–1 | Cmax/dose, ng ⋅ mL–1 ⋅ mg–1 |
| Chachay et al., 2014 (57) | Overweight or obese [mean BMI (kg/m2) = 31.8] humans with hepatic steatosis | trans-Resveratrol capsule | 6 | trans-Resveratrol (no metabolites measured) | 1500 | 705 | 65.7 | 1 | 0.47 | 0.0438 |
| Boocock et al., 2007 (56) | Healthy humans | trans-Resveratrol capsule | 24 | trans-Resveratrol | 1000 | 545 | 117 | 0.76 | 0.54 | 0.117 |
| Glucuronide 1 | NA | 3060 | 474 | 2.25 | 3.06 | 0.475 | ||||
| Glucuronide 2 | NA | 2590 | 673 | 1.75 | 2.59 | 0.673 | ||||
| Sulfate | NA | 10,100 | 2100 | 2 | 10.1 | 2.10 | ||||
| Resveratrol + metabolites | NA | 16,300 | 3370 | — | 16.3 | 3.37 |
Cmax, maximum concentration; NA, not applicable; ref, reference; Tmax, time of maximum concentration.
Dose cells under Boocock et al. are NA because the metabolites listed were generated endogenously and not provided in the dose listed.
Pharmacokinetic analyses in animal models suggest obesity and diabetes affect xenobiotic transport and metabolism.
Table 2 summarizes the literature that suggests that obesity or diabetes may lead to a fundamental alteration in xenobiotic metabolism and transport systems critical for the delivery of phenolic compounds (60). The flavone glycoside baicalin, found in the Chinese herb Scutellaria baicalensis, was reported to be excreted at faster rates in diabetic rats than in healthy rats (61). Although the plasma AUC of baicalin was greater in the diabetic group when provided a 200-mg/kg intragastric dose (48.5 compared with 101 μg ⋅ h ⋅ mL−1; normal compared with diabetic), the normal group had significantly higher concentrations of the compound when given a 12-mg/kg dose intravenously (18.0 compared with 11.2 μg ⋅ h ⋅ mL−1; normal compared with diabetic). Because baicalin is transported across the ileum after the glucuronide moiety is cleaved, both groups of rats were tested for activity of β-glucuronidase, the enzyme that catalyzes the cleavage. There was a 1.7-fold increase in the activity of this enzyme in the duodenum of the diabetic rats, which shows a mechanism for the observed apparent increased bioavailability via the oral dose. Still unanswered in this study is the underlying explanation for the increased systemic clearance that drives the decreased baicalin plasma concentrations in the diabetic rats compared with the normal rats when given the intravenous dose.
TABLE 2.
Summary of preclinical pharmacokinetic studies that used normal and diabetic rodent models1
| Study authors, year (ref) | Model and health status | Source | Time, h | Compound | Dose, mg/kg | AUC, ng ⋅ h ⋅ mL–1 | Cmax,2 ng ⋅ mL–1 | Tmax2 | AUC/dose, ng ⋅ h ⋅ mL–1 ⋅ mg–1 | Cmax/dose, ng ⋅ mL–1 ⋅ mg–1 | Comments |
| Liu et al., 2010 (61) | Male Sprague-Dawley rats | Baicalin, i.v. | 10 | Baicalin | 12 | 18,000 | — | — | 1500 | — | Decreased plasma AUC in diabetic animals with i.v. dose may indicate increased clearance |
| STZ-induced diabetic rats | 11,200 | — | — | 930 | — | ||||||
| Male Sprague-Dawley rats | Baicalin, oral | 24 | 200 | 48,500 | 3390, 3860 | 0.2, 8.4 h | 240 | 17, 19 | Increased plasma AUC in diabetic animals with oral dose may be due to increased brush border enzyme activity | ||
| STZ-induced diabetic rats | 101,00 | 5740, 8630 | 0.2, 8.8 h | 500 | 29, 43 | ||||||
| Yu et al., 2010 (62) | Male Sprague-Dawley rats | Coptidis rhizomea extract | 8 | Jatrorrhizine | 34 | 290 | 150 | 2.88 h | 8.5 | 4.4 | |
| STZ-induced diabetic rats | 2150 | 1050 | 1.88 h | 63.2 | 30.9 | ||||||
| Liu et al., 2012 (63) | Male Wistar rats | Mangiferin | Mangiferin | 400 | 3820 | 720 | 0.73 h | 9.6 | 1.8 | ||
| STZ-induced diabetic rats | 8950 | 2000 | 2.25 h | 22.4 | 5.0 | ||||||
| Kang et al., 2010 (64) | Male Sprague-Dawley rats | Liquiritigenin, i.v. | 12 | Liquiritigenin | 20 | 6400 | — | — | 320 | — | Increase in metabolites with i.v. but not oral dose may indicate tissue-specific alterations in metabolism (e.g., gut vs. liver) |
| Metabolite 1 | 13,100 | 223 | 3 min | 655 | 11.2 | ||||||
| Metabolite 2 | 12,300 | 410 | 3 min | 615 | 20.5 | ||||||
| STZ-induced diabetic rats | Liquiritigenin | 5910 | — | — | 296 | — | |||||
| Metabolite 1 | 15,400 | 322 | 3 min | 770 | 16.1 | ||||||
| Metabolite 2 | 19,300 | 643 | 3 min | 965 | 32.2 | ||||||
| Male Sprague-Dawley rats | Liquiritigenin, oral | Liquiritigenin | 50 | 297 | 26.0 | 7 min | 5.94 | 0.52 | |||
| Metabolite 1 | 34,800 | 133 | 15 min | 697 | 2.7 | ||||||
| Metabolite 2 | 32,000 | 247 | 15 min | 640 | 4.9 | ||||||
| STZ-induced diabetic rats | Liquiritigenin | 443 | 49.7 | 5 min | 8.86 | 1.0 | |||||
| Metabolite 1 | 32,300 | 298 | 15 min | 647 | 6.0 | ||||||
| Metabolite 2 | 33,000 | 558 | 15 min | 660 | 11.2 | ||||||
| Hasegawa et al., 2010 (65) | Male Donryu rats | Morphine | Morphine | 15 | 12,200 | — | — | 813 | — | ||
| STZ-induced diabetic rats | 7000 | — | — | 466 | — |
Cmax, maximum concentration; ref, reference; STZ, streptozocin; Tmax, time of maximum concentration.
The presence of two Tmax and or Cmax values indicates presence of two peaks in plasma concentration during assessment period.
Similar effects have been observed in nonphenolic compounds that rely on similar transport and metabolizing pathways. Yu et al. (62) noted elevated plasma clearance of the phenolic-like alkaloid jatrorrhizine in diabetic Sprague-Dawley rats compared with healthy controls. In this study, a plant extract rich in jatrorrhizine was administered by intestinal perfusion, and plasma from the portal vein was collected. The Cmax in diabetic rats was 9.5-fold greater and the AUC was 7.5-fold greater than in the nondiabetic controls. Probing P-glycoprotein (P-gp) function with Rho123 found it to be less active in the diabetic rats, which correlated to decreased protein concentrations of P-gp in all segments of the small intestine. Compared with the normal group, there was a greater increase in portal vein concentrations of jatrorrhizine in the diabetic rats, but not a corresponding higher amount of compound in the peripheral plasma. This first appears to be a paradox because the compound must be transported to another compartment after reaching the portal vein. However, Liu et al. (61) suggested increased clearance from systemic circulation to urine in diabetic rats due to greater transport of jatrorrhizine across the small intestine but lower concentrations in the periphery.
Preclinical and in vitro data suggest obesity and diabetes affect the phase II and III metabolizing systems.
The observed alterations in xenobiotic metabolism in obese and diabetic humans coincide with animal research by Liu et al. (63) and with additional studies that reported differential expression of key phase II metabolizing enzymes with health status (see Table 3 for a summary). When male diabetic Wistar rats were orally administered 400 mg of the xanthone mangiferin/kg (a C-glycoside found in plants in the Anacardiaceae family), the plasma AUC was significantly greater in the diabetic rat group (3820 compared with 8950 ng ⋅ h ⋅ mL−1; healthy compared with diabetic). Although pharmacokinetic parameters of the metabolites were not determined, liver mRNA expression of select Ugt1a family enzymes increased 2.28- to 3.93-fold, Ugt2b8 increased 4.39-fold, and Sult1a1 increased 1.62-fold in diabetic rats. In contrast, liver mRNA expression of Comt decreased by 72%, Ugt2b family enzymes decreased by ∼100%, and there was an 850% reduction in Sult1c1. Overall, these data indicate that differences in pharmacokinetic parameters in the diabetic state may be due to fundamental alterations in metabolizing systems.
TABLE 3.
Summary of preclinical molecular-based studies that used normal, diabetic, and obese rodent models1
| Study authors, year (ref) | Model and health status | Tissue type | Xenobiotic, tran sport, and metabolizing system | Direction of change |
| Wang et al., 2002 (66) | Male Wistar STZ-induced diabetic rats | Liver | COMT activity | ↓ |
| Braun et al., 1998 (67) | Male B/Wor diabetic rats | Liver | Ugt1a1 mRNA, protein, activity | ↑ |
| Burant et al., 1994 (68) | Male Sprague-Dawley STZ-induced diabetic rats | Enterocytes | Glut2, Glut5, Sglt1 mRNA and protein | ↑ |
| Maeng et al., 2007 (69) | Male Sprague-Dawley STZ-induced diabetic rats | Brain | P-gp protein | ↑ |
| Zhang et al., 2011 (70) | Male Sprague-Dawley STZ-induced diabetic rats | Liver | Abcb1a, Abcb1b mRNA and protein (P-gp) | No change |
| P-gp protein | No change | |||
| Intestinal mucosa | Abcb1a, Abcb1b mRNA and protein (P-gp) | ↓ | ||
| Kidney | Abcb1a mRNA | ↑ | ||
| Abcb1b mRNA | No change | |||
| P-gp protein | ↑ | |||
| Liu et al. 2006 and 2007 (71, 72) | Male Sprague-Dawley and ICR STZ-induced diabetic rats | Brain cortex | P-gp protein | ↓ |
| Nawa et al., 2010 (73) | Male ddY STZ-induced diabetic mice | Ileum | P-gp protein | ↓ |
| van Waarde et al., 2002 (74) | Male Wistar STZ-induced diabetic rats | Liver | Mdr2 mRNA and protein | ↑ |
| Liver | Mrp2 mRNA | No change | ||
| Mrp2 protein | ↓ | |||
| Nikooie et al., 2013 (75) | Male Wistar STZ-induced diabetic rats | Soleus muscle | Mct1 mRNA and protein | ↓ |
| Yu et al., 2010 (62) | Male Sprague-Dawley STZ-induced diabetic rats | Small intestine | P-gp protein | ↓ |
| Liu et al., 2012 (63) | Male Wistar STZ-induced diabetic rats | Duodenum and jejunum | Mdr1a and Mdr1b (P-gp) mRNA | ↓ |
| Ileum | Mdr1a and Mdr1b (P-gp) mRNA | ↑ | ||
| Liver | Comt mRNA | ↓ | ||
| Liver | Ugt1a3, Ugt1a7, Ugt2b3, Ugt2b6, Ugt2b12 mRNA | ↑ | ||
| Liver | Ugt2b3 and Ugt2b8 mRNA | ↓ | ||
| Liver | Sult1b1 mRNA | ↓ | ||
| Kang et al., 2010 (64) | Male Sprague-Dawley STZ-induced diabetic rats | Liver | UDP-glucuronic acid concentrations | ↑ |
| Intestine | UDP-glucuronic acid concentrations | No change | ||
| Hasegawa et al., 2010 (65) | Male Donryu STZ-induced diabetic rats | Glucuronidation probed by using morphine | UGT | ↑ |
| Liver | Ugt2b1 mRNA | ↑ | ||
| Liver | Mrp2 mRNA | ↓ | ||
| Mrp3 mRNA | ↑ | |||
| Kim et al., 2004 (76) | Male Sprague-Dawley vs. obese Zucker rats | Liver | Ugt1a1, Ugt1a6, Ugt2b1, Mrp2 mRNA | ↓ |
| Xu et al., 2012 (77) | Male obese C57BL/6J Lepob/ob mice | Liver | Ugt1a1, Ugt1a6, Ugt1a9, Ugt2a3, Ugt3a1, Ugt3a2 mRNA | ↑ |
| Liver | Ugt2b1 mRNA | ↓ | ||
| Lucas-Teixeira et al., 2002 (78) | Obese Zucker rats | Jejunal mucosa | COMT activity | ↑ |
| Koide et al., 2011 (79) | Male C57BL/6J diet-induced obese rats | Liver | Total UGT activity | ↑ |
| Liver | Sult2a1 protein and activity | ↓ | ||
| Kim et al., 2004 (80) | Female C57BL/6 mice treated with LPS | Liver | Sult2a1 mRNA and total SULT activity | ↓ |
| Shimada et al., 1999 (81) | Male Sprague-Dawley rats treated with LPS | Liver | Total SULT activity | ↓ |
| Ho and Piquette-Miller, 2007 (82) | Male Sprague-Dawley rats treated with LPS | Liver | Mdr1b mRNA | ↑ |
Abc, ATP-binding cassette transporter; COMT, catechol-O-methyltrasferase; Glut, glucose transporter; Mct1, monocarboxylate transporter 1; Mdr, multidrug resistance protein; Mrp, multidrug resistance–associated protein; P-gp, P-glycoprotein; ref, reference; Sglt1, sodium-dependent glucose transporter 1; STZ, streptozocin; SULT, sulfotransferase; UGT, UDP-glucuronyltransferase; ↓, decrease; ↑, increase.
Furthermore, Wang et al. (66) reported that COMT methylation activity measured over 30 min decreased by 28.1% in the liver of male Wistar rats with uncontrolled diabetes, but the enzyme’s function was rescued by restoring blood glucose concentrations to a normal range. This suggests that the downstream effects from chronic elevated blood glucose may contribute to alterations in methylation activity. In contrast, COMT maximal velocity increased by 80.5% in obese Zucker rats in the jejunum compared with lean rats (78). Thus, changes in COMT activity and expression appear to be dependent on tissue type and homeostatic aberrations, such as elevated blood glucose concentrations.
Protein concentrations of SULT enzymes, along with their activity, have also been reported to be affected by obesity, diabetes, and associated comorbidities. For instance, male C57BL/6J mice with diet-induced obesity have decreased protein concentrations of SULT2A1 in the liver (79). In addition, research on human clinical samples has shown similar trends (see summary in Table 4). Both SULT1C4 mRNA and protein expressions have been reported to increase in humans with fatty liver, a common comorbidity of obesity (83); however, liver SULT1A1 protein concentrations are significantly lower in individuals with either liver steatosis or diabetes (84). Thus, it appears that specific isoforms are differentially affected through pathways mediated by inflammatory signaling associated with obesity and diabetes. For example, liver Sult isoforms 1a1, 1b1, and 1c1 mRNA expression and their respective proteins decreased in male rats after administration of the inflammatory agent bacterial LPS (81). However, there was no reduction in Sult mRNA expression in rats pretreated with the anti-inflammatory compound dexamethasone, which coincides with results from another study in female C57BL/6J mice, which showed a decrease in SULT activity under inflammatory conditions (80). As such, both obesity and diabetes, as well as related inflammatory stress from these conditions, appear to have a role in the ultimate status of SULT enzymes and their activities.
TABLE 4.
Summary of human molecular-based studies that used liver, kidney, and skeletal tissue and the Hep3B hepatocyte cell line1
| Study authors, year (ref) | Population and health status | Tissue type | Xenobiotic, transport, and metabolizing system | Direction of change |
| Hardwick et al., 2013 (83) | Nonalcoholic fatty liver disease | Liver | UGT1A9 and 2B10 mRNA | ↑ |
| UGT1A9 and 2B10 protein | No change | |||
| Total UGT activity | No change | |||
| SULTA1C4 mRNA | ↑ | |||
| SULTA1C4 protein | ↑ | |||
| Total SULT activity | ↓ | |||
| Yalcin et al., 2013 (84) | Liver steatosis | Liver | SULT1A1 mRNA | No change |
| SULT1A1 protein | ↓ | |||
| SULT1A1 activity | ↓ | |||
| Liver | SULT1A3 mRNA | No change | ||
| SULT1A3 protein | ↑ | |||
| SULT1A3 activity | ↓ | |||
| Metz et al., 2008 (85) | Male and female, lean [mean BMI (kg/m2) = 22.6] vs. obese (mean BMI = 35.3) | Leg skeletal muscle | MCT1 protein | No change |
| Dostalek et al., 2011 (86) | Male and female diabetics | Liver | UGT1A9, UGT1A1, and UGT2B7 mRNA | ↓ |
| Kidney | UGT1A9, UGT1A1, and UGT2B7 mRNA | ↓ | ||
| Yalcin et al., 2013 (84) | Diabetics | Liver | SULT1A1 mRNA | No change |
| SULT1A1 protein | ↓ | |||
| SULT1A1 activity | ↓ | |||
| Liver | SULT1A3 mRNA | No change | ||
| SULT1A3 protein | No change | |||
| SULT1A3 activity | ↓ | |||
| Juel et al., 2004 (87) | Male type 2 diabetics | Leg skeletal muscle | MCT1 protein | ↓ |
| Kim et al., 2004 (80) | Hep3B cells treated with TNF-α and IL-1 | Hepatocytes | SULT2A1 mRNA | ↓ |
MCT1, monocarboxylate transporter1; ref, reference; SULT, sulfotransferase; UGT, UDP-glucuronyltransferase; ; ↓, decrease; ↑, increase.
Data from animal and human studies provide conflicting information on the impact of obesity and/or diabetes on glucuronidation processes. Male diabetic Donryu rats had ∼2 times the circulating concentrations of morphine-3-glucuronide from 185 to 365 min after an initial 15-mg/kg intravenous morphine dose compared with normal controls (65). In addition, Xu et al. (77) found that obese Lepob/ob compared with normal C57BL/6J mice had significantly increased acetaminophen glucuronidation velocity in hepatocytes (0.15 compared with 0.23 nmol ⋅ min–1 ⋅ mg protein−1), which correlated to an ∼5-fold increase in hepatocyte mRNA expression of Ugt1a1 and Ugt3a1 isoforms in the obese mice. A comparison of male diabetic BB/Wor with normal Wistar rats showed that UGT1A1 enzyme activity increased from 0.231 to 1.82 nmol ⋅ min−1 ⋅mg protein−1 in permeabilized liver microsomes (67). In streptozocin-induced diabetic male Sprague-Dawley rats, the plasma AUC of the licorice-derived flavanone metabolite liquiritigenin-7-O-glucuronide increased from 738 to 1160 μg ⋅ min ⋅ mL−1 and Cmax from 24.6 to 38.6 μg/mL compared with normal controls (64). In contrast, there was a 33–63% decrease in hepatic mRNA expression of the Ugt isoforms1a1, 1a6, and 2b1 in obese Zucker rats compared with lean Sprague-Dawley rats (76). In humans, the diabetic condition has been reported to decrease both glucuronide enzyme activity and mRNA expression of the UGT2B7 isoform of the enzyme by ∼50% (86). However, obese individuals are reported to have increased concentrations of oxazepam glucuronide metabolites (55), and mRNA expression of UGT1A9 and 2B10 isoforms has been shown to be significantly higher in humans with nonalcoholic fatty liver disease (83). Although the data overall are somewhat mixed, evidence does support alteration in glucuronidation processes due to the obese or diabetic state. Because these changes in glucuronidation can affect overall metabolite profiles, the excretion of phenolic compounds, and by extension apparent Cmax values, they may potentially affect reaching the threshold concentrations to elicit a physiologic response.
Disease state may also affect the phase III metabolizing system by affecting function and expression levels of efflux proteins. A comparison of 8-wk-old male Sprague-Dawley rats with diabetic rats treated with insulin showed significantly greater Rho123 concentrations in the liver (69.4 compared with 121 ng/g; normal compared with diabetic), intestinal mucosa (101,000 compared with 210,000 ng/g; normal compared with diabetic), and kidney (422 compared with 578 ng/g; normal compared with diabetic) of diabetic rats that showed impaired P-gp function (70). In ileal tissue of diabetic male ddY mice, P-gp protein concentrations were 60% those of controls (73). Decreased P-gp function has also been shown in the brain of diabetic male Sprague-Dawley rats by showing that the ratio of brain tissue to plasma concentrations of the classical P-gp substrate vincristine is significantly greater in the diabetic group than in the control group (0.023 compared with 0.072 mL/brain; normal compared with diabetic) (71). This has been confirmed in male diabetic ICR mice, with brain concentrations of Rho that were significantly greater in diabetic rats (13.7 ng/g) than in normal controls (10.2 ng/g) (72). In contrast, van Waarde et al. (74) found that the livers of diabetic Wistar rats had a 530% increase in protein concentration of multidrug resistance protein 2 (MDR2) and a corresponding elevation in bile salt secretion compared with control rats.
Efflux transporters have an important role in the excretion of phenolic compounds, and the expression of transporters that drive transport of these compounds into the enterocyte and other cells may also be altered with obesity and diabetes. In diabetic male Wistar rats, monocarboxylate transporter 1 (Mct1) mRNA expression in soleus muscle was significantly lower than in control rats (75), in addition to decreased expression of Mct1 protein in the plantaris muscle (88). Although this pattern has also been reported in male diabetic patients with decreased skeletal muscle expression of MCT1 (87), obese individuals did not show differences in muscle MCT1 protein concentrations compared with controls (85). Although MCT1 in human hepatic or intestinal tissues was not directly measured in these studies, these may similarly be affected in diseased conditions.
Additional potential factors from the obese and diabetic condition may affect pharmacokinetics of phenolic compounds.
Although this review focused on critical xenobiotic metabolizing and transport systems altered by obesity and diabetes, there are additional factors to consider that may influence pharmacokinetic parameters of phenolic compounds under these conditions. One such example is that there is an increase in glomerular filtration rate from diabetes (89), which may decrease plasma concentrations of phenolic compounds by increasing their elimination through urine. In addition, kidney disease that results from late-stage diabetes can alter factors that influence xenobiotic metabolism (90). It is also important to consider that genetics (91) in addition to aging (92) modulate expression and therefore alter the observed function of the xenobiotic metabolizing systems, potentially affecting the bioavailability of phenolic compounds.
Alterations in gut function from the diabetic condition may also partially explain differential absorption of phenolic compounds. The oral bioavailability of the peptide cyclosporin has been observed to be lower in diabetic populations as measured by plasma AUC (93). Ogata et al. (94) therefore tested differences in the absorption of cyclosporin between Goto-Kakizaki diabetic rats, a model for a nonobese diabetic phenotype, compared with normal Wistar rats by administering the compound orally or intravenously. When 10 mg cyclosporin A/kg was administered intravenously, the diabetic and normal groups displayed similar plasma AUCs of the compound (21.9 compared with 20.6 μg ⋅ h ⋅ mL−1; normal compared with diabetic), but when orally administered the diabetic rats showed lower plasma AUCs (11.3 compared with 1.8 μg ⋅ h ⋅ mL−1; normal compared with diabetic). Because this compound is absorbed in the small intestine, gastric emptying was determined by using radiolabeled sodium chromate. Although 90% of the marker could be recovered in the distal section of the ileum of the normal group after 2 h, only 60% could be recovered in the diabetic rats, with 25% of the marker still remaining in the stomach. It is important to point out that although this study compared only 2 different breeds of rats, these results may be applicable in a broader sense to the absorption of phenolic compounds because gut transit time can affect pharmacokinetic parameters.
Another possible factor to consider is that of the intestinal microbiota, because microbiota profiles can change under diabetic or obese conditions (95, 96). Because phenolic compounds have low first-pass bioavailability, they reach the lower gut where they may be catabolized into low-molecular-weight compounds that are potentially absorbed and then enter the systemic circulation where they can exert biological activity (6). Due to the beneficial effects of phenolic compounds potentially being mediated by gut microbiota, disease states that perturb microbiota composition may alter their efficacy (97). For example, Selma et al. (98) found that the production of bioactive catabolites of ellagic acid (urolithins) differed between overweight or obese and normal-weight individuals, decreasing the potential efficacy derived from these compounds.
In the diabetic condition, there are alterations to intestinal tissues that occur due to elevated blood glucose concentrations, which may also affect the transport of phenolic compounds. These changes may emerge because expressions of the small intestinal carbohydrate transporters SGLT1 and glucose transporter 2 (GLUT2) increase in diabetic rats, although controlling blood glucose with proper insulin treatment restores these to normal concentrations (68). This alteration can then potentially increase the uptake of certain phenolic compounds whose transport is associated with glucose transporters.
Potential underlying mechanism for altered xenobiotic metabolizing and transporting systems in obese and diabetic populations.
A potential underlying mechanism for the differences in xenobiotic transport and metabolism observed in obese and diabetic populations involves a chronic, low-grade inflammatory response prevalent in these conditions (99, 100). In a study mentioned previously, the administration of LPS to Sprague-Dawley rats resulted in a 36.8% reduction in hepatic sulfonation activity (81), which aligns with another report that LPS-induced secretion of inflammatory cytokines and stimulation of the inflammatory processes alter drug metabolism (101). Similarly, elevated insulin concentrations from diabetes may also induce alterations that affect phase II enzymes (102). In addition, Thibault et al. (103) reported that individuals with inflammatory bowel disease have decreased expression of the MCT1 transporter, which was replicated in a mechanistic in vitro study that showed that treating the intestinal cell line HT-29 with the inflammatory cytokines IFN-γ and TNF-α dose-dependently decreases mRNA expression and protein concentrations of this transporter. The authors of the study hypothesized that the mechanism underlying this phenomenon may be due to an inflammatory response element in the promoter region of the MCT1 gene. Hence, inflammatory processes mediated through NF-κB signaling or another inflammatory signaling pathway likely play a role in the expression of MCT1 and, by extension, may affect the absorption of certain phenolic compounds.
Furthermore, the inflammatory response mediated through NF-κB induction is known to modulate the expression of P-gp (69). A review on P-gp expression in the intestine suggests that it is increased in diabetic humans due to elevated inflammatory cytokines and blood glucose concentrations (104). In addition, the transporter multidrug resistance–associated protein (MRP) 3 responds to inflammatory stimuli and has been reported to be either up- or downregulated depending on the animal model used in the study (82). More research is needed in humans to provide a definitive answer on how disease states affect these transporters; however, existing data do suggest that the diabetic condition likely increases efflux transporter activity, which could then potentially increase the excretion of phenolic compounds.
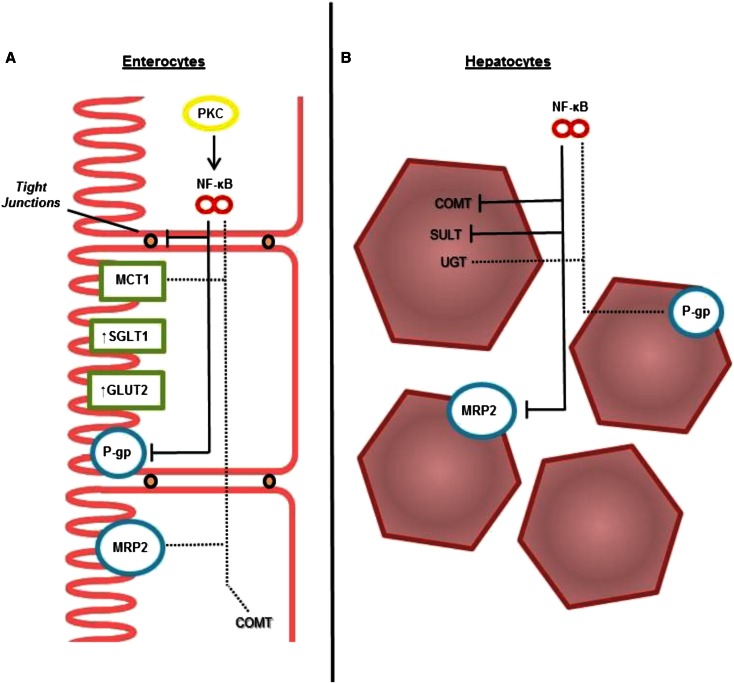
Additional evidence supports the relation between increased inflammatory status and altered xenobiotic metabolizing and transporting systems. Individuals with Crohn disease, a condition of elevated inflammation in the gut, show increased expression of P-gp in intestinal and hepatic tissue (105) and increased permeability of tight junctions, resulting in decreased ability of the gut to maintain barrier function (106). Although the exact mechanisms behind this relation have not been fully elucidated, decreased mRNA expression of pregnane X receptor (PXR), which controls select metabolizing and efflux proteins, has been observed in the ilium of individuals with active Crohn disease (107). MRP2/3 and additional drug metabolizing systems also have both been found to be altered by increased inflammatory status (82, 108, 109). In addition, elevation of protein kinase C in the diabetic state leads to an increase in the p65 subunit of the NF-κB transcription factor, which then may increase P-gp concentrations in the liver (69, 110). Figure 4 synthesizes this information in a potential model for mechanistic control of altered absorption of phenolic compounds under increased inflammatory status.
FIGURE 4.
Potential mechanism for altered xenobiotic transport and metabolizing systems in human intestinal (A) and hepatic (B) tissues. Increased NF-κB–mediated inflammation resulting from the diabetic and obese condition is believed to be involved in the alterations. Dashed lines indicate systems potentially affected by increased inflammatory signaling, and solid lines indicate those negatively affected. COMT, catechol-O-methyltransferase; GLUT2, glucose transporter 2; MCT1, monocarboxylate transporter 1; MRP2, multidrug resistance–associated protein 2; P-gp, P-glycoprotein; Phase II, phase II metabolizing enzymes; PKC, protein kinase C; SGLT1, sodium-dependent glucose transporter 1; SULT, sulfotransferase; UGT, UDP-glucuronyltransferase.
Conclusions and Implications
Absorption, metabolism, and transport of phenolic compounds to target tissues are all essential for effective delivery of their bioactivity from food and dietary supplements; and these processes appear to be affected by health status. The overall effect from these diseases is that they may create a situation in which the application of foods or supplements rich in phenolic compounds may not be as effective as expected due to alteration of their absorption, metabolism, and excretion. Subsequently, formulations and therapies may have to be adjusted to accommodate for altered bioavailability of these phenolic compounds. This may include modifying formulations to include specific macronutrients such as protein and carbohydrate (e.g., starch and sucrose) and micronutrients such as ascorbic acid, which have been previously described to influence the bioavailability of phenolic compounds (21, 111).
Although some evidence does exist for the possible sources of the differences, it is critical to fully understand the underlying mechanism for the observed differences in phenolic compound effectiveness and/or bioavailability and metabolism between healthy and obese or diabetic individuals. Only then will strategies to enhance the effectiveness of preventative ameliorative therapies that use these phenolic compounds be improved. Continued research on the fundamentals of the absorption and metabolism of dietary phenolic compounds can help inform future studies that use these compounds in individuals with obesity or diabetes. One goal of future research should be to specifically compare the differential absorption of phenolic compounds in individuals with disease compared with healthy individuals. The study design will have to be carefully addressed to elucidate the mechanism for the decreased absorption of phenolic compounds in those with obesity or diabetes. Experiments of this nature are needed to confirm differences in absorption parameters of phenolic compounds between normal individuals and those with disease. With evolving research that targets the fundamentals of phenolic compound absorption, researchers and product developers will be able to leverage these findings to develop products and strategies that better target obese or diabetic populations.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABC, ATP-binding cassette; Cmax, maximum concentration; COMT, catechol-O-methyltransferase; GLUT, glucose transporter; MCT, monocarboxylate transporter; MRP, multidrug resistance–associated protein; MDR2, multidrug resistance protein 2; P-gp, P-glycoprotein; PXR, pregnane X receptor; SGLT, sodium-dependent glucose transporter; SULT, sulfotransferase; UGT, UDP-glucuronyl transferase.
References
- 1.Zamora-Ros R, Forouhi NG, Sharp SJ, González CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care 2013;36:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenore GC, Stiuso P, Campiglia P, Novellino E. In vitro hypoglycaemic and hypolipidemic potential of white tea polyphenols. Food Chem 2013;141:2379–84. [DOI] [PubMed] [Google Scholar]
- 3.Tian C, Ye X, Zhang R, Long J, Ren W, Ding S, Liao D, Jin X, Wu H, Xu S, et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARγ-adiponectin pathway. PLoS One 2013;8:e53796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 5.Jia L, Liu X, Bai YY, Li SH, Sun K, He C, Hui R. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2010;92:218–25. [DOI] [PubMed] [Google Scholar]
- 6.Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr 2004;134:613–7. [DOI] [PubMed] [Google Scholar]
- 8.Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the Phenol-Explorer database. J Agric Food Chem 2010;58:4959–69. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur J Clin Nutr 2010;64(Suppl 3):S112–20. [DOI] [PubMed] [Google Scholar]
- 11.Bhagwat S, Haytowitz DB, Wasswa-Kintu S. USDA’s expanded flavonoid database for the assessment of dietary intakes [Internet]. Washington (DC): USDA–Agricultural Research Service; 2014 [cited 2016 Mar 3]. Available from: http://www.ars.usda.gov/nutrientdata.
- 12.Sebastian RS, Wilkinson Enns C, Goldman JD, Martin CL, Steinfeldt LC, Murayi T, Moshfegh AJ. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US adults. J Nutr 2015;145:1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjønneland A, Kyrø C, Fagherazzi G, Boutron-Ruault M-C, et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr 2016;55:1359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jun S, Shin S, Joung H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br J Nutr 2016;115:480–9. [DOI] [PubMed] [Google Scholar]
- 15.Ferruzzi MG. The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiol Behav 2010;100:33–41. [DOI] [PubMed] [Google Scholar]
- 16.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13–27. [DOI] [PubMed] [Google Scholar]
- 17.Xiao J, Högger P. Influence of diabetes on the pharmacokinetic behavior of natural polyphenols. Curr Drug Metab 2014;15:23–9. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Gerspach AC, Wölnerhanssen B, Beglinger B, Nessenius F, Napitupulu M, Schulte FH, Steinert RE, Beglinger C. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav 2014;129:265–71. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz M, O’Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med 2002;19:177–94. [DOI] [PubMed] [Google Scholar]
- 20.Hartstra AV, Bouter KEC, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015;38:159–65. [DOI] [PubMed] [Google Scholar]
- 21.Neilson AP, Ferruzzi MG. Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa. Annu Rev Food Sci Technol 2011;2:125–51. [DOI] [PubMed] [Google Scholar]
- 22.Clifford MN, van der Hooft JJJ, Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr 2013;98(Suppl):1619S–30S. [DOI] [PubMed] [Google Scholar]
- 23.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–42S. [DOI] [PubMed] [Google Scholar]
- 24.Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr 2010;104(Suppl 3):S67–90. [DOI] [PubMed] [Google Scholar]
- 25.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005;81:243S–55S. [DOI] [PubMed] [Google Scholar]
- 26.Croom E. Metabolism of xenobiotics of human environments. Prog Mol Biol Transl Sci 2012;112:31–88. [DOI] [PubMed] [Google Scholar]
- 27.Lambert JD, Sang S, Lu AYH, Yang CS. Metabolism of dietary polyphenols and possible interactions with drugs. Curr Drug Metab 2007;8:499–507. [DOI] [PubMed] [Google Scholar]
- 28.Calani L, Dall’Asta M, Derlindati E, Scazzina F, Bruni R, Del Rio D. Colonic metabolism of polyphenols from coffee, green tea, and hazelnut skins. J Clin Gastroenterol 2012;46(Suppl):S95–9. [DOI] [PubMed] [Google Scholar]
- 29.Banini AE, Boyd LC, Allen JC, Allen HG, Sauls DL. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006;22:1137–45. [DOI] [PubMed] [Google Scholar]
- 30.Dragoni S, Gee J, Bennett R, Valoti M, Sgaragli G. Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro. Br J Pharmacol 2006;147:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otaolaurruchi E, Fernández-Pachón MS, Gonzalez AG, Troncoso AM, García-Parrilla MC. Repeated red wine consumption and changes on plasma antioxidant capacity and endogenous antioxidants (uric acid and protein thiol groups). J Agric Food Chem 2007;55:9713–8. [DOI] [PubMed] [Google Scholar]
- 33.Davison K, Coates AM, Buckley JD, Howe PRC. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32:1289–96. [DOI] [PubMed] [Google Scholar]
- 34.Lampe JW, Chang J-L. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol 2007;17:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos-Bedolla P, Walter FR, Veszelka S, Deli MA. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch Med Res 2014;45:610–38. [DOI] [PubMed] [Google Scholar]
- 36.Ouzzine M, Gulberti S, Ramalanjaona N, Magdalou J, Fournel-Gigleux S. The UDP-glucuronosyltransferases of the blood-brain barrier: their role in drug metabolism and detoxication. Front Cell Neurosci 2014;8:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm 2007;4:819–25. [DOI] [PubMed] [Google Scholar]
- 38.Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, Andres-Lacueva C, Bartolomé B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct 2010;1:233. [DOI] [PubMed] [Google Scholar]
- 39.Spencer JPE, Abd-el-Mohsen MM, Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch Biochem Biophys 2004;423:148–61. [DOI] [PubMed] [Google Scholar]
- 40.Walle T. Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption. Mol Pharm 2007;4:826–32. [DOI] [PubMed] [Google Scholar]
- 41.Chen T-Y, Kritchevsky J, Hargett K, Feller K, Klobusnik R, Song BJ, Cooper B, Jouni Z, Ferruzzi MG, Janle EM. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol Nutr Food Res 2015;59:2432–47. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B, Pan Y, Gowda GAN, Raftery D, Arrieta-Cruz I, et al. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J Neurosci 2012;32:5144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferruzzi MG, Lobo JK, Janle EM, Cooper B, Simon JE, Wu Q-L, Welch C, Ho L, Weaver C, Pasinetti GM. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J Alzheimers Dis 2009;18:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dueñas M, González-Manzano S, González-Paramás A, Santos-Buelga C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J Pharm Biomed Anal 2010;51:443–9. [DOI] [PubMed] [Google Scholar]
- 45.Ishimoto H, Tai A, Yoshimura M, Amakura Y, Yoshida T, Hatano T, Ito H. Antioxidative properties of functional polyphenols and their metabolites assessed by an ORAC assay. Biosci Biotechnol Biochem 2012;76:395–9. [DOI] [PubMed] [Google Scholar]
- 46.Peters C, Schmidt B, Rommerskirch W, Rupp K, Zühlsdorf M, Vingron M, Meyer HE, Pohlmann R, von Figura K. Phylogenetic conservation of arylsulfatases: cDNA cloning and expression of human arylsulfatase B. J Biol Chem 1990;265:3374–81. [PubMed] [Google Scholar]
- 47.Zhang L, Zheng Y, Chow MSS, Zuo Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int J Pharm 2004;287:1–12. [DOI] [PubMed] [Google Scholar]
- 48.Lasa A, Churruca I, Eseberri I, Andrés-Lacueva C, Portillo MP. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol Nutr Food Res 2012;56:1559–68. [DOI] [PubMed] [Google Scholar]
- 49.Sinnott ML. Catalytic mechanism of enzymic glycosyl transfer. Chem Rev 1990;90:1171–202. [Google Scholar]
- 50.Lu D-L, Ding D-J, Yan W-J, Li R-R, Dai F, Wang Q, Yu S-S, Li Y, Jin X-L, Zhou B. Influence of glucuronidation and reduction modifications of resveratrol on its biological activities. Chembiochem 2013;14:1094–104. [DOI] [PubMed] [Google Scholar]
- 51.Ruotolo R, Calani L, Brighenti F, Crozier A, Ottonello S, Del Rio D. Glucuronidation does not suppress the estrogenic activity of quercetin in yeast and human breast cancer cell model systems. Arch Biochem Biophys 2014;559:62–7. [DOI] [PubMed] [Google Scholar]
- 52.Silberberg M, Morand C, Mathevon T, Besson C, Manach C, Scalbert A, Remesy C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur J Nutr 2006;45:88–96. [DOI] [PubMed] [Google Scholar]
- 53.Gwilt PR, Nahhas RR, Tracewell WG. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet 1991;20:477–90. [DOI] [PubMed] [Google Scholar]
- 54.Brill MJE, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CAJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 2012;51:277–304. [DOI] [PubMed] [Google Scholar]
- 55.Abernethy DR, Greenblatt DJ, Divoll M, Shader RI. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med 1983;101:873–80. [PubMed] [Google Scholar]
- 56.Boocock DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 2007;16:1246–52. [DOI] [PubMed] [Google Scholar]
- 57.Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014;12:2092–103. [DOI] [PubMed] [Google Scholar]
- 58.Sabir N, Sermez Y, Kazil S, Zencir M. Correlation of abdominal fat accumulation and liver steatosis: importance of ultrasonographic and anthropometric measurements. Eur J Ultrasound 2001;14:121–8. [DOI] [PubMed] [Google Scholar]
- 59.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–27. [DOI] [PubMed] [Google Scholar]
- 60.Lee JH, Yang SH, Oh JM, Lee MG. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol 2010;62:1–23. [DOI] [PubMed] [Google Scholar]
- 61.Liu L, Deng Y-X, Liang Y, Pang X-Y, Liu X-D, Liu Y-W, Yang J-S, Xie L, Wang G-J. Increased oral AUC of baicalin in streptozotocin-induced diabetic rats due to the increased activity of intestinal β-glucuronidase. Planta Med 2010;76:70–5. [DOI] [PubMed] [Google Scholar]
- 62.Yu S, Yu Y, Liu L, Wang X, Lu S, Liang Y, Liu X, Xie L, Wang G. Increased plasma exposures of five protoberberine alkaloids from Coptidis rhizoma in streptozotocin-induced diabetic rats: is P-GP involved? Planta Med 2010;76:876–81. [DOI] [PubMed] [Google Scholar]
- 63.Liu H, Wu B, Pan G, He L, Li Z, Fan M, Jian L, Chen M, Wang K, Huang C. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab Dispos 2012;40:2109–18. [DOI] [PubMed] [Google Scholar]
- 64.Kang HE, Sohn SI, Baek SR, Lee JW, Lee MG. Liquiritigenin pharmacokinetics in a rat model of diabetes mellitus induced by streptozotocin: greater formation of glucuronides in the liver, especially M2, due to increased hepatic uridine 5′-diphosphoglucuronic acid level. Metabolism 2010;59:1472–80. [DOI] [PubMed] [Google Scholar]
- 65.Hasegawa Y, Kishimoto S, Shibatani N, Nomura H, Ishii Y, Onishi M, Inotsume N, Takeuchi Y, Fukushima S. The pharmacokinetics of morphine and its glucuronide conjugate in a rat model of streptozotocin-induced diabetes and the expression of MRP2, MRP3 and UGT2B1 in the liver. J Pharm Pharmacol 2010;62:310–4. [DOI] [PubMed] [Google Scholar]
- 66.Wang JP, Liu IM, Tzeng TF, Cheng JT. Decrease in catechol-O-methyltransferase activity in the liver of streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol 2002;29:419–22. [DOI] [PubMed] [Google Scholar]
- 67.Braun L, Coffey MJ, Puskás F, Kardon T, Nagy G, Conley AA, Burchell B, Mandl J. Molecular basis of bilirubin UDP-glucuronosyltransferase induction in spontaneously diabetic rats, acetone-treated rats and starved rats. Biochem J 1998;336:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burant CF, Flink S, DePaoli AM, Chen J, Lee WS, Hediger MA, Buse JB, Chang EB. Small intestine hexose transport in experimental diabetes. Increased transporter mRNA and protein expression in enterocytes. J Clin Invest 1994;93:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeng H-J, Kim M-H, Jin H-E, Shin SM, Tsuruo T, Kim SG, Kim D-D, Shim C-K, Chung S-J. Functional induction of P-glycoprotein in the blood-brain barrier of streptozotocin-induced diabetic rats: evidence for the involvement of nuclear factor-kappaB, a nitrosative stress-sensitive transcription factor, in the regulation. Drug Metab Dispos 2007;35:1996–2005. [DOI] [PubMed] [Google Scholar]
- 70.Zhang LL, Lu L, Jin S, Jing X, Yao D, Hu N, Liu L, Duan R, Liu X, Wang G, et al. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 2011;32:956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H, Xu X, Yang Z, Deng Y, Liu X, Xie L. Impaired function and expression of P-glycoprotein in blood-brain barrier of streptozotocin-induced diabetic rats. Brain Res 2006;1123:245–52. [DOI] [PubMed] [Google Scholar]
- 72.Liu H, Zhang D, Xu X, Liu X, Wang G, Xie L, Pang X, Liu L. Attenuated function and expression of P-glycoprotein at blood-brain barrier and increased brain distribution of phenobarbital in streptozotocin-induced diabetic mice. Eur J Pharmacol 2007;561:226–32. [DOI] [PubMed] [Google Scholar]
- 73.Nawa A, Fujita Hamabe W, Tokuyama S. Inducible nitric oxide synthase-mediated decrease of intestinal P-glycoprotein expression under streptozotocin-induced diabetic conditions. Life Sci 2010;86:402–9. [DOI] [PubMed] [Google Scholar]
- 74.van Waarde WM, Verkade HJ, Wolters H, Havinga R, Baller J, Bloks V, Müller M, Sauer PJJ, Kuipers F. Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC-transporters in rats. Gastroenterology 2002;122:1842–52. [DOI] [PubMed] [Google Scholar]
- 75.Nikooie R, Rajabi H, Gharakhanlu R, Atabi F, Omidfar K, Aveseh M, Larijani B. Exercise-induced changes of MCT1 in cardiac and skeletal muscles of diabetic rats induced by high-fat diet and STZ. J Physiol Biochem 2013;69:865–77. [DOI] [PubMed] [Google Scholar]
- 76.Kim M-S, Wang S, Shen Z, Kochansky CJ, Strauss JR, Franklin RB, Vincent SH. Differences in the pharmacokinetics of peroxisome proliferator-activated receptor agonists in genetically obese Zucker and Sprague-Dawley rats: implications of decreased glucuronidation in obese Zucker rats. Drug Metab Dispos 2004;32:909–14. [PubMed] [Google Scholar]
- 77.Xu J, Kulkarni SR, Li L, Slitt AL. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug Metab Dispos 2012;40:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucas-Teixeira VA, Hussain T, Serrão P, Soares-da-Silva P, Lokhandwala MF. Intestinal dopaminergic activity in obese and lean Zucker rats: response to high salt intake. Clin Exp Hypertens 2002;24:383–96. [DOI] [PubMed] [Google Scholar]
- 79.Koide CLK, Collier AC, Berry MJ, Panee J. The effect of bamboo extract on hepatic biotransforming enzymes—findings from an obese-diabetic mouse model. J Ethnopharmacol 2011;133:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim MS, Shigenaga J, Moser A, Grunfeld C, Feingold KR. Suppression of DHEA sulfotransferase (Sult2A1) during the acute-phase response. Am J Physiol Endocrinol Metab 2004;287:E731–8. [DOI] [PubMed] [Google Scholar]
- 81.Shimada M, Watanabe E, Iida Y, Nagata K, Yamazoe Y. Alteration of hepatic sulfation by endotoxin. Jpn J Pharmacol 1999;80:371–3. [DOI] [PubMed] [Google Scholar]
- 82.Ho EA, Piquette-Miller M. KLF6 and HSF4 transcriptionally regulate multidrug resistance transporters during inflammation. Biochem Biophys Res Commun 2007;353:679–85. [DOI] [PubMed] [Google Scholar]
- 83.Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, Slitt AL, Cherrington NJ. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 2013;41:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yalcin EB, More V, Neira KL, Lu ZJ, Cherrington NJ, Slitt AL, King RS. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab Dispos 2013;41:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Metz L, Mercier J, Tremblay A, Alméras N, Joanisse DR. Effect of weight loss on lactate transporter expression in skeletal muscle of obese subjects. J Appl Physiol 2008;104:633–8. [DOI] [PubMed] [Google Scholar]
- 86.Dostalek M, Court MH, Hazarika S, Akhlaghi F. Diabetes mellitus reduces activity of human UDP-glucuronosyltransferase 2B7 in liver and kidney leading to decreased formation of mycophenolic acid acyl-glucuronide metabolite. Drug Metab Dispos 2011;39:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Juel C, Holten MK, Dela F. Effects of strength training on muscle lactate release and MCT1 and MCT4 content in healthy and type 2 diabetic humans. J Physiol 2004;556:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enoki T, Yoshida Y, Hatta H, Bonen A. Exercise training alleviates MCT1 and MCT4 reductions in heart and skeletal muscles of STZ-induced diabetic rats. J Appl Physiol 2003;94:2433–8. [DOI] [PubMed] [Google Scholar]
- 89.Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab 2015;41:5–17. [DOI] [PubMed] [Google Scholar]
- 90.Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 2008;83:898–903. [DOI] [PubMed] [Google Scholar]
- 91.Schoket B, Papp G, Lévay K, Mracková G, Kadlubar FF, Vincze I. Impact of metabolic genotypes on levels of biomarkers of genotoxic exposure. Mutat Res 2001;482:57–69. [DOI] [PubMed] [Google Scholar]
- 92.Cheng X, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos 2009;37:2178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mendonza AE, Gohh RY, Akhlaghi F. Blood and plasma pharmacokinetics of ciclosporin in diabetic kidney transplant recipients. Clin Pharmacokinet 2008;47:733–42. [DOI] [PubMed] [Google Scholar]
- 94.Ogata M, Uchimura T, Iizuka Y, Murata R, Suzuki S, Toyota T, Hikichi N. Effect of non-insulin dependent diabetes on cyclosporin A disposition in Goto-Kakizaki (GK) rats. Biol Pharm Bull 1997;20:1026–9. [DOI] [PubMed] [Google Scholar]
- 95.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Krogh Pedersen H, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015;528:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomás-Barberán FA, García-Villalba R, González-Sarrías A, Selma MV, Espín JC. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 2014;62:6535–8. [DOI] [PubMed] [Google Scholar]
- 98.Selma MV, Romo-Vaquero M, García-Villalba R, González-Sarrías A, Tomás-Barberán FA, Espín JC. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct 2016;7:1769–74. [DOI] [PubMed] [Google Scholar]
- 99.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest 2006;116:33–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 101.Waring RH, Harris RM, Hunter JO, Mitchell SC. Xenobiotic sulphation and its variability during inflammation: a factor in adverse drug reactions? Curr Drug Metab 2013;14:361–5. [DOI] [PubMed] [Google Scholar]
- 102.Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther 2007;113:88–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier J-F, Galmiche J-P, Shirazi-Beechey S, Segain J-P. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 2007;133:1916–27. [DOI] [PubMed] [Google Scholar]
- 104.Kobori T, Harada S, Nakamoto K, Tokuyama S. Functional alterations of intestinal P-glycoprotein under diabetic conditions. Biol Pharm Bull 2013;36:1381–90. [DOI] [PubMed] [Google Scholar]
- 105.Fakhoury M, Lecordier J, Medard Y, Peuchmaur M, Jacqz-Agrain E. Impact of inflammation on the duodenal mRNA expression of CYP3A and P-glycoprotein in children with Crohn’s disease. Inflamm Bowel Dis 2006;12:745–9. [DOI] [PubMed] [Google Scholar]
- 106.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 2008;180:5653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shakhnovich V, Vyhlidal C, Friesen C, Hildreth A, Singh V, Daniel J, Kearns GL, Leeder JS. Decreased pregnane X receptor expression in children with active Crohn’s disease. Drug Metab Dispos 2016;44:1066–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le Vee M, Lecureur V, Stieger B, Fardel O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab Dispos 2009;37:685–93. [DOI] [PubMed] [Google Scholar]
- 109.Vet NJ, Brussee JM, de Hoog M, Mooij MG, Verlaat CWM, Jerchel IS, van Schaik RHN, Koch BCP, Tibboel D, Knibbe CAJ, et al. Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am J Respir Crit Care Med 2016;194:58–66. [DOI] [PubMed] [Google Scholar]
- 110.Kameyama N, Arisawa S, Ueyama J, Kagota S, Shinozuka K, Hattori A, Tatsumi Y, Hayashi H, Takagi K, Wakusawa S Increase in P-glycoprotein accompanied by activation of protein kinase Cα and NF-κB p65 in the livers of rats with streptozotocin-induced diabetes. Biochim Biophys Acta 2008;1782:355–60. [DOI] [PubMed] [Google Scholar]
- 111.Bordenave N, Hamaker BR, Ferruzzi MG. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct 2014;5:18–34. [DOI] [PubMed] [Google Scholar]