Abstract
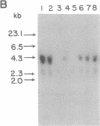
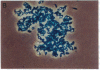
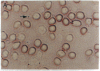
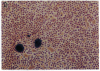
We have used the 1.1 kilobases of the 5' upstream region of the platelet factor four (PF4) gene coupled to the prokaryotic beta-galactosidase gene to generate two lines of transgenic mice that express this construct. Studies of blood, bone marrow, spleen, and thymus reveal that platelets are the only circulating blood cells and megakaryocytes are the only hematopoietic precursor cells that possess the prokaryotic enzyme. The lack of transgene expression in brain, heart, intestine, kidney, liver, lung, and skeletal muscle was established by in situ staining of tissue sections as well as kinetic assay of tissue homogenates. These data suggest that this domain of the PF4 promoter contains most, if not all, of the tissue-specific region of the gene. Unexpectedly, the adrenal gland exhibits approximately 2% of the levels of beta-galactosidase possessed by megakaryocytes and the distribution of the prokaryotic enzyme corresponds to the location of mineralocorticoid-secreting cells. This result implies that either the PF4 gene is transcribed at low levels in specialized adrenal cells or that these specialized endocrine cells possess trans-acting factors similar to those that control the megakaryocyte promoter. The selective high-level expression of transgenes linked to the PF4 promoter should allow us to augment or suppress the in vivo levels of critical components in megakaryocytes and platelets and subsequently ascertain the effects of these modifications.
Full text
PDF

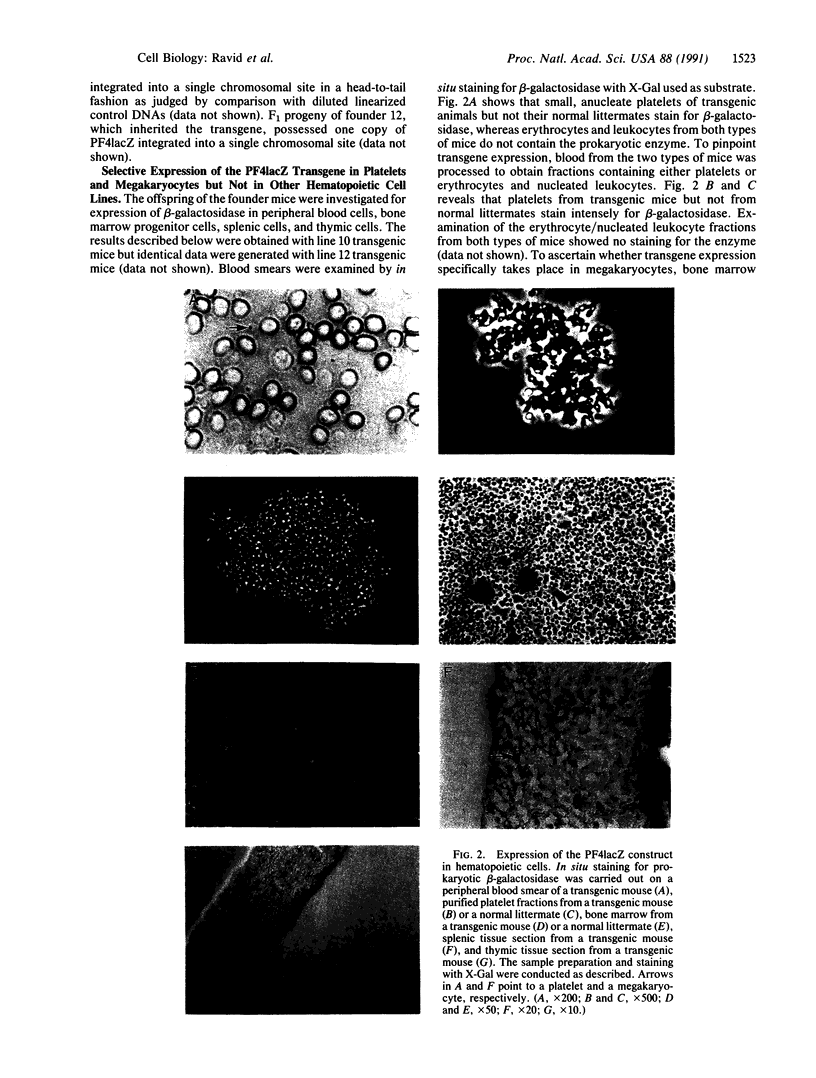


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. J., Käser-Glanzmann R., Jakábová M., Lüscher E. F. Characterization of a chondroitin 4 -sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4 ) released from human blood platelets. Biochim Biophys Acta. 1972 Dec 29;286(2):312–329. [PubMed] [Google Scholar]
- Burstein S. A., Adamson J. W., Erb S. K., Harker L. A. Megakaryocytopoiesis in the mouse: response to varying platelet demand. J Cell Physiol. 1981 Nov;109(2):333–341. doi: 10.1002/jcp.1041090217. [DOI] [PubMed] [Google Scholar]
- Burstein S. A., Adamson J. W., Thorning D., Harker L. A. Characteristics of murine megakaryocytic colonies in vitro. Blood. 1979 Jul;54(1):169–179. [PubMed] [Google Scholar]
- Busch C., Dawes J., Pepper D. S., Wasteson A. Binding of platelet factor 4 to cultured human umbilical vein endothelial cells. Thromb Res. 1980 Jul 1;19(1-2):129–137. doi: 10.1016/0049-3848(80)90412-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Greenberg S. M., Rosenberg R. D. Structure of the rat platelet factor 4 gene: a marker for megakaryocyte differentiation. Mol Cell Biol. 1987 Feb;7(2):898–904. doi: 10.1128/mcb.7.2.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBBE S., STOHLMAN F., Jr MEGAKARYOCYTOPOIESIS IN THE RAT. Blood. 1965 Jul;26:20–35. [PubMed] [Google Scholar]
- Gewirtz A. M., Calabretta B. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science. 1988 Dec 2;242(4883):1303–1306. doi: 10.1126/science.2461588. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hiti-Harper J., Wohl H., Harper E. Platelet factor 4: an inhibitor of collagenase. Science. 1978 Mar 3;199(4332):991–992. doi: 10.1126/science.203038. [DOI] [PubMed] [Google Scholar]
- Katz I. R., Thorbecke G. J., Bell M. K., Yin J. Z., Clarke D., Zucker M. B. Protease-induced immunoregulatory activity of platelet factor 4. Proc Natl Acad Sci U S A. 1986 May;83(10):3491–3495. doi: 10.1073/pnas.83.10.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lonky S. A., Marsh J., Wohl H. Stimulation of human granulocyte elastase by platelet factor 4 and heparin. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1113–1118. doi: 10.1016/0006-291x(78)90657-5. [DOI] [PubMed] [Google Scholar]
- Maione T. E., Gray G. S., Petro J., Hunt A. J., Donner A. L., Bauer S. I., Carson H. F., Sharpe R. J. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990 Jan 5;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W. Polyploidy and maturation of rat megakaryocytes. Blood. 1968 Jul;32(1):102–110. [PubMed] [Google Scholar]
- Paulus J. M. DNA metabolism and development of organelles in guinea-pig megakaryocytes: a combined ultrastructural, autoradiographic and cytophotometric study. Blood. 1970 Mar;35(3):298–311. [PubMed] [Google Scholar]
- Ryo R., Nakeff A., Huang S. S., Ginsberg M., Deuel T. F. New synthesis of a platelet-specific protein: platelet factor 4 synthesis in a megakaryocyte-enriched rabbit bone marrow culture system. J Cell Biol. 1983 Feb;96(2):515–520. doi: 10.1083/jcb.96.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma J. J., Wester J. The hemostatic plug. Semin Hematol. 1977 Jul;14(3):265–299. [PubMed] [Google Scholar]
- Vinci G., Tabilio A., Deschamps J. F., Van Haeke D., Henri A., Guichard J., Tetteroo P., Lansdorp P. M., Hercend T., Vainchenker W. Immunological study of in vitro maturation of human megakaryocytes. Br J Haematol. 1984 Apr;56(4):589–605. doi: 10.1111/j.1365-2141.1984.tb02184.x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]