Abstract
A number of long non-coding RNAs (lncRNAs) have been found to play critical roles in oncogenesis and tumor progression. We aimed to investigate whether lncRNAs could act as prognostic biomarkers for papillary thyroid cancer (PTC) that may assist us in evaluating disease status and prognosis for patients. We found 220 lncRNAs with expression alteration from the annotated 2773 lncRNAs approved by the HUGO gene nomenclature committee in The Cancer Genome Atlas (TCGA) dataset, of which FAM41C, CTBP1-AS2, LINC00271, HAR1A, LINC00310 and HAS2-AS1 were associated with recurrence. After adjusting classical clinicopathogical factors and BRAFV600E mutation, LINC00271 was found to be an independent risk factor for extrathyroidal extension, lymph node metastasis, advanced tumor stage III/IV and recurrence in multivariate analyses. Additionally, LINC00271 expression was significantly downregulated in PTCs versus adjacent normal tissues (P < 0.001). The Gene Set Enrichment Analysis (GSEA) revealed that genes associated with cell adhesion molecules, cell cycle, P53 signaling pathway and JAK/STAT signaling pathway were remarkably enriched in lower-LINC00271 versus higher-LINC00271 tumors. In conclusion, LINC00271 was identified as a possible suppressor gene in PTC in our study, and it may serve as a potential predictor of poor prognoses in PTC.
Differentiated thyroid cancer (DTC), arising from thyroid follicular epithelial cells, accounts for the vast majority of thyroid cancers. Of the DTCs, papillary thyroid cancer (PTC) is the most common histological type1. The yearly incidence of thyroid cancer in the United States has nearly tripled from 4.9 per 100,000 in 1975 to 14.3 per 100,000 in 2009, and almost the entire change has been attributed to an increase in the incidence of PTC2,3. In general, most PTCs are indolent in biological processes and can be cured with thyroidectomy and radioiodine therapy. However, approximately 5–20% of patients can suffer disease recurrence4, and occasionally some progress to aggressive and lethal outcomes. The wide spectrum of PTC behaviors may attribute to variable genetic backgrounds and molecular events.
Recent efforts on DNA and RNA sequencing data have mainly focused on protein coding exons and transcripts to provide deep insights into genomic characteristics of PTC5. Interestingly, areas of non-coding transcripts that account for ~50% of all transcribed RNAs6,7 are poorly explored. Of the non-coding transcripts, long non-coding RNAs (lncRNAs) refer to the ones that vary from 200 bp to tens of kilobases in length. So far, an overwhelming number of lncRNAs have been identified8,9, and a certain subset of lncRNAs have been found to play critical roles in oncogenesis and tumor progression10,11,12,13. Increasing evidences indicate that, in many types of cancers, lncRNAs are aberrantly expressed and are involved in regulation of diverse biological functions14,15,16,17,18, suggesting their potential as biomarkers for cancer. Moreover, recent reports18,19,20 have uncovered the potential of lncRNAs as biomarkers for clinical outcomes in many cancers. However, the possible roles and prognostic values of lncRNA signature and specific lncRNAs have not been elucidated clearly in PTC. Older age at diagnosis, poor histological subtypes, extrathyroidal extension (ETE), lymph node metastasis (LNM), and advanced tumor stage are conventional clinicopathological parameters considered as prognostic factors for poor clinical outcomes. The present study aimed to identify novel markers from the annotated lncRNAs for prediction of poor prognoses that may assist us in evaluating disease status and prognosis for PTC patients.
Results
LncRNAs with expression alteration in PTC
The annotated 2773 lncRNAs were searched for expression data in PTC at The cBioPortal for Cancer Genomics (Supplementary Table S1). Of the 2773 lncRNAs, 220 lncRNAs with over-expression or under-expression alteration in PTC patients were selected based on the Onco Query Language criteria “EXP < = −2, EXP > = 2” from The Cancer Genome Atlas (TCGA) dataset, and the alteration frequencies were listed in Supplementary Figure S1. According to the cBioPortal for Cancer Genomics, the expression alteration frequency of the 220 lncRNAs ranged from 1% to 7% in 486 PTC patients with RNA sequencing data.
Clinicopathological data of patients in the TCGA and FUSCC cohorts
A total of 471 PTC patients (126 males and 345 females; mean age: 47.02 ± 15.89 years, range: 15–89 years) with detailed clinicopathological data and expression data of lncRNAs from the TCGA cohort were enrolled in this study. We also included the Fudan University Shanghai Cancer Center (FUSCC) cohort comprising 185 PTC patients (142 females and 43 males) with mean age of 43.30 ± 11.09 years (range: 16–75 years). Tumor characteristics including multifocality, histological subtypes, coexistence of Hashimoto’s thyroiditis (HT), ETE, tumor-node-metastasis (TNM) stage and BRAFV600E mutation were summarized in Table 1.
Table 1. Clinicopathological characteristics of PTC patients in the FUSCC and TCGA cohorts.
| Variables | TCGA (N = 471) |
FUSCC (N = 185) |
P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | 0.099 | ||||
| <45 | 217 | 46.1 | 99 | 53.5 | |
| ≥45 | 254 | 53.9 | 86 | 46.5 | |
| Gender | 0.374 | ||||
| Male | 126 | 26.8 | 43 | 23.2 | |
| Female | 345 | 73.2 | 142 | 76.8 | |
| Multifocality | <0.001 | ||||
| Unifocal | 250 | 54.2 | 135 | 73.0 | |
| Multifocal | 211 | 45.8 | 50 | 27.0 | |
| Histological type | <0.001 | ||||
| Classical PTC | 329 | 70.9 | 183 | 98.9 | |
| Follicular PTC | 99 | 21.3 | 2 | 1.1 | |
| Tall-cell PTC | 36 | 7.8 | 0 | 0 | |
| Coexistent HT | 0.004 | ||||
| Yes | 66 | 14.0 | 44 | 23.8 | |
| No | 405 | 86.0 | 141 | 76.2 | |
| ETE | <0.001 | ||||
| Yes | 313 | 66.5 | 168 | 90.8 | |
| No | 144 | 30.6 | 17 | 9.2 | |
| NA | 14 | 3.0 | 0 | 0 | |
| T Stage | <0.001 | ||||
| T1-T2 | 282 | 59.9 | 166 | 89.7 | |
| T3-T4 | 188 | 39.9 | 19 | 10.3 | |
| NA | 1 | 0.2 | 0 | 0 | |
| LNM | <0.001 | ||||
| N0 | 215 | 45.6 | 85 | 45.9 | |
| N1 | 210 | 44.6 | 100 | 54.1 | |
| NA | 46 | 9.8 | 0 | 0 | |
| M | <0.001 | ||||
| M0 | 263 | 55.8 | 185 | 100 | |
| M1 | 8 | 1.7 | 0 | 0 | |
| NA | 200 | 42.5 | 0 | 0 | |
| TNM Stage | <0.001 | ||||
| I-II | 312 | 66.2 | 152 | 82.1 | |
| III-IV | 157 | 33.3 | 33 | 17.9 | |
| NA | 2 | 0.4 | 0 | 0 | |
| BRAFV600E | <0.001 | ||||
| Mutation | 222 | 37.6 | 103 | 55.7 | |
| Wild-type | 177 | 47.1 | 79 | 42.7 | |
| NA | 72 | 15.3 | 3 | 1.6 | |
Notes: Italic and bold type indicates statistical significance.
Abbreviations: PTC, papillary thyroid cancer; FUSCC, Fudan University Shanghai Cancer Center; TCGA, The Cancer Genomics Atlas; HT, Hashimoto’s thyroiditis; ETE, extrathyroidal extension; LNM, lymph node metastasis; M, metastasis; TNM, tumor-node-metastas.
Association of LINC00271 with aggressive PTC
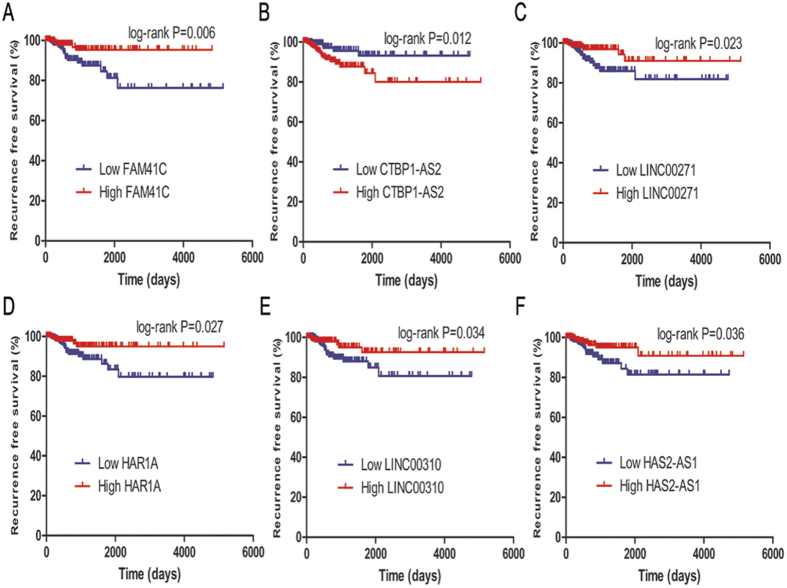
To investigate whether lncRNAs can act as potential biomarkers for recurrence in PTC, we performed kaplan-Meier analyses on the 220 lncRNAs and recurrence free survival (RFS) in the TCGA cohort. As shown in Fig. 1, six lncRNAs including FAM41C (Fig. 1A, P = 0.006), CTBP1-AS2 (Fig. 1B, P = 0.012), LINC00271 (Fig. 1C, P = 0.023), HAR1A (Fig. 1D, P = 0.027), LINC00310 (Fig. 1E, P = 0.034) and HAS2-AS1 (Fig. 1F, P = 0.036) were screened out to show significant correlations with RFS. The signatures and annotations of the six lncRNAs were summarized in Fig. 2A. Table 2 showed, in univariate hazards proportional analysis calculated by hazard ratio (HR) and 95% confidence interval (CI), FAM41C (HR = 3.394, 95%CI:1.354–8.507, P = 0.009), LINC00271 (HR = 2.655, 95%CI:1.109–6.359, P = 0.028), HAR1A (HR = 2.699, 95%CI:1.077–6.761, P = 0.034), LINC00310 (HR = 2.494, 95%CI: 1.041–5.972, P = 0.040) and HAS2-AS1 (HR = 2.389, 95%CI: 1.030–5.541, P = 0.043) at low expression levels were risk factors for poor RFS, and overexpression of CTBP1-AS2 (HR = 3.045, 95%CI: 1.215–7.633, P = 0.018) increased the risk of recurrence.
Figure 1. Identification of the annotated lncRNAs associated with recurrence of PTC.
(A–F) Kaplan–Meier plots of recurrence free survival in the TCGA cohort are shown according to FAM41C, CTBP1-AS2, LINC00271, HAR1A, LINC00310 and HAS2-AS1 expression, respectively.
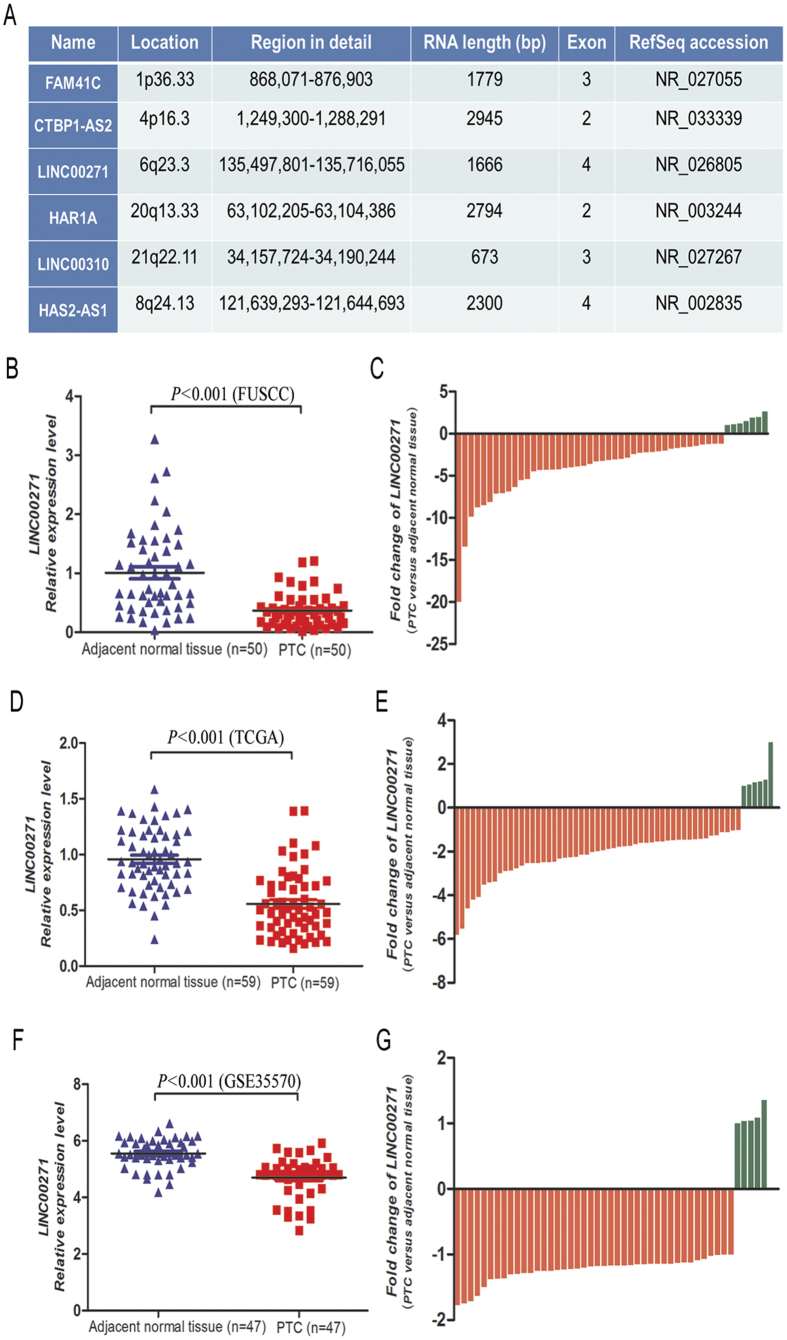
Figure 2. Annotations of the six identified lncRNAs and comparison of LINC00271 expression between PTC and adjacent normal tissues.
(A) Organizations of the six lncRNAs and chromosome locations. (B–G) (B,D,F) showed LINC00271 expression was significantly downregulated in PTC compared with the level in the adjacent normal tissues in the FUSCC, TCGA and GSE35570 cohorts. (C,E,G) indicated 86.0% (43/50), 89.8% (53/59) and 89.4% (42/47) of the cases with negative fold changes of LINC00271 expression in PTCs compared with adjacent normal tissues in the FUSCC, TCGA and GSE35570 cohorts, respectively.
Table 2. Association of the six lncRNAs with recurrence and high-risk clinicopathological factors of PTC in the TCGA cohort.
| lncRNA | RFS |
ETE |
LNM |
T3-T4 stage |
TNM stage: III–IV |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| FAM41C (low vs high) | 3.394 (1.354–8.507) | 0.009 | 1.078 (0.726–1.599) | 0.711 | 0.836 (0.571–1.223) | 0.357 | 0.572 (0.621–1.301) | 0.572 | 0.938 (0.639–1.377) | 0.744 |
| CTBP1-AS2 (high vs low) | 3.045 (1.215–7.633) | 0.018 | 1.287 (0.866–1.913) | 0.211 | 1.267 (0.865–1.854) | 0.224 | 1.264 (0.873–1.830) | 0.214 | 1.488 (1.011–2.189) | 0.044 |
| LINC00271 (low vs high) | 2.655 (1.109–6.359) | 0.028 | 2.523 (1.673–3.805) | <0.001 | 1.753 (1.193–2.574) | 0.004 | 2.377 (1.628–3.472) | <0.001 | 2.464 (1.656–3.665) | <0.001 |
| HAR1A (low vs high) | 2.699 (1.077–6.761) | 0.034 | 1.627 (1.091–2.426) | 0.017 | 1.682 (1.146–2.468) | 0.008 | 1.377 (0.951–1.994) | 0.090 | 1.17 (00.890–1.922) | 0.171 |
| LINC00310 (low vs high) | 2.494 (1.041–5.972) | 0.040 | 1.898 (1.274–2.830) | 0.002 | 1.552 (1.077–2.236) | 0.018 | 1.587 (1.092–2.308) | 0.016 | 1.471 (0.996–2.173) | 0.052 |
| HAS2-AS1 (low vs high) | 2.389 (1.030–5.541) | 0.043 | 0.633 (0.425–0.942) | 0.024 | 0.560 (0.381–0.822) | 0.003 | 0.726 (0.501–1.052) | 0.090 | 0.67 (0.457–0.989) | 0.044 |
Notes: Italic and bold type indicates statistical significance; Abbreviations: lncRNA, long non-coding RNA; PTC, papillary thyroid cancer; TCGA, The Cancer Genomics Atlas; RFS, recurrence free survival; HR, hazards ratio; OR, odds ratio; CI, confidence interval; ETE, extrathyroidal extension; LNM, lymph node metastasis; TNM, tumor-node-metastasis.
In addition, we conducted a further analysis for associations of the six lncRNAs with ETE, LNM, T3-T4 and III-IV stage. As shown in Table 2, among the six lncRNAs, decreased expression of LINC00271 significantly increased risks of ETE (odds ratio (OR) = 2.523, 95%CI: 1.673–3.805, P < 0.001), LNM (OR = 1.753, 95%CI: 1.193–2.574, P = 0.004), T3/T4 stage (OR = 2.377, 95%CI: 1.628–3.472, P < 0.001) and advanced TNM stage III/IV (OR = 2.464, 95%CI: 1.656–3.665, P < 0.001) in univariate logistic regression analysis, suggesting it as an optimal biomarker for aggressive behaviors of PTC. To validate the correlation of LINC00271 with the clinicopathological characteristics of PTC patients, LINC00271 expression in the FUSCC cohort was analyzed. Low LINC00271 expression was found to correlate with male gender (P = 0.006), larger tumor size (P = 0.038) and LNM (P = 0.024), showing a lower expression level in PTC with aggressive behaviors (Supplementary Table S2).
LINC00271 was downregulated in PTC compared with adjacent normal tissues
To determine the role of LINC00271 in PTC, we initially detected LINC00271 expression in the carcinoma specimens and the paired normal tissues in 50 PTC patients, and the results suggested LINC00271 was significantly downregulated in PTC compared with the level in the adjacent normal tissues (P < 0.001, Fig. 2B,C). While confirmed in the validation cohorts including the TCGA and GSE35570 cohorts, LINC00271 expression was also found to be suppressed in tumor tissues in comparison with normal tissues (Fig. 2D–G). Additionally, significantly repressed expression of LINC00271 was observed in breast invasive carcinoma (BRCA, Supplementary Figure S2A), lung adenocarcinoma (LUAD, Supplementary Figure S2B), kidney renal papillary cell carcinoma (KIRP, Supplementary Figure S2C) and head and neck squamous cell carcinoma (HNSCC, Supplementary Figure S2>D) when we performed an analysis of LINC00271 expression in BRCA, LUAD, KIRP, HNSCC, liver hepatocellular carcinoma (LIHC) and prostate adenocarcinoma (PRAD) from the TCGA database (Supplementary Figure S2).
LINC00271 as an independent risk factor for poor clinical outcomes of PTC
Multivariate analyses were performed to confirm whether associations of LINC00271 with high-risk pathological outcomes (ETE, LNM and III/IV stage) and recurrence were independent of classical clinicopathogical factors and BRAFV600E mutation. Table 3 showed that LINC00271 < cutoff remained significantly correlated with ETE, LNM and III/IV stage in the TCGA cohort in multivariate logistic regression analyses though LINC00271 < cutoff was only found to statistically increase the risk of LNM in the FUSCC cohort. In Table 4, the TCGA cohort indicated advanced T stage (T3-T4, HR = 2.294, 95%CI: 1.010–5.211, P = 0.047) and LINC00271 < cutoff (HR = 3.688, 95%CI: 1.384–9.829, P = 0.009) were risk factors for poor RFS in univariate cox proportional hazards analysis, and female gender was a protective factor for improved RFS. Multivariate analysis adjusted by age, gender, histological subtypes, T stage, TNM stage, showed female gender (HR = 0.386, 95%CI: 0.172–0.868, P = 0.021) and LINC00271 (HR = 3.182, 95%CI: 1.160–8.726, P = 0.025) were still statistically significant. Then, we performed a ROC analysis to evaluate the predictive values of LINC00271, gender and TNM stage for PTC recurrence. After combining LINC00271, the AUC values of gender and TNM stage for predicting recurrence were significantly elevated from 0.611 to 0.697 (P = 0.020) and 0.597 to 0.677 (P = 0.016), respectively (Supplementary Figure S3).
Table 3. Multivariate analyses of associations of LINC00271 with high-risk clinicopathogical factors in the TCGA and FUSCC cohorts.
| Variables | TCGA |
FUSCC |
||||
|---|---|---|---|---|---|---|
| P valuea | OR | 95.0% CI for OR | P valuea | OR | 95.0% CI for OR | |
| ETEb | ||||||
| LINC00271 ≥cutoff | 1 | 1 | ||||
| <cutoffc | 0.003 | 2.299 | 1.324–3.992 | 0.107 | 2.506 | 0.820–7.663 |
| LNMd | ||||||
| LINC00271 ≥cutoff | 1 | 1 | ||||
| <cutoffe | 0.037 | 1.713 | 1.033–2.841 | 0.007 | 2.554 | 1.300–5.018 |
| III/IV stagef | ||||||
| LINC00271 ≥cutoff | 1 | 1 | ||||
| <cutoffg | 0.011 | 2.180 | 1.200–3.959 | 0.608 | 1.297 | 0.480–3.502 |
Notes: aItalic and bold type indicates statistical significance; badjusted by age, gender, multifocality, histological subtypes, HT, LNM and BRAFV600E mutation in multivariate analysis; cLINC00271 expression level less than the best cutoff value for prediction of ETE; dadjusted by age, gender, multifocality, histological subtypes, HT, ETE, T stage and BRAFV600E mutation in multivariate analysis; fLINC00271 expression level less than the best cutoff value for prediction of LNM; fadjusted by gender, multifocality, histological subtypes, HT, ETE, LNM, T stage and BRAFV600E mutation in multivariate analysis; gLINC00271 expression level less than the best cutoff value for prediction of III/IV stage; Abbreviations: PTC, papillary thyroid cancer; TCGA, The Cancer Genomics Atlas; FUSCC, Fudan University Shanghai Cancer Center; OR, odds ratio; CI, confidence interval; HT, Hashimoto’s thyroiditis; ETE, extrathyroidal extension; LNM, lymph node metastasis; TNM, tumor-node-metastasis.
Table 4. Cox proportional hazards analysis of factors associated with RFS for PTC patients in TCGA cohort.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| P valuea | HR | 95.0% CI for HR | P valuea | HR | 95.0% CI for HR | |
| Ageb | 0.195 | 1.016 | 0.992–1.040 | 0.977 | 1.000 | 0.968–1.034 |
| Genderb | ||||||
| Male | 1 | 1 | ||||
| Female | 0.009 | 0.350 | 0.159–0.769 | 0.021 | 0.386 | 0.172–0.868 |
| Multifocality | ||||||
| Unifocal | 1 | |||||
| Multifocal | 0.106 | 1.933 | 0.868–4.300 | |||
| Histological subtypesb | ||||||
| Classical PTC | 1 | 1 | ||||
| Follicular PTC | 0.913 | 0.941 | 0.318–2.786 | 0.871 | 1.095 | 0.367–3.269 |
| Tall-cell PTC | 0.202 | 2.229 | 0.650–7.641 | 0.886 | 1.098 | 0.306–3.949 |
| Coexistent HT | ||||||
| No | 1 | |||||
| Yes | 0.740 | 1.729 | 0.648–4.616 | |||
| ETE | ||||||
| Yes | 1 | |||||
| No | 0.182 | 1.712 | 0.777–3.773 | |||
| T stageb | ||||||
| T1-T2 | 1 | 1 | ||||
| T3-T4 | 0.047 | 2.294 | 1.010–5.211 | 0.508 | 1.401 | 0.516–3.806 |
| LNM | ||||||
| No | 1 | |||||
| Yes | 0.111 | 2.077 | 0.846–5.100 | |||
| TNM stageb | ||||||
| Stage I-II | 1 | 1 | ||||
| Stage III-IV | 0.052 | 2.176 | 0.992–4.773 | 0.664 | 1.326 | 0.371–4.738 |
| BRAFV600E | ||||||
| Wild-type | 1 | |||||
| Mutation | 0.365 | 1.557 | 0.597–4.057 | |||
| LINC00271b | ||||||
| ≥cutoff | 1 | 1 | ||||
| <cutoffc | 0.009 | 3.688 | 1.384–9.829 | 0.025 | 3.182 | 1.160–8.726 |
Notes: aItalic and bold type indicates statistical significance; badjusted by age, gender, histological subtypes, T stage, TNM stage and LINC00271 in multivariate analysis; c, LINC00271 expression level less than the best cutoff value for prediction of recurrence; Abbreviations: PTC, papillary thyroid cancer; TCGA, The Cancer Genomics Atlas; OR, odds ratio; CI, confidence interval; HT, Hashimoto’s thyroiditis; ETE, extrathyroidal extension; LNM, lymph node metastasis; TNM, tumor-node-metastasis.
Furthermore, to investigate independent factors that might affect LINC00271 expression in PTC, we conducted a multivariate logistic regression analysis in the TCGA and FUSCC cohorts (Supplementary Table S3). LNM was shown to be significantly correlated with low expression of LINC00271 in both TCGA and FUSCC cohorts.
Identification of LINC00271-associated biological pathways by GSEA
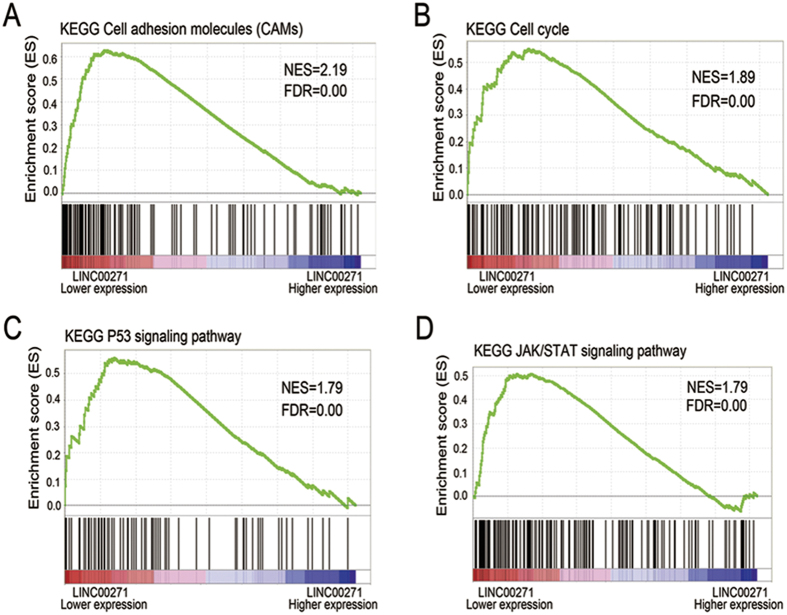
To identify LINC00271-associated biological signaling pathways on an unbiased basis, we performed Gene Set Enrichment Analysis (GSEA) using high throughput RNA-sequencing data of the TCGA cohort. The expression level of LINC00271 was used as the phenotype label. Among all the predefined KEGG pathways gene sets, cell adhesion molecules (CAMs), cell cycle, P53 signaling pathway and JAK/STAT signaling pathway were found to be significantly associated with LINC00271 expression in the TCGA cohort (Fig. 3), suggesting that LINC00271 may be involved in PTC development and progression through the above cancer-associated signaling pathways.
Figure 3. LINC00271-associated biological signaling pathways.
(A–D) Based on the TCGA dataset, GSEA showed genes associated with CAMs, cell cycle, P53 signaling pathway and JAK/STAT signaling pathway were significantly enriched in lower-LINC00271 vs higher-LINC00271 tumors.
Discussion
Large numbers of lncRNAs have been identified through genome-wide transcriptome analyses21,22. A series of studies have revealed that lncRNAs can act as regulators of diverse biological functions including X-chromosome silencing23, transcription regulation24, P53 function25, and cell growth control26. Recently, roles and functions of more annotated specific lncRNAs in cancer have been characterized. HOTAIR is shown to promote breast cancer metastasis by reprograming chromatin state, and HOTAIR expression level has prognostic value for metastasis and survival27. Leukemia-induced non-coding activator RNA-1 (LUNAR1) is revealed as a novel regulator of IGF1 signaling and T-ALL cell growth and may be a potential biomarker and therapeutic target in acute leukemia15. Ewing sarcoma-associated transcript 1 (EWSAT1) is found to facilitate Ewing sarcoma oncogenesis by mediating EWS-FLI1 suppression pathways16. BRAF-regulated lncRNA (BANCR) induced by BRAFV600E gene can regulate carcinoma cell migration in melanoma14. Furthermore, the potential of lncRNAs as biomarkers for cancer have been confirmed in many cancers. Wang GH et al.28 revealed that LINC01207 overexpression was associated with advanced TNM stage and shorter survival in lung adenocarcinoma patients. Zhang EB et al.19 found that ANRIL, as a growth regulator, may serve as a candidate prognostic biomarker and target for new therapies in human gastric cancer. Jin Meng et al.29 reported that a four-long non-coding RNA signature can be predictive of breast cancer survival, and Liu HR18 also identified several lncRNAs with prognostic values for breast cancer. Though recent efforts on protein coding exons and transcripts provided deep insights into genomic characteristics of PTC5, the possible roles and prognostic values of lncRNA signature and specific lncRNAs have been poorly characterized in PTC.
As reported in the previous study18, the cancer dataset of TCGA at cBioPortal were available for investigation of expression and clinical significance of lncRNAs. In the present study, the TCGA dataset was used to screen out the potential annotated lncRNAs with prognostic values for PTC. Initially, we performed an associative analysis of the annotated lncRNAs with patients’ clinicopathological parameters by using the PTC data of TCGA, and then validated the preliminary findings through our own data analysis at FUSCC. As shown in the study, the 220 lncRNAs were found to be altered at expression levels in PTC patients at cBioportal. Of the 220 lncRNAs, FAM41 C, CTBP1-AS2, LINC00271, HAR1A, LINC00310 and HAS2-AS1 were associated with RFS for PTC patients, and decreased LINC00271 expression also significantly increased risks of ETE, LNM, advanced T stage and TNM stage while the other five lncRNAs failed to correlate with these poor outcomes of PTC accordantly, which indicated LINC00271 as the optimal biomarker for aggressive behaviors of PTC. The associative analysis of LINC00271 expression with clinicopathological factors in the FUSCC cohort confirmed the role of enhancing aggressiveness of LINC00271 in PTC though there was no recurrent case in our cohort due to the limited follow-up time.
PTC is usually curable with thyroidectomy followed by radioiodine therapy, but many patients suffer disease recurrence, and some cases even advance to be incurable and fatal4,30. Therefore, specific biomarkers for risk stratification are helpful to identify patients at high recurrence so active treatment and careful monitoring can be provided. As we know, the conventional risk stratification is based on patient age, gender, tumor size, histological subtypes, the presence or absence of ETE and LNM and tumor stage finally defined by pathology. However, these criteria are always dependent on postoperative pathology and histological outcomes are not defined before surgery, and there exist heterogeneity and uncertainty in the risk evaluation based on these criteria.
As a prognostic marker, BRAFV600E mutation has received great attention in the past decades for its potential utility in the risk stratification and management of PTC31. However, as mentioned in the 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer2, BRAFV600E has limited values in predicting recurrence of PTC and should not impact on the decision for prophylactic central neck dissection in primary tumor2,31. Likewise, in our study, BRAFV600E had no predictive values for recurrence and LNM in PTC. Our multivariate analyses showed that associations of LINC00271 with ETE, LNM, advanced tumor stage and recurrence were independent of classical clinicopathogical factors and BRAFV600E mutation in the TCGA cohort, which suggested that it could act as a potential prognostic predictor for PTC. We had to admit that the limited number of cases and follow-up time and the difference in sample distribution in the FUSCC cohort were responsible for the negative statistical effects of LINC00271 on ETE and advanced tumor stage although the ORs of LINC00271 for the two parameters revealed positive trends.
Furthermore, the comparison of LINC00271 expression between tumor and adjacent normal tissues in the FUSCC, TCGA and GSE35570 cohorts suggested that LINC00271 could act as a suppressor gene in PTC. Likewise, the suppressive role of LINC00271 is observed in many common cancer types including BRCA, LUAD, KIRP and HNSCC. Diverse signaling pathways including cell cycle, P53 signaling and cell adhesion signaling cooperate to initiate and sustain oncogenesis and progression through maintaining proliferative signaling, evading growth suppressors and activating invasion and metastasis32. The pathways that LINC00271 may mediate in PTC remain unclear, so we performed GSEA to identify LINC00271-associated biological signaling pathways. GSEA showed low LINC00271 expression was positively associated with CAMs, cell cycle, P53 signaling pathway and JAK/STAT signaling pathway, which may suggest that LINC00271 is involved in PTC development and progression through these cancer-associated pathways.
In spite of observing the role of decreased LINC00271 expression firstly in PTC in our study, the possible mechanism mediated by LINC00271 has not been illuminated. Basic experiments in vitro and in vivo and large samples of patients with long-term follow-up outcomes are needed to confirm the effects of LINC00271 in PTC in the future.
In conclusion, LINC00271 was identified as a possible suppressor gene in PTC in our study, and it was an independent risk factor for LNM and recurrence of PTC. LINC00271 may serve as a potential predictor for poor clinical outcomes in PTC.
Materials and Methods
Annotated lncRNA database search
We performed a primary search for annotated lncRNAs at the website of HUGO gene nomenclature committee (HGNC) (http://www.Genenames.org/) according to a previous study18, and a total of 2773 lncRNAs were obtained from the HGNC database (http://www.genenames.org/cgi-bin/statistics). The 2773 lncRNAs were searched for expression data in PTC at The cBioPortal for Cancer Genomics34,35 (http://www.cbioportal.org/), of which 220 known lncRNAs with upregulation or downregulation were found finally by using Onco Query Language “EXP < = −2 EXP > = 2” from the TCGA data. Expression data of the 220 lncRNAs and the corresponding clinicopathological data from TCGA dataset were downloaded from the website of The cBioPortal for Cancer Genomics (http://www.cbioportal.org/) and Cancer Genomics Browser of University of California Santa Cruz (https://genome-cancer. ucsc. edu/). Paired LINC00271 expression data in tumor and normal tissues were retrieved from TCGA RNA-sequencing data of PTC, BRCA, LUAD, KIRP, HNSCC, LIHC and PRAD by employing The Atlas of Noncoding RNAs in Cancer (TANRIC)33 (http://ibl.mdanderson.org/tanric/design/basic/index.html) and Gene Expression Omnibus data with accession number GSE3557036 (https://www.ncbi.nlm.nih.gov/geo/).
Patients and clinicopathological data
A total of 471 primary PTC patients with clinicopthogical data, detailed expression of the 220 lncRNAs and BRAFV600E mutation were collected from the updated TCGA database according to the methods in the previous studies37,38. In addition, consecutive samples were selected from 185 patients diagnosed as PTC by pathology at the FUSCC from Mar 2012 to Jan 2015. The data on patients’ clinicopathological features including gender, age at diagnosis, maximum size of tumor, multifocality, HT, histological subtypes, ETE and cervical LNM were retrospectively abstracted from patient records. All the patients were staged using the 2009 TNM classification of American Joint Committee on Cancer/International Union Against Cancer. The selected samples were subjected to repeated evaluation to confirm diagnosis of the above histological characteristics.
Ethics statement
Each patient provided a written informed consent for his/her specimens and information to be used for research and stored in the hospital database, and this study was approved by the Ethical Committee of the FUSCC (Reference number: 050432-4-1212B). All procedures performed in our study were in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
BRAF V600E mutation detection
According to the manufacturer’s instructions, genomic DNA was extracted from the resected specimens using the QIAamp DNA Mini Kit (QIAGEN, Chatsworth, California). DNA templates were amplified for analysis of mutations in exon 15 of BRAF gene using the polymerase chain reaction (PCR) protocol as previously mentioned39, followed by a Big Dye (Applied Biosystems, Foster City, CA) reaction for Sanger sequencing. We recognized BRAFV600E mutation on sequencing electropherograms.
RNA extraction and real-time qRT-PCR analysis
We extracted total RNA from the specimens using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The extracted RNA was reversely transcribed for cDNA, followed by real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as previously described20. The primers for LINC00271 were as follows: GCTATTGGTGGGAGGCTTCAG (Sense), TGGGCTGGACTTAATGACTTGC (Antisense). GAPDH was used as a housekeeping gene. qRT-PCR assays were performed in triplicate for each sample, and the mean value was used for calculation of mRNA expression levels. The relative mRNA expression levels of LINC00271 were determined by the comparative Ct (2−∆Ct) method. The amount of target gene expression levels was given as ratios to GAPDH mRNA level.
Gene Set Enrichment Analysis
GSEA was performed using GSEA software, Version 2.0.1, which was obtained from the Broad Institute (http://www.broad.mit.edu/gsea), as previously described40,41. Enrichment map was used for visualization of the GSEA results. False discovery rate (FDR) value and normalized enrichment score (NES) were used to sort the pathways enriched in each phenotype after gene set permutations were performed 1000 times for each analysis.
Statistical Analysis
Categorical data were summarized with frequencies and percentages. The continuous results were expressed as the mean ± standard deviation. Paired-t and independent-t test was used to compare continuous variables in two groups. Associations between continuous variables and categorical variables were evaluated using Mann-Whitney U tests for two groups and Kruskal-Wallis tests for more than two groups. χ2 and Fisher’s exact test were used for categorical variables. The Kaplan-Meier method was used to construct RFS curves, and the univariate survival difference was determined by the log-rank test. To analyze the association between lncRNAs and clinicopathological parameters, patients were divided into two subgroups (Low expression and High expression) according to the median value of lncRNA expression levels. Nonparametric receiver operating characteristic (ROC) analyses were performed to calculate the best cutoff value for LINC00271 expression level that would be predictive of LNM and recurrence. Moreover, univariate and multivariate analysis were performed to determine risk factors for recurrence in PTC using cox proportional hazards models calculated by HR and 95% CI. OR with 95% CI was calculated by logistic regression analysis. A p-value < 0.05 was considered significant. Statistical analyses were performed using the GraphPad Prism 6.0 and SPSS for Windows (SPSS Inc., Chicago, IL).
Additional Information
How to cite this article: Ma, B. et al. Long intergenic non-coding RNA 271 is predictive of a poorer prognosis of papillary thyroid cancer. Sci. Rep. 6, 36973; doi: 10.1038/srep36973 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The research is supported by the Science and Technology Commission of Shanghai Municipality (14ZR1407300) and the National Science Foundation of China (No. 81572622).
Footnotes
Author Contributions Yu Wang designed the research and finalized the study conclusions; Ben Ma, Tian Liao and Duo Wen performed the experiments and wrote the paper; Li Zhou and Shuwen Yang collected the samples and clinicopathogical data of patients; Ben Ma and Chuanpeng Dong were responsible for all bioinformatics and statistical analysis; Qinghai Ji supervised all data analysis and provided critical suggestions for manuscript writing. All authors read and approved the manuscript.
References
- Aschebrook-Kilfoy B., Ward M. H., Sabra M. M. & Devesa S. S. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 21, 125–134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitoia F. & Miyauchi A. 2015 American Thyroid Association Guidelines for Thyroid Nodules and Differentiated Thyroid Cancer and Their Implementation in Various Care Settings. Thyroid 26, 319–321 (2016). [DOI] [PubMed] [Google Scholar]
- Davies L. & Welch H. G. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140, 317–322 (2014). [DOI] [PubMed] [Google Scholar]
- Schlumberger M. J. Papillary and follicular thyroid carcinoma. N Engl J Med 338, 297–306 (1998). [DOI] [PubMed] [Google Scholar]
- Integrated genomic characterization of papillary thyroid carcinoma. Cell 159, 676–690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T. R., Dinger M. E. & Mattick J. S. Long non-coding RNAs: insights into functions. Nat Rev Genet 10, 155–159 (2009). [DOI] [PubMed] [Google Scholar]
- Kapranov P. et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316, 1484–1488 (2007). [DOI] [PubMed] [Google Scholar]
- Volders P. J. et al. An update on LNCipedia: a database for annotated human lncRNA sequences. Nucleic Acids Res 43, 4363–4364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C. et al. NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Res 42, D98–D103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X. et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene 35, 2746–2755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J. F. et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 24, 513–531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty D. et al. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 5, 5383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. et al. Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/beta-catenin signaling. Hepatology 58, 739–751 (2013). [DOI] [PubMed] [Google Scholar]
- Flockhart R. J. et al. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res 22, 1006–1014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi T. et al. Genome-wide mapping and characterization of Notch-regulated long noncoding RNAs in acute leukemia. Cell 158, 593–606 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques H. M. et al. Long noncoding RNA EWSAT1-mediated gene repression facilitates Ewing sarcoma oncogenesis. J Clin Invest 124, 5275–5290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. A. et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. et al. Long non-coding RNAs as prognostic markers in human breast cancer. Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E. B. et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 5, 2276–2292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Chen H. & Liu J. The long noncoding RNA LINC01207 promotes proliferation of lung adenocarcinoma. Am J Cancer Res 5, 3162–3173 (2015). [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I. & Bartel D. P. lincRNAs: genomics, evolution, and mechanisms. Cell 154, 26–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. V. & Mattick J. S. The rise of regulatory RNA. Nat Rev Genet 15, 423–437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Sun B. K., Erwin J. A., Song J. J. & Lee J. T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750–756 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C. & Maquat L. E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 470, 284–288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M. et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T. et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43, 621–629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. A. et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Chen H. & Liu J. The long noncoding RNA LINC01207 promotes proliferation of lung adenocarcinoma. Am J Cancer Res 5, 3162–3173 (2015). [PMC free article] [PubMed] [Google Scholar]
- Meng J., Li P., Zhang Q., Yang Z. & Fu S. A four-long non-coding RNA signature in predicting breast cancer survival. J Exp Clin Cancer Res 33, 84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboulleux S. et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab 90, 5723–5729 (2005). [DOI] [PubMed] [Google Scholar]
- Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol 321, 86–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- Nohata N., Abba M. C. & Gutkind J. S. Unraveling the oral cancer lncRNAome: Identification of novel lncRNAs associated with malignant progression and HPV infection. Oral Oncol 59, 58–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 5, pl1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handkiewicz-Junak D. et al. Gene signature of the post-Chernobyl papillary thyroid cancer. Eur J Nucl Med Mol Imaging 43, 1267–1277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. Z., Yu K. D., Zuo W. J., Peng W. T. & Shao Z. M. GATA3 mutations define a unique subtype of luminal-like breast cancer with improved survival. Cancer 120, 1329–1337 (2014). [DOI] [PubMed] [Google Scholar]
- Lu X., Wan F., Zhang H., Shi G. & Ye D. ITGA2B and ITGA8 are predictive of prognosis in clear cell renal cell carcinoma patients. Tumour Biol 37, 253–262 (2016). [DOI] [PubMed] [Google Scholar]
- Lang B. H. et al. Is BRAFV600E mutation a marker for central nodal metastasis in small papillary thyroid carcinoma? Endocr Relat Cancer 21, 285–295 (2014). [DOI] [PubMed] [Google Scholar]
- Kapoor A. et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158, 185–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. H. et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 25, 666–681 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.