Abstract
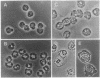
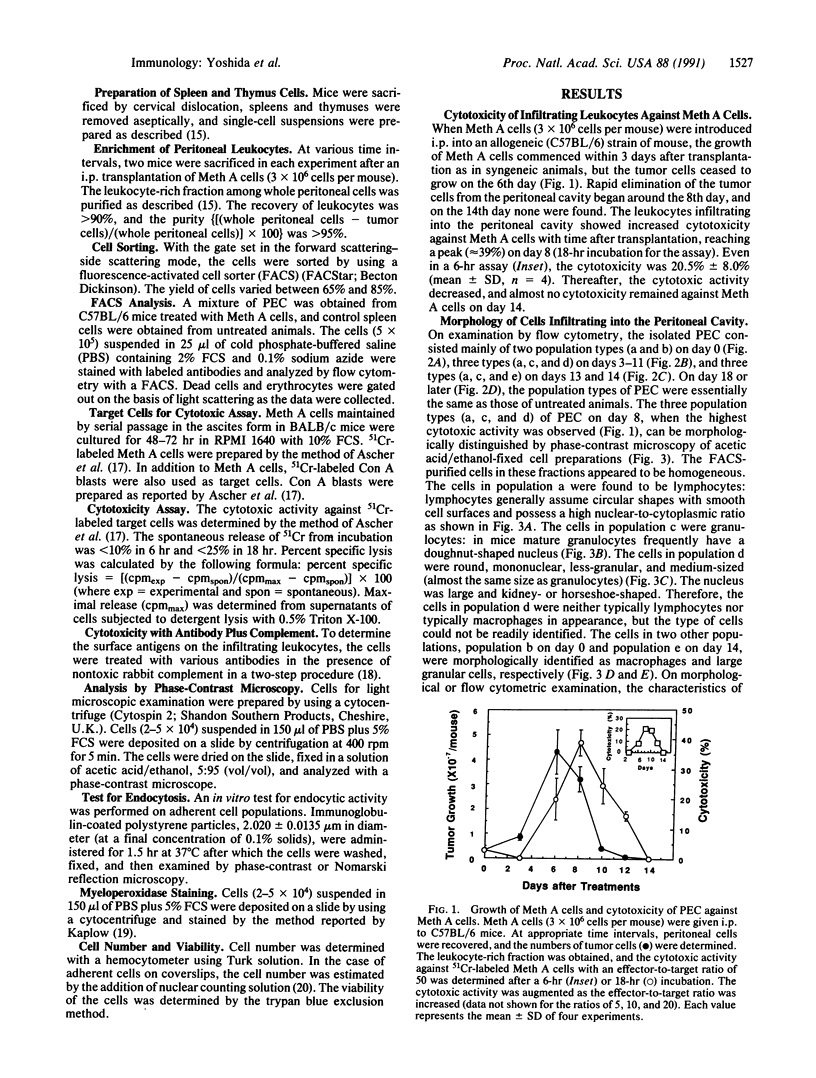
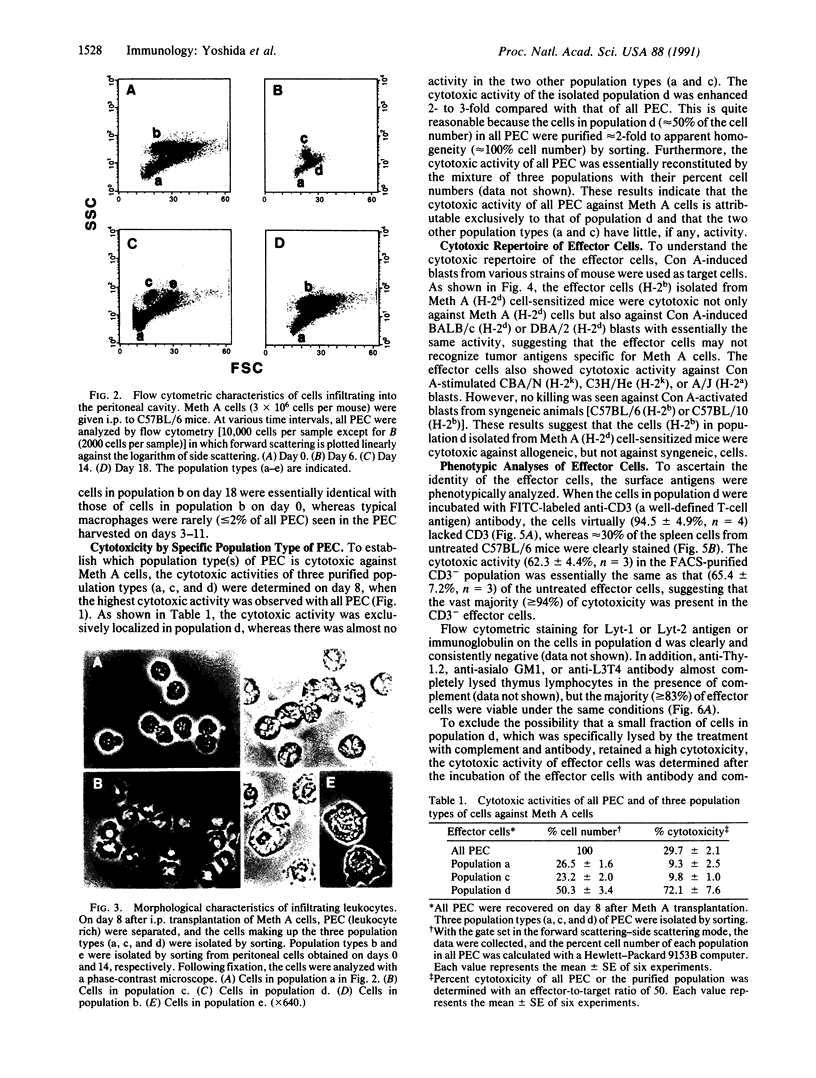
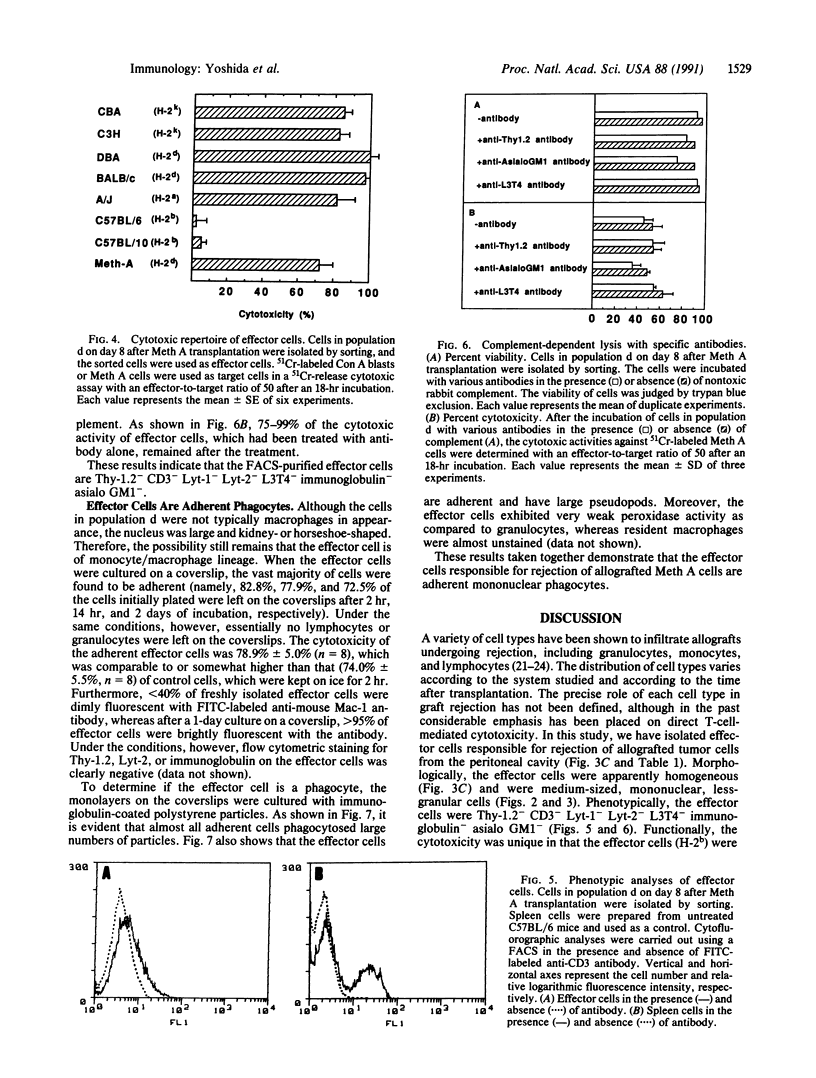
To understand the in situ mechanism of immunological response of recipient animals to allografted tumor cells, the types of cells that infiltrated into the rejection site were examined. When Meth A cells (H-2d) were given i.p. to an allogeneic [C57BL/6 (H-2b)] strain of mouse, the tumor cells ceased to grow on the 6th day, accompanied by an i.p. infiltration of leukocytes. The tumor cells were totally eliminated from the peritoneal cavity around the 12th day. The highest cytotoxic activity against Meth A cells was obtained with the peritoneal exudate cells harvested on day 8. On this day, the exudate cells consisted of three populations when examined by flow cytometry, and each was isolated by sorting. Each of them appeared to be homogeneous, and they were morphologically identified as lymphocytes; granulocytes; and medium-sized, mononuclear, less-granular cells. The cytotoxic activity was confined exclusively to the last population. The effector cells (H-2b) were cytotoxic against not only Meth A cells (H-2d) but also concanavalin A-stimulated allogeneic spleen cells [C3H/He (H-2k), CBA/N (H-2k), A/J (H-2a), BALB/c (H-2d), and DBA/2 (H-2d) strains of mouse]. The effector cells were totally inert against concanavalin A-activated syngeneic spleen cells [C57BL/6 (H-2b) and C57BL/10 (H-2b) strains of mouse]. The effector cells were phenotypically (Thy-1.2- CD3- Lyt-1- Lyt-2- L3T4- immunoglobulin- asialo GM1-), morphologically, and functionally distinct from cytotoxic T cells, natural killer cells, and lymphokine-activated killer cells but were adherent mononuclear phagocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNASON B. G., JANKOVIC B. D., WAKSMAN B. H., WENNERSTEN C. Role of the thymus in immune reactions in rats. II. Suppressive effect of thymectomy at birth on reactions of delayed (cellular) hypersensitivity and the circulating small lymphocyte. J Exp Med. 1962 Aug 1;116:177–186. doi: 10.1084/jem.116.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acha-Orbea H., Groscurth P., Lang R., Stitz L., Hengartner H. Characterization of cloned cytotoxic lymphocytes with NK-like activity. J Immunol. 1983 Jun;130(6):2952–2959. [PubMed] [Google Scholar]
- Ascher N. L., Ferguson R. M., Hoffman R., Simmons R. L. Partial characterization of cytotoxic cells infiltrating sponge matrix allografts. Transplantation. 1979 Apr;27(4):254–259. doi: 10.1097/00007890-197904000-00010. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Urdal D. L., Henney C. S. Lymphokine-driven "differentiation" of cytotoxic T-cell clones into cells with NK-like specificity: correlations with display of membrane macromolecules. Immunol Rev. 1983;72:43–72. doi: 10.1111/j.1600-065x.1983.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Aleksijevic A., Scuderi P., Hersh E. M., Grimes W. J. Phenotypic and functional analysis of lymphokine-activated killer (LAK) cell clones. Ability of CD3+, LAK cell clones to produce interferon-gamma and tumor necrosis factor upon stimulation with tumor targets. Cancer Immunol Immunother. 1989;29(4):270–278. doi: 10.1007/BF00199215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. M., Dorsch S., Roser B. The cellular basis of allograft rejection in vivo. I. The cellular requirements for first-set rejection of heart grafts. J Exp Med. 1978 Oct 1;148(4):878–889. doi: 10.1084/jem.148.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock W. W., Thomson N. M., Atkins R. C. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983 May;35(5):458–463. doi: 10.1097/00007890-198305000-00013. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Differential induction of activation markers in macrophage cell lines by interferon-gamma. Cell Immunol. 1989 May;120(2):401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., Hogarth P. M., Ceredig R., McKenzie I. F. Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J Exp Med. 1981 May 1;153(5):1044–1057. doi: 10.1084/jem.153.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDAWAR P. B. The homograft reaction. Proc R Soc Lond B Biol Sci. 1958 Dec 4;149(935):145–166. doi: 10.1098/rspb.1958.0058. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Asherson G. L., Chandler P., Colizzi V., Watkins M. C., Zembala M. Nonspecific inhibitor of DNA synthesis elaborated by T acceptor cells. I. Specific hapten- and I-J-driven liberation of an inhibitor of cell proliferation by Lyt-1-2+ cyclophosphamide-sensitive T acceptor cells armed with a product of Lyt-1+2+-specific suppressor cells. J Immunol. 1983 Feb;130(2):785–790. [PubMed] [Google Scholar]
- Medawar P. B. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944 Oct;78(Pt 5):176–199. [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Reitamo S., Konttinen Y. T., Ranki A., Häyry P. The relation of different inflammatory cell types to the various parenchymal components of rejecting kidney allografts. Histopathology. 1980 Sep;4(5):517–532. doi: 10.1111/j.1365-2559.1980.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Roberts K., Moore M. A clonal analysis of human peripheral blood lymphocytes displaying natural killer-like activity. Eur J Immunol. 1985 May;15(5):448–456. doi: 10.1002/eji.1830150507. [DOI] [PubMed] [Google Scholar]
- Rothwell T. L., Papadimitriou J. M. The cellular infiltrate in skin allografts in mice. J Pathol. 1972 Aug;107(4):235–243. doi: 10.1002/path.1711070403. [DOI] [PubMed] [Google Scholar]
- Sasaki D. T., Dumas S. E., Engleman E. G. Discrimination of viable and non-viable cells using propidium iodide in two color immunofluorescence. Cytometry. 1987 Jul;8(4):413–420. doi: 10.1002/cyto.990080411. [DOI] [PubMed] [Google Scholar]
- Shortman K., Wilson A., Scollay R. Loss of specificity in cytolytic T lymphocyte clones obtained by limit dilution culture of Ly-2+ T cells. J Immunol. 1984 Feb;132(2):584–593. [PubMed] [Google Scholar]
- Swain S. L., Panfili P. R. Helper cells activated by allogeneic H-2K or H-2D differences have a Ly phenotype distinct from those responsive to I differences. J Immunol. 1979 Feb;122(2):383–391. [PubMed] [Google Scholar]
- Tabata Y., Uno K., Ikada Y., Muramatsu S. Potentiation of antitumor activity of macrophages by recombinant interferon alpha A/D contained in gelatin microspheres. Jpn J Cancer Res. 1988 May;79(5):636–646. doi: 10.1111/j.1349-7006.1988.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesole D. H., Dart G. A., Talmage D. W. Rejection of stable cultured allografts by active or passive (adoptive) immunization. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1626–1628. doi: 10.1073/pnas.79.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIENER J., SPIRO D., RUSSELL P. S. AN ELECTRON MICROSCOPIC STUDY OF THE HOMOGRAFT REACTION. Am J Pathol. 1964 Feb;44:319–347. [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Park S. W., Yasui H., Takikawa O. Tryptophan degradation in transplanted tumor cells undergoing rejection. J Immunol. 1988 Oct 15;141(8):2819–2823. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- von Willebrand E., Häyry P. Composition and in vitro cytotoxicity of cellular infiltrates in rejecting human kidney allografts. Cell Immunol. 1978 Dec;41(2):358–372. doi: 10.1016/0008-8749(78)90233-2. [DOI] [PubMed] [Google Scholar]