Abstract
Lung inflammation in premature infants contributes to the development of bronchopulmonary dysplasia (BPD), a chronic lung disease with long-term sequelae. Pilot studies administering budesonide suspended in surfactant have found reduced BPD without the apparent adverse effects that occur with systemic dexamethasone therapy. Our objective was to determine budesonide potency, stability, and antiinflammatory effects in human fetal lung. We cultured explants of second-trimester fetal lung with budesonide or dexamethasone and used microscopy, immunoassays, RNA sequencing, liquid chromatography/tandem mass spectrometry, and pulsating bubble surfactometry. Budesonide suppressed secreted chemokines IL-8 and CCL2 (MCP-1) within 4 hours, reaching a 90% decrease at 12 hours, which was fully reversed 72 hours after removal of the steroid. Half-maximal effects occurred at 0.04–0.05 nM, representing a fivefold greater potency than for dexamethasone. Budesonide significantly induced 3.6% and repressed 2.8% of 14,500 sequenced mRNAs by 1.6- to 95-fold, including 119 genes that contribute to the glucocorticoid inflammatory transcriptome; some are known targets of nuclear factor-κB. By global proteomics, 22 secreted inflammatory proteins were hormonally regulated. Two glucocorticoid-regulated genes of interest because of their association with lung disease are CHI3L1 and IL1RL1. Budesonide retained activity in the presence of surfactant and did not alter its surface properties. There was some formation of palmitate-budesonide in lung tissue but no detectable metabolism to inactive 16α-hydroxy prednisolone. We concluded that budesonide is a potent and stable antiinflammatory glucocorticoid in human fetal lung in vitro, supporting a beneficial antiinflammatory response to lung-targeted budesonide:surfactant treatment of infants for the prevention of BPD.
Keywords: inflammatory, chemokines, surfactant, RNA sequencing, proteomics
Clinical Relevance
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of premature infants that is associated with later asthma and adverse neurodevelopmental outcome. Postnatal systemic dexamethasone therapy reduces the incidence of BPD, in part because of its antiinflammatory action; however, this therapy increases the risk of neurodevelopmental impairment. Pilot studies instilling budesonide suspended in surfactant have found reduced BPD without apparent adverse effects, but gene responses and the optimal treatment regimen are not known. In explants of human fetal lung, budesonide at low concentrations rapidly and reversibly suppressed chemokines and other inflammatory mediators acting at the level of transcription. Budesonide retained activity in the presence of surfactant and did not alter the surface properties of surfactant, supporting this approach to lung-targeted glucocorticoid therapy.
Bronchopulmonary dysplasia (BPD) is a chronic lung disease in premature infants that is associated with later respiratory disease, including asthma, and adverse neurodevelopmental outcome (1, 2). The pathogenesis of this disorder involves an immature lung with inadequate defense mechanisms, plus exposure to hyperoxia, barotrauma, and often infection leading to an inflammatory process, respiratory failure, arrested lung development, and airway abnormalities (3). Currently, there is no safe and highly effective preventative therapy.
Postnatal systemic dexamethasone therapy reduces the incidence of BPD, likely because of its antiinflammatory action and because it promotes lung maturation. However, this therapy increases the risk of long-term neurodevelopmental impairment and currently is used generally for short durations only in selected infants to improve respiratory status (4, 5). Because of safety issues with systemic dexamethasone, lung-targeted alternatives to corticosteroid therapy have been explored. Administration of inhaled budesonide has generally been ineffective (6), likely because of inefficient delivery to the lung parenchyma, although a recent trial with prolonged treatment demonstrated reduced BPD occurrence (7). Pilot studies performed by Yeh and colleagues (8, 9) used intratracheal instillation of budesonide suspended in surfactant to improve the delivery of budesonide to the lung periphery. They reported a substantially reduced incidence of BPD with no observed immediate or long-term adverse effects (8–10).
Budesonide is a nonfluorinated synthetic glucocorticoid that is used extensively via inhalation for asthma control. Budesonide can be conjugated with fatty acids to form an ester that subsequently is hydrolyzed to release free budesonide (11, 12); this reservoir effect occurs in adult lung tissue and likely improves the duration of local glucocorticoid action.
To explore budesonide's antiinflammatory effects in fetal lung, as well as to inform the most appropriate dosing regimen for budesonide:surfactant in human trials, we performed studies using explants of human fetal lung. We characterized budesonide suppression of chemokines IL-8 and CCL2 (MCP1) and determined the profile of inflammatory genes that are transcriptionally regulated by budesonide. Our results also provide new information on budesonide metabolism in fetal lung and its interactive effects with surfactant.
Materials and Methods
Tissue
Midgestation (19- to 23-wk-old) human fetal lungs were collected with institutional review board approval from elective pregnancy terminations in healthy consenting women. Explants were prepared as described previously (13) and treated with dexamethasone (Sigma-Aldrich, St. Louis, MO), budesonide (Sigma-Aldrich), or diluent (control) added to the medium after 24 hours of culture. In some experiments, budesonide at various concentrations was combined with calfactant, and the mixture was added to explants (10 μl surfactant/ml of medium). Fresh medium was added every 24 hours in most experiments, unless noted. Tissue and medium were harvested at various times during culture.
Protein Analysis
Western analyses were performed and quantitated as described previously (14). Chemokines were quantified by ELISA using Human CCL2/MCP-1 and CXCL8/IL-8 Quantikine ELISA Kits (R&D Systems, Minneapolis, MN) per manufacturer’s protocols and a Victor 3 multiwell plate reader (Perkin Elmer, Waltham, MA). The limit of detection was 10 pg/ml for CCL2 and 7.5 pg/ml for IL-8. Proteomic studies of explant culture medium analyzed digested peptides by liquid chromatography and tandem mass spectrometry (LC-MS/MS) (Thermo Q Exactive plus; Thermo Scientific, Waltham, MA) at the University of California, San Francisco (UCSF) Mass Spectrometry Facility, with spectral data analysis performed using Protein Prospector (15) software.
mRNA Analysis
Total RNA was extracted from three different lungs before culture (preculture) and after 96-hour culture (control and budesonide, 30 nM) using RNeasy kits (QIAGEN, Valencia CA) and was quantified and quality checked using the Bioanalyzer 2100 (Agilent Technologies Inc., Santa Clara, CA) and the Nano Drop (Thermo Scientific). mRNA sequencing was performed by the UCSF SABRE Functional Genomics Core using HiSeq 2500 machines (Illumina, San Diego, CA) and multiplexing four samples per lane with100 base-paired end reads. Quantitative polymerase chain reaction for selected mRNAs was performed as described (14).
Per gene differential expression on the basis of RNA-Seq read counts was performed using the R/Bioconductor package limma (16) and the voom method (17). Control of false discovery rates (FDRs) for multiple comparisons was performed via the Benjamini-Hochberg correction (18). Prefiltering to exclude genes possessing ≤5 counts per million when summed over all experiments was performed. Post-processing of the top differentially expressed genes (FDR < 0.05; fold change >1.6) used enrichment analyses by Database for Annotation, Visualization, and Integrated Discovery (DAVID) (19) and the Ingenuity Pathway Analysis platform (QIAGEN). Complete RNA sequencing (RNA-seq) data are available at Gene Expression Omnibus (accession number GSE83888) of the National Center of Biotechnology (www.ncbi.nlm.nig.gov/geo/).
Metabolism
We used LC-MS/MS and positive electrospray ionization detection as described (20) to investigate the levels of budesonide and selected major metabolites in the explants and culture medium. Medium was extracted as described previously (20); for tissue extraction, 30 mg lung was sonicated in ethanol and shaken for 5 hours at 4°C. Samples were reconstituted in 30:70 MeOH:H2O for analysis. The limit of quantification was 0.5 ng/ml for budesonide palmitate and 16α-hydroxyprednisolone, and 2.0 ng/ml for budesonide.
Surface Tension Measurements
Surface activity of calfactant supplemented with budesonide (at 0.5 and 1% of phospholipid by weight) was assayed in a pulsating bubble surfactometer (Electronectics Corp., Buffalo, NY) as described (21). Pulsation was at 20 cycles per minute, and an equilibrium surface tension of 23–25 mN/m was reached instantaneously for all samples.
Results
Chemokine Secretion
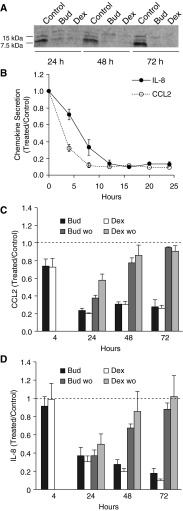
To characterize the antiinflammatory effects of budesonide in fetal lung, we chose two secreted chemokines, IL-8 and CCL2, which are known to be both glucocorticoid responsive and elevated in infants with early respiratory failure (13, 22). Explants were treated with serum-free control medium or medium containing 30 nM budesonide or dexamethasone, which resulted in similar morphological changes of epithelial cells by light microscopy (see online supplement). Figure 1A shows a representative Western blot for secreted CCL2, which was markedly suppressed by both corticosteroids at all three 24-hour time points, whereas there was no significant change for controls; in the same experiments, neither chemokine was detected in explant tissue (not shown). To further define the kinetics of budesonide response, media were collected and replaced every 4 hours. By 12 hours, both chemokines were suppressed by ∼90% compared with control samples, with a faster initial response observed for CCL2 (Figure 1B).
Figure 1.
Time course of chemokine release from fetal lung explants cultured in Bud or Dex. (A) Representative CCL2 Western blot of culture media ± 30 nM Bud or Dex collected every 24 hours. At this concentration of corticosteroid, CCL2 was similarly suppressed by both Bud and Dex at all time points (7.5 and 15 kD bands) with minimal effect on the upper nonspecific band. (B) IL-8 and CCL2 levels by ELISA in culture media collected and changed every 4 hours, maintaining the original treatment conditions. Bud reduced levels by 4 hours with ∼90% suppression within 12–16 hours and 8–12 hours for CCL2 and IL-8, respectively (n = 4, 30 nM Bud). (C and D) Effect of removing corticosteroid after 4 hours of exposure. Explants were exposed to 3 nM Bud or Dex continuously (solid bars) or for 4 hours only (gray bars) followed by a saline rinse and replacement with steroid-free medium. For CCL2, the differences between washout levels and continuous exposure were statistically significant at all time points for both steroids, whereas IL-8 levels were significantly different at 48 and 72 hours. Dashed line indicates control level. Data are presented as mean ± SE (n = 4). Bud, budesonide; Dex, dexamethasone; wo, steroid washout. CCL2, C-C motif chemokine ligand 2.
To examine the reversibility of hormone treatment, explants were cultured with 3 nM budesonide or dexamethasone; this lower concentration was chosen to facilitate the removal of steroid when changing to the control medium. After 4 hours of exposure to hormone, the glucocorticoid-containing media of some culture dishes were collected and replaced with hormone-free control media for 20 hours (washout); this procedure was repeated at 24, 48, and 72 hours. As shown in Figure 1C (CCL2) and Figure 1D (IL-8), suppression was maintained in the continuing presence of corticosteroid and was partially reversed at 24 hours (CCL2) or 48 hours (IL-8); at 72 hours after hormone washout, levels were similar to those of the control. There was a trend toward more rapid reversibility for dexamethasone compared with budesonide (e.g., CCL2 at 24 h).
We determined the potency of budesonide and dexamethasone in dose–response experiments using 10 concentrations of steroid (0.02–100 nM) with media collection and replacement every 24 hours. Results were similar at each time point, and summary half-maximal data are presented in Table 1. For both CCL2 and IL-8, budesonide caused half-maximal suppression at 0.04–0.05 nM and was five- to six-fold more potent than dexamethasone (P < 0.003). There was no difference in half-maximal values for the two chemokines. In separate dose–response experiments, we tested responses to budesonide suspended in calfactant, which gave results equivalent to those of budesonide alone (data not shown).
Table 1.
Half-Maximal Repression
| Chemokine | Budesonide (nM) | Dexamethasone (nM) | P Value |
|---|---|---|---|
| IL-8 | 0.04 ± 0.02 | 0.27 ± 0.11 | 0.003 |
| CCL2 | 0.05 ± 0.02 | 0.24 ± 0.04 | 0.001 |
Half-maximal repression values were calculated from dose–response experiments (mean ± SD (n = 7). Budesonide is five- to sixfold more potent than dexamethasone. There are no significant differences between IL-8 and CCL2 for half-maximal values. CCL2, C-C motif chemokine ligand 2.
Expression Profiling
We performed RNA-seq studies to evaluate the global effects of budesonide on gene expression. In three experiments examining the mRNA content of explants after 4 days of culture, we found a significant signal for ∼14,500 genes using a statistical limit of <0.05 for FDR. There were 530 (3.6%) mRNAs induced and 400 (2.8%) genes repressed with fold changes of ≥1.6-fold. Genes with highly increased expression (>25-fold) included PGC, APOD, IL1RL1, SULT1E1, FKBP5, F13A1, DIP3, FCN3, SFTPC, PEBP4, and eight surfactant-related genes, confirming previous observations in fetal lung epithelial cells treated with dexamethasone plus cAMP (23). Highly repressed genes (>25-fold) included ANXA10, SERPIND1, IL6, CCL7, B3GNT6, and TDO2.
Further analysis of the RNA-seq data focused on budesonide’s effects on the inflammatory process, which is likely the major domain of action of postnatal corticosteroid treatment for infant lung disease. By functional annotation, induced genes were enriched for the categories Acute Inflammatory Response (P = 1.2 × 10−5) and Response to Wounding (P = 4.7 × 10−4), and repressed genes were enriched for categories Regulation of Apoptosis (P = 5.1 × 10−7), Immune Response (P = 4.8 × 10−5), and Inflammatory Response (P = 5.8 × 10−5). By pathway analysis (KEGG) the most highly significant category was Cytokine-Cytokine Receptor Interaction (P = 1.3 × 10−6) with 24 genes; using the category “upstream regulator” (Ingenuity) to evaluate all regulated genes, the five most significantly enriched categories (all with P < 10−15) were TNF, dexamethasone, LPS, TGFβ1, and IL1β. We identified 81 repressed genes with inflammatory biological function by gene ontology annotation (24), as presented in Table 2. Repression by budesonide ranged from 1.6- to 39.6-fold. This list includes IL-8 (CXCL8, 11.5-fold repression) and CCL2 (5.4-fold), findings that were confirmed by quantitative polymerase chain reaction analysis (6.4 ± 3.6-fold and 3.7 ± 2.2-fold, mean ± SD, respectively); these results indicate that budesonide acts primarily at the level of transcription to decrease production of these chemokines. Of the 81 repressed genes, 14 are known to be nuclear factor-κB (NF-κB) targets, including three components (NFKB1, REL, and RELB) of the NF-κB complex.
Table 2.
Inflammatory Genes Repressed by Budesonide
| Gene Symbol | Gene Expression |
Secreted Protein |
||
|---|---|---|---|---|
| Fold Decreased | Expression (log2cpm) | Fold Decreased | P Value | |
| IL6* | 39.6 | 5.35 | 33.3 | 0.007 |
| CCL7 | 31.8 | 0.15 | ND | |
| DMBT1 | 20.4 | 2.97 | BLQ | |
| BDKRB1* | 18.6 | 1.16 | ND | |
| CXCL5* | 14.3 | 4.80 | 3.2 | 0.05 |
| CHI3L1 | 12.1 | 7.26 | 2.1 | 0.004 |
| CXCL8* | 11.5 | 7.56 | 2.7 | 0.001 |
| FGF23 | 8.8 | 0.14 | ND | |
| PTPRN | 8.5 | 1.80 | ND | |
| IL1B* | 7.9 | 2.85 | ND | |
| CCL22 | 6.7 | 2.07 | ND | |
| MYCN | 5.8 | 2.40 | ND | |
| APLN | 5.6 | 6.85 | ND | |
| IL13RA2 | 5.4 | 2.04 | ND | |
| CCL2* | 5.4 | 6.18 | 6.7 | 0.14 |
| EGF | 4.9 | 1.94 | ND | |
| ADORA1 | 4.6 | 1.68 | ND | |
| CFI | 4.5 | 7.64 | 1.8 | 0.01 |
| CFTR | 4.5 | 7.21 | ND | |
| CARD11 | 4.4 | 2.25 | ND | |
| TNFSF11 | 4.4 | 2.13 | ND | |
| IER3* | 4.4 | 7.47 | ND | |
| VCAM1* | 4.1 | 4.71 | BLQ | |
| PIK3CG | 4.0 | 3.00 | ND | |
| FGF7 | 3.8 | 5.90 | ND | |
| CCL21 | 3.6 | 3.43 | 1.9 | 0.01 |
| TGFB3 | 3.4 | 5.79 | ND | |
| AMBP | 3.4 | 0.07 | BLQ | |
| KITLG | 3.4 | 8.18 | ND | |
| TLR7 | 3.3 | 0.19 | ND | |
| TGFB2 | 3.3 | 6.06 | ND | |
| TNFSF15 | 3.3 | 1.92 | ND | |
| TNFSF14 | 3.2 | 3.23 | ND | |
| ITGA2 | 3.2 | 7.34 | ND | |
| C3 | 3.1 | 11.37 | 1.6 | 0.01 |
| TRIM22 | 3.0 | 5.93 | ND | |
| GJA1 | 3.0 | 9.10 | ND | |
| ITGB6 | 3.0 | 8.16 | ND | |
| JAK3 | 3.0 | 3.99 | ND | |
| C5 | 2.9 | 6.78 | 1.6 | 0.02 |
| HBEGF | 2.8 | 6.05 | ND | |
| IL1RAP | 2.8 | 6.49 | 1.4 | 0.08 |
| HRH1 | 2.7 | 4.56 | ND | |
| TNFRSF21 | 2.7 | 6.78 | ND | |
| CCR7* | 2.7 | 0.92 | ND | |
| CD74* | 2.6 | 6.40 | ND | |
| IFITM1 | 2.6 | 5.87 | ND | |
| ICAM1* | 2.5 | 8.16 | 1.4 | 0.12 |
| RELB* | 2.5 | 3.93 | ND | |
| PVRL1 | 2.5 | 4.65 | ND | |
| MAP2K6 | 2.4 | 1.51 | ND | |
| CASP1 | 2.3 | 4.06 | ND | |
| NEK6 | 2.2 | 6.01 | ND | |
| ROBO1 | 2.2 | 5.89 | ND | |
| ULBP1 | 2.2 | 4.07 | ND | |
| SEMA3C | 2.2 | 6.57 | BLQ | |
| PCSK5 | 2.1 | 4.53 | ND | |
| IL33 | 2.1 | 5.32 | ND | |
| VEGFA | 2.1 | 10.02 | ND | |
| CLU | 2.1 | 7.90 | 1.5 | 0.05 |
| BCL3* | 2.0 | 5.04 | ND | |
| SEMA7A | 2.0 | 4.96 | 1.3 | 0.07 |
| CARD16 | 2.0 | 2.97 | ND | |
| APOBEC3C | 2.0 | 2.58 | ND | |
| CFH | 1.9 | 7.60 | 1.4 | 0.11 |
| DDX60 | 1.9 | 4.61 | ND | |
| ERAP2 | 1.9 | 5.32 | ND | |
| DUSP10 | 1.8 | 3.76 | ND | |
| NFKB2* | 1.8 | 5.95 | ND | |
| BTN3A3 | 1.8 | 4.91 | ND | |
| IL13RA1 | 1.8 | 7.59 | ND | |
| MALT1 | 1.8 | 5.79 | ND | |
| ETV6 | 1.8 | 3.49 | ND | |
| REL | 1.7 | 2.98 | ND | |
| RUNX1 | 1.7 | 7.01 | ND | |
| SMAD6 | 1.7 | 4.13 | ND | |
| GPI | 1.7 | 7.73 | 0.9 | NS |
| IGFBP4 | 1.7 | 8.68 | 1.2 | 0.12 |
| TRAF3 | 1.7 | 4.55 | ND | |
| IFNAR2 | 1.7 | 4.37 | ND | |
| BTN2A2 | 1.6 | 4.31 | ND | |
Definition of abbreviations: BLQ, below limit of quantitation (mean peptide count < 5); cpm, counts per million; ND, not detected; NS, nonsignificant.
Repressed genes are classified as inflammatory related on the basis of gene ontology annotation as inflammatory process, cytokine, chemokine, and/or immune process. Data are presented as the mean from three experiments using RNA sequencing for tissue and liquid chromatography and tandem mass spectrometry for culture medium of explants cultured for 72 hours with or without 30 nM budesonide. All RNA sequencing data are P < 0.05 and false discovery rate < 0.05. Full proteomic results will be published separately.
Nuclear factor-κB target gene designation from compilation at http://bioinfo.lifl.fr/NF-KB/.
There were 40 budesonide-induced genes with assigned inflammatory function by gene ontology (Table 3). Induction by budesonide ranged from 1.6- to 95-fold. Of the 40 induced genes, 12 are known to be antiinflammatory, and 2 (AGER and BCL2L1) are targets of NF-κB.
Table 3.
Inflammatory-Related Genes Induced by Budesonide
| Gene Symbol | Gene Expression |
Secreted Protein |
||
|---|---|---|---|---|
| Fold Increased | Expression (cpm) | Fold Increased | P Value | |
| PGC* | 95.0 | 19.9 | 3.7 | 0.01 |
| APOD* | 72.6 | 5.3 | 5.0 | 0.23 |
| IL1RL1* | 56.6 | 23.4 | 24.6 | 0.001 |
| FCN3 | 28.3 | 1.6 | ND | |
| DUOX1 | 20.1 | 17.4 | ND | |
| VSIG4* | 10.1 | 3.6 | 2.4 | 0.04 |
| SERPINA3* | 9.2 | 27.9 | 6.6 | 0.001 |
| C1QA | 7.7 | 4.9 | BLQ | |
| CD163 | 7.2 | 15.4 | 4.7 | 0.005 |
| SCGB3A2* | 6.3 | 589.5 | 3.9 | 0.08 |
| CMKLR1* | 4.2 | 5.9 | ND | |
| CFD | 4.1 | 2.4 | 3.4 | 0.05 |
| CEACAM6* | 4.0 | 55.9 | ND | |
| MASP1* | 3.8 | 1.1 | ND | |
| PER1* | 3.8 | 48.5 | ND | |
| TNFRSF4* | 3.7 | 1.5 | ND | |
| PDE2A | 3.6 | 12.1 | ND | |
| ADCY5 | 3.4 | 4.9 | ND | |
| AGER | 3.3 | 46.8 | 2.5 | 0.01 |
| FGFR4 | 3.3 | 5.3 | ND | |
| CORO1A | 2.8 | 1.4 | ND | |
| PDGFRA | 2.8 | 215.3 | ND | |
| GIMAP1 | 2.7 | 6.5 | ND | |
| CAMK1D | 2.6 | 9.8 | ND | |
| IGF2* | 2.6 | 240.4 | BLQ | |
| LRRC32* | 2.6 | 40.8 | ND | |
| AIF1 | 2.4 | 3.2 | ND | |
| BCL2L1 | 2.4 | 88.6 | ND | |
| HYAL2 | 2.4 | 53.4 | ND | |
| IL6R* | 2.4 | 57.5 | ND | |
| IRS2 | 2.4 | 41.9 | ND | |
| SERPINF1* | 2.4 | 65 | 1.1 | NS |
| ISG20 | 2.3 | 14.5 | ND | |
| ADARB1 | 2.1 | 38.6 | ND | |
| MGLL | 2.1 | 96.9 | ND | |
| SFTPB* | 2.1 | 7129 | 1.1 | NS |
| ALDH1A2 | 2.0 | 14.6 | ND | |
| ALOX5AP | 2.0 | 2.6 | ND | |
| CTNNBIP1 | 2.0 | 27.4 | ND | |
| RPS6KA2 | 1.7 | 200 | ND | |
For a list of abbreviations, see Table 2.
Induced genes are classified as inflammatory related on the basis of gene ontology annotation as inflammatory process, cytokine, chemokine, and/or immune process, or by literature search. Data are from experiments as described in Table 2.
Genes reported to have antiinflammatory action.
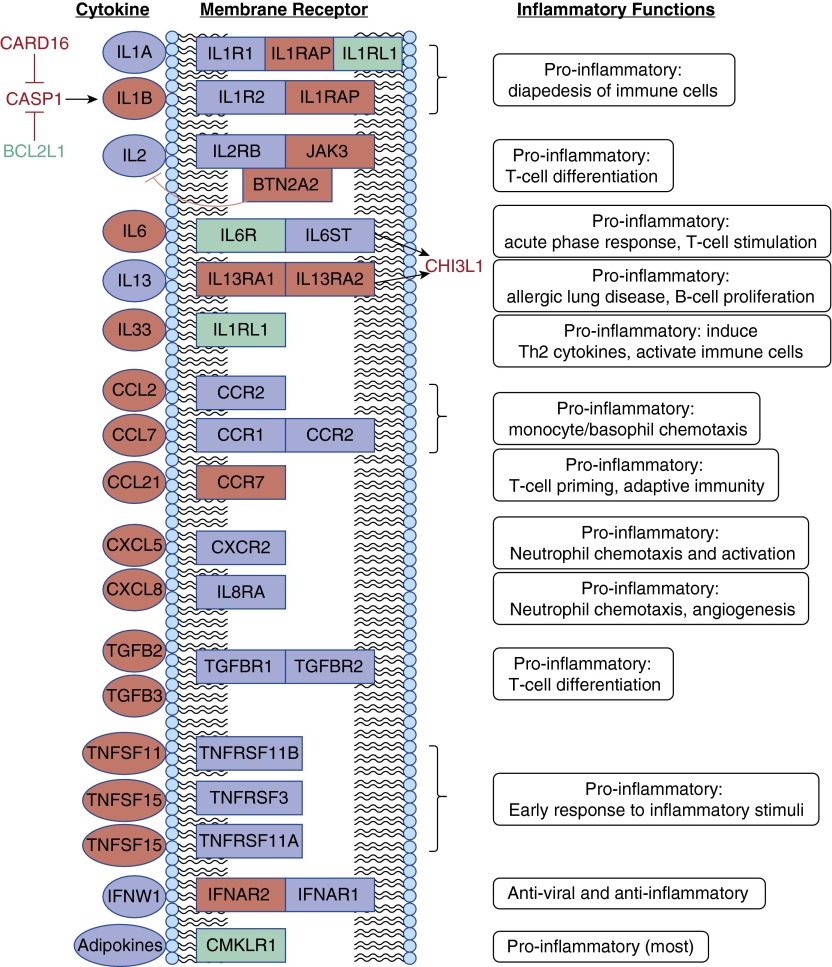
Figure 2 is a schematic overview of cytokine-related inflammatory genes that were regulated by budesonide in fetal lung. Of the 28 genes shown, mRNA levels for 23 were repressed and 5 were increased; however, the functional impact was antiinflammatory, with one apparent exception (IFNW1 signaling). The listed proinflammatory cytokines are chemotactic for circulating immune cells, promote diapedesis, activate T and B cells, stimulate other cytokine production, and/or increase angiogenesis. It is of interest to note that most of the identified genes with known inflammatory action are either cytokines or their receptors rather than downstream signaling molecules.
Figure 2.
Cytokine-related genes regulated by budesonide. Twenty-eight budesonide-regulated genes for cytokines, their receptors, or interacting proteins from Tables 2 and 3 are shown. Ovals designate cytokines (n = 13), boxes indicate membrane receptors (11), green designates up-regulation of mRNA by budesonide (5), and red represents suppression (23). Related proteins include CARD16 and BCL2L1, which both inhibit CASP1 and its activation of IL1, and CHI3L1, which mediates some effects of IL6 and IL13. Some of the known inflammatory-related functions of each cytokine are listed to the right; with two possible exceptions (down-regulation of IFNAR2 [receptor for IFNW1] and inhibition of CARD16), budesonide effects are antiinflammatory.
In the same experiments, we also performed RNA-seq analysis of explants before culture. Using the same cutoffs for fold-change and FDR, the expression of only 14 genes changed significantly during 4 days of control culture: 5 genes (PDE4A, LRP2, RGCC, COMP, and SFTPA1) were induced 2.2- to 16.4-fold, and 9 genes (GPCPD1, DUSP4, STAT4, CPM, RASGRF1, ABCG2, RARRES1, IL1RL1, and DMBT1) were repressed 2- to 16.7-fold. This list includes two genes that were induced by budesonide (CPM and IL1RL1) and one (DMBT1) that was repressed by budesonide. Thus, there was a very limited response to explant culture (0.1% of genes had altered expression) compared with budesonide treatment.
We used LC-MS/MS to evaluate secreted proteins regulated by budesonide. On proteomic analysis of culture medium, we found that 564 total proteins were detected reliably. With budesonide treatment, content was decreased for 14 inflammatory-related proteins as listed in Table 2 and was increased for 8 proteins as listed in Table 3. Most proteins in both tables were not detected likely because of either low expression levels or lack of secretion. These results identify candidate proteins (e.g., IL6, IL8, CCL2, IL1RL1, and CHI3L1) for assessing antiinflammatory responses to budesonide using lung fluid from treated patients.
Surface Tension
The effect of budesonide supplementation on calfactant surface tension properties in vitro was examined by pulsating bubble surfactometry at 3.5 and 1.75 mg PL/ml and with budesonide at 0.5 and 0.25% by weight; results were equivalent under both sets of conditions, and combined data are shown in Table 4. Minimal surface tension (STmin) (<5 mN/m) and time to STmin (<13 s) were in the normal range as reported for unsupplemented calfactant (21). With storage of supplemented calfactant at room temperature for 24 or 48 hours, STmin increased slightly but significantly, but remained in the normal range, whereas there was no significant change in time to STmin. Thus, there was no apparent adverse effect of budesonide on calfactant activity, in agreement with other observations (8, 25), or on stability.
Table 4.
In Vitro Surface Tension Properties of Calfactant Supplemented with Budesonide
| Time (h) | STmin (mN/m) | Time to STmin (s) |
|---|---|---|
| 0 | 0.3 ± 0.7 | 10.5 ± 1.6 |
| 24 | 1.6 ± 0.3* | 12.7 ± 3.3 |
| 48 | 1.2 ± 0.5* | 12.6 ± 2.4 |
Definition of abbreviation: STmin, minimal surface tension.
Calfactant supplemented with budesonide was tested in a pulsating bubble surfactometer. Data are presented as mean ± SD (n = 6 for time 0, and n = 12 for 24 and 48 hours of exposure at room temperature). All values are within the normal range for unsupplemented calfactant (21).
P < 0.01 versus time 0.
Budesonide Metabolism
In vivo, budesonide is metabolized in the liver to 16α-hydroxyprednisolone and other minor metabolites and has a half-life of ∼4 hours in both infants and lambs (8, 20, 26). We used LC-MS/MS to examine the metabolism of budesonide in cultured fetal lung, testing both the culture medium and tissue after exposure to 30 nM budesonide for 48–72 hours. In the medium, concentration levels of both 16α-hydroxyprednisolone and the fatty acid derivative of budesonide were below the limits of detection. In tissue, budesonide was present at 0.03–0.08 ng/mg and fatty acid ester was detected at low levels, but 16α-hydroxyprednisolone was not detected. These results indicate low metabolism of budesonide by fetal lung cells and some esterification with fatty acid.
Discussion
Inflammation is a key component of the pathogenesis of chronic lung disease in premature infants. In this study, we examined issues related to the instillation of budesonide in surfactant to provide lung-targeted antiinflammatory treatment, which is a promising approach for the prevention of BPD in pilot clinical studies. We found that budesonide is a potent antiinflammatory glucocorticoid in second-trimester fetal lung tissue acting at the level of transcription. Although there is considerable information on the maturational effects of glucocorticoids in fetal lung, to our knowledge, the current results using next-generation RNA-seq provide the first comprehensive data on the glucocorticoid inflammatory transcriptome in the human fetal lung and identify new putative glucocorticoid target genes. The results establish that fetal lung cells synthesize a variety of glucocorticoid-responsive inflammatory mediators.
Glucocorticoids act by multiple mechanisms to restrain inflammation. There are nongenomic effects on second messenger pathways, direct suppression of inflammatory genes by glucocorticoid receptor (GR) interaction with a negative glucocorticoid response element (GRE), induction of antiinflammatory genes via a positive GRE, and GR-mediated repression of proinflammatory genes by interfering with the NF-κB pathway. Within our limits for statistical significance of gene profiling, we found evidence for budesonide action via the last two mechanisms and only one example of suppression of a fetal lung gene known to have a negative GRE (IL1B, sevenfold decrease, FDR = 0.16) (27). Among the repressed genes, some are known targets for the transcription factor NF-κB (Table 2); the inhibitory mechanism of corticosteroids related to NF-κB target genes involves direct interaction of the GR-glucocorticoid complex with NF-κB and the inhibition of its transcriptional activation activity at promoter regulatory sites (27, 28).
The list of budesonide-repressed genes (Table 2) includes 15 chemokines/cytokines (IL1B, IL6, CCL7, CXCL5, CXCL8, CCL2, CCL21, CCL22, TGFB2, TGFB3, IL33, CHI3L1, TNFSF11, TNFSF14, and TNFSF15), some of which activate NF-κB. Of interest, budesonide inhibition of IL1, which is a key initiator of inflammation, involves suppression of the cytokine and of CASP1 but not of NOD-like receptors that complex with procaspase1 to form the NLRP3 inflammasome that is critical in the pathogenesis of BPD (29). IL6 mRNA was repressed 40-fold, and this effect likely results in decreased IL6 signaling despite a twofold increase in its receptor mRNA. Other categories of repressed genes in the inflammatory process include components of NF-κB (NFKB2, REL, and RELB), receptors and accessory proteins of inflammatory mediators (IL1RAP, CCR7, BDKRB1, ADORA1, TRAF3, IL13RA1, and IFNAR2), components of signaling pathways (SMAD6, PTPRN, APLN, KITLG, ITGB6, and MAP2K6), molecules involved in apoptosis and activation of pro-cytokines (EGF, IER3, CASP1, NEK6, CARD11, and CARD16), three members of the complement system, and molecules that promote inflammatory cell influx (ICAM1, VCAM1, and PIK3CG). Although most of these responses to glucocorticoid are antiinflammatory, some repression may represent a proinflammatory effect (e.g., SMAD6, which inhibits NF-κB action [30] and the receptor for antiinflammatory IL13 and IL4). Although we focused our analysis on inflammatory-related functions of regulated genes, it should be pointed out that some of these repressed genes may also influence lung differentiation. For example, TGFβ suppresses surfactant components in fetal lung, and VEGFA, GPI, and NF-κB promote the angiogenesis that is essential for alveogenesis in lung development. Clearly, the spectrum of inflammatory effects of glucocorticoids in the fetal lung is broad and complex.
A target gene of interest is chitinase 3-like 1, cartilage glycoprotein-39, YKL-40 (CHI3L1), which was repressed 12-fold by RNA-seq and 2.1-fold by protein analysis. CHI3L1 lacks chitinase activity and is expressed in numerous cell types including macrophages, endothelial cells, and airway epithelium. It is induced by IL-6, IL-13, and IFNγ and mediates some of the proinflammatory effects of these cytokines. Other than clinical observations related to corticosteroid therapy, suppression by glucocorticoid has not been reported previously, to our knowledge. Dysregulated levels of CHI3L1 are associated with a variety of chronic inflammatory diseases including asthma, and studies of variants in the gene support a disease susceptibility role. Studies in transgenic mice indicate that the effects of CHI3L1 in the lung are highly dependent on levels, with injury occurring at both low levels (with hyperoxia) and with overexpression (31). These observations support a potential role of CHI3L1 as a causative agent in asthma and premature infant lung disease and as a target for the beneficial effects of glucocorticoid therapy.
Examples of induced antiinflammatory genes (Table 3) include SFTPB, a surfactant protein with antiinflammatory action (32, 33) and PGC, an aspartic protease involved in SFTPB processing (34). CEACAM6, which is induced fourfold, is a secreted protein that protects surfactant and reduces inflammation by promoting clearance of gram-negative bacteria (35). As in our previous report (23), budesonide strongly up-regulated mRNA and protein levels of IL1RL1 (ST2), which is a receptor for IL33. Production of proinflammatory molecules induced by IL33 is considered to be a critical event in atopic diseases including asthma. The secreted B form of IL1RL1, which is elevated in inflammatory disease (e.g., asthma), acts as a decoy for IL-33 and decreases signaling via form A receptor (36). These observations suggest that IL1RL1 may represent a clinically important target for corticosteroid therapy in lung disease. Apolipoprotein D (APOD), which was the second highest induced, is a negative regulator of cytokine production, including CCL2. Ficolin (FCN2), which is induced 28-fold, may activate the lectin/complement pathway and may enhance innate immunity in the lung (37). IL6R, a component of the IL6 receptor, is induced but appears to be proinflammatory as a mediator of IL6 signaling. The specific role of IL6R and other induced genes in pro- versus antiinflammatory effects will require additional understanding of function.
A strength of our approach is that the gestational age of the fetal lung studied was comparable to those of extremely premature infants who are at highest risk of BPD. The use of explants allows profiling of budesonide responses in all tissue cell types, but likely not in circulating immune cells that leach from the tissue during culture. A corresponding limitation is the inability to assign responses to specific lung cell types. Using RNA-seq of airway smooth muscle cells from adults with asthma, Yick and colleagues (38) found differential expression of 15 genes with prednisolone therapy. Most of these genes were expressed in fetal lung with significant repression for one (GRB14, 4.7-fold decrease). Himes and colleagues (39) cultured airway smooth muscle cells from adult lung with/without dexamethasone; of the top 15 up-regulated genes, of which 10 were related to inflammation, 11 were significantly induced in fetal lung. In our previous study with adult lung type 2 cells, dexamethasone plus cAMP repressed six of the genes listed in Table 2 (14). It is likely that a number of lung cell types respond to the antiinflammatory action of glucocorticoids.
It is known that infants with more severe early lung disease have elevated levels of a variety of inflammatory mediators (22), and that postnatal instilled budesonide suppresses several cytokines and chemokines (IL1B, IL6, and CXCL8) in lung fluid (9). Our findings confirm the in vivo suppression of these three cytokines; however, additional studies in infants are required to document other responses that we observed in explant cultures. Because of the limited sample size in our study, the list of suppressed inflammatory genes likely underestimates the number of budesonide-regulated genes; for example, expression of LCN2, CCL20, CXCL1, CXCL3, and C4BPA were decreased in each of the three lungs but did not meet the cutoff for statistical significance because of variable levels of basal expression. In our studies, it is uncertain whether the level of inflammatory-related gene expression in explants represents basal (nonstressed) conditions or an elevated inflammatory state secondary to tissue acquisition. Our RNA-seq data with preculture tissue appear to rule out an inflammatory response induced by in vitro culture. Future studies must address gene responses in the presence of inflammatory stimuli such as infection (e.g., LPS and bacteria), hyperoxia, and stretch, and must also evaluate genetic factors (e.g., race and sex and future identified BPD-associated gene variants) that contribute to the severity of lung injury in vivo. Another limitation of our experimental model is the uncertainty about extrapolating all findings to in vivo treatment of infants where other physiological factors may affect budesonide delivery, clearance, and responses.
We found that budesonide is ∼fivefold more potent for repression of IL-8 and CCL2 than is dexamethasone, which is in general agreement with most previously reported studies in other human cell types (40–42). The half maximal value of dexamethasone for repression of chemokines is lower than that which we found for the induction of surfactant-related genes in fetal lung epithelial cells (43, 44) but similar to the potency reported for a human fetal lung fibroblast cell line (45). The difference in potency for induction versus repression effects may reflect, at least in part, a higher affinity of GR for NF-κB and c-jun/c-fos (activator protein 1) transcription factors than for the GRE in promoters of induced genes (46, 47).
The time course experiments indicated that budesonide repression began by 4 hours, which is consistent with the time required for cellular uptake of steroid, inhibition of chemokine mRNA synthesis, and degradation of previously secreted chemokine. Consistent with the high potency of budesonide, a lag period (∼20 h) for reversibility was observed on removal of the steroid. These latter results suggest that the antiinflammatory effects of budesonide in vivo after a single dose will last considerably longer than predicted by the observed plasma half-life of 4.3 hours (8). Esterification of budesonide by fatty acids in the lung, which we observed but could not quantitate with our LC-MS/MS approach, is consistent with previous studies in adult lung in vitro (48) and fetal lamb in vivo (20) and could provide a reservoir of budesonide that would extend the biological effect in lung cells.
Our studies with mixtures of budesonide and calfactant indicate a lack of interaction, supporting the use of this approach to lung-targeted delivery of corticosteroid. Delivery of glucocorticoid to infants by aerosolization has been studied extensively, with only one report of decreased BPD after prolonged therapy (6, 7). These largely negative results may reflect the doses used, duration of therapy, and ineffective delivery of steroid to the lung periphery because of both droplet size and atelectasis. The major advantages of budesonide suspended in surfactant are the high solubility of lipophilic steroid in surfactant lipids and the ability of instilled surfactant to disburse throughout the lung parenchyma because of its surface-active properties, providing delivery of steroid to both aerated and atelectatic regions. The improved distribution and retention time of budesonide in surfactant, compared with budesonide suspended in saline, has been demonstrated in the rat (9). If more effective nebulization is developed eventually, it is possible that an inhaled, rather than an instilled, surfactant:budesonide mixture will provide an effective and less invasive approach to delivery of steroid to the injured lung.
On the basis of our in vitro findings of high budesonide potency and slow reversibility, we suspect that the dose of instilled budesonide used by Yeh and colleagues (9) (0.25 mg/kg) is excessive. This dose in infants resulted in a peak plasma concentration of 20 ng/ml (47 nM), compared with a half-maximal concentration of 0.05 nM for chemokine suppression, and it is reported that lung levels of inhaled budesonide exceed plasma levels (49). We are planning a dose escalation pilot study of budesonide:surfactant in premature infants at high risk of chronic lung disease to address the issues related to dose–response and the timing of antiinflammatory effects in vivo. Large randomized clinical trials are needed in extremely premature infants, who are at highest risk of BPD, to document that lung-targeted delivery of budesonide using surfactant is both beneficial and safe. This form of corticosteroid therapy also has potential application in children and adults with acute respiratory distress syndrome or severe asthma.
Acknowledgments
Acknowledgments
The authors thank Hart Horneman and David Leaffer for technical assistance, Ron Clyman for resource support, and Roberta Keller for technician funding. mRNA sequencing was performed by the UCSF SABRE Functional Genomics Core. Mass spectrometry analysis was performed by the Bio-Organic Biomedical Mass Spectrometry Resource at UCSF (A. L. Burlingame, Director). The authors also thank both ONY Inc. and IKARIA Inc. for research support; these supporting sponsors had no input in study design, execution, data analysis, or manuscript preparation.
Footnotes
This work was supported by the National Institutes of Health (NIH) grants HL024075 and HL129910 (P.L.B.); the Department of Pediatrics University of California, San Francisco; the Biomedical Technology Research Centers program of the NIH National Institute of General Medical Sciences grants NIH NIGMS 8P41GM103481 and NIH 1S10OD016229; and unrestricted research grants from ONY Inc. and IKARIA Inc.
Author Contributions: A.M.B., E.A.E., and P.L.B.: conception and design; P.L.B. and A.L.B.: project coordination; A.M.B., J.K.R., C.C., E.A.E., J.A.O.-P., and S.C.: acquisition of data and data analysis; M.R.S.: statistical analysis; A.M.B. and P.L.B.: drafting manuscript; and all authors: review and revision of manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0068OC on June 9, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, Laptook AR, Bell EF, Stoll BJ, Newman N, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88:509–515. doi: 10.1016/j.earlhumdev.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98:217–224. doi: 10.1159/000286210. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98:289–296. doi: 10.1159/000286212. [DOI] [PubMed] [Google Scholar]
- 6.Onland W, Offringa M, van Kaam A. Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2012;4:CD002311. doi: 10.1002/14651858.CD002311.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V, Van den Anker JN, Meisner C, Engel C, Schwab M, et al. NEUROSIS Trial Group. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med. 2015;373:1497–1506. doi: 10.1056/NEJMoa1501917. [DOI] [PubMed] [Google Scholar]
- 8.Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, Pyati S, Tsai CH. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008;121:e1310–e1318. doi: 10.1542/peds.2007-1973. [DOI] [PubMed] [Google Scholar]
- 9.Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, Tsai CH, Lin HC. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193:86–95. doi: 10.1164/rccm.201505-0861OC. [DOI] [PubMed] [Google Scholar]
- 10.Kuo HT, Lin HC, Tsai CH, Chouc IC, Yeh TF. A follow-up study of preterm infants given budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants. J Pediatr. 2010;156:537–541. doi: 10.1016/j.jpeds.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Miller-Larsson A, Mattsson H, Hjertberg E, Dahlbäck M, Tunek A, Brattsand R. Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos. 1998;26:623–630. [PubMed] [Google Scholar]
- 12.Wieslander E, Delander EL, Järkelid L, Hjertberg E, Tunek A, Brattsand R. Pharmacologic importance of the reversible fatty acid conjugation of budesonide studied in a rat cell line in vitro. Am J Respir Cell Mol Biol. 1998;19:477–484. doi: 10.1165/ajrcmb.19.3.3195. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–L951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- 14.Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, et al. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol. 2010;299:L36–L50. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalkley RJ, Baker PR, Medzihradszky KF, Lynn AJ, Burlingame AL. In-depth analysis of tandem mass spectrometry data from disparate instrument types. Mol Cell Proteomics. 2008;7:2386–2398. doi: 10.1074/mcp.M800021-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth GK.Limma: linear models for microarray data Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W.editors. Bioinformatics and computational biology solutions using R and bioconductor. New York, NY: Springer 2005397–420. [Google Scholar]
- 17.Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Coc B. 1995;57:289–300. [Google Scholar]
- 19.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JK, Stockmann C, Dahl MJ, Albertine KH, Egan E, Lin Z, Reilly CA, Ballard PL, Ballard RA, Ward RM. Pharmacokinetics of budesonide administered with surfactant in premature lambs: implications for neonatal clinical trials. Curr Clin Pharmacol. 2016;11:53–61. doi: 10.2174/1574884710666150929100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notter RH, Wang Z, Egan EA, Holm BA. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids. 2002;114:21–34. doi: 10.1016/s0009-3084(01)00197-9. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev. 2013;14:173–179. doi: 10.1016/j.prrv.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Wade KC, Guttentag SH, Gonzales LW, Maschhoff KL, Gonzales J, Kolla V, Singhal S, Ballard PL. Gene induction during differentiation of human pulmonary type II cells in vitro. Am J Respir Cell Mol Biol. 2006;34:727–737. doi: 10.1165/rcmb.2004-0389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. The Gene Ontology Consortium. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danhaive O, Chapin C, Horneman H, Cogo PE, Ballard PL. Surface film formation in vitro by infant and therapeutic surfactants: role of surfactant protein B. Pediatr Res. 2015;77:340–346. doi: 10.1038/pr.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore CD, Roberts JK, Orton CR, Murai T, Fidler TP, Reilly CA, Ward RM, Yost GS. Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos. 2013;41:379–389. doi: 10.1124/dmd.112.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 28.Alvira CM. Nuclear factor-κ-B signaling in lung development and disease: one pathway, numerous functions. Birth Defects Res A Clin Mol Teratol. 2014;100:202–216. doi: 10.1002/bdra.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao J, Kapadia VS, Brown LS, Cheong N, Longoria C, Mija D, Ramgopal M, Mirpuri J, McCurnin DC, Savani RC. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun. 2015;6:8977. doi: 10.1038/ncomms9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi KC, Lee YS, Lim S, Choi HK, Lee CH, Lee EK, Hong S, Kim IH, Kim SJ, Park SH. Smad6 negatively regulates interleukin 1-receptor-Toll-like receptor signaling through direct interaction with the adaptor Pellino-1. Nat Immunol. 2006;7:1057–1065. doi: 10.1038/ni1383. [DOI] [PubMed] [Google Scholar]
- 31.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol. 2001;63:555–578. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- 33.Epaud R, Ikegami M, Whitsett JA, Jobe AH, Weaver TE, Akinbi HT. Surfactant protein B inhibits endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2003;28:373–378. doi: 10.1165/rcmb.2002-0071OC. [DOI] [PubMed] [Google Scholar]
- 34.Gerson KD, Foster CD, Zhang P, Zhang Z, Rosenblatt MM, Guttentag SH. Pepsinogen C proteolytic processing of surfactant protein B. J Biol Chem. 2008;283:10330–10338. doi: 10.1074/jbc.M707516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolla V, Gonzales LW, Bailey NA, Wang P, Angampalli S, Godinez MH, Madesh M, Ballard PL. Carcinoembryonic cell adhesion molecule 6 in human lung: regulated expression of a multifunctional type II cell protein. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1019–L1030. doi: 10.1152/ajplung.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhabir L, Sandford A. Genetics of interleukin 1 receptor-like 1 in immune and inflammatory diseases. Curr Genomics. 2010;11:591–606. doi: 10.2174/138920210793360907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endo Y, Nakazawa N, Iwaki D, Takahashi M, Matsushita M, Fujita T. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J Innate Immun. 2010;2:33–42. doi: 10.1159/000227805. [DOI] [PubMed] [Google Scholar]
- 38.Yick CY, Zwinderman AH, Kunst PW, Grünberg K, Mauad T, Fluiter K, Bel EH, Lutter R, Baas F, Sterk PJ. Glucocorticoid-induced changes in gene expression of airway smooth muscle in patients with asthma. Am J Respir Crit Care Med. 2013;187:1076–1084. doi: 10.1164/rccm.201210-1886OC. [DOI] [PubMed] [Google Scholar]
- 39.Himes BE, Jiang X, Wagner P, Hu R, Wang Q, Klanderman B, Whitaker RM, Duan Q, Lasky-Su J, Nikolos C, et al. RNA-Seq transcriptome profiling identifies CRISPLD2 as a glucocorticoid responsive gene that modulates cytokine function in airway smooth muscle cells. PLoS One. 2014;9:e99625. doi: 10.1371/journal.pone.0099625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whelan CJ, Payne AN, Planquois JM. A comparison of the inhibitory effects of budesonide, beclomethasone dipropionate, dexamethasone, hydrocortisone and tixocortol pivalate on cytokine release from leukocytes recovered from human bronchoalveolar lavage. Inflamm Res. 1999;48:224–228. doi: 10.1007/s000110050450. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y, Leung PC, Woo KS, Chen GG, Wong YO, Liu SX, van Hasselt CA. Inhibitory effects of budesonide, desloratadine and dexamethasone on cytokine release from human mast cell line (HMC-1) Inflamm Res. 2004;53:664–669. doi: 10.1007/s00011-004-1309-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Moilanen E, Kankaanranta H. Beclomethasone, budesonide and fluticasone propionate inhibit human neutrophil apoptosis. Eur J Pharmacol. 2001;431:365–371. doi: 10.1016/s0014-2999(01)01437-6. [DOI] [PubMed] [Google Scholar]
- 43.Liley HG, White RT, Warr RG, Benson BJ, Hawgood S, Ballard PL. Regulation of messenger RNAs for the hydrophobic surfactant proteins in human lung. J Clin Invest. 1989;83:1191–1197. doi: 10.1172/JCI114000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol. 1996;14:599–607. doi: 10.1165/ajrcmb.14.6.8652188. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Nelson A, Weiler ZM, Patil A, Sato T, Kanaji N, Nakanishi M, Michalski J, Farid M, Basma H, et al. Anti-inflammatory effects of budesonide in human lung fibroblast are independent of histone deacetylase 2. J Inflamm Res. 2013;6:109–119. doi: 10.2147/JIR.S43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 47.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 48.Nave R, Fisher R, McCracken N. In vitro metabolism of beclomethasone dipropionate, budesonide, ciclesonide, and fluticasone propionate in human lung precision-cut tissue slices. Respir Res. 2007;8:65. doi: 10.1186/1465-9921-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van den Bosch JM, Westermann CJ, Aumann J, Edsbäcker S, Tönnesson M, Selroos O. Relationship between lung tissue and blood plasma concentrations of inhaled budesonide. Biopharm Drug Dispos. 1993;14:455–459. doi: 10.1002/bdd.2510140511. [DOI] [PubMed] [Google Scholar]