Abstract
Intratracheal instillation of bacterial LPS is a well-established model of acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS). Because the myristoylated alanine-rich C kinase substrate (MARCKS) protein is involved in neutrophil migration and proinflammatory cytokine production, we examined whether an aerosolized peptide that inhibits MARCKS function could attenuate LPS-induced lung injury in mice. The peptide, BIO-11006, was delivered at 50 μM via inhalation either just before intratracheal instillation of 5 μg of LPS into Balb/C mice, or 4, 12, 24, or 36 hours after LPS instillation. Effects of BIO-11006 were evaluated via analysis of mouse disease-related behavior, lung histology, bronchoalveolar lavage fluid total protein, neutrophil counts and percentages, cytokine (KC [CXCl1, mouse IL-8 equivalent] and TNF-α) expression, and activation of NF-κB in lung tissue. Treatment with aerosolized BIO-11006 at 0, 4, 12, 24, and even 36 hours after LPS instillation reversed the disease process: mouse behavior returned to normal after two treatments 12 hours apart with the inhaled peptide after LPS injury, whereas control LPS-instilled animals treated with PBS only remained moribund. Histological appearance of inflammation, bronchoalveolar lavage fluid protein levels, leukocyte and neutrophil numbers, KC and TNF-α gene and protein expression, and NF-κB activation were all significantly attenuated by inhaled BIO-11006 at all time points. These results implicate MARCKS protein in the pathogenesis of ALI/ARDS and suggest that MARCKS-inhibitory peptide(s), delivered by inhalation, could represent a new and potent therapeutic treatment for ALI/ARDS, even if administered well after the disease process has begun.
Keywords: myristoylated alanine-rich C kinase substrate, acute lung injury, LPS, neutrophils
Clinical Relevance
These studies show that treatment of mice with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) with an inhaled inhibitor of myristoylated alanine-rich C kinase substrate (MARCKS) protein reverses disease progression, even when treatment is initiated well into the disease process, a time when the animals are moribund. These results implicate MARCKS protein in the pathogenesis of ALI/ARDS and suggest that MARCKS-inhibitory peptide(s), delivered by inhalation, could represent a new and potent therapeutic treatment for ALI/ARDS, even if administered well after the disease process has begun.
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are major causes of respiratory failure; worldwide, over 1 million cases occur annually, with over 200,000 adult and 20,000 pediatric cases per year in the United States. This disorder is characterized by a large influx of leukocytes, especially neutrophils, to the lung as part of an acute inflammatory response, with injury to epithelial and endothelial cell barriers and pulmonary edema, all of which lead to respiratory failure. Treatment options remain limited to mechanical ventilation, a supportive therapy that still results in an in-hospital mortality rate of approximately 40% in the United States. There are currently no effective pharmacological therapies to counter disease progression in patients with ARDS (1, 2).
A major component of the initial phases of the pathophysiology of ARDS is recruitment of neutrophils into the lung (1, 3). In previous studies, we have shown that a key molecule regulating directed in vitro migration of neutrophils (4–7) and other inflammatory cells (8, 9) is myristoylated alanine-rich C kinase substrate (MARCKS) protein. These reports used a peptide that inhibits MARCKS function called the myristoylated N-terminal sequence (MANS) peptide (10). Additional studies demonstrated that a smaller analog of MANS, BIO-11006, has the same MARCKS-inhibitory properties as MANS (11), and its smaller size and high solubility make it more suitable for use as a drug; in fact, BIO-11006, administered as an inhaled aerosol, has already been tested in a phase 2a clinical trial in human patients with chronic obstructive pulmonary disease. Given that BIO-11006 attenuates neutrophil motility and migration, its effects as a potential therapy in ALI/ARDS were investigated in an animal model of the disease.
The model of ALI/ARDS used in this study was intratracheal instillation of endotoxin (LPS from Escherichia coli) into mice, a widely used and well characterized model of inflammation and lung injury that involves massive neutrophil infiltration, enhanced production of proinflammatory cytokines and chemokines, edema, and tissue damage (12–15). BIO-11006 was administered to LPS-instilled mice as an inhaled aerosol (similar to its use in the clinical trial mentioned previously here on patients with chronic obstructive pulmonary disease). Several agents and treatments can prevent ALI in LPS-instilled mice when administered previous to, simultaneously with, or within a few hours after LPS instillation (12–15). However, human patients with ALI/ARDS clearly do not present until the disease has progressed to a point where there are clear symptoms, well into the course of the disease. To mimic this in the mouse model, we started aerosolized treatments (two treatments 12 h apart) at various times after LPS instillation: 0, 4, 12, 24, and as long as 36 hours. The results showed that administration of aerosolized BIO-11006, even as long as 36 hours after LPS instillation, a time when the mice were quite moribund, resulted in reversal of the disease process and full recovery of the animals. Parameters related to ALI, including mouse disease-related behavior, neutrophil influx, chemokine/cytokine production, and NF-κB activation, were all significantly ameliorated by treatment with BIO-11006, regardless of the time after LPS instillation that peptide treatment was initiated.
Materials and Methods
LPS Lung Injury Model
Animals were handled in accordance with the policies of the North Carolina State University (Raleigh, NC) Institutional Animal Care and Use Committee. Briefly, 7- to 10-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were kept quarantined for 7 days. Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally, and then instilled intratracheally with 5 μg of LPS (E. coli endotoxin no. L2630; Sigma-Aldrich, St. Louis, MO) in 50 μl PBS.
BIO-11006
BIO-11006 is an analog of the MANS peptide, which is identical to the first 24 amino acids of the MARCKS molecule (10). BIO-11006 contains the active site of MANS (the first 10 amino acids) and is more suitable as a drug because of its smaller size and enhanced solubility; it is identical to MANS in its MARCKS-inhibitory actions (11). BIO-11006 was synthesized by PolyPeptide (Torrence, CA). A concentration of 50 µM for N-terminal MARCKS inhibitors was shown to have optimal effects on attenuating neutrophil motility in vitro (4, 5), and showed efficacy in preliminary in vivo studies in LPS-injured mice, so this concentration of BIO-11006 (equivalent to ∼0.75 mg/kg) was chosen for time-related studies detailed subsequently here.
Treatment Groups
Mice were divided into seven treatment groups containing six mice each;
Group 1: No LPS or peptide (negative control).
Group 2: LPS; treated only with inhaled PBS (positive control for each group).
Group 3: LPS; treatment started with aerosolized BIO-11006 just before LPS instillation (0 time); treated again at 12 hours.
Group 4: LPS; treatment started with aerosolized BIO-11006 4 hours after LPS instillation; treated again at 16 hours.
Group 5: LPS; treatment started with aerosolized BIO-11006 12 hours after LPS instillation; treated again at 24 hours.
Group 6: LPS; treatment started with aerosolized BIO-11006 24 hours after LPS instillation; treated again at 36 hours.
Group 7: LPS; treatment started with aerosolized BIO-11006 36 hours after LPS instillation, treated again at 48 hours.
The experimental groups were treated at the indicated times using 50 μM BIO-11006 aerosolized in PBS and administered via inhalation. The nebulizer (TREK S portable aerosol system; PARI Respiratory Equipment, Inc., Midlothian, VA) and the exposure chamber were placed inside a biological safety cabinet. Mice (three in the exposure chamber at a time) were exposed to aerosolized PBS or BIO-11006 for 30 minutes, with treatments repeated 12 hours later such that each group was exposed to the aerosolized peptide or PBS control twice over the course of the experiment. All animals were killed at 72 hours after exposure and lung injury and inflammation measured as described subsequently here.
Disease-Related Behavior
Mice were recorded by video for at least 30 seconds directly after they had received two treatments of BIO-11006. Videos of mice were analyzed blindly by a laboratory animal veterinarian for parameters related to disease severity and behavior using a scoring system similar to those described previously (12, 13). Each disease-related parameter was quantified via a scoring of 0 (no disease apparent, normal behavior) to 3+ (extremely sick). The disease-related parameters were: (1) activity in cage; (2) “hunching”; (3) ptosis; (4) piloerection; and (5) labored/rapid breathing.
Analysis of Lung Injury
At 72 hours after LPS instillation, mice were killed with an overdose of ketamine /xylazine. One lobe of the left lung was tied off and fixed, sectioned, and stained using standard hematoxylin and eosin for assessment of inflammation.
Bronchoalveolar lavage (BAL) was performed by cannulating the trachea with a 23-gauge catheter and lavaging the lungs with 850 μl sterile PBS solution. The collected lavage fluid was centrifuged (300 × g for 15 min), and the supernatant stored at −80°C. Protein concentrations were measured with a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Protein levels of two prominent proinflammatory cytokines related to ALI (KC [CXCl1; the murine IL-8 equivalent] and TNF-α) were measured by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The cell pellet was resuspended in 1 ml of PBS and cytospin preparations performed. Slides were hematoxylin and eosin stained for differential cell counts, allowing for quantification of total leukocytes and neutrophils.
RNA was isolated from the right lung using a Qiagen RNeasy kit (Qiagen Sciences, Germantown, MD), per the manufacturer’s instructions. Gene expression levels of KC and TNF-α were measured by RT-PCR. LPS-induced NF-κB activation was measured in cytoplasmic extracts from right lung tissue using a Western blotting technique to visualize phospho–NF-κB p65 levels, which were quantified with ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
Data were analyzed for significance using one-way ANOVA with Bonferroni posttest corrections. Differences between treatments were considered significant at P less than 0.05.
Results
Disease-Related Behavior
As shown in Table 1 and in Figure E1 (see Figure E1 in the online supplement), the effects of BIO-11006 on the mice were dramatic, regardless of time after LPS instillation that the peptide was administered. Treatment of mice with aerosolized BIO-11006 at 0 time prevented the onset of injury. Administration of BIO-11006 at 4, 12, 24, or 36 hours after LPS instillation actually reversed the disease process: mice treated with LPS plus PBS only at these times appeared sick and moribund, but mice treated with BIO-11006 showed behavior and disease parameters returning to normal after postinjury treatment initiated at each of these time points (see Table 1). The dramatic effect on the mice was apparent when their behavior after treatment with BIO-11006 is visualized (see Figure E1).
Table 1.
Blinded Analysis of Mouse Disease-Related Behavior Parameters by a Veterinarian after Two Treatments with BIO-11006 or PBS 12 Hours Apart at Indicated Times
| Treatment Group | Disease-Related Behavior |
||||
|---|---|---|---|---|---|
| Lack of Activity | Hunched Posture | Ptosis | Piloerection | Labored Breathing | |
| LPS + PBS | +++ | +++ | +++ | +++ | +++ |
| LPS + BIO-11006; 0, 12 h | 0 | 0 | 0 | 0 | 0 |
| LPS + BIO-11006; 4, 16 h | 0 | 0 | 0 | 0 | 0 |
| LPS + BIO-11006; 12, 24 h | 0 | 0 | 0 | + | 0 |
| LPS + BIO-11006; 24, 36 h | 0 | 0 | + | + | 0 |
| LPS + BIO-11006; 36, 48 h | 0 | 0 | + | + | 0 |
BIO-11006 ameliorates each of these acute lung injury–associated effects. Key: 0, normal; +, mild; ++, moderate; +++, severe.
Histological Assessment
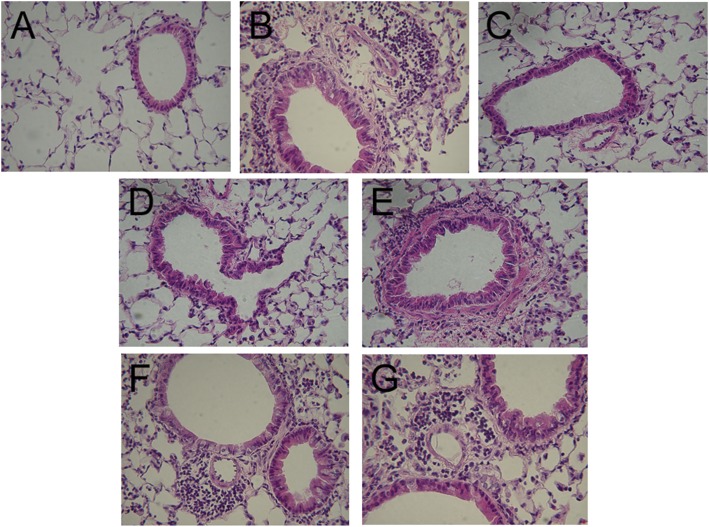
As illustrated in Figure 1, lungs of LPS-instilled mice treated only with PBS showed extensive infiltration of neutrophils and edema around airways and into the parenchyma when analyzed 72 hours after LPS instillation. Treatment with BIO-11006 appeared to ameliorate this response, with no apparent inflammation at 0 time, and increasing levels of neutrophilia as the time from LPS administration to initial treatment increased. Even with treatment starting at 36 hours, there clearly was less inflammation and edema apparent than in the LPS-instilled mice treated with PBS only.
Figure 1.
Lung histology (hematoxylin and eosin) at 72 hours after LPS instillation, with BIO-11006 administered at different times after LPS. BIO-11006 was administered via inhalation at 50 μM either just before (0 h) intratracheal instillation of LPS, or 4, 12, 24, or 36 hours after. Inhaled BIO-11006 ameliorated inflammation, even when administered as long as 36 hours after LPS instillation. (A) PBS only (negative control; No LPS); lungs appear normal with no apparent neutrophilia nor inflammation. (B) LPS plus PBS only (positive control). Extensive neutrophilia and inflammation are apparent. (C) LPS plus BIO-11006, treatment started at 0 hours; very sparse neutrophilia or inflammation are apparent. (D–G) LPS plus BIO-11006, treatment started at (D) 4 hours, (E) 12 hours, (F) 24 hours, and (G) 36 hours. Neutrophilia and inflammation appear to be increased with increasing time before initiation of treatment with BIO-11006 after LPS; however, even with treatment starting at 36 hours after LPS instillation (G), there appears to be much less neutrophilia and inflammation than is seen in (B). Original magnification, ×400.
BAL Protein Analysis
LPS instillation resulted in an approximate fourfold increase in total protein content when measured at 72 hours. BIO-11006 decreased total protein content, measured at 72 hours after LPS, by approximately 25–30% when treatment was begun at 0, 4, and 12 hours, and by approximately 15–20% when treatment was begun at 24 and 36 hours after LPS. Decrease in protein content at each time point was significant (P < 0.05) compared with LPS plus PBS. There were no statistical differences in total protein content among any of the time points themselves.
Leukocyte Influx
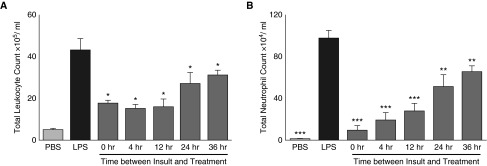
As illustrated in Figure 2, numbers of total leukocytes, and especially neutrophils, measured in BAL fluid (BALF) at 72 hours after LPS were decreased inversely proportional to the time after LPS instillation when treatment was initiated (i.e., the earlier the time after LPS that the peptide was given, the fewer leukocytes and neutrophils in the lung were observed).
Figure 2.
Treatment with aerosolized BIO-11006 attenuates total leukocyte and neutrophil influx into the lung. Cells measured in bronchoalveolar lavage fluid (BALF) 72 hours after LPS instillation. Numbers of total leukocytes (A), and especially neutrophils (B), are inversely proportional to the time after LPS instillation when peptide treatment was initiated. *P < 0.05, **P < 0.01, and ***P > 0.001 compared with LPS-instilled, PBS-treated animals. Data are presented as mean (±SEM).
Proinflammatory Cytokine Expression
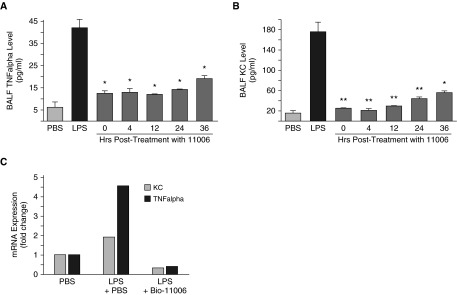
As illustrated in Figures 3A and 3B, BALF levels of the proinflammatory cytokines, TNF-α and KC, were significantly attenuated at 72 hours after LPS instillation, inversely proportional to the time after LPS instillation when treatment was initiated. Message levels for both cytokines at 72 hours after LPS were also significantly attenuated by treatment with BIO-11006 (Figure 3C), shown here with treatment initiated at 4 hours after LPS instillation.
Figure 3.
Protein levels of proinflammatory cytokines measured in BALF. (A) TNF-α and (B) KC (murine IL-8 equivalent) are significantly attenuated in mice treated with aerosolized BIO-11006, inversely proportional to the time after LPS instillation when peptide treatment was initiated. *P < 0.05; **P < 0.001 compared with LPS plus PBS–treated animals. (C) mRNA levels of TNF-α and KC in lung tissue are attenuated in mice treated with aerosolized BIO-11006. In this example, mice were instilled with LPS and then treated with BIO-11006 after 4 hours, and then again at 16 hours. The mice were killed and mRNA expression of TNF-α and KC measured at 72 hours after LPS via real-time PCR. Treatment with BIO-11006 clearly decreased mRNA levels of both TNF-α and KC. Data are presented as mean (±SEM).
NF-κB Activation
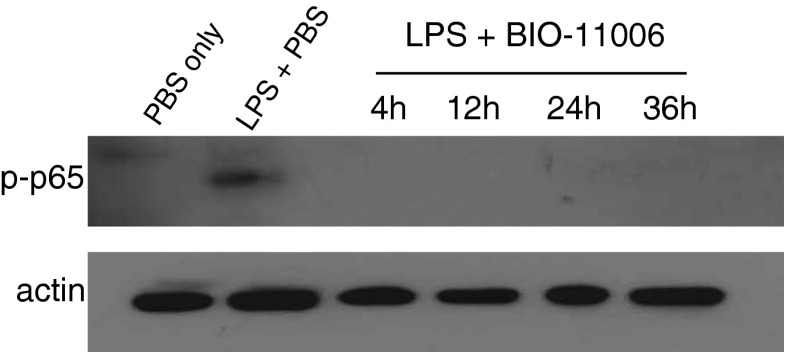
As illustrated in Figure 4, BIO-11006 attenuated LPS-induced NF-κB activation, as assessed by measurement of phosphorylated p65 (p-p65) in the lung, when administered at each of the time points (4, 12, 24, or 36 h after LPS). Total p65 in cytoplasmic extracts increased over time (data not shown).
Figure 4.
BIO-11006 attenuates LPS-induced NF-κB activation in lung, measured 72 hours after LPS instillation, at each time point of initiation of treatment. NF-κB activation was assessed via phospho–NF-κB p65 analysis by immunoblotting. Actin was used as a loading control (lower panel). BIO-11006 clearly attenuates NF-κB activation in response to LPS at each time point.
Discussion
The results of this study indicate that inhaled, aerosolized BIO-11006, a peptide that inhibits the function of MARCKS protein, attenuates LPS-induced neutrophil influx into the lung, activation of NF-κB, and expression of the proinflammatory cytokines, KC and TNF-α, and thus reverses the development of lung injury in mice instilled intratracheally with LPS. Although it is known that several agents can affect the onset of ALI when given to mice before or even a few hours after LPS instillation (14–16), this is the first time, to our knowledge, that a treatment demonstrates the ability to stop, and even reverse, ALI, even when administered well into the course of the disease process. This reversal took place as long as 36 hours after LPS instillation, a time when the mice appeared quite moribund before treatment. These findings could potentially be extrapolated to the human situation, where patients usually present with ALI/ARDS that is already well established. There currently are no effective pharmacological treatments for ALI/ARDS, but targeting MARCKS protein with BIO-11006 or similar peptides could represent a novel pharmacological approach.
Neutrophil infiltration and inflammation are major contributors to tissue damage in ALI (3, 17). Neutrophils are recruited to the injured lung within minutes after the initial insult, and neutrophil influx and the resultant cytokine “storm” are considered hallmarks of ALI. As shown in Figure 2, treatment of LPS-instilled mice with aerosolized BIO-11006 appears to rapidly inhibit further influx of neutrophils into the lung. The mechanisms through which this might occur were then investigated. One possibility is that BIO-11006 directly inhibits neutrophil motility, thereby blocking neutrophil migration to the injured lung. Indeed, a number of published studies from our laboratory and others show that treatment of isolated neutrophils and other cell types with N-terminal MARCKS-inhibitory peptides attenuates their motility/migration (4–9), probably through effects on the actin-binding function of MARCKS (4). Thus, an obvious explanation for the effect of inhaled BIO-11006 is that it directly inhibits movement of neutrophils, not allowing them to migrate to the lung after injury. However, based on other published work describing a role for MARCKS in release of cytokines and chemokines (18), we looked at other potential mechanisms that might be involved in attenuating influx of neutrophils into the lung and cytokine/chemokine expression in response to LPS. We focused this study on the potent neutrophil chemoattractant, KC, or CXCL1, the mouse IL-8 equivalent, and the pleiotropic proinflammatory cytokine, TNF-α. Indeed, analysis of BALF at 72 hours after LPS showed that treatment with inhaled BIO-11006 significantly decreased levels of both cytokines (Figures 3A and 3B). We also investigated, using RT-PCR, whether this effect could be on the expression of KC and TNF-α at the mRNA level. As illustrated in Figure 3C, treatment with BIO-11006 provoked a significant decrease in mRNA expression of these cytokines in the lung, suggesting effects at the message level.
MARCKS also has been shown to be involved in activation of NF-κB in different cells (19–21), perhaps through a protein kinase C δ–related mechanism (22), and it is known that KC and TNF-α– contain NF-κB–binding sites in their promoter regions, so a logical next step was to look at whether or not BIO-11006 could affect NF-κB. Using phosphorylation of the p65 subunit of NF-κB in lung cytoplasmic extracts as an indicator of NF-κB activation, inhalation of BIO-11006 at any of the time points in this study inhibited LPS-induced NF-κB activation (Figure 4).
Given that BIO-11006 can inhibit the above proinflammatory parameters, a possible explanation is that each of these effects can contribute to attenuation of neutrophil influx into the lung after LPS injury. For example, if the initial event is blocking NF-κB activation, this would, in turn, attenuate production of the potent neutrophil chemoattractant, KC, which would result in decreased neutrophil migration to the lung. This could occur on top of the direct effect of the MARCKS-inhibitory peptide on neutrophil motility, further decreasing neutrophil influx. At this point, the exact contribution of each of these potential inhibitory events is not known, nor is their a known temporal sequence, but it could be that decreased chemoattraction/inflammation and direct inhibition of neutrophil motility both play a role in the ameliorating effects of BIO-11006 on LPS-induced inflammation in the lung. The exact contribution of each of these parameters is a topic of ongoing study.
To ascertain that the effects of aerosolized BIO-11006 were not restricted to LPS-induced lung injury, we also tested the effects of inhaled BIO-11006 in two other well characterized murine models of ALI: (1) intratracheal instillation of bacterial lipotetrachoic acid (LTA) (23); and (2) the bacterial pneumonia model with intranasal instillation of Streptococcus pneumonia (24). Inhaled BIO-11006 inhibited neutrophil influx in both of these ALI models as efficiently as in the LPS-instilled mice (data not shown).
The results of this study clearly indicate that MARCKS protein is involved integrally in the pathogenesis of LPS-induced ALI/ARDS. Inhibition of MARCKS in ALI/ARDS with an inhaled N-terminal inhibitor could represent a potential pharmacological approach to treat patients with ALI/ARDS and other forms of ALI (e.g., smoke inhalation, trauma) characterized by neutrophil influx into the lung. The finding that the drug was effective even when administered fairly deeply into disease progression indicates that it may be useful for treating patients who present with the disease process already well underway. Clinical trials of BIO-11006 in patients with ARDS are being planned.
Acknowledgments
Acknowledgments
The authors thank Gabriel McKeon, D.V.M., for the blinded analysis of mouse disease-related behavior reflected in Table 1. BIO-11006 is covered by U.S. patent #8,999,915.
Footnotes
This work was supported by National Institutes of Health grants R37 HL36982 and R41 HL124817.
Author Contributions: Q.Y., S.F., J.P., A.L.C., I.P., and K.B.A. all provided input into experimental design and analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0236RC on August 24, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol. 2010;42:586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott LE, Sung EJ, Melvin AT, Sheats MK, Haugh JM, Adler KB, Jones SL. Fibroblast migration is regulated by myristoylated alanine-rich C-kinase substrate (MARCKS) protein. PLoS One. 2013;8:e66512. doi: 10.1371/journal.pone.0066512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheats MK, Pescosolido KC, Hefner EM, Sung EJ, Adler KB, Jones SL. Myristoylated alanine rich C kinase substrate (MARCKS) is essential to β2-integrin dependent responses of equine neutrophils. Vet Immunol Immunopathol. 2014;160:167–176. doi: 10.1016/j.vetimm.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheats MK, Sung EJ, Adler KB, Jones SL. In vitro neutrophil migration requires protein kinase C-delta (δ-PKC)–mediated myristoylated alanine-rich C-kinase substrate (MARCKS) phosphorylation. Inflammation. 2015;38:1126–1141. doi: 10.1007/s10753-014-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green TD, Park J, Yin Q, Fang S, Crews AL, Jones SL, Adler KB. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J Leukoc Biol. 2012;92:633–639. doi: 10.1189/jlb.1211604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JD, Lankford SM, Adler KB, Brody AR. Mesenchymal stem cells require MARCKS protein for directed chemotaxis in vitro. Am J Respir Cell Mol Biol. 2010;43:253–258. doi: 10.1165/rcmb.2010-0015RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–40990. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A, Murphy EC, III, Park J, Adler KB, Parikh I. Aerosolized BIO-11006, a novel MARCKS-related peptide, improves airway obstruction in a mouse model of mucus hypersecretion. J Epithel Biol Pharmacol. 2011;4:1–6. [Google Scholar]
- 12.Calderon Toledo C, Rogers TJ, Svensson M, Tati R, Fischer H, Svanborg C, Karpman D. Shiga toxin–mediated disease in MyD88-deficient mice infected with Escherichia coli O157:H7. Am J Pathol. 2008;173:1428–1439. doi: 10.2353/ajpath.2008.071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkholder T, Foltz C, Karlsson E, Linton CG, Smith JM. Health evaluation of experimental laboratory mice. Curr Protoc Mouse Biol. 2012;2:145–165. doi: 10.1002/9780470942390.mo110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, Matthay MA. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol. 2015;195:875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- 15.Üstün S, Lassnig C, Preitschopf A, Mikula M, Müller M, Hengstschläger M, Weichhart T. Effects of the mTOR inhibitor everolimus and the PI3K/mTOR inhibitor NVP-BEZ235 in murine acute lung injury models. Transpl Immunol. 2015;33:45–50. doi: 10.1016/j.trim.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin M-H, Chen M-C, Chen T-H, Chang H-Y, Chou T-C. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-γ–dependent inhibition of NF-kB activation. Int Immunopharmacol. 2015;28:270–278. doi: 10.1016/j.intimp.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 17.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4) suppl:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 18.Li J, D’Annibale-Tolhurst MA, Adler KB, Fang S, Yin Q, Birkenheuer AJ, Levy MG, Jones SL, Sung EJ, Hawkins E, et al. A myristoylated alanine-rich C kinase substrate–related peptide suppresses cytokine mRNA and protein expression in LPS-activated canine neutrophils. Am J Respir Cell Mol Biol. 2013;48:314–321. doi: 10.1165/rcmb.2012-0278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Liu H, Gong YS, Liu SF. LPS down-regulates specificity protein 1 activity by activating NF-κB pathway in endotoxemic mice. PLoS One. 2015;10:1–14. doi: 10.1371/journal.pone.0130317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaver CM, Grove BS, Putz ND, Clune JK, Lawson WE, Carnahan RH, Mackman N, Ware LB, Bastarache JA. Regulation of alveolar procoagulant activity and permeability in direct acute lung injury by lung epithelial tissue factor. Am J Respir Cell Mol Biol. 2015;53:719–727. doi: 10.1165/rcmb.2014-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S-M, Suk K, Lee W-H. Myristoylated alanine-rich C kinase substrate (MARCKS) regulates the expression of proinflammatory cytokines in macrophages through activation of p38/JNK MAPK and NF-κB. Cell Immunol. 2015;296:115–121. doi: 10.1016/j.cellimm.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Imran M, Lim IK. Regulation of Btg2(/TIS21/PC3) expression via reactive oxygen species–protein kinase C–ΝFκΒ pathway under stress conditions. Cell Signal. 2013;25:2400–2412. doi: 10.1016/j.cellsig.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Lin X, Koga K, Takahashi K, Linge HM, Mello A, Laragione T, Gulko PS, Miller EJ. Strain differences in alveolar neutrophil infiltration and macrophage phenotypes in an acute lung inflammation model. Mol Med. 2011;17:780–789. doi: 10.2119/molmed.2010.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, Doerschuk CM. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med. 2011;183:1391–1401. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]