Abstract
Increased dietary fiber (DF) intake elicits a wide range of physiologic effects, not just locally in the gut, but systemically. DFs can greatly alter the gut milieu by affecting the gut microbiome, which in turn influences the gut barrier, gastrointestinal immune and endocrine responses, and nitrogen cycling and microbial metabolism. These gut-associated changes can then alter the physiology and biochemistry of the body’s other main nutrient management and detoxification organs, the liver and kidneys. The molecular mechanisms by which DF alters the physiology of the gut, liver, and kidneys is likely through gut-localized events (i.e., bacterial nitrogen metabolism, microbe-microbe, and microbe–host cell interactions) coupled with specific factors that emanate from the gut in response to DF, which signal to or affect the physiology of the liver and kidneys. The latter may include microbe-derived xenometabolites, peptides, or bioactive food components made available by gut microbes, inflammation signals, and gut hormones. The intent of this review is to summarize how DF alters the gut milieu to specifically affect intestinal, liver, and kidney functions and to discuss the potential local and systemic signaling networks that are involved.
Keywords: xenobiotic, microbiota, fiber, chronic kidney disease, nonalcoholic fatty liver disease
Introduction
The consumption of dietary fiber (DF)8 can positively affect gut health (1) as well as non-gastrointestinally related conditions such as diabetes (2), cardiovascular disease (3), nonalcoholic fatty liver disease (NAFLD) (4), and chronic kidney disease (CKD) (5). DF has a variety of physiologic effects (6–8), such as fostering the growth of select gut microbes (9), altering the production of host factors such as hormones (10) and cytokines (11), as well as the production of microbe-derived metabolites (xenometabolites) (12). With respect to specific target organs, poor gut health is increasingly recognized as an important contributor in regulating the physiology and biochemistry of nutrient management and detoxification; this concept has given rise to terms such as the gut-liver axis (13) and the gut-kidney axis (14). The intent of this review is to focus on these systems 1) by considering how various DFs affect the gut milieu to alter intestine, liver, and kidney function and 2) to provide examples of how “omics”-based technologies can be leveraged to gain novel insights into potential mechanisms and the therapeutic potential of DF. To place these topics into proper context, a brief overview of DF is presented.
Defining and classifying DF.
The definitions and nutritional aspects of DF have been comprehensively reviewed elsewhere (7, 15), and thus only the key highlights are described herein. The term “dietary fiber” encompasses a wide range of nondigestible carbohydrates. Several definitions and classification systems for DF exist, as the highly varied nature of DF has made it difficult to define and classify. The Institute of Medicine divides fiber into 2 categories: 1) DF, which consists of nondigestible carbohydrates and lignin that are intrinsic and intact in plants, and 2) functional fiber, which consists of isolated, nondigestible carbohydrates that have beneficial physiologic effects in humans. Total fiber is defined as the sum of DF and functional fiber (16). DF has been categorized on the basis of solubility, viscosity, susceptibility to fermentation by gastrointestinal bacteria, and whether the fiber occurs naturally in plants or is isolated or synthetic. The term “viscosity” (gel-forming ability) is preferred over solubility because it is a better predictor of physiologic outcomes than is solubility (7). Establishing consistent viscosity values has been difficult because viscosity can differ on the basis of the concentration of fiber, diet matrix, pH, and temperature (17). The susceptibility of fermentation by the gut microbiota has also been used to classify DF as fermentable or nonfermentable. The degree of fermentation is often assessed by the microbial production of SCFAs or the disappearance of fiber from the feces (18). Each classification system includes fibers that vary greatly in composition and structure (19), and these differences in monosaccharide content, glycosidic linkages, degree of polymerization (length of molecule), degree of substitution (side chains), and fiber preparation can contribute to the different physiologic outcomes associated with certain fiber types (20). It is important to acknowledge these differences in order to attribute health outcomes with fiber type; this information can then be leveraged to develop more specific fiber recommendations to achieve desired outcomes (i.e., reduced hepatic lipid accumulation in NAFLD, lowered serum creatinine to ameliorate CKD, etc.). A list of common fiber types, structure, and food sources is shown in Table 1.
TABLE 1.
Common fiber types, structure, and food sources
| Fiber type | Structure | Sources |
| Lignin | Cross-linked aromatic rings (21) | Ubiquitous in plant cell walls |
| Cellulose | β-(1,4)-Linked glucose units (22) | Ubiquitous in plant cell walls |
| Arabinoxylan | β-(1,4)-Linked xylose backbone with arabinose side chains (23) | Cereal grains |
| Inulin | β-(2,1)-Linked fructose units typically with terminal glucose ends (24) | Onions, Jerusalem artichokes, and chicory root isolates added to processed foods to increase fiber content (25) |
| β-Glucan | β-(1,3)-Linked glucose units (26) | Cereals and mushrooms |
| Guar gum | β-(1,4)-Linked mannose residues with α-(1,6)-linked galactose side chains (27) | Guar bean |
| Gum acacia (gum arabic) | β-(1,3)-Linked galactose backbone with highly branched arabinose and rhamnose side chains and glycoproteins (28) | Hardened Acacia tree sap |
| Pectin | Complex chemical structures generally consisting of an α-(1,4)-linked galacturonic acid backbone with arabinose, galactose, and/or xylose side chains (29) | Apples, pears, peaches, and cherries (30) |
| Psyllium | β-(1,4)-Linked xylose backbone with arabinose and xylose side chains (31) | Seeds from the genus Plantago |
| Fructo-oligosaccharides | Two to 10 β-(1,2)-linked fructose units (32, 33) | Inulin degradation or transfructosylation of sucrose |
| Resistant starch (5 types) | α-(1,4)-Linked glucose molecules (34, 35) | Type 1: whole kernel grains |
| Type 2: green bananas, high-amylose corn starch | ||
| Type 3: cooked then cooled potatoes and rice | ||
| Type 4: chemically cross-linked | ||
| Type 5: lipid interactions |
DF alters gut microbiota and xenometabolites.
A well-known effect of DF is the alteration of the gut microbiota. The term “gut microbiota” refers to all of the archaea, bacteria, eukaryotes (i.e., fungi and parasites), and viruses present in the gut. Bacteria are the most intensely studied and characterized; however, emerging evidence indicates that other gut microbes (fungi, viruses, and yeasts) are also important modulators of host phenotype (36, 37). The human gut microbiome is characterized by trillions of microbes that possess 150-fold more protein-coding genes than the human genome (38, 39). These microbial genes greatly expand the metabolic potential of the host by providing enzymes the host lacks, such as those that degrade various DFs (40). Unlike the human genome, which is largely fixed, the gut microbiome is plastic and can be affected by diet (12), past and present diseases (41), lifestyle factors such as exercise (42) and stress (43), or environmental exposures (44). These factors contribute to the high interindividual variation observed in the gut microbiota (45), which has made it difficult to establish consistent DF-induced bacterial changes, at least in humans.
Fermenter systems that mimic human digestion in vitro have been used to overcome the challenges of interindividuality. One such study compared the fermentation of inulin and apple pectin and found that apple pectin gave rise to a more diverse bacterial community (46). This is likely due to the complex structural and chemical nature of pectin (47). Thus, DF complexity plays an important role in microbial diversity. This is further supported by the finding that resistant starch (RS) decreases microbial diversity (48). RS has a simple structure and chemical composition [composed solely of α-(1,4)-linked glucose molecules] (49) and may therefore select for a more homogeneous microbiome than a fiber with a complex chemical structure, such as pectin. Typically, diets that consist of a variety of fiber-rich foods give rise to a more diverse gut microbiota (50, 51), and this is generally associated with better health outcomes (52, 53). It has been proposed that individuals with more diverse gut microbiota are more adept at responding to environmental challenges (54), such as resisting colonization of gut pathogens by competitive exclusion (i.e., commensal bacteria take over niches and/or consume substrates to inhibit the growth of pathogenic bacteria) (55). Interestingly, mice fed low-fiber diets showed decreased microbial diversity, which could be recovered after the introduction of a high-fiber diet; however, after generations of feeding a low-fiber diet, microbial diversity could not be recovered after the re-introduction of fiber (56). This finding may have implications to our current population, because DF consumption has decreased since the industrial revolution (57).
In summary, DF encompasses a wide variety of carbohydrates that vary greatly in chemical composition and structure. This inherent variability along with preparation method and differences in resident host microbiota contribute to the range of responses observed with the consumption of different fiber types. In general, increased DF consumption has been attributed to improved health outcomes, especially in relation to gut health.
Impact of DF on the Gut, the Gatekeeper of the Body
The gut has the dual and opposing roles of allowing nutrients to enter the body while excluding the entry of harmful substances. Both gut barrier function and nutrient absorption have been shown to be altered by DF. One example of DF-induced changes to the gut barrier is an increase in mucins and the cells that produce them, goblet cells (58, 59). Mucins are large glycoproteins that, along with water, ions, proteins, lipids, antibodies, antimicrobial peptides, and bacteria, form what is known as mucus (60). Mucus acts to protect the gut epithelium from mechanical stress, to lubricate the intestine to ease transit of digested material, and to prevent the translocation of harmful substances. A study comparing a standard rodent diet (fiber from wheat, corn, and oats comprising 4.3% of the diet by weight) with a diet devoid of any fiber showed that mice fed the fiber-deficient diet had a thinner mucus layer, thus allowing microbes to come in closer proximity to the gut epithelium (61). Without sufficient amounts of DF in the gut, bacteria may degrade the host mucus layer in order to provide themselves with the substrates necessary to survive, thus breaking down one of the host’s physical barriers.
SCFAs resulting from the fermentation of DF have been shown to bolster gut barrier function by increasing gut cell proliferation and differentiation (62). SCFAs decrease intestinal pH, which can alter the gut microbiota by inhibiting the growth of pathogens and reduce the expression of microbial virulence genes (63). Recently, it was shown that epithelial cell lines metabolize the SCFA butyrate (and to a lesser extent propionate and acetate), resulting in oxygen reduction that leads to stabilization of the transcription factor, hypoxia-inducible factor 1α (Hif-1α) (64). In the intestine, this transcription factor has been implicated in gut barrier function by regulating inflammation (65) and apoptosis (66). A microarray study found increased levels of Hif-1α expression along with increases in genes related to cell growth, proliferation, differentiation, and apoptosis in the cecal tissue of rats supplemented with 30% RS compared with rats fed an equivalent amount of energy from a low-fiber diet (67). Another component of the gut barrier affected by DF is that of tight junction proteins. One study found that feeding a standard rodent diet supplemented with 10% fructo-oligosaccharides (FOSs) increased gene expression of the jejunal tight junction proteins occludin and ZO1, reduced intestinal permeability, and lowered plasma LPS concentrations. With regard to mechanism of action, these changes were ablated by injections of glucagon-like peptide 2 (GLP-2) antagonist over 4 wk (68). GLP-2 has been shown to regulate both transcellular and paracellular gut permeability (69, 70), increase epithelial cell proliferation (71), and promote intestinal wound healing through a TGF-β–mediated mechanism (72). Notably, rats fed 2.5% pectin for 2 wk showed increased cecal SCFAs and increased plasma GLP-2 (73). Butyrate, in a Caco-2 cell culture model, was also shown to activate AMP-activated protein kinase, resulting in tight junction protein assembly and improved barrier function indicated by increased transepithelial electrical resistance (TEER) (74). Another study that used Caco-2 cells found that butyrate increased lipoxygenase activity by inhibiting histone deacetylation, resulting in increased TEER (75). The importance of fiber has also been recognized in critical care settings, because the use of total enteral or parenteral diets that lack fiber were found to induce gut atrophy and to increase gut permeability; this could be recovered with the addition of fiber or SCFAs (76, 77). Together, these studies highlight the importance of microbe derived SCFAs in bolstering the physical components of the gut barrier (mucus, cellularity, and tight junctions) through the regulation of specific cell signal pathways and transcription factors.
In addition to affecting physical barriers, DF can also alter gut immune factors. The gut is the largest immune organ in the body (78), harboring 70–80% of the body’s immune cells (79), and has been implicated as a major source of inflammation suggested to contribute to diseases such as NAFLD (80, 81) and CKD (82). Several studies have shown immunomodulatory activities for a variety of DFs, including FOSs (83), arabinoxylans (84), and β-glucans (85). FOSs (0.06% in the diet for 15 d) have been shown to increase the production of the immunoglobulin IgA in the cecum of rodents. Efficacy was dependent on FOS chain length, with shorter chain lengths resulting in higher cecal IgA concentrations (86). Shorter chain lengths resulted in higher viscosity and enhanced microbial fermentation (7, 87). IgA plays an important role in maintaining gut barrier function by binding to microbes and preventing adhesion and translocation of bacteria across the gut barrier (88). Mice supplemented with a 150-mM mix of SCFAs in the drinking water daily for 2 wk showed increased intestinal regulatory T cells (89), which are responsible for limiting intestinal inflammation. One way in which SCFAs have been shown to increase colonic regulatory T cells is by reducing histone deacetylase 6 (HDAC6) and HDAC9 gene expression, thereby increasing histone acetylation which allows for increased gene transcription. This process required the presence of the SCFA receptor, FFA receptor 2 (GPCR 43) (90). DF has been recognized as a potential dietary treatment for inflammatory bowel diseases because fiber can favorably affect gut microbe and gut immune factors found to be altered in diseases such as Crohn disease and ulcerative colitis (91). In summary, DF can bolster the gut barrier by maintaining host physical barriers (mucosal layer and cellular tight junctions) as well as by altering host immune factors. Such outcomes serve to minimize systemic proinflammatory insults that would otherwise gain access to tissues such as liver and kidneys.
In addition to altering physical barriers and intestinal immune function to minimize harm from microbe-derived proinflammatory factors, DF can also protect key organs such as the liver and kidney from metabolic insults. It has long been recognized that the consumption of nondigestible carbohydrates, in lieu of rapidly digestible carbohydrates, reduces increases in blood glucose and insulin. Another carbohydrate regulatory pathway affected by DF consumption was described: intestinal gluconeogenesis (92). Intestinal production of glucose is thought to increase glucose sensing in the portal vein, leading to decreases in hepatic glucose production and altered signaling to the brain, resulting in increased satiation. Fiber is thought to play a role via microbial fermentation of DF to propionate, which can then serve as a gluconeogenic precursor (93). One study found that mice supplemented with FOSs (10% by weight of the diet) for ∼2 wk showed increased mRNA expression of intestinal gluconeogenic enzymes [glucose-6-phosphatase catalytic subunit (G6pc), phosphoenolpyruvate carboxykinase 1 (Pck1)] and these changes were concurrent with reductions in body weight gain and improved glucose and insulin sensitivity despite no change in food intake; furthermore, these changes were ablated when FOSs were fed to intestine-specific G6pc (I-G6pc) knockout mice (93). Mice lacking I-G6pc are unable to convert propionate into glucose in the intestine; instead, the propionate is converted to glucose in the liver. The authors proposed that glucose production in the liver, rather than in the intestine, bypasses the gut-brain glucose-sensing system, ultimately resulting in impaired glucose and insulin homeostasis and increased adiposity in the I-G6pc knockout mice. Maintaining proper glucose and insulin homeostasis and preventing the accumulation of advanced glycation end-products is an important component for delaying disease progression in both NAFLD (94, 95) and CKD (96, 97). As we will see, beyond carbohydrate regulation through gut-derived events and signals, DF also plays an important role in fat and protein metabolism relevant to liver and kidneys.
Liver Responses to DF
The liver receives blood from the gut through the portal vein, and therefore this organ is a logical target of gut-derived factors influenced by diet and microbiome shifts. Indeed, DF is being considered as a potential treatment option for nongastrointestinal diseases, such as NAFLD (98). It is likely that the hepatic effects of DF involve alteration of microbiome ecology and hence gut permeability, systemic inflammation, and circulating gut-derived hormone and metabolite signals. Supporting the link between liver and gut health, patients with NAFLD have been found to exhibit an altered gut microbiota (80) and increased gut permeability (99), and several studies have found detectable levels of bacterial DNA in the serum (100) and in ascites fluid (excessive fluid accumulation in peritoneal cavity) of patients with cirrhosis (101). DFs have been shown to reduce translocation of bacterial products such as LPS (102); this would serve to reduce hepatic exposure to LPS and other microbe-derived proinflammatory products. This might reduce the likelihood of fatty liver progressing to the inflammatory form known as non-alcoholic steatohepatitis (NASH). The transition from fatty liver to NASH is thought to occur in 2 stages and is referred to as the “2-hit hypothesis.” The “first hit” is the accumulation of fat in the liver, making the liver more vulnerable to the “second hit,” which induces hepatic inflammation. The “second hit” is thought to come from a variety of sources, including bacterial overgrowth (103). In addition to affecting the gut barrier, DFs have also been shown to decrease erythrocyte lipid peroxidation and increase antioxidant enzyme activity (i.e., hepatic and erythrocyte superoxide dismutase and catalase) (104) and alter detoxifying enzymes in the liver (i.e., increase protein expression of cytochrome p450 1A2) (105, 106). Reduced concentrations of cytochrome p450 1A2 have been observed in human and NAFLD animal models (106). Increasing the activity of antioxidant and detoxification enzymes may be useful in preventing the transition from fatty liver to NASH. Animal models that examined the effects of fiber in NAFLD have shown promising results (98); however, to our knowledge, to date there have been no randomized controlled trials to determine the effectiveness of fiber on NAFLD in humans.
DF may also affect liver metabolism by altering bile acid pools. Bile acids aid in the absorption of dietary fat and fat-soluble vitamins (107) as well as serve as signaling molecules (108). Primary bile acids are made by the host in the liver and secondary bile acids are generated by the gut microbiota. Examples of gut microbiota–derived modulations to bile acids include the following: dehydration, deconjugation, desulfation, epimerization, and oxidation (109–111). Patients with cirrhosis showed lower fecal concentrations of secondary bile acids (lithocholic and deoxycholic acid decreased by an average of 23% and 68%, respectively) than did healthy controls (112). Decreased concentrations of secondary bile acids may be protective because secondary bile acids can destabilize membranes, potentially increasing intestinal permeability (113). The primary bile acid chenodeoxycholic acid serves as the strongest ligand for the farnesoid X receptor (FXR) (108). Bile acid activation of FXR and another bile acid receptor, TGR5, has been shown to decrease hepatic lipid accumulation and inflammation (114). A study in humans found decreased expression of FXR and increased expression of LXR, SREBP-1, and FAS proteins in the liver of patients with NAFLD compared with healthy controls (115). Not all bile acids serve as agonists for FXR: tauro-conjugated β- and α-muricholic acids serve as FXR antagonists and their generation is dependent on the types of gut microbes present (116). These observations support the idea that specific DFs may be a useful tool for fostering the growth of desired gut microbes to generate the types of bile acids that can favorably modulate metabolism (117, 118). However, more work needs to be done to determine how DF affects bile acid signaling pathways and to associate any observed changes with overall host phenotype (i.e., liver TG accumulation and inflammation).
DF, Kidney Function, and Nitrogen Metabolism
The kidney is another important organ affected by DF. For instance, DF may reduce nitrogen burden and systemic inflammatory insult in CKD. Just as with NAFLD, patients and animal models with CKD often exhibit an altered gut microbiota (119, 120), increased intestinal permeability (121), intestinal inflammation (122, 123), and increased blood concentrations of microbe-derived metabolites (e.g., indoxyl sulfate and p-cresol sulfate) (124). Epidemiologic studies have shown that increased DF intake reduces all-cause mortality in patients with CKD (125). The mechanisms are not clear, but one likely scenario involves maintenance of substrate delivery to the lower gut, which modifies bacterial metabolism. If sufficient amounts of nondigestible carbohydrates do not reach the colon, then other substrates such as amino acids will be fermented, resulting in the production of potentially harmful metabolites such as indoles and p-cresol, which stress the kidney (93, 126). Yet, patients with CKD are often advised to limit their consumption of many common fiber-rich foods to prevent the blood accumulation of potassium and phosphorus, minerals that can lead to cardiac arrhythmias and bone mineral disorders, respectively (127, 128).
Studies have begun to test if regimens that increase DF intake without increasing potassium and phosphorous load (i.e., through DF supplementation) can improve kidney function through the alteration of bacteria that metabolize uremic retention solutes and other kidney-relevant metabolites. A study of the fecal microbiota in patients with end-stage renal disease found increases in bacterial families possessing the enzymatic capacity to produce indole, p-cresol, urease, and uricase and decreases in families capable of producing butyrate (129). Microbial metabolism of urea by the enzyme urease generates ammonia, which can be further converted to ammonium hydroxide. Ammonium hydroxide increases intestinal pH and can lead to the disruption and loss of the intestinal tight junction proteins and thereby increase gut permeability (130, 131). Indoxyl sulfate and p-cresol sulfate are derived from microbial metabolite derivatives of tryptophan and tyrosine (indole and cresol), respectively, and have been associated with increased cardiovascular disease and all-cause mortality (132, 133). A study in hemodialysis patients supplemented with 15 g RS/d for 6 wk found reductions in plasma concentrations of indoxyl sulfate (134). Another study conducted by our group found that supplementing rats with adenine-induced CKD with 59% high-amylose RS (by weight of the diet) for 3 wk significantly improved kidney function and gut permeability indexes [i.e., decreased serum creatinine, increased creatinine clearance, improved tubulo-interstitial injury score, and restored colonic tight junction proteins (occludin and claudin-1)] (135). This treatment also markedly altered the systemic metabolome, including reducing serum concentrations of toxic metabolites known to accumulate in the blood of patients with CKD (e.g., indoxyl sulfate, p-cresol-sulfate) (135). Indole and p-cresol metabolites undergo O-sulfonation in the liver to enhance excretion and aid in detoxification (136). Excessive amounts of these metabolites may reduce the liver’s capacity to detoxify other metabolites, as evidenced by a pharmacometabolomic study in humans that found that individuals with higher urinary concentrations of p-cresol sulfate had a reduced capacity to sulfonate the common drug acetaminophen (137). Another study found that supplementing patients with chronic renal failure with 50 g guar gum/d for 4 wk increased fecal nitrogen excretion and decreased serum urea; these changes were not found with an equivalent amount of pectin supplementation (5). Recently, it was shown that 12 mo of combined probiotic and prebiotic treatment improved glomerular filtration rate better than a low protein diet (138). A reduced glomerular filtration rate can lead to the accumulation of toxins in the body (139).
One mechanism underlying kidney effects of DF may involve a decrease in the nitrogen load on the liver and the kidneys by increasing microbial biomass, which serves to sequester nitrogen in the gut and reduce the amount that enters the portal circulation (140). A study that compared germ-free and conventional mice found lower concentrations of amino acids entering the hepatic portal vein in the conventional mice; this decrease in portal amino acids was attributed to increased nitrogen demands for microbial synthesis (141). Increased microbial demand for nitrogen may also divert urea away from the liver into the intestine where it can be used for microbial synthesis (142). In addition to nitrogen sequestration in the gut, another novel mechanism by which DF could affect kidney function involves SCFAs. It has been proposed that SCFAs can modulate kidney blood flow through the activation of olfactory receptor 78, a G-coupled protein receptor located in the renal juxtaglomerular apparatus that increases renin secretion, which is responsible for controlling blood pressure (143). The modulation of blood pressure control is an important component of managing the progression of CKD (144). Taken together, these studies show that DF can improve CKD outcomes and kidney function by causing shifts in the microbiome that enhance or maintain the gut barrier (thus reducing bacterial translocation and subsequent inflammation), altering microbial nitrogen and uremic solute metabolism, and possibly influencing renal blood flow.
Systems Biology Approaches Expand Our Understanding of DF-Induced Changes in Host Metabolism
The molecular signals involved in DF-associated alterations in host systems remain largely unknown, and the integrated networks that modulate diet-microbiome-host crosstalk remain to be fully elaborated. Some aspects of signaling were discussed above for well-known molecules (i.e., SCFAs, GLP-2, and proinflammatory factors such as LPS), but there are certainly many more that remain to be discovered. Exploring the comprehensive effects of DF-induced outcomes has been made possible by the advent and widespread adoption of “omics”-based technologies (e.g., transcriptomics, proteomics, and metabolomics). These tools have allowed researchers to move beyond measuring a few classic biomarkers and to begin to explore, in an unbiased manner, how DF affects body-wide systems and the molecular events in specific tissues. This approach will prove valuable in uncovering new and unanticipated DF-related mechanisms of action and potential therapeutic targets (145).
Transcriptomics has highlighted that fibers elicit differential metabolic effects on the gut, depending on DF type. For instance, one study compared the colon mucosal transcriptome of mice fed 5 different fibers (arabinoxylan, FOSs, inulin, guar gum, or RS at 10% by weight of the diet) for 10 d. Some unique properties associated with each were as follows: arabinoxylan increased tryptophan metabolism gene expression, FOSs increased the unfolded protein response transcripts, inulin increased β-oxidation pathway mRNAs, and guar gum increased cholesterol and arachidonic acid metabolism gene expression (146). These fibers increased PPAR-γ, which is known to affect gut inflammation (147). Thus, the molecular pathways engaged by DF differ significantly depending on the specific fiber used. The disparate effects of specific DFs could be leveraged, in theory, for comparative “omics” studies to identify DF-specific microbes or microbe-derived metabolites that correlate with molecular phenotype outcomes. These would serve as candidates that could explain DF specificity on outcomes in the intestine and other tissues.
With respect to the liver, a study that supplemented rats with an inulin-rich fiber (10% of diet by weight for 4 wk) on the background of a high-fructose diet found decreased liver TGs along with differential expression of 147 hepatic genes including genes related not only to lipid metabolism but also to fibrosis and inflammation (148). For example, inulin supplementation downregulated the expression of connective tissue growth factor and decorin, which are known to play a role in fibrosis (148). These findings may shed light on new hepatic regulators relevant to NASH and NAFLD that are influenced by DF. Another study that supplemented high fat–fed mice with the nonfermentable viscous DF hydroxypropyl-methylcellulose (6% by weight of the diet for 5 wk) also found decreases in hepatic lipid accumulation and altered expression of hepatic genes involved in glucocorticoid metabolism, steroid metabolism, androgen and estrogen hormone synthesis, methylation, and oxidation reduction (149). Although the specific signals that link DF-associated changes in the gut to liver fat metabolism and gene expression remain to be determined, these experiments have identified new potential downstream targets sensitive to DF feeding.
With the use of a multi-omics approach, our group recently discovered that mice fed different forms of DF [enzyme-treated wheat bran (ETWB) or high-amylose-maize RS type 2 (HAMRS2)] at 20% of the diet exhibited marked changes in the liver transcriptome and metabolome (150, 151). For example, metabolomics revealed almost-uniformly reduced hepatic amino acid concentrations in HAMRS2-fed mice despite normal blood concentrations, suggestive of a DF-associated shift in liver amino acid intermediary metabolism. Several bacterial taxa tended to shift along with changes in liver amino acid abundances (i.e., Ruminococcaceae was negatively correlated with many liver amino acids, whereas Lachnospiraceae was positively correlated). These unexpected findings point to new biology associated with DF feeding; namely, hepatic nitrogen and amino acid metabolism can be dramatically altered in response to DF. Another unexpected observation was that livers in ETWB-fed mice also showed significantly altered metabolite and gene expression patterns that, in some ways, mimicked the fasting state. For instance, the rate-limiting enzyme for gluconeogenesis, Pck1, was upregulated in ETWB-fed mice; this enzyme is increased during fasting (152). Liver and blood concentrations of the ketone body β-hydroxybutyrate were also increased in ETWB-fed mice. As with the HAMRS2 effects in the liver, many of these changes were significantly correlated with DF-associated alterations in specific gut microbiota, suggesting that factors emanating from these microbes were involved.
Metabolomics has been used to identify metabolite shifts that occur in CKD (153). Recently, this approach was extended to uncover the effects of different rhubarb extracts in rats with CKD. The authors found that treatment with various rhubarb extracts restored urine metabolite abnormalities (i.e., increased creatinine, decreased pyrimidine) and improved renal function and kidney histopathology (154). This study did not study the effects of DF; however, this same approach can be used to determine which metabolic pathways are affected by CKD and whether these perturbations are normalized by treatment. Note that many sources of DF are accompanied by phytochemicals and it may be the phytochemicals, and not the DF per se, that have an impact. The administration of the intact fiber source and isolated phytochemicals should prove useful in untangling the specific beneficial effects of DF.
Summary and Future Directions
“Omics” studies have begun to successfully catalog the changes in hundreds of variables in response to DF (i.e., DF-associated alterations in microbes, metabolites, and transcripts). Some of these variables are significantly correlated with biological phenotypes. However, the field is rapidly moving beyond this descriptive phase, and future efforts will consider how the identified variables affect target organs at a molecular level. For example, it is increasingly appreciated that simply characterizing the types of bacteria present may not provide adequate evidence to draw reliable conclusions with regard to gut microbiota influences on host phenotype; it may be more biologically relevant to identify the metabolic activities of the gut microbiota via meta-transcriptomics (bacterial gene expression), rather than simply quantifying the bacteria that are present. Stated another way, what the bacteria are doing is more important than which bacteria are there (155). Meta-transcriptomics will likely aid in the identification of bacterial species that are responsible for producing specific xenometabolites. Effects of the identified xenometabolites can then be studied in host cell culture systems as well as in germ-free and humanized animal models to unravel how the gut microbiota communicates with the intestines, liver, kidney, and other organs.
These technologies can also be used to identify patterns of interindividual variability. Fiber supplementation studies in humans have often generated inconsistent results that may be accounted for by differences in resident gut microbes. The ability to classify people as responders or nonresponders by assessing variables such as fecal SCFAs or breath hydrogen can aid in identifying gut microbe communities that are necessary to elicit a response to DF supplementation (156). These microbes can then be supplied to nonresponders along with the DF to determine if supplementing the “missing microbe” does indeed result in an enhanced response to the DF intervention.
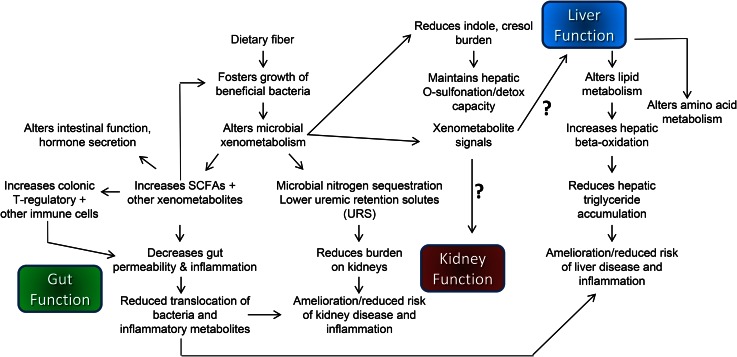
In conclusion, DFs alter the gut environment by a number of mechanisms, including fostering the growth of select bacteria, which leads to altered microbial metabolite production and host immune response. An overview of fiber-induced changes in gut, liver, and kidney is shown in Figure 1. Fiber-induced gut changes can result in enhanced gut barrier function that protects the liver and kidney from translocation of proinflammatory bacteria and bacterial products. This could allow the liver and kidneys to devote more capacity to metabolism-associated processes rather than controlling inflammation (104). In addition, DF increases microbial sequestration of nitrogen in the gut, resulting in increased fecal nitrogen excretion and reduced concentrations of nitrogenous metabolites in the blood. Reduced nitrogenous burden on the kidneys is desired for the treatment of diseases such as CKD. However, not all fibers behave the same way, with differences in fiber structure and preparation resulting in varied outcomes; fiber from a diverse range of sources is likely to provide the most health benefits. Unfortunately, most Americans do not consume enough fiber (157), prompting the 2015 Dietary Guidelines Advisory Committee to name fiber a nutrient of concern (158). Fiber intake has declined over time as has the diversity of our microbiomes (159), and this decreased diversity is generally associated with poor health outcomes (54). Increasing the amount of fiber in the diet is likely one way to increase diversity and ameliorate many diseases beyond the gut. An in-depth understanding of the specific microbes and signals that are altered in response to fibers (and the host tissue molecular targets) will support the development of evidence-based strategies to improve health and thwart diseases such as gastrointestinal disorders, NAFLD, and CKD.
FIGURE 1.
Schematic overview of the major mechanisms by which dietary fiber affects gut, liver, and kidneys.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CKD, chronic kidney disease; DF, dietary fiber; ETWB, enzyme-treated wheat bran; FOS, fructo-oligosaccharide; FXR, farnesoid X receptor; GLP-2, glucagon like peptide 2; G6pc, glucose-6-phosphatase catalytic subunit; HAMRS2, high-amylose-maize resistant starch type 2; HDAC, histone deacetylase; Hif-1α, hypoxia-inducible factor 1α NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; Pck1, phosphoenolpyruvate carboxykinase 1; RS, resistant starch.
References
- 1.Burkitt DP. Colonic-rectal cancer: fiber and other dietary factors. Am J Clin Nutr 1978;31(Suppl):S58–64. [DOI] [PubMed] [Google Scholar]
- 2.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med 2010;27:391–7. [DOI] [PubMed] [Google Scholar]
- 3.Threapleton DE, Greenwood DC, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2013;347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int 2012;32:701–11. [DOI] [PubMed] [Google Scholar]
- 5.Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr 1996;63:392–8. [DOI] [PubMed] [Google Scholar]
- 6.Roberfroid M. Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit Rev Food Sci Nutr 1993;33:103–48. [DOI] [PubMed] [Google Scholar]
- 7.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5:1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahouny GV, Kritchevsky D. Dietary fiber in health and disease. New York: Springer Science & Business Media; 2013. [Google Scholar]
- 9.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 2012;6:1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 2008;295:E1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aliasgharzadeh A, Dehghan P, Gargari BP, Asghari-Jafarabadi M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: a randomised controlled clinical trial. Br J Nutr 2015;113:321–30. [DOI] [PubMed] [Google Scholar]
- 12.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compare D, Coccoli P, Rocco A, Nardone O, De Maria S, Cartenì M, Nardone G. Gut–liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2012;22:471–6. [DOI] [PubMed] [Google Scholar]
- 14.Rossi M, Johnson DW, Campbell KL. The kidney–gut axis: implications for nutrition care. J Ren Nutr 2015;25:399–403. [DOI] [PubMed] [Google Scholar]
- 15.Turner ND, Lupton JR. Dietary fiber. Adv Nutr 2011;2:151–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): Institute of Medicine; 2002. [DOI] [PubMed] [Google Scholar]
- 17.Dikeman CL, Fahey GC. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr 2006;46:649–63. [DOI] [PubMed] [Google Scholar]
- 18.Bliss DZ, Weimer PJ, Jung H-JG, Savik K. In vitro degradation and fermentation of three dietary fiber sources by human colonic bacteria. J Agric Food Chem 2013;61:4614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeVries J, Prosky L, Li B, Cho S. A historical perspective on defining dietary fiber. Cereal Foods World 1999;44:367–9. [Google Scholar]
- 20.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2010;2:1266–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatakeyama H, Hatakeyama T. Lignin structure, properties, and applications. In: Abe A, Dusek K, Kobayashi S, editors. Biopolymers: lignin, proteins, bioactive nanocomposites. Berlin, Heidelberg (Germany): Springer Berlin Heidelberg; 2010. p. 1–63. [Google Scholar]
- 22.O’Sullivan AC. Cellulose: the structure slowly unravels. Cellulose 1997;4:173–207. [Google Scholar]
- 23.Izydorczyk MS, Biliaderis CG. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr Polym 1995;28:33–48. [Google Scholar]
- 24.Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WLJ. Inulin, a flexible oligosaccharide I: review of its physicochemical characteristics. Carbohydr Polym 2015;130:405–19. [DOI] [PubMed] [Google Scholar]
- 25.Moshfegh AJ, Friday JE, Goldman JP, Ahuja JKC. Presence of inulin and oligofructose in the diets of Americans. J Nutr 1999;129(Suppl):1407S–11S. [DOI] [PubMed] [Google Scholar]
- 26.Izydorczyk M, Dexter J. Barley β-glucans and arabinoxylans: molecular structure, physicochemical properties, and uses in food products—a review. Food Res Int 2008;41:850–68. [Google Scholar]
- 27.Mudgil D, Barak S, Khatkar BS. Guar gum: processing, properties and food applications—a review. J Food Sci Technol 2014;51:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nussinovitch A. Plant gum exudates of the world: sources, distribution, properties, and applications. Boca Raton (FL): CRC Press; 2009. [Google Scholar]
- 29.Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol 2008;11:266–77. [DOI] [PubMed] [Google Scholar]
- 30.Marlett JA. Content and composition of dietary fiber in 117 frequently consumed foods. J Am Diet Assoc 1992;92:175–86. [PubMed] [Google Scholar]
- 31.Fischer MH, Yu N, Gray GR, Ralph J, Anderson L, Marlett JA. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr Res 2004;339:2009–17. [DOI] [PubMed] [Google Scholar]
- 32.Crittenden R, Playne MJ. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol 1996;7:353–61. [Google Scholar]
- 33.IUB-IUPAC Joint Commission on Biochemical Nomenclature (JCBN). Abbreviated terminology of oligosaccharide chains: recommendations 1980. J Biol Chem 1982;257:3347–51. [PubMed] [Google Scholar]
- 34.Birt DF, Boylston T, Hendrich S, Jane J-L, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M. Resistant starch: promise for improving human health. Adv Nutr 2013;4:587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, Martin RJ. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr 2015;6:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 2014;14:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadwell K. Expanding the role of the virome: commensalism in the gut. J Virol 2015;89:1951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science 2006;312:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464(7285):59–65. [cited 2016 Jan 21]. Available from: http://www.nature.com/nature/journal/v464/n7285/suppinfo/nature08821_S1.html. [DOI] [PMC free article] [PubMed]
- 40.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 2012;10:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen L. Dysbiosis of the gut microbiota in disease. Microbial ecology in health and disease 2015;26:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Sullivan O, Cronin O, Clarke SF, Murphy EF, Molloy MG, Shanahan F, Cotter PD. Exercise and the microbiota. Gut Microbes 2015;6:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011;25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Nichols RG, Correll J, Murray IA, Tanaka N, Smith P, Hubbard TD, Sebastian A, Albert I, Hatzakis E. Persistent organic pollutants modify gut microbiota–host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environmental Health Perspectives 2015;123(7):679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol 2009;587:4153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung WSF, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol 2016;14:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voragen AG, Coenen G-J, Verhoef RP, Schols HA. Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem 2009;20:263–75. [Google Scholar]
- 48.Umu ÖC, Frank JA, Fangel JU, Oostindjer M, da Silva CS, Bolhuis EJ, Bosch G, Willats WG, Pope PB, Diep DB. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 2015;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annison G, Topping DL. Nutritional role of resistant starch: chemical structure vs physiological function. Annu Rev Nutr 1994;14:297–320. [DOI] [PubMed] [Google Scholar]
- 50.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 2014;426:3838–50. [DOI] [PubMed] [Google Scholar]
- 52.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 53.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl 1997;222:3–9. [DOI] [PubMed] [Google Scholar]
- 56.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016;529:212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005;81:341–54. [DOI] [PubMed] [Google Scholar]
- 58.Satchithanandam S, Vargofcak-Apker M, Calvert RJ, Leeds AR, Cassidy MM. Alteration of gastrointestinal mucin by fiber feeding in rats. J Nutr 1990;120:1179–84. [DOI] [PubMed] [Google Scholar]
- 59.Piel C, Montagne L, Sève B, Lallès J-P. Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation. J Nutr 2005;135:86–91. [DOI] [PubMed] [Google Scholar]
- 60.Lamont JT. Mucus: the front line of intestinal mucosal defense. Ann N Y Acad Sci 1992;664:190–201. [DOI] [PubMed] [Google Scholar]
- 61.Earle K A, Billings G, Sigal M, Lichtman J S, Hansson G C, Elias J E, Amieva M R, Huang K C, Sonnenburg JL. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 2015;18(4):478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodlad RA, Ratcliffe B, Fordham JP, Wright NA. Does dietary fibre stimulate intestinal epithelial cell proliferation in germ free rats? Gut 1989;30:820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, O’Riordan MXD. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv Appl Microbiol 2013;85:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly C J, Zheng L, Campbell Eric L, Saeedi B, Scholz Carsten C, Bayless Amanda J, Wilson Kelly E, Glover Louise E, Kominsky Douglas J, Magnuson A, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015;17:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004;114:1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 2010;139:2093–101. [DOI] [PubMed] [Google Scholar]
- 67.Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, Brown IL, Birkett A, Newman SS, Skaf J, Hegsted M, Tulley RT, et al. A microarray study indicates high-amylose resistant starch increases hormones and improves structure and function of the GI tract. J Nutrigenet Nutrigenomics 2012;5:26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benjamin MA, McKay DM, Yang P-C, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 2000;47:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hadjiyanni I, Li KK, Drucker DJ. Glucagon-like peptide-2 reduces intestinal permeability but does not modify the onset of type 1 diabetes in the nonobese diabetic mouse. Endocrinology 2009;150:592–9. [DOI] [PubMed] [Google Scholar]
- 71.Jasleen J, Ashley SW, Shimoda N, Zinner MJ, Whang EE. Glucagon-like peptide 2 stimulates intestinal epithelial proliferation in vitro. Dig Dis Sci 2002;47:1135–40. [DOI] [PubMed] [Google Scholar]
- 72.Bulut K, Meier JJ, Ansorge N, Felderbauer P, Schmitz F, Hoffmann P, Schmidt WE, Gallwitz B. Glucagon-like peptide 2 improves intestinal wound healing through induction of epithelial cell migration in vitro—evidence for a TGF-β-mediated effect. Regul Pept 2004;121:137–43. [DOI] [PubMed] [Google Scholar]
- 73.Fukunaga T, Sasaki M, Araki Y, Okamoto T, Yasuoka T, Tsujikawa T, Fujiyama Y, Bamba T. Effects of the soluble fibre pectin on intestinal cell proliferation, fecal short chain fatty acid production and microbial population. Digestion 2003;67(1–2):42–9. [DOI] [PubMed] [Google Scholar]
- 74.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009;139:1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohata A, Usami M, Miyoshi M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005;21:838–47. [DOI] [PubMed] [Google Scholar]
- 76.Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med 2002;30:396–402. [DOI] [PubMed] [Google Scholar]
- 77.Koruda MJ, Rolandelli RH, Settle RG, Zimmaro DM, Rombeau JL. Effect of parenteral nutrition supplemented with short-chain fatty acids on adaptation to massive small bowel resection. Gastroenterology 1988;95:715–20. [DOI] [PubMed] [Google Scholar]
- 78.Alverdy JC. Effects of glutamine-supplemented diets on immunology of the gut. JPEN J Parenter Enteral Nutr 1990;14(4, Suppl):109S–13S. [DOI] [PubMed] [Google Scholar]
- 79.Castro GA, Arntzen CJ. Immunophysiology of the gut: a research frontier for integrative studies of the common mucosal immune system. Am J Physiol 1993;265:G599–610. [DOI] [PubMed] [Google Scholar]
- 80.Alisi A, Ceccarelli S, Panera N, Nobili V. Causative role of gut microbiota in non-alcoholic fatty liver disease pathogenesis. Front Cell Infect Microbiol 2012;2:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brun P, Castagliuolo I, Leo VD, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2007;292:G518–25. [DOI] [PubMed] [Google Scholar]
- 82.Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron 2015;130:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bornet FR, Brouns F. Immune-stimulating and gut health-promoting properties of short-chain fructo-oligosaccharides. Nutr Rev 2002;60(10, Part 1):326–34. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S, Li W, Smith CJ, Musa H. Cereal-derived arabinoxylans as biological response modifiers: extraction, molecular features, and immune-stimulating properties. Crit Rev Food Sci Nutr 2015;55:1035–52. [DOI] [PubMed] [Google Scholar]
- 85.Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by β-glucans. Physiol Behav 2008;94:276–84. [DOI] [PubMed] [Google Scholar]
- 86.Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, Conlon MA, Morita T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J Agric Food Chem 2011;59:5771–8. [DOI] [PubMed] [Google Scholar]
- 87.Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, Dilawari J, Goff DV, Metz GL, Alberti KG. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. BMJ 1978;1:1392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kagnoff MF. Immunology of the intestinal tract. Gastroenterology 1993;105:1275–80. [DOI] [PubMed] [Google Scholar]
- 89.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341(6145):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012;4:1095–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mithieux G, Andreelli F, Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Curr Opin Clin Nutr Metab Care 2009;12:419–23. [DOI] [PubMed] [Google Scholar]
- 93.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2013;156(1):84–96. [DOI] [PubMed] [Google Scholar]
- 94.Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): treatment. Best Pract Res Clin Gastroenterol 2004;18:1105–16. [DOI] [PubMed] [Google Scholar]
- 95.Takino J-i, Nagamine K, Hori T, Sakasai-Sakai A, Takeuchi M. Contribution of the toxic advanced glycation end-products-receptor axis in nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol 2015;7:2459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis 2005;45:978–93. [DOI] [PubMed] [Google Scholar]
- 97.Stinghen AEM, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol 2016;27:354–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int 2012;32(5):701–11. [DOI] [PubMed] [Google Scholar]
- 99.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Masciana R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877–87. [DOI] [PubMed] [Google Scholar]
- 100.Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez‐Valera F, Pascual S, Sola‐Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture‐negative, nonneutrocytic ascites. Hepatology 2002;36:135–41. [DOI] [PubMed] [Google Scholar]
- 101.Sugihara T, Koda M, Maeda Y, Matono T, Nagahara T, Mandai M, Ueki M, Murawaki Y. Rapid identification of bacterial species with bacterial DNA microarray in cirrhotic patients with spontaneous bacterial peritonitis. Intern Med 2009;48:3–10. [DOI] [PubMed] [Google Scholar]
- 102.Lecerf JM, Depeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 2012;108:1847–58. [DOI] [PubMed] [Google Scholar]
- 103.Paschos P, Paletas K. Non alcoholic fatty liver disease two-hit process: multifactorial character of the second hit. Hippokratia 2009;13:128. [PMC free article] [PubMed] [Google Scholar]
- 104.Ban SJ, Rico CW, Um IC, Kang MY. Antihyperglycemic and antioxidative effects of hydroxyethyl methylcellulose (HEMC) and hydroxypropyl methylcellulose (HPMC) in mice fed with a high fat diet. Int J Mol Sci 2012;13:3738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nugon-Baudon L, Roland N, Flinois J-P, Beaune P. Hepatic cytochrome P450 and UDP-glucuronosyl transferase are affected by five sources of dietary fiber in germ-free rats. J Nutr 1996;126:403–9. [DOI] [PubMed] [Google Scholar]
- 106.Merrell MD, Cherrington NJ. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev 2011;43:317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Haen P. Recent progress in pharmacological studies on bile acids. J Am Pharm Assoc 1944;33:161–9. [Google Scholar]
- 108.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 1999;284:1362–5. [DOI] [PubMed] [Google Scholar]
- 109.Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47:241–59. [DOI] [PubMed] [Google Scholar]
- 110.Eyssen H, Van Eldere J, Parmentier G, Huijghebaert S, Mertens J. Influence of microbial bile salt desulfation upon the fecal excretion of bile salts in gnotobiotic rats. J Steroid Biochem 1985;22:547–54. [DOI] [PubMed] [Google Scholar]
- 111.Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2013;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: Unraveling a complex relationship. Gut Microbes 2013;4:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y, Jadhav K, Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol 2013;86:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int 2010;4:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17:225–35. [DOI] [PubMed] [Google Scholar]
- 117.Vahouny GV, Khalafi R, Satchithanandam S, Watkins DW, Story JA, Cassidy MM, Kritchevsky D. Dietary fiber supplementation and fecal bile acids, neutral steroids and divalent cations in rats. J Nutr 1987;117:2009–15. [DOI] [PubMed] [Google Scholar]
- 118.Alberts DS, Einspahr JG, Earnest DL, Krutzsch MF, Lin P, Hess LM, Heddens DK, Roe DJ, Martínez ME, Salen G, et al. Fecal bile acid concentrations in a subpopulation of the Wheat Bran Fiber Colon Polyp Trial. Cancer Epidemiol Biomarkers Prev 2003;12:197–200. [PubMed] [Google Scholar]
- 119.Mafra D, Fouque D. Gut microbiota and inflammation in chronic kidney disease patients. Clin Kidney J 2015;8:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013;83:308–15. [DOI] [PubMed] [Google Scholar]
- 121.Wang F, Jiang H, Shi K, Ren YI, Zhang PAN, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 2012;17:733–8. [DOI] [PubMed] [Google Scholar]
- 122.Zuckerman GR, Cornette GL, Clouse RE, Harter HR. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med 1985;102:588–92. [DOI] [PubMed] [Google Scholar]
- 123.Margolis DM, Saylor JL, Geisse G, DeSchryver-Kecskemeti K, Harter HR, Zuckerman GR. Upper gastrointestinal disease in chronic renal failure: a prospective evaluation. Arch Intern Med 1978;138:1214–7. [PubMed] [Google Scholar]
- 124.Wu I-W, Hsu K-H, Lee C-C, Sun C-Y, Hsu H-J, Tsai C-J, Tzen C-Y, Wang Y-C, Lin C-Y, Wu M-S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 2011;26:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krishnamurthy VMR, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 2012;81:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, Campbell KL. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis 2015;25(9):860–5. [DOI] [PubMed] [Google Scholar]
- 127.Eknoyan G, Levin A, Levin NW. Bone metabolism and disease in chronic kidney disease. American Journal of Kidney Diseases. 2003;42(Supp 3):1–201. [Google Scholar]
- 128.Sanghavi S, Whiting S, Uribarri J. Potassium balance in dialysis patients. Semin Dial 2013;26:597–603. [DOI] [PubMed] [Google Scholar]
- 129.Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014;39:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bourke E, Milne MD, Stokes GS. Caecal pH and ammonia in experimental uraemia. Gut 1966;7:558–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant 2012;27:2686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu I-W, Hsu K-H, Hsu H-J, Lee C-C, Sun C-Y, Tsai C-J, Wu M-S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol Dial Transplant 2012;27:1169. [DOI] [PubMed] [Google Scholar]
- 133.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009;4:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 2014;9:1603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vaziri ND, Liu S-M, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 2014;9:e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pang KS, Schwab AJ, Goresky CA, Chiba M. Transport, binding, and metabolism of sulfate conjugates in the liver. Chem Biol Interact 1994;92:179–207. [DOI] [PubMed] [Google Scholar]
- 137.Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci USA 2009;106:14728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pavan M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol 2014;68;222–6. [PubMed] [Google Scholar]
- 139.Siddharth M, Datta SK, Bansal S, Mustafa M, Banerjee BD, Kalra OP, Tripathi AK. Study on organochlorine pesticide levels in chronic kidney disease patients: Association with estimated glomerular filtration rate and oxidative stress. J Biochem Mol Toxicol 2012;26:241–7. [DOI] [PubMed] [Google Scholar]
- 140.Younes H, Alphonse J-C, Behr SR, Demigné C, Rémésy C. Role of fermentable carbohydrate supplements with a low-protein diet in the course of chronic renal failure: experimental bases. Am J Kidney Dis 1999;33:633–46. [DOI] [PubMed] [Google Scholar]
- 141.Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, Backhed F, Nielsen J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 2015;11:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bergen WG. Small-intestinal or colonic microbiota as a potential amino acid source in animals. Amino Acids 2015;47:251–8. [DOI] [PubMed] [Google Scholar]
- 143.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 2013;110:4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl 2005;68:S57–65. [DOI] [PubMed] [Google Scholar]
- 145.Duffy LC, Raiten DJ, Hubbard VS, Starke-Reed P. Progress and challenges in developing metabolic footprints from diet in human gut microbial cometabolism. J Nutr 2015;145(Suppl):1123S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lange K, Hugenholtz F, Jonathan MC, Schols HA, Kleerebezem M, Smidt H, Müller M, Hooiveld GJEJ. Comparison of the effects of five dietary fibers on mucosal transcriptional profiles, and luminal microbiota composition and SCFA concentrations in murine colon. Mol Nutr Food Res 2015;59:1590–602. [DOI] [PubMed] [Google Scholar]
- 147.Speca S, Dubuquoy L, Desreumaux P. Peroxisome proliferator–activated receptor gamma in the colon: inflammation and innate antimicrobial immunity. J Clin Gastroenterol 2014;48:S23–7. [DOI] [PubMed] [Google Scholar]
- 148.Chang WC, Jia H, Aw W, Saito K, Hasegawa S, Kato H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus) in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br J Nutr 2014;112:709–17. [DOI] [PubMed] [Google Scholar]
- 149.Kim H, Bartley GE, Young SA, Seo K-H, Yokoyama W. Altered hepatic gene expression profiles associated with improved fatty liver, insulin resistance, and intestinal permeability after hydroxypropyl methylcellulose (HPMC) supplementation in diet-induced obese mice. J Agric Food Chem 2013;61:6404–11. [DOI] [PubMed] [Google Scholar]
- 150.Kieffer DA, Piccolo BD, Marco ML, Kim EB, Goodson ML, Keenan MJ, Dunn TN, Knudsen KNE, Adams SH, Martin RJ. Obese mice fed a diet supplemented with enzyme-treated wheat bran display marked shifts in the liver metabolome concurrent with altered gut bacteria. J Nutr 2016 Dec (Epub ahead of print); DOI: 10.3945/jn.116.238923). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kieffer DA, Piccolo BD, Marco ML, Kim EB, Goodson ML, Keenan MJ, Dunn TN, Knudsen KEB, Martin RJ, Adams SH. Mice fed a high-fat diet supplemented with resistant starch display marked shifts in the liver metabolome concurrent with altered gut bacteria. J Nutr 2016 Dec (Epub ahead of print); DOI: 10.3945/jn.116.238931). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol 2000;20:6508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao YY. Metabolomics in chronic kidney disease. Clin Chim Acta 2013;422:59–69. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Z-H, Wei F, Vaziri ND, Cheng X-L, Bai X, Lin R-C, Zhao Y-Y. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci Rep 2015;5:14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 2011;6:209–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Korpela K, Flint HJ, Johnstone AM, Lappi J, Poutanen K, Dewulf E, Delzenne N, de Vos WM, Salonen A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS One 2014;9:e90702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.King DE, Mainous AG, Lambourne CA. Trends in dietary fiber intake in the United States, 1999–2008. J Acad Nutr Diet 2012;112:642–8. [DOI] [PubMed] [Google Scholar]
- 158.USDA. Scientific report of the 2015 Dietary Guidelines Advisory Committee. Washington (DC): USDA; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014;20:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]