Rheumatic fever licks at the joints, but bites at the heart.
Laseque, (1884)
1. INTRODUCTION
In March 1942, a 33-year-old woman in the USA was hospitalized for a month with a life-threatening streptococcal infection at a New Haven, Connecticut, hospital. She had streptococcal septicemia from childbirth. She was delirious and her temperature reached almost 107°F (41.6°C). Treatments with sulfa drugs, blood transfusions, and surgery had no effect. As a last resort, her doctors injected her with a tiny amount of an obscure experimental drug called penicillin. Her hospital chart, now at the Smithsonian Institution, indicates a sharp overnight drop in temperature; by the next day, she was no longer delirious. She survived to marry, raise a family, and meet Sir Alexander Fleming, the scientist who discovered penicillin. The patient died in June 1999 at the age of 90 years.[1]
The rest of the penicillin story is history. Its use revolutionized the world of medicine. It enabled physicians to treat formerly severe and life-threatening illnesses such as bacterial endocarditis, meningitis, pneumococcal pneumonia, gonorrhea, and syphilis. Doctors finally had a tool that could completely cure their patients of deadly infectious diseases.
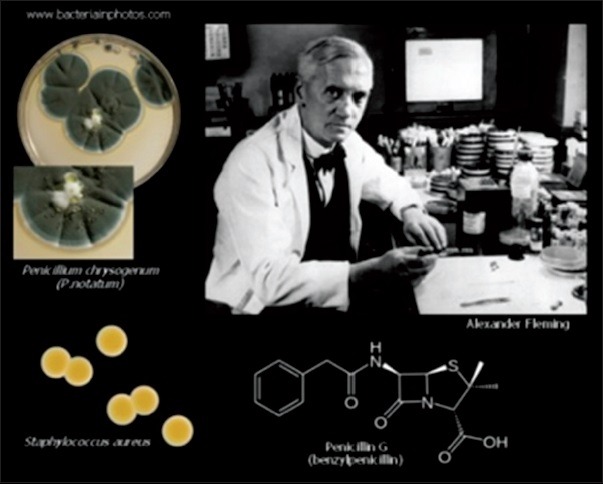
Alexander Fleming, who is credited with discovering penicillin in 1928
One of the dreaded infections that were modified by penicillin was streptococcal pharyngitis (rheumatic fever [RF]), which had a high mortality rate.
In the early years of the last century, RF was a big killer even in the developed countries of that era. In the USA in the 1920s, RF was the leading cause of death in individuals between 5 and 20 years of age and was second only to tuberculosis in those between 20 and 30 years.[2] The only treatment was salicylates and bed rest. The majority remained at home for weeks, more often for months, with a smoldering illness; in the USA and Europe, the sicker children were managed in foster homes or special institutions for the chronically ill.[2]
RF is caused by a preceding Group A streptococcal (strep) infection. Acute RF (ARF) primarily affects the heart, joints, and central nervous system. Of these symptoms, the autoimmune cardiac sequelae are the most dreaded for it causes fibrosis of heart valves, leading to crippling valvular heart disease, heart failure, strokes, endocarditis, and death. Generations of medical students have been taught that treating strep throat with antibiotics can prevent RF. Moreover, regular antibiotics (usually monthly injections) can prevent patients with RF from contracting further strep infections and causing progression of valve damage.
Hence, rheumatic heart disease (RHD) is a chronic heart condition caused by RF that can be prevented and controlled.
Since the early 1900s, the incidence and prevalence of ARF and RHD have been decreasing in developed nations and this trend is believed to be the result of improved living conditions and availability of antibiotics for the treatment of Group A streptococcal infection. However, both RF and RHD continue to be major causes of morbidity and mortality among young people in developing nations. According to WHO, overcrowding, poor housing conditions, undernutrition, and lack of access to healthcare play a role in the persistence of this disease in developing countries.[1] Therefore, RF has been called a disease of poverty.[3] It is estimated that there are over 15 million cases of RHD worldwide, with 282,000 new cases and 233,000 deaths annually.[4]

The miasma theory: Illustration shows that some of fatal diseases polluted air could bring pleurisy, bronchitis, and asthma
2. HISTORICAL INSIGHTS
2.1. Miasma (bad air) theory of disease
Microorganisms
Microorganisms (bacterium, fungus, and virus) have existed long before humanity. Physicians used to believe that “bad air” were the cause of infectious diseases such as cholera or the Black Death.
In the 9th century, the Abbasid Caliph Al-Mu'tadid in Baghdad asked the great Moslem physician Al-Razi (864–923) to choose a site for building a hospital in the city. Al-Razi hung pieces of meat in various quarters of the city. He chose a location where meat has remained longer without spoiling as the location with “good” air and suitability for the hospital.
They ancient physicians believed that epidemics were caused by miasma (Greek meaning “pollution”) emanating from rotten organic matter.
The miasma theory[5] of disease was accepted in ancient times in Europe, India, and China. The existence of microorganisms unseen by the human eyes as causing disease was unknown.

The Germ Theory of Disease
2.2. Theory of contagion
In the time of Hippocrates (c. 460–c. 370 BC)[6] however the concept of contagion took root. He no longer believed in miasma as a cause for disease. According to medical historians, many of the clinical descriptions presented in the Corpus Hippocraticum (Hippocratic collection) are still the prototypes of the natural history of certain infectious diseases and their collective interplay with the environment, climate, and society. Galen, who lived in AD 130–200 and whose ideas influenced medical thought for thousands of years admired Hippocrates but he asserted that the formation of pus as essential for wound healing (!), an assumption that was unquestioned for 15 centuries and that delayed medical progress.[6]

2003 Italian stamp from San Marino bears image of Girolamo Fracastoro, with ancient Roman V erona Bridge in the background
In 1546, the Italian physician Girolamo Fracastoro[7] proposed that epidemic diseases are caused by transferable tiny particles or “spores” that could transmit infection by direct or indirect contact or even without contact over long distances. In his writing, the “spores” of diseases may refer to chemicals rather than to any living entities. It is called the Germ Theory of disease, but scientific evidence in support of this accumulated slowly and the miasma theory remained dominant among scientists and doctors.
The work of Louis Pasteur and Robert Koch provided convincing evidence of microorganisms causing certain diseases. However, by 1880, miasma theory was still competing with the Germ Theory of disease.
By the 19th century (the golden era of bacteriology), many organisms were identified as causing many diseases. This was due mainly to improvements in microscope technology which enabled generations of microbiologists to investigate further the world of previously unseen disease-causing organisms.
2.3. History of clinical manifestations and diagnosis of rheumatic fever
The major clinical manifestations of RF were not well recognized until Thomas Sydenham (1624–1689), an English physician distinguished an acute, febrile polyarthritis in 1685. He was recognized as a founder of clinical medicine and epidemiology in Britain. He was the author of Observationes Medicae that became a standard textbook of medicine for two centuries. He became known as “The English Hippocrates.”[8] He recognized that it was “chiefly attacking the young and vigorous” and different from gout. One year later, he described “St. Vitus' dance” the neurological disorder that is now called “Sydenham's chorea.”[9]
Richard Bright (1789–1858), a British physician, who was the first to described the clinical manifestations of the kidney disorder known as Bright's disease or nephritis, was also the first, who in 1839 connected the febrile polyarthritis with RF.[10]
In 1797, Matthew Baillie (1761–1783) a Scottish-born physician and pathologist and pupil of his uncle, the anatomist John Hunter, had noted in 1797 a thickening of some heart valves in autopsies of patients who had had acute rheumatism. Four years later, William Charles Wells (1757–1817), a Scottish-American physician and printer, published a series of 16 cases of “rheumatism of the heart” (median age 15 years) and addled the description of subcutaneous nodules. However, the occurrence of nodules was largely ignored until a comprehensive study by Thomas Barlow (1845–1945), a British royal physician known for his research on infantile scurvy. He and Francis Warner published the information in 1881.[11]
RHD was recognized from abnormalities of the pulse, respiration, and palpation of the chest in the presence or recent history of fever and joint pains until the introduction of auscultation by the French physician, inventor of the stethoscope in 1818, Rene T. Laennec (1781–1826). Laennec had described murmurs caused by deformities of the mitral valves. A few years later in 1835 James Hope (1801–1841) an English physician known for discovering the early diastolic murmur of mitral stenosis in 1829, who has been called “the first cardiologist” according to Wikipedia[12] described murmurs that originated from the other valves and concluded that RF is the most frequent cause.[13]
A French physician Jean-Baptiste Bouillaud (1796–1881) soon confirmed this opinion. Bouillaud, who performed studies of “heart sounds” is known for providing a correlation between rheumatism and heart disease, and a French medical dictionaries still refer to acute rheumatic carditis as “Bouillaud's disease.”[14]
The “Aschoff nodule,” the myocardial granuloma that came to be considered pathognomonic of rheumatic carditis, had been recognized as early as 1883 but was described definitively by a German physician and pathologist Ludwig Aschoff (1866–1942) in 1904.[11]
There is no single confirmatory clinical sign or laboratory test for the diagnosis of ARF. In 1944, T. Duckett Jones, who was Director of research in RF and RHD at the House of Good Samaritan Hospital in Boston for 20 years, established the first clinical criteria for its diagnosis.[16,17] These criteria known as the “Jones Criteria” remained the benchmark for ARF diagnosis for over 50 years.
2.4. Bacterial etiology
Streptococcus
Streptococcus, a type of bacteria that cause pharyngitis and hence RF, was first identified and named in 1874 by Theodor Billroth,[15] a Viennese surgeon who was studying RF. Besides pharyngitis, it was found to cause scarlet fever, impetigo, toxic shock syndrome, cellulitis, and Streptococcus necrotizing fasciitis (flesh-eating disease). As other streptococci were identified, it became apparent that some species were pathogenic while others were beneficial. In 1918, the different species of streptococci were classified into two groups. Group A streptococci, which included Streptococcus pyogenes was specific to humans and human disease, including pharyngitis. Group B strep can cause blood infections, pneumonia, and meningitis in newborns. Adults can also get Group B strep infections, especially if they are elderly or already have health problems. Strep B can cause urinary tract infections, blood infections, skin infections, and pneumonia in adults.
2.5. Pharyngitis (“Strep throat”)
Pharyngitis has been documented as a common ailment throughout history, and doubtless many cases were caused by streptococcal infections.
Most “strep throat” is caused by S. pyogenes, a Group A Streptococcus that also causes scarlet fever, RF, and impetigo. ARF and RHD are significant public health concerns around the world. Despite decreasing incidence, there is still a significant disease burden, especially in developing nations. Results of echocardiography studies to screen for RHD in developing nations led to a realization in the marked increase in its prevalence in the developing world.
3. DIAGNOSIS OF RHEUMATIC FEVER: THE JONES CRITERIA
The entire clinical spectrum of ARF (from tonsillitis to carditis) was first described by Cheadle in 1889[16] but it was not until 1944 that criteria for its diagnosis were established by Dr. T. Duckett Jones.[16,17] The criteria are divided into major and minor criteria plusevidence of recent streptococcal infection. The diagnosis of ARF requires the existence of two major criteria or one major and two minor plus a history of a streptococcal throat infection.
The original Jones criteria have been the primary guideline for diagnosing acute ARF but sometimes in applying these guidelines, the diagnosis is missed in some populations. In addition, the global epidemiology of ARF and our knowledge regarding the variability of its presentation have changed.
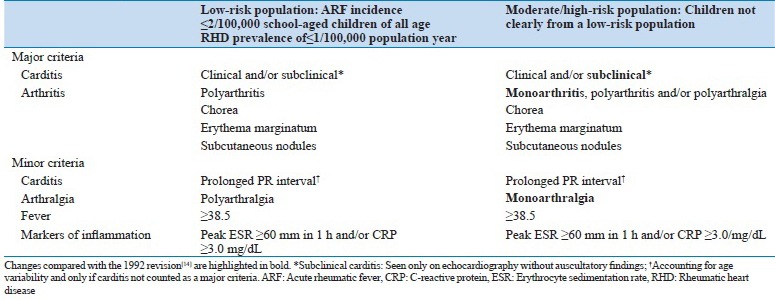
Considering its devastating health sequelae, the criteria have been refined through the years, with expert committees issuing guidelines. The American Heart Association (AHA) in 2015[17] revised the original Jones criteria taking into consideration current epidemiology of the disease and evidence supporting the use of Doppler echocardiography in the diagnosis of carditis as major manifestation of ARF. The revised guidelines consider the risk within a population and offer two separate diagnostic pathways that prioritize specificity among those at low risk and sensitivity among those at moderate/high risk. Echocardiography is now recommended in all patients with suspected or confirmed ARF and subclinical carditis can fulfill a major criterion for ARF in all populations. The new guidelines also provide new and specific criteria for the diagnosis of ARF recurrences.
Table 1.
Major and minor Jones criteria for the diagnosis of acute rheumatic fever

Table 2.
3.1. Role of Echocardiography in the diagnosis of rheumatic heart disease
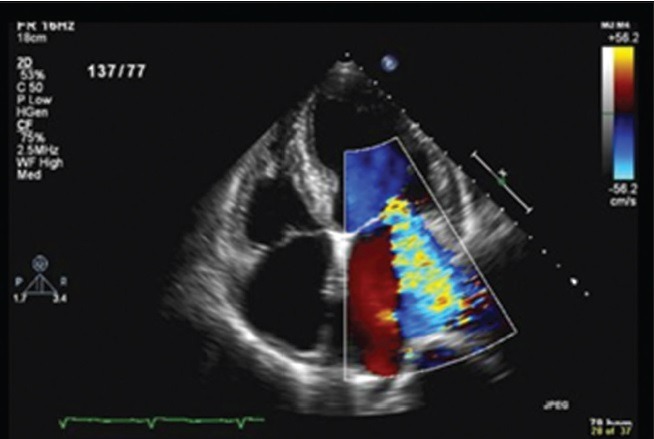
Mitral regurgitation by color Doppler echocardiography
The value of Doppler echocardiography in RF is its ability to detect subclinical carditis. Since carditis may lead to acute heart failure and chronic valve disease, it is the most severe major finding in ARF.
Clinical carditis is defined as an audible murmur consistent with aortic or mitral regurgitation.
Doppler echocardiography has been shown to detect such valvular pathology before our ears could hear such murmurs. This phenomenon became known as subclinical carditis. Many studies have shown the prevalence of subclinical carditis among patients with ARF. A 2007 meta-analysis that included 23 studies from five continents demonstrated that patients with ARF showed a weighted pooled prevalence of subclinical carditis of 16.8% and 44.7% of these patients showed worsening of valvular involvement over time.[20]
ARF and consequent RHD remain extremely common in low- and middle-income countries, with social and economic as well as medical consequences. Secondary prophylaxis based on monthly penicillin injections in children identified to have evidence of RHD has been shown to be the most efficient and cost-effective strategy to reduce RHD burden.[18] Thus, early detection is needed to reduce the burden of RHD.
Hence, the 2015 Jones revision[19] by the AHA included echocardiography for the diagnosis of ARF, since cardiac ultrasound is known to be more sensitive than auscultation for the detection of pathologic valve disease. The recent availability of high-quality portable ultrasound equipment makes it possible to screen large numbers of school children at schools in developing nations. The criteria recommend that when possible, all patients with confirmed or suspected ARF undergo echocardiography to evaluate for carditis. Those who are negative at first evaluation should undergo repeated study to assess for evolving cardiac disease.
4. REFLECTIONS ON THE PRESENT AND FUTURE OF RHEUMATIC HEART DISEASE
ARF used to be a fatal disease but much has changed over the last half-century. The incidence and prevalence of ARF and RHD have been decreasing in developed nations since the early 1900. They however, continue to be major causes of morbidity and mortality among young people in developing nations. It is estimated that there are over 15 million cases of RHD worldwide, with 282,000 new cases and 233,000 deaths annually.[22]
The AHA revised the Jones criteria to incorporate new technology such as Doppler echocardiography and new knowledge on the epidemiology of the disease, formulating and publishing new guidelines in 2015 to better identify patients at risk. The exact incidence and prevalence of ARF and RHD are unknown, but recent data using echocardiography to screen for RHD in developing nations led to a marked increase in the recognized prevalence.[23,24,25,26,27] These data clearly demonstrate that ARF and RHD still exist in substantial numbers worldwide. Consequently, there has been an increased awareness and interest in ARF and RHD. That the incidence and prevalence of ARF and RHD are still high in developing nations are a big disappointment. We need to eliminate ARF to minimize the burden RHD. Hopefully, application of the 2015 revised Jones Criteria by the AHA will simplify the diagnosis of ARF so that cases can be picked up early and therapy instituted.
Developing nations should learn from wealthy nations where RF has become a rarity. These rich nations have used penicillin for the primary prevention but experts say most of the reduction is attributable to improved living conditions, which have resulted in less overcrowding and better hygiene with consequent reductions in transmission of Group A streptococci.[28]
The control of RF remains the treatment of group A streptococcal pharyngitis with penicillin (primary prophylaxis) and administration of penicillin G benzathine injections every 3–4 weeks for many years in people with a history of RF to prevent recurrent episodes (secondary prophylaxis).[29,30] Secondary prophylaxis has been proved to be cost-effective and practical even in the poorest countries, and the WHO has advocated and coordinated such strategy but experts say most developing countries still do not have effective secondary prophylaxis programs.[28] Fortunately, Group A streptococcal is still sensitive to penicillin but there is a concern about the development of resistance in the future.
It has been argued that the use of echocardiography to screen cases is too expensive and represents inappropriate use of much needed funds and resources in poor nations. The study by Marijon et al.[23] point out that if clinical diagnosis alone had been relied on, approximately 90% of echocardiographically detected cases would have been missed. Of course, it is not acceptable to leave these cases undiagnosed and untreated and at risk for recurrence of RF simply because echocardiographic screening is seen as too being too expensive and inappropriate use of modern technology in a developing country. Cheaper models of echocardiographic screening are needed. Perhaps, the use of college-educated technicians for screening is too expensive. Maybe, high-school graduates who understand cardiac anatomy can be used. Such model would bring down the cost of an echocardiographic screening considerably. Handheld cardiac ultrasound has come down in price considerably. Further, research is needed to define models of echocardiographic screening that are practical, affordable, and widely applicable.
A vaccine that protects against RF is ideal, and a number of vaccines are in development. It is predicted that a safe and effective vaccine maybe available within one or two decades. Until then, each developing nation should have a strategy to implement secondary prophylaxis. RF warrants prevention in poor regions of the world to ease the burden of RHD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1. [Last accessed on 2016 Sep 06]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4829a1.htm .
- 2.Bland EF. Rheumatic fever: The way it was. Circulation. 1987;76:1190–5. doi: 10.1161/01.cir.76.6.1190. [DOI] [PubMed] [Google Scholar]
- 3.Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–41. doi: 10.1056/NEJMp078039. [DOI] [PubMed] [Google Scholar]
- 4.Mendis S, Puska P, Norrving B, editors. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization (in Collaboration with the World Heart Federation and World Stroke Organization); 2011. [Google Scholar]
- 5. [Last accessed on 2016 Sep 06]. Available from: http://www.carlsterner.com/research/files/History_of_Miasmic_Theory_2007.pdf .
- 6.Pappas G, Kiriaze IJ, Falagas ME. Insights into infectious disease in the era of Hippocrates. Int J Infect Dis. 2008;12:347–50. doi: 10.1016/j.ijid.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7. [Last accessed on 2016 Sep 06]. Available from: http://www.infectiousdiseases.edwardworthlibrary.ie/theory-of-contagion/
- 8. [Last accessed on 2016 Sep 06]. Available from: https://www.en.wikipedia.org/wiki/Thomas_Sydenham .
- 9.Murphy GE. The evolution of our knowledge of rheumatic fever. An historical survey with particular emphasis on rheumative heart disease. Bull Hist Med. 1943;14:123–47. [Google Scholar]
- 10.Schechter DC. St. Vitus' dance and rheumatic disease. N Y State J Med. 1975;75:1091–102. [PubMed] [Google Scholar]
- 11.Benedek TG. Subcutaneous nodules and the differentiation of rheumatoid arthritis from rheumatic fever. Semin Arthritis Rheum. 1984;13:305–21. doi: 10.1016/0049-0172(84)90011-8. [DOI] [PubMed] [Google Scholar]
- 12. [Last accessed on 2016 Sep 06]. Available from: https://www.en.wikipedia.org/wiki/James_Hope_(physician)
- 13.Hope J. A Treatise on the Diseases of the Heart and Great Vessels, and on the Affections which may be Mistaken for Them. 3rd London edition. USA, Philadelphia: 1846. [Google Scholar]
- 14. [Last accessed on 2016 Sep 06]. Available from: https://www.en.wikipedia.org/wiki/Jean-Baptiste_Bouillaud .
- 15.Ferretti J, Köhler W. History of streptococcal research. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. [Last accessed on 2016 Feb 10]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK333430/ [PubMed] [Google Scholar]
- 16.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones TD. The diagnosis of rheumatic fever. JAMA. 1944;126:481–4. [Google Scholar]
- 18.Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069–73. [PubMed] [Google Scholar]
- 19.Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: A scientific statement from the American Heart Association. Circulation. 2015;131:1806–18. doi: 10.1161/CIR.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 20.Beaton A, Carapetis J. The 2015 revision of the Jones criteria for the diagnosis of acute rheumatic fever: Implications for practice in low-income and middle-income countries. Heart Asia. 2015;7:7–11. doi: 10.1136/heartasia-2015-010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069–73. [PubMed] [Google Scholar]
- 22.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 23.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 24.Carapetis JR, Hardy M, Fakakovikaetau T, Taib R, Wilkinson L, Penny DJ, et al. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nat Clin Pract Cardiovasc Med. 2008;5:411–7. doi: 10.1038/ncpcardio1185. [DOI] [PubMed] [Google Scholar]
- 25.Sadiq M, Islam K, Abid R, Latif F, Rehman AU, Waheed A, et al. Prevalence of rheumatic heart disease in school children of urban Lahore. Heart. 2009;95:353–7. doi: 10.1136/hrt.2008.143982. [DOI] [PubMed] [Google Scholar]
- 26.Paar JA, Berrios NM, Rose JD, Cáceres M, Peña R, Pérez W, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol. 2010;105:1809–14. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhaya M, Panwar S, Beniwal R, Panwar RB. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography. 2010;27:448–53. doi: 10.1111/j.1540-8175.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 28.Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–41. doi: 10.1056/NEJMp078039. [DOI] [PubMed] [Google Scholar]
- 29.Stollerman GH, Rusoff JH, Hirschfeld I. Prophylaxis against group A streptococci in rheumatic fever; the use of single monthly injections of benzathine penicillin G. N Engl J Med. 1955;252:787–92. doi: 10.1056/NEJM195505122521901. [DOI] [PubMed] [Google Scholar]
- 30.Denny FW, Wannamaker LW, Brink WR, Rammelkamp CH, Jr, Custer EA. Prevention of rheumatic fever; treatment of the preceding streptococcic infection. J Am Med Assoc. 1950;143:151–3. doi: 10.1001/jama.1950.02910370001001. [DOI] [PubMed] [Google Scholar]


