Abstract
We performed a meta-analysis to elucidate the associations of the clinicopathological characteristics and prognostic factors of papillary thyroid cancer (PTC) with TERT promoter mutations. A literature search was performed of the PubMed and EMBASE databases using Medical Subject Headings and keywords. Individual study-specific odds ratios (ORs) and confidence intervals (CIs) were calculated. The average prevalence rate of TERT promoter mutations was 10.1%. TERT promoter mutations occurred more frequently in patients with larger tumors (p = 0.003). TERT promoter mutations were associated with advanced stage (OR = 3.11, 95% CI = 2.22–4.36), lymph node metastasis (OR = 1.82, 95% CI = 1.12–2.96), distant metastasis (OR = 4.18, 95% CI = 1.61–10.81), BRAF mutation positivity (OR = 2.71, 95% CI = 1.45–3.24), recurrence (OR = 3.91, 95% CI = 1.83–8.34), and mortality (OR = 8.13, 95% CI = 3.77–17.53). The associations of TERT promoter mutations with extrathyroidal invasion (OR = 1.98, 95% CI = 0.96–4.07), unifocality (OR = 1.36, 95% CI = 0.90–2.07), and vascular invasion (OR = 1.45, 95% CI = 0.92–2.30) were not significant. TERT promoter mutations are closely associated with aggressive clinicopathological characteristics and poorer prognosis in PTC.
Thyroid cancer is the most common malignant tumor of the endocrine system, and its global incidence has rapidly increased in recent decades1. The most common thyroid malignancy is papillary thyroid cancer (PTC), which is derived from the follicular epithelium2. Although conventional surgery with adjuvant radioiodine (131I) therapy has been the main treatment for PTC, it is often not curative. Recently, improved understanding of the molecular pathogenesis of PTC has increased the prospects for developing more effective therapies for this cancer.
Telomerase is responsible for the elongation of telomeric DNA. However, it can also lead to the infinite proliferation of malignant cells by stabilizing telomere length3,4. Telomerase reverse transcriptase (TERT) is considered a predominant determinant for controlling the activity of telomerase. Derepression of TERT transcription is known to induce telomerase activation and consequent malignant transformation5,6. In recent years, the mechanism underlying TERT transcriptional activation in cancer cells has been an active area of investigation.
More recently, a few studies have demonstrated the presence of TERT promoter mutations in PTC6,7. However, the diagnostic and prognostic utility of TERT promoter mutations in PTC remains controversial and requires additional investigation7,8. Therefore, we performed a systematic review and meta-analysis to elucidate the association of TERT promoter mutations with clinicopathological characteristics and prognosis in PTC.
Results
Literature Searches and Study Characteristics
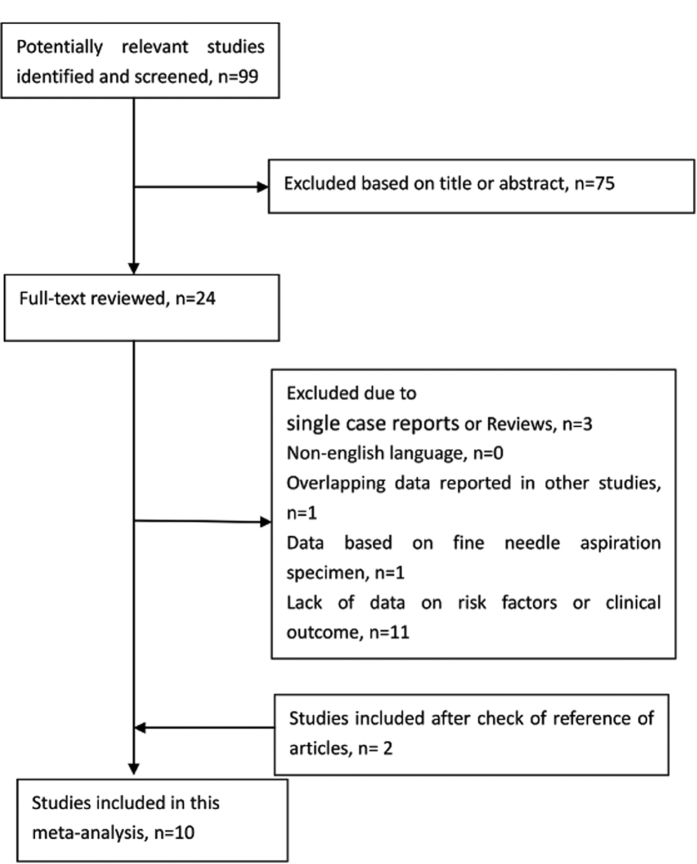
Figure 1 shows the study selection process. A total of 99 abstracts and titles were obtained through electronic searches. Of these abstracts and papers, 24 full-text papers were deemed relevant and examined in detail. Following this detailed review, 10 studies met our inclusion/exclusion criteria6,7,8,9,10,11,12,13,14,15, and these studies contributed 2537 patients with PTC to the meta-analysis. The main features of the 10 eligible studies that investigated prognostic factors are summarized in Table 1. Among these studies, six studies evaluated extrathyroidal invasion, and three studies evaluated tumor size. Data regarding TNM stage, multifocality, vascular invasion, lymph node metastasis, distant metastasis, and BRAF V600E mutations were reported in seven, four, four, nine, four, and five studies, respectively (Tables 1 and 2). The funnel plots for each outcome did not suggest the presence of publication bias (data not shown).
Figure 1. The study selection process.
Table 1. Summary of the 10 Included Studies Comparing TERT Promoter Mutation Rates According to the Clinical and Pathological Risk Factors of PTC.
| Study | Country | Ethnicity (A: Asians, C: Caucasians) | No. of patients with PTC | % Female | Age (TERT mutation-positive, TERT mutation-negative) | TERT Mutation Rate, % | TERT promoter mutation sequence | Clinicopathological features |
|---|---|---|---|---|---|---|---|---|
| Liu (T) 2013 | Sweden | C | 51 | 70.6 | 68 (49–84) 36 (22–97) | 25.50% | N/A | LNM |
| Liu 2014 | USA | A | 408 | 78.2 | 53.40 ± 16.14, 43.66 ± 12.91 | 11.30% | N/A | Extrathyroidal invasion, tumor size, TNM stage, LNM, mortality |
| Gandolfi 2015 | Italy | C | 121 | 68.6 | 59.8 ± 17.1, 45.6 ± 17.6 | 17.40% | 5′-GGATTCGCGGGCACAGAC-3′ 5′-AGCGCTGCCTGAAACTCG-3′ | TNM stage, vascular invasion, LNM, DM, BRAF mutation, recurrence, mortality |
| Melo 2014 | Portugal | C | 332 | 77.7 | 58.4 ± 13.2, 43.6 ± 15.3 | 7.50% | N/A | Extrathyroidal invasion, tumor size, TNM stage, vascular, invasion, LNM, DM, BRAF mutation, mortality |
| Xing 2014 | USA | C | 507 | 72 | 51.7 ± 15.7, 45.1 ± 13.6 | 12.00% | N/A | Extrathyroidal invasion, TNM stage, unifocality, vascular invasion, LNM, DM, recurrence |
| Lee 2015 | Korea | A | 137 | 84.7 | N/A | 6.60% | 5′-AGTGGATTC GCGGGCACAGA-3′ 5′-CAGCGCTGC CTG AAA CTC-3′ | Extrathyroidal invasion, LNM, |
| Muzza 2015 | Italy | C | 182 | 77 | 57.6 (29–82), 44.2 (14–80) | 12% | 5′-AGTGGATTCGCGGGCACAGA-3′ 5′-GCAGCGCTGCCTGAAACTC-3′ | Extrathyroidal invasion, TNM stage, unifocality, LNM, BRAF mutation, recurrence |
| Liu (XL) 2013 | USA | C | 257 | N/A | N/A | 11.70% | 5′-AGTGGATTCGCGGGCACAGA-3′ 5′-CAGCGCTGCCTGAAACTC-3′ | BRAF mutation |
| Qasem 2015 | Arabia | C | 138 | 75.8 | 37.4 ± 18.7, 30.8 ± 15.7 | 7.00% | 5′-AGTGGATTCGCGGGCACAGA-3′ 5′-CAGCGCTGCCTGAAACTC-3′ | Extrathyroidal invasion, tumor size, TNM stage, unifocality, vascular invasion, LNM, DM, recurrence |
| Biase 2015 | Italy | C | 404 | 78.2 | 50.2 ± 11.5, 47.8 ± 12.8 | 4.70% | 5′-GGCTCCCAG TGG ATTCG-3′ 5′-AAGGAAGGG GAG GGG C-3′ | TNM stage, unifocality, LNM, BRAF mutation, recurrence |
N/A, not available; PTC, papillary thyroid cancer; LNM, lymph node metastasis; DM, distant metastasis.
Table 2. Summary of the Seven Included Studies Comparing the TERT promoter Mutation Rate in Patients with Poor Outcomes.
| Study | Country | No. of Follow-up Cases | TERT promoter mutation Rate, % | Median Follow-up, months | Stage of Disease | Initial Treatment | Poor Outcome | Confirmation Method |
|---|---|---|---|---|---|---|---|---|
| Liu (T) 2013 | Sweden | 51 | 25.5% | N/A | III/IV 24.4 | N/A | Mortality | N/A |
| Gandolfi 2015 | Italy | 121 | 17.4% | 124.1 | III/IV 43.0 | TT, ipsilateral central neck dissection | Recurrence, persistent disease, and cancer-specific death | Pathology |
| Melo 2014 | Portugal | 284 | 7.5% | N/A | III/IV 64.7 | N/A | Mortality | Radioiodine scan, Tg, |
| Xing 2014 | USA | 507 | 12.0% | 24 | III/IV 17.5 | Therapeutic neck dissection and RAI ablation | Recurrence | Pathology, Radioiodine scan |
| Muzza 2015 | Italy | 182 | 12% | 74.5 | III/IV 20.6 | N/A | Recurrence and persistence | Pathology, Radioiodine scan, Tg |
| Qasem 2015 | Arabia | 256 | 7.0% | 34 | III/IV 12.7 | Near TT or TT, unilateral or bilateral neck dissection, RAI ablation | Recurrence | Pathology, Radioiodine scan, Tg |
| Biase 2015 | Italy | 288 | 4.7% | N/A | III/IV | N/A | Recurrence | N/A |
N/A, not available; RAI, radioactive iodine; Tg, thyroglobulin; TT, total thyroidectomy; US, ultrasonography.
Meta-analysis of the Effects of TERT promoter mutations on Clinicopathological Features
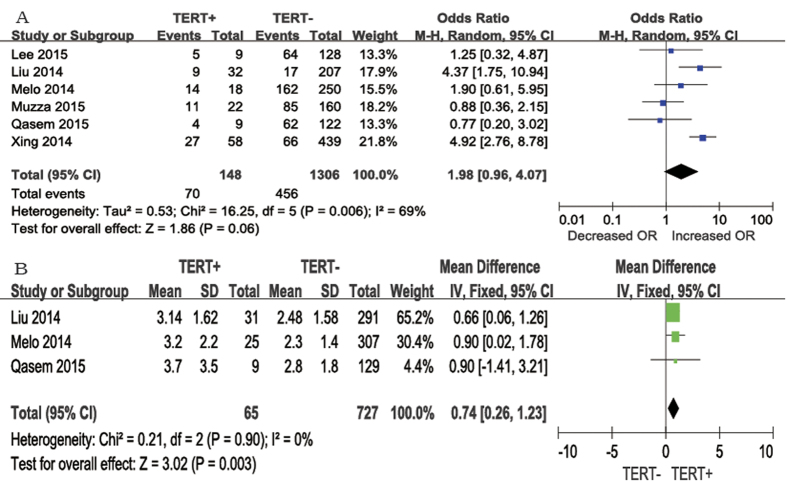
In each category of prognostic factors, the pooled ORs were higher in patients with TERT promoter mutations than in those with the wild-type gene. Six studies presented clinical data including extrathyroidal invasion. Extrathyroidal invasion was present in 70 (47.3%) of 148 patients with TERT promoter mutations and in 456 (34.9%) of 1306 patients without TERT promoter mutations (Fig. 2A). The average odds ratio (OR) from the six studies was 1.98 (95% confidence interval [CI] = 0.96–4.07). The heterogeneity of the data was significant (P = 0.006), and the I2 estimate of the variance between the studies was 69%. According to our analysis, the difference between the occurrence of extrathyroidal invasion according to TERT promoter mutation positivity was not significant (P = 0.06).
Figure 2.
The odds ratios (ORs), Mean Difference (MD) with 95% confidence intervals (CIs) for the association between TERT mutation and extrathyroidal invasion (A) and larger tumor size (cm) (B) respectively in patients with PTC. M-H, Mantel-Haenszel; IV, inverse variance.
We analyzed three studies that reported tumor size (Fig. 2B). A fixed-effects model was adopted, as heterogeneity was not significant between tumor size and TERT promoter mutations (P = 0.90, I2 = 0%). According to our analysis, tumor size was larger in patients with TERT promoter mutations than in those without TERT promoter mutations. The mean difference was 0.74 cm (95% CI = 0.26–1.23, P = 0.003).
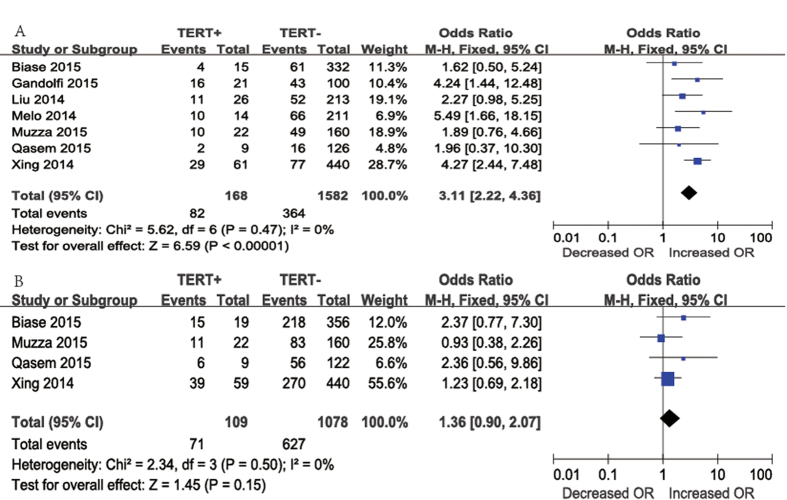
TNM stage was reported for patients in seven studies (Fig. 3A). Advanced TNM stage (III/IV) was identified in 82 (48.8%) of 168 patients with TERT promoter mutations and in 364 (23.0%) of 1582 patients without TERT promoter mutations. A fixed-effects model was adopted because heterogeneity was not significant between TNM stage and TERT promoter mutations (P = 0.47, I2 = 0%). According to our analysis, advanced TNM stage (III/IV) occurred more frequently in patients with TERT promoter mutations than in those without TERT promoter mutations. The overall OR was 3.11 (95% CI = 2.22–4.36, P < 0.00001).
Figure 3.
The odds ratios (ORs) with 95% confidence intervals (CIs) for the association between TERT mutation and advanced TNM stage(III/IV) (A) and unifocality (B) in patients with PTC. M-H, Mantel-Haenszel.
Regarding patients with unifocality (Fig. 3B), four studies were included in the meta-analysis. Unifocality was present in 71 (65.1%) of 109 patients with TERT promoter mutations and in 627 (58.2%) of 1078 patients without TERT promoter mutations. The overall OR was 1.36 (95% CI = 0.90–2.07). A fixed-effects model was adopted, as heterogeneity was not significant between multifocality and TERT promoter mutations (P = 0.50, I2 = 0%). According to our results, there was no significant association between TERT promoter mutations and tumor foci (P = 0.15).
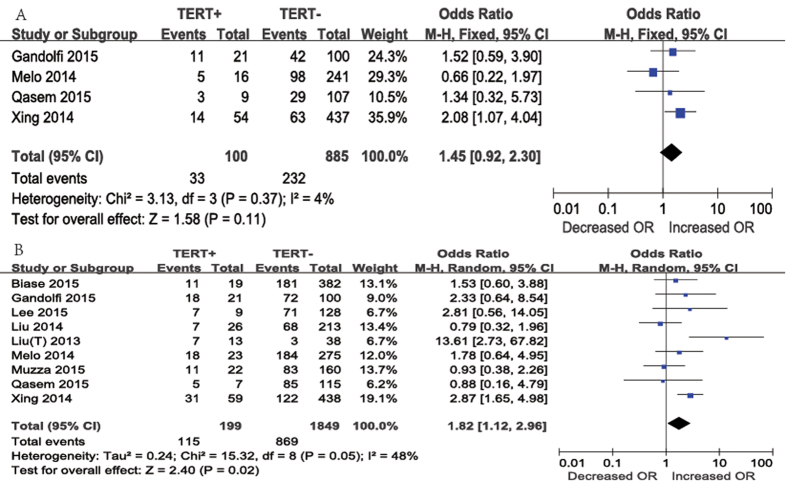
Vascular invasion was reported in four studies (Fig. 4A). Vascular invasion was present in 33 (33%) of 100 patients with TERT promoter mutations and in 232 (26.2%) of 885 patients without TERT promoter mutations. A fixed-effects model was adopted, as heterogeneity was not significant between vascular invasion and TERT promoter mutations (P = 0.37, I2 = 4%). According to our results, the difference in the occurrence of vascular invasion according to TERT promoter mutation positivity was not significant. The overall OR was 1.45 (95% CI = 0.92–2.30, P = 0.11).
Figure 4.
The odds ratios (ORs) with 95% confidence intervals (CIs) for the association between TERT mutation and vascular invasion (A) and lymph node metastasis (B) in patients with PTC. M-H, Mantel-Haenszel.
Regarding cases of lymph node metastasis (Fig. 4B), nine studies were included in the meta-analysis. Lymph node metastasis was present in 115 (57.8%) of 199 patients with TERT promoter mutations and in 869 (47.0%) of 1849 patients without TERT promoter mutations. The overall OR was 1.82 (95% CI, 1.12–2.96). A random-effects model was adopted because the heterogeneity of the data was significant (P = 0.05), and the I2 estimate of the variance between the studies was 48%. According to our analysis, lymph node metastasis occurred more frequently in patients with TERT promoter mutations than in those without TERT promoter mutations (P = 0.02).
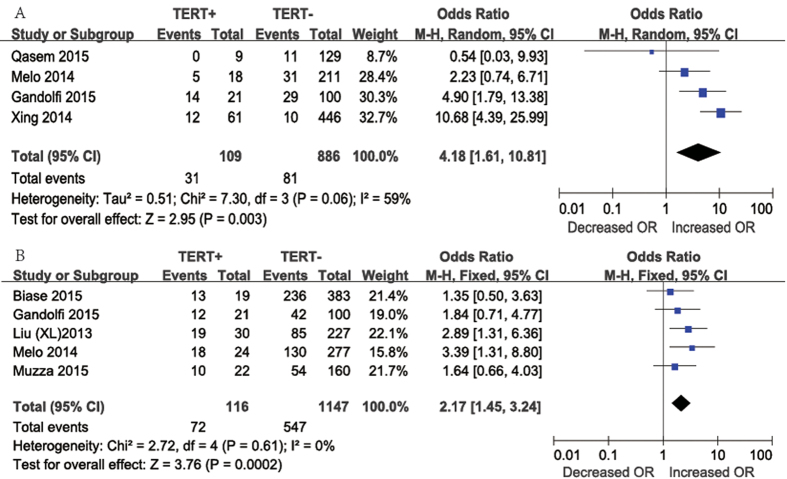
Concerning the presence of distant metastasis (Fig. 5A), four studies were included in the meta-analysis. Distant metastasis was present in 31 (28.4%) of 109 patients with TERT promoter mutations and in 81 (9.1%) of 886 patients without TERT promoter mutations. The overall OR was 4.18 (95% CI, 1.61–10.81). A random-effects model was adopted because the heterogeneity of the data was significant (P = 0.06), and the I2 estimate of the variance between the studies was 59%. According to our analysis, distant metastasis occurred more frequently in patients with TERT promoter mutations than in those without TERT promoter mutations (P = 0.003).
Figure 5.
The odds ratios (ORs) with 95% confidence intervals (CIs) for the association between TERT mutation and distant metastasis (A) and positive BRAF mutation (B) in patients with PTC. M-H, Mantel-Haenszel.
Regarding patients with BRAF mutations (Fig. 5B), five studies were included in our meta-analysis. BRAF mutation positivity was present in 72 (62.1%) of 116 patients with TERT promoter mutations and in 547 (47.7%) of 1147 patients without TERT promoter mutations. The overall OR was 2.17 (95% CI = 1.45–3.24). A fixed-effects model was adopted because the heterogeneity of the data was not significant (P = 0.61), and the I2 estimate of the variance between the studies was 0%. According to our analysis, BRAF mutations occurred more frequently in patients with TERT promoter mutations than in those without TERT promoter mutations (P = 0.0002).
Meta-analysis of the Effect of TERT Mutation on Persistent/Recurrence and Mortality
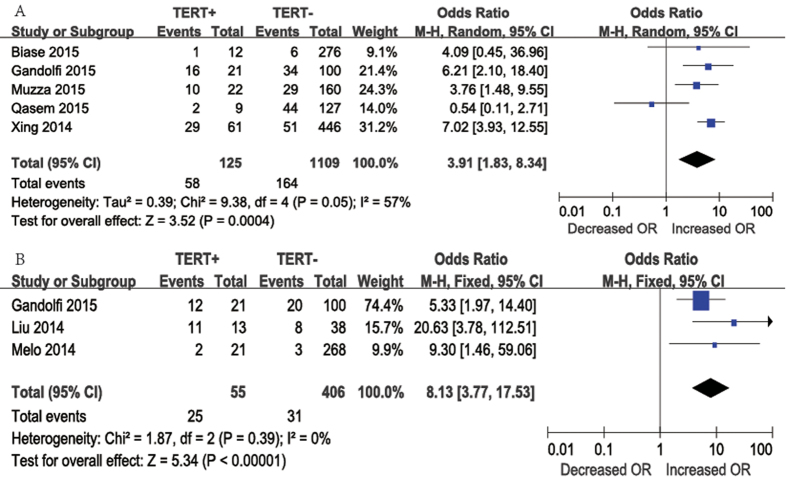
Concerning persistence/recurrence, five studies were included in this meta-analysis. Persistence/recurrence occurred in 58 (46.4%) of 125 patients with TERT promoter mutations and in 164 (14.8%) of 1109 patients without TERT promoter mutations. A random-effects model was adopted because the heterogeneity of the data was significant (P = 0.05), and the I2 estimate of the variance between the studies was 57%. According to our analysis, persistent/recurrence was more frequent in patients with TERT promoter mutations than in those without mutations. The overall OR was 3.91 (95% CI = 1.83–8.34), and the P-value was 0.0004 (Fig. 6A). Furthermore, individual studies were assessed further by sequentially excluding each study from our meta-analysis. In this manner, we found that I2 was 0% when we excluded the study by Qasem, but this did not occur when other articles were excluded; thus, we concluded that the heterogeneity was mainly caused by this particular study.
Figure 6.
The odds ratios (ORs) with 95% confidence intervals (CIs) for the association between TERT mutation and persistence/recurrence (A) and mortality (B) in patients with PTC. M-H, Mantel-Haenszel.
Mortality data were reported in three studies (Fig. 6B). Mortality occurred in 25 (45.5%) of 55 patients with TERT promoter mutations and in 31 (7.6%) of 406 patients without TERT promoter mutations. A fixed-effects model was adopted, as heterogeneity was not significant between mortality and TERT promoter mutations (P = 0.39, I2 = 0%). According to our analysis, mortality was more frequent among patients with TERT promoter mutations than among those without mutations. The overall OR was 8.13 (95% CI = 3.77–17.53, P < 0.00001).
Subgroup Analyses of the Effects of TERT promoter mutations on Aggressive Clinicopathological Features and Prognostic Factors
Subgroup analysis was conducted according to the patients’ country of origin to investigate potential sources of heterogeneity and to assess whether the effects of TERT promoter mutations on aggressive clinicopathological features and poor prognosis of PTC were associated with geographic regions (Table 3). The effect estimates were broadly consistent among the subgroups that were analyzed. Heterogeneity was markedly decreased in the subgroup analyses of extrathyroidal invasion, distant metastasis, and recurrence. However, this may have been caused by two Asian countries (Saudi Arabia and Korea) being distantly located geographically and being different in many aspects. The two studies involved were among the smallest in our meta-analysis, and the total number of cases and relatively low prevalence of TERT promoter mutations led to a lack of statistical power. The results of the subgroup analyses also indicated that TERT promoter mutations were not significantly associated with some high-risk tumor features (e.g., extrathyroidal extension and lymph node metastasis) of PTC in patients from Asia.
Table 3. Subgroup Analysis of the Effects of TERT promoter mutations on the Aggressive Clinicopathological Features and Poor Prognosis of PTC According to Country of Origin.
| Subgroup | OR | 95% CI | I2 (%) | Model used |
|---|---|---|---|---|
| Extrathyroidal invasion | ||||
| USA (one study) | 4.92 | 2.76–8.78 | 0 | — |
| Asia (three studies) | 1.78 | 0.58–5.44 | 61 | Random-effects |
| Europe (two studies) | 1.21 | 0.61–2.40 | 8 | Fixed-effects |
| Lymph node metastasis | ||||
| USA (one study) | 2.87 | 1.65–4.98 | 0 | — |
| Asia (three studies) | 1.07 | 0.54–2.13 | 0 | Fixed-effects |
| Europe (five studies) | 2.01 | 0.98–4.13 | 53 | Random-effects |
| Distant metastasis | ||||
| USA (one study) | 10.68 | 4.39–25.99 | 0 | — |
| Asia (one study) | 0.54 | 0.03–9.93 | 0 | — |
| Europe (two studies) | 3.53 | 1.72–7.26 | 7 | Fixed-effects |
| Recurrence | ||||
| USA (one study) | 7.02 | 3.93–12.55 | 0 | — |
| Asia (one study) | 0.54 | 0.11–2.71 | 0 | — |
| Europe (three studies) | 4.76 | 2.43–9.30 | 0 | Fixed-effects |
OR, odds ratio; CI, confidence interval.
Discussion
To some extent, molecular alterations such as genetic and epigenetic alterations in signaling pathways have changed the treatment of thyroid cancer through the development of targeted therapies. Many somatic genetic alterations including those in BRAF, HRAS, KRAS, NRAS, PTEN, and HER1 have been revealed to play fundamental roles in the tumorigenesis of thyroid carcinoma. The close association of TERT promoter somatic mutations with tumorigenesis is widely recognized. TERT expression confers infinite proliferation potential and many other biological activities on cancer cells by maintaining telomere length16,17,18. In addition, recent data also demonstrated that the functional TERT rs2736100 SNP as a novel genetic component of PTC etiology19.
TERT promoter mutations, which might contribute to telomerase activation, have been reported in various cancers including melanoma, anaplastic oligodendroglioma, and bladder cancer20,21,22,23,24. Recently, the presence of TERT promoter somatic mutations has been reported in PTC; however, their diagnostic and prognostic significance remains unclear7,8. The present study aimed to determine the influence of TERT promoter somatic mutations on the clinicopathological outcomes and prognosis of PTC via a meta-analysis.
Larger tumor size, extrathyroidal invasion, stage III or IV disease, lymph node metastasis, and distant metastasis were correlated with poor prognostic features such as recurrence and short overall survival (OS)15,25. Several authors suggested that TERT promoter somatic mutations were associated with these aggressive clinicopathological characteristics7,14. This view is mainly in line with the results of our meta-analysis. Our findings illustrated that TERT promoter mutations were more likely to be present in patients with larger tumors, stage III or IV disease, lymph node metastasis, and distant metastasis. However, extrathyroidal invasion was at a critical level in terms of an association with TERT promoter mutations (P = 0.06).
One interesting finding in the current study was that the associations of TERT promoter mutations with multifocality and vascular invasion were not significant. This finding may be explained by the fact that the studies including data on focus number and vascular invasion were relatively small.
Most authors previously demonstrated that recurrence and poor OS were more likely to occur in patients with TERT promoter mutations than in those without mutations. By contrast, Qasem et al. found that the relationships of recurrence and short OS with TERT promoter mutations were not significant14. In our meta-analysis, however, patients with PTCs with TERT promoter mutations were more likely to experience recurrence and worse OS. Therefore, we conclude that TERT promoter mutations may represent an independent prognostic factor for prognosis in PTC.
A classic alteration found in PTC is the V600E mutation in the serine/threonine kinase BRAF9,26. Multicenter studies confirmed the association of BRAFV600E with poor clinicopathological outcomes such as aggressive pathological features, increased recurrence, and resistance to radioiodine therapy in PTC27. Recently, a positive correlation between TERT promoter mutations and the BRAFV600E mutation was reported in advanced PTCs, suggesting functional cooperation, and Xing et al. suggested that patients with PTC and coexistent TERT promoter and BRAFV600E mutations tend to have poorer prognosis compared with patients harboring single mutations or wild-type TERT promoter and BRAF7. However, evidence reported by Biase et al. illustrated that TERT promoter mutations do not predict unfavorable features in papillary thyroid microcarcinoma, either alone or in combination with the BRAF V600E mutation8, and Melo et al. found no differences in persistence between tumors with TERT promoter mutations alone and those harboring both TERT and BRAF mutations10. Furthermore, Gandolfi et al. observed a positive association between BRAF V600E and TERT C228T mutations in a cohort of patients with PTC and distant metastases11. However, the associations of BRAF V600E with TERT C250T and all TERT promoter mutations were not significant. Our meta-analysis illustrated that TERT promoter mutations occurred more frequently in patients with BRAF mutations. Whether the coexistence of these two mutations predicts a poor prognosis is unclear and needs further investigation.
Our systematic review and meta-analysis assessed the associations of TERT promoter mutations with clinicopathological outcomes and prognosis in PTC. Although our study cannot identify a causal relationship between TERT promoter mutations and outcomes and TERT promoter mutations may be correlated with some other mutation or some confounder that may be even more useful, our findings may still be of great value in evidence-based clinical decision-making. In other words, our analysis should be interpreted with caution because of the heterogeneity of the data. Possible explanations for this heterogeneity include patient demographics as well as thyroidectomy, approaches to lymph node dissection, radioactive iodine (RAI) treatment, and the duration of follow-up. The population included patients from the USA, Portugal, Italy, and China, among other countries. Details of the approaches used for thyroidectomy, lymph node dissection, and RAI treatment were not all provided; furthermore, the outcomes might be interrelated. For instance, patients with more advanced disease tend to have extrathyroidal invasion, and thus, disease stage may confound the association between TERT promoter mutations and extrathyroidal invasion.
Apart from potential heterogeneity caused by varied management practices, differences in pathology reporting, follow-up duration, and the definition of remission may affect the conclusions of this meta-analysis. Another limitation is that the number of included articles is relatively small, and relevant unpublished data could not be obtained for further analysis. Therefore, our conclusions should be used cautiously. Larger studies would be helpful to definitely address the role of TERT promoter mutations in the prognosis of PTC. Furthermore, we did not distinguish between the C228T and C250T mutations from collective mutations in some studies9,11, and in the study by de Biase et al.8, only microcarcinomas were analyzed, which may have interfered with our results.
Conclusion
Our meta-analysis demonstrated that TERT promoter mutations are closely associated with aggressive clinicopathological factors and poorer prognosis in PTC. TERT promoter mutations may be considered poor diagnostic and prognostic markers in PTC, and patients with such mutations may require aggressive management. More studies are needed to better evaluate the role of TERT promoter mutations in PTC.
Materials and Methods
Search strategy and literature selection
A systematical literature search was performed using online electronic databases (PubMed, EMBASE, and ISI Web of Science) for published papers through November 2015, and the search was supplemented by manual searching and reference backtracking using the following Medical Subject Headings and keywords: “TERT”, “ telomerase reverse transcriptase”, “mutation”, “thyroid”, “neoplasm(s)”, “tumor”, “cancer”, and “carcinoma”. The searches were limited to studies conducted in humans and written in English. Furthermore, the reference lists of retrieved articles were also reviewed to identify additional studies. Relevant unpublished data that were presented at international meetings such as the American Thyroid Association meeting were also included. We contacted the authors for additional tabular data when necessary13.
The following criteria were applied into the literature selection for studies that examined the association of TERT promoter mutations with high-risk clinic pathological factors and prognostic outcomes: (1) the studies had a randomized controlled trial or retrospective comparative study (cohort or case-control study) design; (2) the studies compared the TERT mutation-positive and TERT mutation-negative groups for patients with primary PTC; (3) the study investigated at least one outcome of interest; and (4) weighted mean differences (WMDs) and ORs with 95% CIs were reported or were available to be calculated. Studies lacking a control population, duplicates of previous publication, animal studies, abstracts, single-case reports, reviews, and unpublished reports were excluded. Additionally, studies that included TERT mutation analysis for patients with preoperative fine-needle aspiration biopsies were excluded to avoid false-negative results for the cytologic specimens. For studies with the same or overlapping data published by the same investigators, we selected studies with complete designs and larger sample sizes in our meta-analysis.
Data extraction
All data were extracted independently by two authors (CP Liu and ZM Liu) and cross-checked to resolve any discrepancies. The following information was extracted from each included study regarding the association of TERT promoter mutations with clinical and pathological risk factors of PTC: first author, publication year, study location, ethnicity, number of cases, the percentage of females, age in both the TERT mutation-positive and TERT mutation-negative groups, TERT mutation rate, and the incidence rate of clinicopathological features in both the TERT mutation-positive and TERT mutation-negative groups. Regarding the associations of TERT promoter mutations with recurrent/persistent disease and mortality, the following data were extracted: first author, publication year, study location, number of follow-up cases, TERT mutation rate, stage of disease, initial treatment, poorer outcome, and the incidence rates of persistent/recurrence and mortality in both the TERT mutation-positive and TERT mutation-negative groups.
The following outcomes were extracted to compare the TERT mutation-positive and TERT mutation-negative groups for patients with primary PTC: the presence of extrathyroidal invasion, larger tumor size, advanced TNM stage (stage III or IV), unifocality, vascular invasion, lymph node metastasis, distant metastasis, BRAF mutation positivity, persistent/recurrent disease, and cancer-specific mortality. All of the procedures conformed to the guidelines for the meta-analysis of observational studies in epidemiology28.
Statistical Analysis
The summary ORs with 95% CIs and WMDs with 95% CIs were calculated to compare dichotomous and continuous variables, respectively. The χ2-based Cochran’s Q statistic test and I2 statistics were used to evaluate heterogeneity between the studies, and heterogeneity was considered significant when P < 0.1 for the Q statistic or for an I2 statistic >50%29. A fixed-effects model (Mantel-Haenszel method) was used when no significant heterogeneity was detected; otherwise, a random-effects model (DerSimonian-Laird method) was applied. Subgroup analyses were also performed according to geographic region. In addition, we performed sensitivity analysis to assess the influence of each study on the overall estimate. Moreover, the potential publication bias was assessed using Egger’s test and funnel plot analysis. All meta-analyses were performed using Review Manager (version 5, Cochrane Collaboration, Oxford, UK). The P-values were two-tailed with a level of significant at 0.05.
Additional Information
How to cite this article: Liu, C. et al. TERT promoter Mutation and Its Association with Clinicopathological Features and Prognosis of Papillary Thyroid Cancer: A Meta-analysis. Sci. Rep. 6, 36990; doi: 10.1038/srep36990 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Author Contributions All authors contributed to the design of the study and writing of the manuscript. C.P.L. and Z.M.L. undertook the research and performed the analyses. All authors reviewed and approved the final version of the manuscript.
References
- Jemal A. et al. Global cancer statistics. CA: a cancer journal for clinicians 61, 69–90, doi: 10.3322/caac.20107 (2011). [DOI] [PubMed] [Google Scholar]
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nature reviews. Cancer 13, 184–199, doi: 10.1038/nrc3431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Greider C. W. & Szostak J. W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature medicine 12, 1133–1138, doi: 10.1038/nm1006-1133 (2006). [DOI] [PubMed] [Google Scholar]
- Cong Y. S., Wright W. E. & Shay J. W. Human telomerase and its regulation. Microbiology and molecular biology reviews: MMBR 66, 407–425, table of contents (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M., Peek G. W. & Tollefsbol T. O. Regulation of the human catalytic subunit of telomerase (hTERT). Gene 498, 135–146, doi: 10.1016/j.gene.2012.01.095 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 33, 4978–4984, doi: 10.1038/onc.2013.446 (2013). [DOI] [PubMed] [Google Scholar]
- Xing M. et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 32, 2718–2726, doi: 10.1200/jco.2014.55.5094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Biase D. et al. TERT Promoter Mutations in Papillary Thyroid Microcarcinomas. Thyroid: official journal of the American Thyroid Association 25, 1013–1019, doi: 10.1089/thy.2015.0101 (2015). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. The Journal of clinical endocrinology and metabolism 99, E1130–E1136, doi: 10.1210/jc.2013-4048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo M. et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. The Journal of clinical endocrinology and metabolism 99, E754–E765, doi: 10.1210/jc.2013-3734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi G. et al. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. European journal of endocrinology/European Federation of Endocrine Societies 172, 403–413, doi: 10.1530/eje-14-0837 (2015). [DOI] [PubMed] [Google Scholar]
- Muzza M. et al. Telomerase in differentiated thyroid cancer: promoter mutations, expression and localization. Molecular and cellular endocrinology 399, 288–295, doi: 10.1016/j.mce.2014.10.019 (2015). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocrine-related cancer 20, 603–610, doi: 10.1530/erc-13-0210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasem E. et al. TERT promoter mutations in thyroid cancer: a report from a Middle Eastern population. Endocrine-related cancer 22, 901–908, doi: 10.1530/erc-15-0396 (2015). [DOI] [PubMed] [Google Scholar]
- Lee J. et al. GLI1 Transcription Factor Affects Tumor Aggressiveness in Patients With Papillary Thyroid Cancers. Medicine 94, e998, doi: 10.1097/md.0000000000000998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 32, 4203–4213, doi: 10.1038/onc.2012.441 (2013). [DOI] [PubMed] [Google Scholar]
- Park J. I. et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72, doi: 10.1038/nature08137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. A. et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America 99, 12606–12611, doi: 10.1073/pnas.182407599 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge M. et al. Functional evaluation of TERT-CLPTM1L genetic variants associated with susceptibility of papillary thyroid carcinoma. Scientific reports 6, 26037, doi: 10.1038/srep26037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinagre J. et al. Frequency of TERT promoter mutations in human cancers. Nature communications 4, 2185, doi: 10.1038/ncomms3185 (2013). [DOI] [PubMed] [Google Scholar]
- Huang F. W. et al. Highly recurrent TERT promoter mutations in human melanoma. Science (New York, N.Y.) 339, 957–959, doi: 10.1126/science.1229259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotsch D. et al. Prognostic significance of telomerase-associated parameters in glioblastoma: effect of patient age. Neuro-oncology 15, 423–432, doi: 10.1093/neuonc/nos329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. et al. TERT Promoter Mutations Lead to High Transcriptional Activity under Hypoxia and Temozolomide Treatment and Predict Poor Prognosis in Gliomas. PloS one 9, e100297, doi: 10.1371/journal.pone.0100297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griewank K. G. et al. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PloS one 8, e80354, doi: 10.1371/journal.pone.0080354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J. et al. Risk factors for recurrence after therapeutic lateral neck dissection for primary papillary thyroid cancer. Annals of surgical oncology 21, 1884–1890, doi: 10.1245/s10434-014-3507-y (2014). [DOI] [PubMed] [Google Scholar]
- Kim T. H. et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118, 1764–1773, doi: 10.1002/cncr.26500 (2012). [DOI] [PubMed] [Google Scholar]
- Xing M. et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. The Journal of clinical endocrinology and metabolism 90, 6373–6379, doi: 10.1210/jc.2005-0987 (2005). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 327, 557–560, doi: 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]