Abstract
Heart failure (HF) in children differs from that in adults in many respects. The causes and clinical presentations may differ considerably among children of different age groups and between children and adults. The time of onset of HF holds the key to the etiological diagnosis. Clinical presentation of HF in younger children can be nonspecific requiring heightened degree of suspicion. The overall outcome with HF is better in children than in adults as HF in children is commonly due to structural heart disease and reversible conditions which are amenable to therapy. The principles of management include treatment of the cause, correction of any precipitating event, and treatment of systemic or pulmonary congestion. Though HF in adults has been the subject of extensive research and generation of evidence-based guidelines, there is a scarcity of evidence base in pediatric HF.
Key words: Cardiomyopathy, congenital heart disease, heart failure, heart transplantation
INTRODUCTION
Heart failure (HF) has been defined as an abnormality of cardiac structure or function leading to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolizing tissues, despite normal filling pressures (or only at the expense of increased filling pressures).[1]
HF in adults has been the subject of extensive research and generation of evidence-based guidelines; it has received much less attention in children because of several difficulties. The causes of HF in children are significantly different from those usually responsible for the condition in adults, which include coronary artery disease and hypertension. In children, cardiac failure is most often caused by congenital heart disease (CHD) and cardiomyopathy. Hsu and Pearson have given a good working definition of HF in children as a progressive clinical and pathophysiological syndrome caused by cardiovascular and noncardiovascular abnormalities that result in characteristic signs and symptoms including edema, respiratory distress, growth failure, and exercise intolerance, and accompanied by circulatory, neurohormonal, and molecular derangements.[2]
There is a large amount of research published on the management of HF in adults, whereas there is minimal research on pediatric HF and those which do exist are often small, retrospective studies. As a result, the management of cardiac failure in children has largely evolved based on clinical experience and the extrapolation of adult data, supported by the more limited pediatric literature. Given the significant differences in etiology of HF between the adult and pediatric populations, this may not be ideal. This review aims at providing a concise picture of pediatric HF with special emphasis on diagnosis and management.
EPIDEMIOLOGY
In children, the causes of HF are significantly different from adults and many cases are due to congenital malformations which usually result in high output cardiac failure. Some children suffer from low output cardiac failure such as cardiomyopathy. CHD occurs in around 8/1000 live births. HF associated with CHD occurs in approximately 20% of all patients.
Many of the children with CHD receive early surgical intervention and it has been estimated that the yearly incidence of HF as a result of congenital defects is between 1 and 2 per 1000 live births.[3] The outcome of HF related to CHD has changed dramatically following the introduction of early surgical interventions. The incidence of symptomatic HF has also declined in the “early surgical era.” Massin et al. reported that only 10% of their patients in a tertiary care pediatric cardiology care setting developed symptomatic HF.[4]
Cardiomyopathy also contributes significantly to the number of pediatric patients who present with the symptoms of cardiac failure. Rossano et al. from the United States report that 10,000–14,000 children are hospitalized every year with HF as one of their diagnoses and of those approximately 27% (approximately 3000) have abnormalities of the heart muscle as an underlying cause.[5] The incidence of cardiomyopathies in developed countries is about 0.8–1.3 cases per 100,000 children in the 0–18 years age group but is ten times higher in the 0- to 1-year old age group.[6,7] Ninety percent of all cardiomyopathies in children are of the dilated variety.
In contrast to HF secondary to CHD, the outcome of children with cardiomyopathy remains poor, with a 5-year risk for death or cardiac transplantation of around 50% for patients with dilated cardiomyopathy (DCM).[8]
Another major group of diseases causing HF in children in developing countries is rheumatic fever and rheumatic heart disease. While the incidence and prevalence of rheumatic fever and chronic rheumatic heart disease are well documented, there are scanty data on presentation with HF in this group. A significant number of acute rheumatic carditis and established juvenile mitral stenosis present with features of HF.[9]
CAUSES OF HEART FAILURE IN INFANTS AND CHILDREN
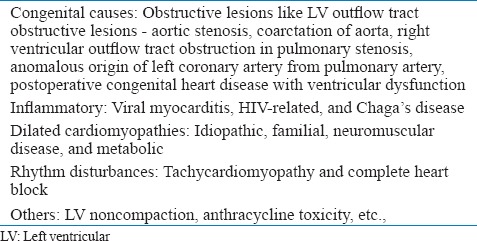
HF in children can be divided into two groups. Over-circulation failure [Table 1] and pump failure [Table 2]. Over-circulation includes conditions that result in volume overload of cardiac chambers. The left ventricular (LV) function is either normal or LV is hypercontractile in them. Pulmonary venous or arterial hypertension may be present to a variable degree. Causes of pump failure include both congenital and acquired conditions. LV or systemic ventricle function is abnormal and most patients have pulmonary venous hypertension in that group.
Table 1.
Causes of over circulation heart failure
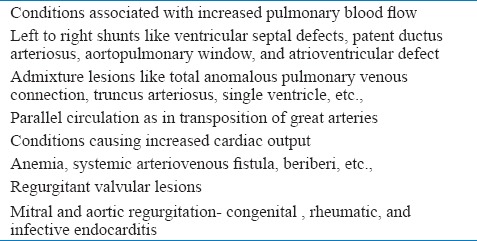
Table 2.
Causes of pump failure
DIAGNOSIS OF HEART FAILURE IN CHILDREN
History and examination
The time of onset of CHF holds the key to the etiological diagnosis. Causes of HF in the fetus include supraventricular tachycardia, severe bradycardia due to complete heart block, severe tricuspid regurgitation due to Ebstein's anomaly of the tricuspid valve, mitral regurgitation from atrioventricular canal defect, systemic arteriovenous fistula, myocarditis, etc., HF presenting on the 1st day of life are commonly due to metabolic abnormalities such as hypoglycemia, hypocalcemia, asphyxia, or sepsis.
Structural diseases that produce fetal cardiac failure can present on the 1st day. Conditions which present in the 1st week of life include critical obstructive lesions such as severe aortic stenosis, coarctation of the aorta (COA), obstructed total anomalous pulmonary venous connection (TAPVC), the great arteries (TGA) with intact ventricular septum (IVS), and hypoplastic left heart syndrome.
Development of HF due to left- to right-shunts usually occurs with the fall in pulmonary vascular resistance at 4–6 weeks, though large ventricular septal defect (VSD), patent ductus arteriosus (PDA), atrio-VSD and aortopulmonary window can cause HF in the 2nd week of life. Other conditions such as truncus arteriosus, unobstructed TAPVC also present in the 2nd week of life. As premature infants have a poor myocardial reserve and their pulmonary vascular resistance falls faster PDA may result in HF in the 1st week in them.
DCM is also a common cause of HF in infants. Causes of DCM in infancy include idiopathic, inborn errors of metabolism, and malformation syndromes. Older children (usually beyond 2 years) are likely to have other causes for HF like acute rheumatic fever with carditis, decompensated chronic rheumatic heart disease, myocarditis, cardiomyopathies, rhythm disturbances, and palliated CHD.
Clinical features suggestive of HF in infants include tachypnea, feeding difficulty, diaphoresis, etc., Feeding difficulty ranges from prolonged feeding time (>20 min) with decreased volume intake to frank intolerance and vomiting after feeds. Irritability with feeding, sweating, and even refusal of feeds are also common.
Established HF presents with poor weight gain and in the longer term, failure in linear growth can also result. Edema of face and limbs is very uncommon in infants and young children. The clinical features of HF in a newborn can be fairly nonspecific and a high index of suspicion is required. Tachycardia > 150/min, respiratory rate >50/min, gallop rhythm, and hepatomegaly are features of HF in infants. Primary cardiac arrhythmia should be considered if heart rate is more than 220/min. Duct dependent pulmonary circulation present with severe cyanosis and acidosis, whereas duct dependent systemic circulation present with HF and shock.
Features of HF in older children and adolescents include fatigue, effort intolerance, dyspnea, orthopnea, abdominal pain, dependent edema, ascites, etc.
Unequal upper and lower limb pulses, peripheral bruits, or raised/asymmetric blood pressure indicating aortic obstruction should always be looked for in a child with unexplained HF at any age. COA in neonates can have normal femoral pulsations in the presence of PDA. COA usually does not cause HF after 1 year of age, when sufficient collaterals have developed. Central cyanosis, even if mild, associated with HF and soft or no murmurs in a newborn suggests TGA with intact IVS, obstructed TAPVC, etc.
An atrial septal defect or VSD does not lead to CHF in the first 2 weeks of life and therefore an additional cause like TAPVC or COA should be ruled out. Older children with tetralogy of Fallot physiology can develop HF due to complications such as anemia, infective endocarditis, aortic regurgitation, or overshunting from aortopulmonary shunts.
The well-established New York Heart Association (NYHA) HF classification is not applicable to most of the pediatric population. The Ross HF classification was developed to assess severity in infants and has subsequently been modified to apply to all pediatric ages. The modified Ross classification for children [Table 3] provides a numeric score comparable with the NYHA classification for adults.[10]
Table 3.
Modified ross heart failure classification for children

Investigations
Basic investigations such as chest radiography (CXR), electrocardiography (ECG), and echocardiography are indicated in all patients with suspected HF.
Chest radiography
Cardiomegaly on pediatric CXR is suggested by a cardiothoracic ratio of >60% in neonates and >55% in older children. Cardiomegaly is highly predictive of ventricular dilation on echocardiography, with high specificity and negative predictive value, but low sensitivity and positive predictive value.[11] Cardiomegaly on CXR indicates poor prognosis in children with DCM.[12] A large thymus can mimic cardiomegaly in CXR of infants and neonates. Left to right shunts usually present with cardiomegaly, enlarged main and branch pulmonary arteries, and pulmonary plethora. CXR is useful in certain cyanotic CHD that presents with typical radiographic features such as egg-on-side appearance in transposition of great arteries, snowstorm appearance in obstructed TAPVC, and figure of eight appearances in unobstructed TAPVC.
Electrocardiography
Most common ECG findings in pediatric HF patients are sinus tachycardia, LV hypertrophy, ST-T changes, myocardial infarction patterns, and conduction blocks. In idiopathic DCM, ECG findings of the left bundle branch block and left atrial enlargement correlated with mortality.[13] Myocardial infarction pattern with inferolateral Q waves indicates anomalous left coronary artery from the pulmonary artery. ECG is particularly useful in the diagnosis of tachycardiomyopathy and other arrhythmic causes of HF like an atrioventricular block. Ambulatory ECG monitoring is useful in the diagnosis of tachycardiomyopathy as well as risk stratification of sudden death in HF resulting from primary cardiomyopathy.[13]
Echocardiography
Transthoracic echocardiography is indicated in all cases of pediatric HF to exclude possible structural disease. Baseline echocardiography will be required for future comparison. LV systolic dysfunction in children is currently defined by an ejection fraction (EF) <55%. Echocardiography is also useful for the screening of cancer patients undergoing treatment with anthracycline chemotherapy, patients with storage disorders, neuromuscular diseases, etc., Periodic echocardiographic evaluation is required in the first-degree relatives of patients with various genetic forms of cardiomyopathy. Periodic echocardiography follow-up is useful in HF patients for the surveillance of disease progression and to assess the response to therapy.[14]
Biomarkers
The natriuretic peptides (brain natriuretic peptide [BNP] or amino terminal [NT]-proBNP) are useful in the acute settings for differentiation of HF from pulmonary causes of respiratory distress.[15] Elevated natriuretic peptide levels might be associated with worse outcome in HF.[16] Plasma BNP elevation is a reliable test for recognizing ventricular dysfunction in children with a variety of CHD.[17] In chemotherapy patients, higher NT-proBNP levels are associated with higher treatment doses of doxorubicin and abnormal echocardiographic parameters including ventricular dysfunction.[18,19] The use of serial BNP or NT-proBNP measurements in children with HF to guide therapeutic intervention or to monitor HF status shows some promise.
Blood glucose and serum electrolytes like calcium, phosphorous should be measured in all children with HF as their abnormalities can cause reversible ventricular dysfunction. Screening for hypoxia and sepsis should be done in newborn with HF.
Antistreptolysin O and C-reactive protein measurement should be done in cases of HF with suspected acute rheumatic fever or reactivation of chronic rheumatic heart disease. Metabolic and genetic testing may be considered in primary cardiomyopathy as recent reports suggest a genetic cause for more than 50% of patients with DCM.[20]
Special investigations
Endomyocardial biopsy (EMB) may be considered for the diagnosis of myocarditis in selected patients presenting with HF when a specific diagnosis is suspected that would influence therapy. A diagnosis of acute myocarditis should be suspected in all children with new-onset HF without a history of decreased functional capacity, especially if ventricular dilation is less than expected for the degree of systolic dysfunction. The prognosis in pediatric myocarditis is significantly better than DCM with a higher probability of recovery in children. However, the diagnostic accuracy of RV EMB for suspected myocarditis tends to be low, and there is limited evidence to support the use of immunosuppressive or immune-modulating therapy in children.
In patients with a clinical diagnosis of myocarditis typical viral pathogen screening using polymerase chain reaction may be considered. Cardiac magnetic resonance imaging (CMRI) is a less invasive alternative to EMB in myocarditis to identify inflammation by showing myocardial edema on T2-weighted images.[21] CMRI might provide additional information in cardiomyopathy by tissue and scar characterization though the prognostic value is uncertain in children.
Treatment
The principles of management include treatment of the cause, correction of any precipitating event, and treatment of systemic or pulmonary congestion. Wherever possible, the cause of CHF should be identified and treated.
In large left to right shunts, prompt surgical therapy should be considered after initiating medical therapy. Other conditions requiring prompt surgery or catheter intervention include severe AS or COA, TGA with IVS, obstructed TAPVC, etc.
A precipitating event such as intercurrent infections, anemia, electrolyte imbalances, arrhythmia, rheumatic reactivation, infective endocarditis, drug interactions, drug toxicity, or drug noncompliance should be identified and corrected if present. Acute HF patients can have symptoms related to fluid overload, underperfusion, or both. The early management of children with HF should address these problems.
In neonates, several causes of HF can present with acute circulatory collapse or progress to shock if not recognized early. Many of these conditions require maintenance of duct patency with prostaglandin infusion or emergency procedures such as ductal stenting and balloon atrial septostomy. Indiscriminate administration of intravenous fluid resuscitation is contra-indicated and will worsen the condition of children with HF. In acute decompensation general measures such as bed rest, propped up position, humidified oxygen sodium, and if required, volume restriction are followed routinely. Infants with CHF require 120–150 Kcal/kg/day of caloric intake and 2–3 mEq/kg/day of sodium.
PHARMACOLOGICAL THERAPY
Drug therapy is aimed at reducing the pulmonary or systemic congestion by the use of diuretics, increasing contractility by inotropes, and reducing the disproportionately elevated afterload by vasodilators and other measures. Routinely used drugs in the management of cardiac failure in children include diuretics, digoxin, angiotensin-converting enzyme inhibitors (ACEIs), spironolactone, beta-blockers, and inotropes. The drugs which are still investigational include natriuretic peptides, vasopressin antagonists, renin inhibitors, endothelin antagonists, oral phosphodiesterase inhibitors, anti-inflammatory molecules, nitric oxide agonists, and neuropeptidase antagonists, etc.
Diuretics
Diuretics are the first line agents to reduce systemic and pulmonary congestion. Loop diuretics have been the most widely used in HF although in some situations other types of diuretics have a role. Frusemide is given intravenously at a dose of 1–2 mg/kg or 1–2 mg/h infusion. For chronic use 1–4 mg/kg of frusemide or 20–40 mg/kg of chlorothiazide in divided doses are used. Patients who are unresponsive to loop diuretic agents alone might benefit from the addition of a thiazide agent like metolazone. Continuous infusion of diuretics is recommended in cases of acute decompensated HF. Diuretic-induced hypokalemia and hypontremia are rare in children. Secondary hyperaldosteronism does occur in children with HF and addition of spironolactone 1 mg/kg single dose to other diuretics conserves potassium.
Digoxin
In the setting of chronic HF, digoxin use decreased the rate of hospitalization and improved the quality of life but not survival in adults.[22] Digoxin is widely used in pediatric cardiac failure despite the lack of trial evidence. Digoxin has a very narrow safety window and it should be avoided in premature babies, those with renal failure and those with acute myocarditis. Electrolyte imbalance like hypokalemia and hypomagnesemia should be promptly corrected to avoid potentiation of toxicity and development of arrhythmias. Rapid digitalization is generally not required. In most circumstances, starting with an oral maintenance dose (8–10 µg/kg/day) with no loading dose is adequate. Dose reduction is required in HF patients on carvedilol and amiodarone targeting lower serum digoxin concentrations (e.g., 0.5–0.9 ng/ml).[23]
Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers
In adult HF patients, ACEI therapy reduces symptoms and improves survival. In children, survival benefit remains unvalidated by any randomized controlled trial. For the treatment of symptomatic LV dysfunction in children, ACEIs are routinely used unless contra-indicated. In children with cardiac failure, the ACEIs which have been most studied are captopril and enalapril.[24,25] Clinical improvement is demonstrated with these agents in left to right shunts with HF as well.[26,27] They should be started at low doses and should be up-titrated to a maximum tolerated, safe dose. ACEIs should be avoided in HF caused by pressure overload lesions as they might interfere with compensatory hypertrophy. Captopril is preferred in neonates (0.4–1.6 mg/kg/day in 3 divided doses) and infants (0.5–4 mg/kg/day in three divided doses). Enalapril is the first choice for those older than 2 years of age (0.1–0.5 mg/kg/day in two divided doses).
Children treated with ACEIs should be watched for deterioration in renal function and hypotension. Other adverse effects include cough and angioedema. Angiotensin receptor blockers are generally reserved for those children with systemic ventricular systolic dysfunction who would benefit from renin-angiotensin-aldosterone system blockade but are intolerant of ACEIs.
Aldosterone antagonists
Aldosterone blockade is a well-established therapy in adults with systolic HF with reduction of mortality in selected patients with HF. The literature supporting the role of spironolactone in pediatric HF is however limited. Aldosterone antagonist therapy is reasonable in children with chronic systolic HF. It is contra-indicated in patients with renal dysfunction and hyperkalemia. Spironolactone is used most often in children because experience with eplerenone in children is limited. Usual starting dose of spironolactone is 1 mg/kg/day and the target maximum dose is 2 mg/kg/day. Male gynecomastia can occur with spironolactone requiring replacement with eplerenone. Monitoring of renal function and serum potassium is required when coadministered with ACEIs.
Beta-blockers
The benefits of beta-blocking agents in HF are well established in adults whereas published experience of their use in children with HF is much less robust and has not yet proven conclusive. According to the guidelines, it is reasonable to consider β-blockers in children with moderate to severe systolic dysfunction of the systemic ventricle, particularly if the systemic ventricle has an LV morphology.[28,29]
In children with HF and related conditions, carvedilol has been the most widely studied beta-blocker. Carvedilol is started at 0.05 mg/kg/dose (twice daily) and increased to 0.4–0.5 mg/kg/dose (twice daily) by doubling the dose every 2 weeks. In many small scale and retrospective studies, carvedilol was found to be effective in improving clinical and echocardiographic parameters and preventing transplantation.[30,31] However, a multicenter, randomized, double-blind, placebo-controlled study of 161 children and adolescents with symptomatic systolic HF did not show an improvement in their composite clinical status after 8 months of treatment with carvedilol compared to placebo.[32] These findings may have been due to an unexpected level of improvement in the placebo arm, inclusion of children with the systemic right ventricle and single ventricle physiology, the low dose of carvedilol used, and the study being underpowered.[33]
Metoprolol (0.1–0.2 mg/kg/dose twice daily and increased to 1 mg/kg/dose twice daily) or bisoprolol may be used as an alternative to carvedilol. Beta-blockers should not be administered in acute decompensated HF. Therapy should be started at a small dose and slowly up-titrated.
Inotropes
A number of inotropes have been used in acute HF; the most common among these are the catecholaminergic drugs such as dopamine and dobutamine, and the phosphodiesterase inhibitors such as milrinone and amrinone.
There are no controlled clinical trial data to guide the use of inotropic agents in the setting of acute HF in children. Even in adults a long-term survival benefit was not demonstrated by trials. However, many cases of acute cardiovascular collapse in childhood result from reversible disease states, including acute severe myocarditis and inotropes are a lifesaving temporary measure.
On the basis of a lack of any pediatric data and lack of data supporting improved outcomes in adults, the use of intermittent or chronic inotropic therapy for chronic HF, other than as a bridge to transplant, is not recommended.[28] Catecholaminergic drugs commonly used are dopamine 5–20 mcg/kg/min and dobutamine 5–20 mcg/kg/min. Epinephrine and norepinephrine are more commonly associated with arrhythmias and increased myocardial oxygen demand.
Milrinone, a phosphodiesterase inhibitor is an inotrope and vasodilator that has been shown to prevent low cardiac output syndrome after cardiac surgery in infants and children.[34] The loading dose of milrinone is 25–50 mcg/kg/min and maintenance dose is 0.25–1 mcg/kg/min. Milrinone might cause peripheral vasodilation and should be used with caution in hypotensive patients. Levosimendan is another inotrope with vasodilatory property by a calcium-sensitizing effect and opening up of vascular ATP-dependent K+ channels. Initial reports of the use of levosimendan in children are promising.[35,36]
Device therapy
Device therapy in HF predominantly includes pacemaker therapy, cardiac resynchronization therapy (CRT), and mechanical circulatory support. Permanent pacemaker implantation is recommended for advanced second- or third-degree atrioventricular block associated with ventricular dysfunction. Implantable cardioverter defibrillator (ICD) implantation is indicated for patients with a history of cardiac arrest or symptomatic sustained ventricular tachycardia in association with CHD.[37]
ICD is reasonable for patients with CHD with recurrent syncope in the presence of ventricular dysfunction or inducible ventricular arrhythmia at electrophysiological study. CRT can be useful for pediatric patients with a systemic LV with an EF < 35%, complete left bundle branch block pattern, QRS duration more than the upper limit of normal for age, NYHA Class II-IV on guideline-directed medical therapy.[28]
Extracorporeal membrane oxygenation (ECMO) has been widely used in the setting of cardiopulmonary arrest. For a child with isolated cardiac failure that is believed to be reversible, ECMO or a ventricular assist device (VAD) may be considered as a temporizing measure as a bridge to recovery of function. ECMO is also used in emergency perioperative salvage. Children with fulminant myocarditis with a high chance of recovery, do well when put on ECMO and VADs.[38,39] For those patients requiring a long-term mechanical support to bridge to cardiac transplantation, VADs are being used with increasing frequency. These devices can offer univentricular or biventricular circuit support.
Cardiac transplantation
Heart transplantation remains the therapy of choice for end-stage HF in children refractory to surgical and medical therapy. There were 9,566 pediatric heart transplants reported to the international society for heart and lung transplantation from 1982 to 2009.[40] The most common indication is the end-stage heart disease due to cardiomyopathies.
Other causes include CHDs such as hypoplastic heart syndrome and other complex CHD, single ventricle, and palliated heart disease. The data show an improved early (1 year) survival for all age groups in the more recent era of transplantation, averaging 80% in children and 90% in infants. Systemic viral infections such as cytomegalovirus, Epstein–Barr virus, and adenovirus, acute cellular rejection, allograft vasculopathy with graft failure, renal dysfunction, hypertension, and malignancy like lymphoproliferative disorders are the major complications in children.
Ringewald et al. reported a high rate of rejection in nonadherent adolescent pediatric heart transplant recipients, which led to a high rate of death in this population.[41] The advances in immunosuppression coupled with a better understanding of rejection have resulted in improved survival, quality of life, and fewer adverse effects after transplantation. However, heart transplantation can be a solution for only a minority of end-stage HF patients owing to the scarcity of donor hearts and expert centers.
CONCLUSION
The causes and clinical presentation of HF are different from adults. The overall outcome with HF is better in children than that in adults. There has been a significant advance in the evidence base for the management of HF in adults. While the general principles of management are similar to those in adults, there is a compelling need for larger and higher quality studies on the treatment of cardiac failure in children to provide a more robust evidence base.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–89. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Hsu DT, Pearson GD. Heart failure in children: Part I: History, etiology, and pathophysiology. Circ Heart Fail. 2009;2:63–70. doi: 10.1161/CIRCHEARTFAILURE.108.820217. [DOI] [PubMed] [Google Scholar]
- 3.Kay JD, Colan SD, Graham TP., Jr Congestive heart failure in pediatric patients. Am Heart J. 2001;142:923–8. doi: 10.1067/mhj.2001.119423. [DOI] [PubMed] [Google Scholar]
- 4.Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol. 2008;31:388–91. doi: 10.1002/clc.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossano JW, Kim JJ, Decker JA, Price JF, Zafar F, Graves DE, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: A population-based study. J Card Fail. 2012;18:459–70. doi: 10.1016/j.cardfail.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 7.Andrews RE, Fenton MJ, Ridout DA, Burch M. British Congenital Cardiac Association. New-onset heart failure due to heart muscle disease in childhood: A prospective study in the United Kingdom and Ireland. Circulation. 2008;117:79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 8.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi V, Saxena A. Heart failure in children: Clinical aspect and management. Indian J Pediatr. 2009;76:195–205. doi: 10.1007/s12098-009-0050-0. [DOI] [PubMed] [Google Scholar]
- 10.Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–5. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- 11.Satou GM, Lacro RV, Chung T, Gauvreau K, Jenkins KJ. Heart size on chest x-ray as a predictor of cardiac enlargement by echocardiography in children. Pediatr Cardiol. 2001;22:218–22. doi: 10.1007/s002460010207. [DOI] [PubMed] [Google Scholar]
- 12.Azevedo VM, Santos MA, Albanesi Filho FM, Castier MB, Tura BR, Amino JG. Outcome factors of idiopathic dilated cardiomyopathy in children – A long-term follow-up review. Cardiol Young. 2007;17:175–84. doi: 10.1017/S1047951107000170. [DOI] [PubMed] [Google Scholar]
- 13.Cianfrocca C, Pelliccia F, Nigri A, Critelli G. Resting and ambulatory ECG predictors of mode of death in dilated cardiomyopathy. J Electrocardiol. 1992;25:295–303. doi: 10.1016/0022-0736(92)90035-x. [DOI] [PubMed] [Google Scholar]
- 14.Van der Hauwaert LG, Denef B, Dumoulin M. Long-term echocardiographic assessment of dilated cardiomyopathy in children. Am J Cardiol. 1983;52:1066–71. doi: 10.1016/0002-9149(83)90534-9. [DOI] [PubMed] [Google Scholar]
- 15.Hammerer-Lercher A, Geiger R, Mair J, Url C, Tulzer G, Lechner E, et al. Utility of N-terminal pro-B-type natriuretic peptide to differentiate cardiac diseases from noncardiac diseases in young pediatric patients. Clin Chem. 2006;52:1415–9. doi: 10.1373/clinchem.2005.060608. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande SR. B-type natriuretic peptide at presentation of dilated cardiomyopathy in children predicts outcome. Congenit Heart Dis. 2011;6:539–40. [Google Scholar]
- 17.Law YM, Keller BB, Feingold BM, Boyle GJ. Usefulness of plasma B-type natriuretic peptide to identify ventricular dysfunction in pediatric and adult patients with congenital heart disease. Am J Cardiol. 2005;95:474–8. doi: 10.1016/j.amjcard.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Hongkan W, Soongswang J, Veerakul G, Sanpakit K, Punlee K, Rochanasiri W, et al. N-terminal pro brain natriuretic peptide and cardiac function in doxorubicin administered pediatric patients. J Med Assoc Thai. 2009;92:1450–7. [PubMed] [Google Scholar]
- 19.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi Med J. 2005;26:1197–202. [PubMed] [Google Scholar]
- 20.Kindel SJ, Miller EM, Gupta R, Cripe LH, Hinton RB, Spicer RL, et al. Pediatric cardiomyopathy: Importance of genetic and metabolic evaluation. J Card Fail. 2012;18:396–403. doi: 10.1016/j.cardfail.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: Predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–15. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 23.Ratnapalan S, Griffiths K, Costei AM, Benson L, Koren G. Digoxin-carvedilol interactions in children. J Pediatr. 2003;142:572–4. doi: 10.1067/mpd.2003.160. [DOI] [PubMed] [Google Scholar]
- 24.Lewis AB, Chabot M. The effect of treatment with angiotensin-converting enzyme inhibitors on survival of pediatric patients with dilated cardiomyopathy. Pediatr Cardiol. 1993;14:9–12. doi: 10.1007/BF00794837. [DOI] [PubMed] [Google Scholar]
- 25.Leversha AM, Wilson NJ, Clarkson PM, Calder AL, Ramage MC, Neutze JM. Efficacy and dosage of enalapril in congenital and acquired heart disease. Arch Dis Child. 1994;70:35–9. doi: 10.1136/adc.70.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw NJ, Wilson N, Dickinson DF. Captopril in heart failure secondary to a left to right shunt. Arch Dis Child. 1988;63:360–3. doi: 10.1136/adc.63.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frenneaux M, Stewart RA, Newman CM, Hallidie-Smith KA. Enalapril for severe heart failure in infancy. Arch Dis Child. 1989;64:219–23. doi: 10.1136/adc.64.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirk R, Dipchand AI, Rosenthal DN, Addonizio L, Burch M, Chrisant M, et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary.[Corrected] J Heart Lung Transplant. 2014;33:888–909. doi: 10.1016/j.healun.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Kantor PF, Lougheed J, Dancea A, McGillion M, Barbosa N, Chan C, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29:1535–52. doi: 10.1016/j.cjca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Blume ED, Canter CE, Spicer R, Gauvreau K, Colan S, Jenkins KJ. Prospective single-arm protocol of carvedilol in children with ventricular dysfunction. Pediatr Cardiol. 2006;27:336–42. doi: 10.1007/s00246-005-1159-1. [DOI] [PubMed] [Google Scholar]
- 31.Azeka E, Franchini Ramires JA, Valler C, Alcides Bocchi E. Delisting of infants and children from the heart transplantation waiting list after carvedilol treatment. J Am Coll Cardiol. 2002;40:2034–8. doi: 10.1016/s0735-1097(02)02570-6. [DOI] [PubMed] [Google Scholar]
- 32.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA. 2007;298:1171–9. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 33.Foerster SR, Canter CE. Pediatric heart failure therapy with beta-adrenoceptor antagonists. Paediatr Drugs. 2008;10:125–34. doi: 10.2165/00148581-200810020-00007. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 35.Egan JR, Clarke AJ, Williams S, Cole AD, Ayer J, Jacobe S, et al. Levosimendan for low cardiac output: A pediatric experience. J Intensive Care Med. 2006;21:183–7. doi: 10.1177/0885066606287039. [DOI] [PubMed] [Google Scholar]
- 36.Namachivayam P, Crossland DS, Butt WW, Shekerdemian LS. Early experience with Levosimendan in children with ventricular dysfunction. Pediatr Crit Care Med. 2006;7:445–8. doi: 10.1097/01.PCC.0000235251.14491.75. [DOI] [PubMed] [Google Scholar]
- 37.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 38.Duncan BW, Bohn DJ, Atz AM, French JW, Laussen PC, Wessel DL. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122:440–8. doi: 10.1067/mtc.2001.115243. [DOI] [PubMed] [Google Scholar]
- 39.Stiller B, Dähnert I, Weng YG, Hennig E, Hetzer R, Lange PE. Children may survive severe myocarditis with prolonged use of biventricular assist devices. Heart. 1999;82:237–40. doi: 10.1136/hrt.82.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirk R, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirteenth official pediatric heart transplantation report – 2010. J Heart Lung Transplant. 2010;29:1119–28. doi: 10.1016/j.healun.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Ringewald JM, Gidding SS, Crawford SE, Backer CL, Mavroudis C, Pahl E. Nonadherence is associated with late rejection in pediatric heart transplant recipients. J Pediatr. 2001;139:75–8. doi: 10.1067/mpd.2001.115067. [DOI] [PubMed] [Google Scholar]


