Abstract
Polycythemia vera (PV) and essential thrombocythemia (ET) are the two most common myeloproliferative neoplasms. The same JAK2V617F mutation can be found in both disorders and is able to recapitulate many of the phenotypic abnormalities of these diseases in the murine models. The disease phenotype is also influenced by other unknown genetic or epigenetic factors. MicroRNAs (miRNA) are 18–24 nucleotides single-stranded non-protein-coding RNAs that function primarily as gene repressors by binding to their target messenger RNAs. We performed miRNA expression profiling by oligonucleotide microarray analysis in purified peripheral blood CD34+ cells from eight JAK2V617F-positive PV patients and six healthy donors. A quantitative reverse-transcription polymerase chain reaction assay was used to verify differential miRNA expression. Since erythrocytosis is the only feature that distinguishes PV from ET, we also compared specific miRNA expression in the nucleated erythroid cells directly descended from the early erythroid progenitor cells of PV and ET patients. Our data indicate that significant miRNA deregulation occurs in PV CD34+ cells and confirm a genetic basis for the gender-specific differences that characterize PV with respect to miRNA. The results of our study also suggest that deregulated miRNAs may represent an important mechanism by which the PV erythrocytosis and ET thrombocytosis phenotypes are determined.
Keywords: MicroRNA, Myeloproliferative neoplasm, Polycythemia vera, Essential thrombocythemia, Hematopoiesis
Introduction
The chronic myeloproliferative neoplasms (MPN), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal stem cell disorders characterized by deregulated stem cell expansion and production of red cells, white cells and platelets alone or in combination. The discovery of a somatic mutation (V617F) in the tyrosine kinase, Janus Kinase 2 (JAK2) [1–5], was the most important advance in MPN since the discoveries 30 years ago that hematopoiesis in these disorders was both autonomous and clonal [6–8]. Although the murine models have provided unequivocal evidence that JAK2V617F is able to cause MPNs, there is significant heterogeneity in disease phenotypes between different murine lines and even within the same line, suggesting that disease phenotype is affected by other unknown genetic or epigenetic factors [2,9–12]. In particular, PV is characterized by raised red cell mass and sometimes increased platelet and white cell counts, while ET is defined by an elevated platelet count alone and a normal red cell mass. As the erythroid and megakaryocytic lineages are closely associated during differentiation and are generated from a common progenitor cell, additional mechanisms must exist to regulate erythropoiesis and megakaryopoiesis differently between JAK2V617F-positive PV and JAK2V617F-positive ET patients [13] and further characterization of these molecular differences in PV and ET should lead to a better understanding of the development of these diseases, their clinical manifestations, and their treatment.
MicroRNAs (miRNA) are 18–24 nucleotides single-stranded non-protein-coding RNAs that function primarily as gene repressors by binding to the target messenger RNAs to regulate gene expression [14–16]. There is growing evidence that miRNAs regulate hematopoiesis in both hematopoietic stem cells and committed progenitor cells [17–25]. Deregulated miRNA profiles have been reported in MPN patients during in vitro erythroid differentiation as well as in peripheral blood granulocytes, platelets, and reticulocytes [26,27]. Since the MPNs are stem cell disorders, comprehensive miRNA analysis in MPN stem cells is needed to further delineate their roles in MPN pathogenesis but so far there is only limited data in this area [26,28–30].
We performed miRNA expression profiling by microarray analysis in purified peripheral blood CD34+ cells from six healthy donors and eight JAK2V617F-positive PV patients. Since erythrocytosis is the only feature that distinguishes PV from ET, we also compared specific miRNA expression in the nucleated erythroid cells directly descended from the burst-forming unit erythroid (BFU-E) progenitor cells of PV and ET patients. Our data indicate that significant miRNA deregulation occurs in the CD34+ cells of PV patients and confirm a genetic basis for the gender-specific differences that characterize PV [31–34]. The results of our study also suggest that deregulated miRNAs may represent an important mechanism by which the PV erythrocytosis and ET thrombocytosis phenotypes are determined.
Materials and methods
Patients
Thirteen PV patients and 4 ET patients from the Johns Hopkins University Hospital and the James J. Peters VA Medical Center Hematology clinics were studied. The diagnosis of PV and ET was based on the Polycythemia Vera Study Group Criteria [35]. All patients gave written consent for venipuncture and allowed their clinical and laboratory data (e.g. cell counts, family history, chemotherapy, and spleen size) to be recorded in a dataset for later analysis. Splenomegaly was considered present if the spleen was palpable by physical exam. The study protocol was approved by the Institutional Review Boards of both institutions.
Mononuclear cell (MNC) and CD34+ cell isolation
Peripheral blood MNCs were isolated using Ficoll-Paque (Sigma, St Louis, MO) gradient centrifugation. Peripheral blood CD34+ cells were isolated from the Ficoll buffy coats using immunomagnetic beads (Miltenyi, Auburn, CA). CD34+ cells were analyzed for expression of CD34, CD38, CD117, and CD41 using commercially available labeled antibodies. Fluorescence of at least 10,000 cells was measured on a FACS Caliber and analyzed with CELLQuest and Paint-a-gate software (BD Biosciences, San Jose, CA, USA). Similar to other published reports of the CD34+ cell phenotype in the MPN, the peripheral blood CD34+ cells were >95% positive both for CD34 and CD38, were dimly positive for CD117, were CD41 negative, and were primarily in G0/G1 of the cell cycle [31]. Peripheral blood CD34+ cells from healthy controls were purchased from AllCells™.
JAK2V617F determination
The CD34+ cell JAK2V617F allele burdens were measured using an allele-specific, quantitative real-time polymerase chain reaction assay sensitive to 10% of either the wild-type or mutant JAK2 allele as previously described [31].
Methocult colony formation assay
Peripheral blood MNCs (3 × 105 cells) was cultured using Methocult semisolid media with growth factors (StemCell Technologies, Vancouver, Canada) according to manufacturer's recommendations and incubated in a humidified environment at 37 °C in the presence of 5% carbon dioxide for 14 days. Burst Forming Unit-Erythroid (BFU-E) colonies, identified by visual inspection, were plucked. Up to 30 colonies were pooled for total RNA extraction for specific miRNA expression analysis.
Microarray analysis
Total RNA from the peripheral blood CD34+ cells of healthy controls and PV patients was prepared using the miRNeasy Mini Kit (Qiagen) with on-column DNase I digestion. All microarray samples were processed at the Sidney Kimmel Comprehensive Cancer Center Microarray Core Facility at Johns Hopkins University, Baltimore, USA. RNA was tested for integrity using the Bioanalyzer 2100 (Agilent, Santa Clara, CA) and RNA integrity (RIN) scores above 8.0 were present in all samples. Human miRNA Microarray Version 3 Kit (Agilent Technologies, Santa Clara, CA),which contains probes for 866 human miRNAs from the Sanger database v12.0, was used for microarray assays to examine the global miRNA expression. Briefly, 150 ng total RNA from each sample was labeled using the miRNA Labeling Reagent & Hybridization Kit (Agilent Technologies) as described in the instruction manual. All arrays were hybridized at 55 °C for 20 hours followed by wash procedure according to the miRNA Microarray System Protocol, Version 1.5, December 2007 (Agilent Technologies). Fluorescent signals were obtained by scanning with an Agilent scanner controlled by Agilent Scan Control 7.0 software. Data were acquired with Agilent Feature Extraction 9.5.3.1 software for miRNA microarray. Data analysis was performed using GeneSpring GX 11 software. The Student's unpaired t Test and multiple comparison adjustment were used to select differentially expressed miRNAs between sample groups with a p value<0.05 considered statistically significant. The statistical analysis was performed independently by W.Y. and A.W.
Quantitative real-time polymerase chain reaction
The TaqMan MicroRNA Assay (Applied Biosystems, Foster City, CA) was used for quantitative real-time polymerase chain reaction (qRT-PCR) to verify differential miRNA expression on an ABI ViiA™ 7 Real-Time PCR machine (Applied Biosystems). The miRNA expression levels was normalized to RNU6B (Applied Biosystems) and relative fold changes of gene expression was calculated by the 2ΔΔCT method. All assays were performed in triplicate. The Student's unpaired t test was used to test the significance of differences using a p value≤0.05 as the threshold for significance.
Results
Distinct miRNA expression in PV patients' peripheral blood CD34+ cells in comparison to healthy controls
Eight JAK2V617F-positive PV patients (four females and four males, median CD34+ cell JAK2V617F allele burden = 70%, range 16–100%) and six healthy controls (three females and three males) were included in the miRNA expression profiling by microarray analysis. Table 1 shows the clinical and laboratory features of the eight JAK2V617F-positive PV patients. Overall, in the eight PV patients as a group, there was differential regulation of 71 miRNAs compared to healthy controls (p<0.05) (36 up-regulated and 35 down-regulated) (Table 2). All the 71 deregulated miRNAs were concordantly up or down-regulated in both the male and female patients. There was no correlation between the miRNA microarray expression and the CD34+ cell JAK2V617F allele burden (not shown).
Table 1.
Clinical and laboratory characteristics of the eight polycythemia vera patients in microRNA microarray expression analysis.
| PV | |
|---|---|
| N | 8 |
| % Female | 50% |
| % Antecedent MPNa | 25% |
| Disease duration (years), median (range) | 5.5 (1–12) |
| Age (years) at diagnosis, median (range) | |
| Female | 56 (30–59) |
| Male | 66 (60–68) |
| % with palpable splenomegaly | 50% |
| Therapy at the time of sample collection | |
| Phlebotomy, n (%) | 7 (88) |
| Aspirin, n (%) | 4 (50) |
| Hydroxyurea, n (%) | 4 (50) |
| Mean CD34 + cell JAK2V617F allele burden (%) | |
| Female (range) | 55 (16–100) |
| Male (range) | 80 (68–100) |
PV = Polycythemia vera, MPN = Myeloproliferative neoplasm.
Two patients (one female and one male) had a prior history of ET.
Table 2.
Microarray analysis identified 71 significantly deregulated microRNAs in polycythemia vera patients' peripheral blood CD34+ cells.
| Up-regulated miRNAs in PV |
Fold change (patient/ normal) |
p Value |
Down-regulated miRNAs in PV |
Fold change (patient/ normal) |
p Value |
|---|---|---|---|---|---|
| hsa-miR-575 | 7.69 | 0.0001 | hsa-miR-769-5p | 0.51 | 0.0003 |
| hsa-miR-887 | 3.70 | 0.0003 | hsa-miR-551b | 0.21 | 0.0014 |
| hsa-miR-630 | 10.00 | 0.0003 | hsa-miR-181c* | 0.29 | 0.0023 |
| hsa-miR-370 | 3.23 | 0.0006 | hsa-miR-128 | 0.67 | 0.0036 |
| hsa-miR-1207-5p | 2.78 | 0.0013 | hsa-miR-181d | 0.37 | 0.0038 |
| hsa-miR-135a* | 2.63 | 0.0014 | hsa-miR-874 | 0.62 | 0.0042 |
| hsa-miR-1246 | 2.94 | 0.0020 | hsa-miR-648 | 0.37 | 0.0043 |
| hsa-miR-198 | 3.57 | 0.0024 | hsa-miR-342-5p | 0.27 | 0.0046 |
| hsa-miR-486-5p | 5.88 | 0.0030 | hsa-miR-196b | 0.19 | 0.0058 |
| hsa-miR-134 | 2.17 | 0.0032 | hsa-miR-181c | 0.33 | 0.0071 |
| hsa-miR-188-5p | 2.33 | 0.0034 | hsa-miR-181a-2* | 0.44 | 0.0074 |
| hsa-miR-1268 | 2.08 | 0.0034 | hsa-miR-223* | 0.53 | 0.0075 |
| hsa-miR-298 | 2.44 | 0.0045 | hsa-miR-361-5p | 0.70 | 0.0136 |
| hsa-miR-422a | 1.79 | 0.0055 | hsa-miR-361-3p | 0.52 | 0.0148 |
| hsa-miR-516a-5p | 2.27 | 0.0058 | hsa-miR-10b | 0.32 | 0.0152 |
| hsa-miR-1225-5p | 2.33 | 0.0068 | hsa-miR-512-3p | 0.70 | 0.0167 |
| hsa-miR-490-5p | 2.17 | 0.0079 | hsa-miR-222 | 0.54 | 0.0170 |
| hsa-miR-451 | 10.99 | 0.0081 | hsa-miR-25 | 0.68 | 0.0229 |
| kshv-miR-K12-3 | 2.50 | 0.0091 | hsa-miR-181a | 0.51 | 0.0267 |
| hcmv-miR-US33-5p | 1.92 | 0.0092 | hsa-miR-454 | 0.51 | 0.0282 |
| hsa-miR-145 | 2.63 | 0.0117 | hsa-miR-133b | 0.31 | 0.0314 |
| hsv1-miR-H1 | 2.63 | 0.0129 | hsa-miR-150 | 0.20 | 0.0315 |
| hsa-miR-654-5p | 2.56 | 0.0153 | hsa-let-7f-1* | 0.54 | 0.0324 |
| ebv-miR-BART7 | 1.96 | 0.0160 | hsa-miR-374b | 0.69 | 0.0346 |
| hsa-miR-659 | 2.63 | 0.0170 | hsa-miR-19b-1* | 0.50 | 0.0346 |
| hsa-miR-483-5p | 2.78 | 0.0258 | hsa-miR-221* | 0.55 | 0.0351 |
| hiv1-miR-H1 | 1.59 | 0.0261 | hsa-miR-489 | 0.43 | 0.0377 |
| hsa-miR-520b | 1.54 | 0.0275 | hsa-miR-935 | 0.42 | 0.0383 |
| hsa-miR-125a-3p | 2.22 | 0.0283 | hsa-miR-363 | 0.45 | 0.0393 |
| hsa-miR-1182 | 2.22 | 0.0284 | hsa-let-7 g | 0.68 | 0.0412 |
| kshv-miR-K12-10b | 2.44 | 0.0350 | hsa-miR-149 | 0.63 | 0.0421 |
| hsa-miR-490-3p | 2.78 | 0.0387 | hsa-miR-30e* | 0.63 | 0.0428 |
| hsa-miR-1290 | 2.08 | 0.0457 | hsa-miR-501-3p | 0.39 | 0.0475 |
| hsa-miR-1471 | 2.44 | 0.0462 | hsa-miR-770-5p | 0.55 | 0.0485 |
| hsa-miR-425 | 1.28 | 0.0463 | hsa-miR-614 | 0.47 | 0.0499 |
| hsa-miR-409-3p | 4.55 | 0.0494 |
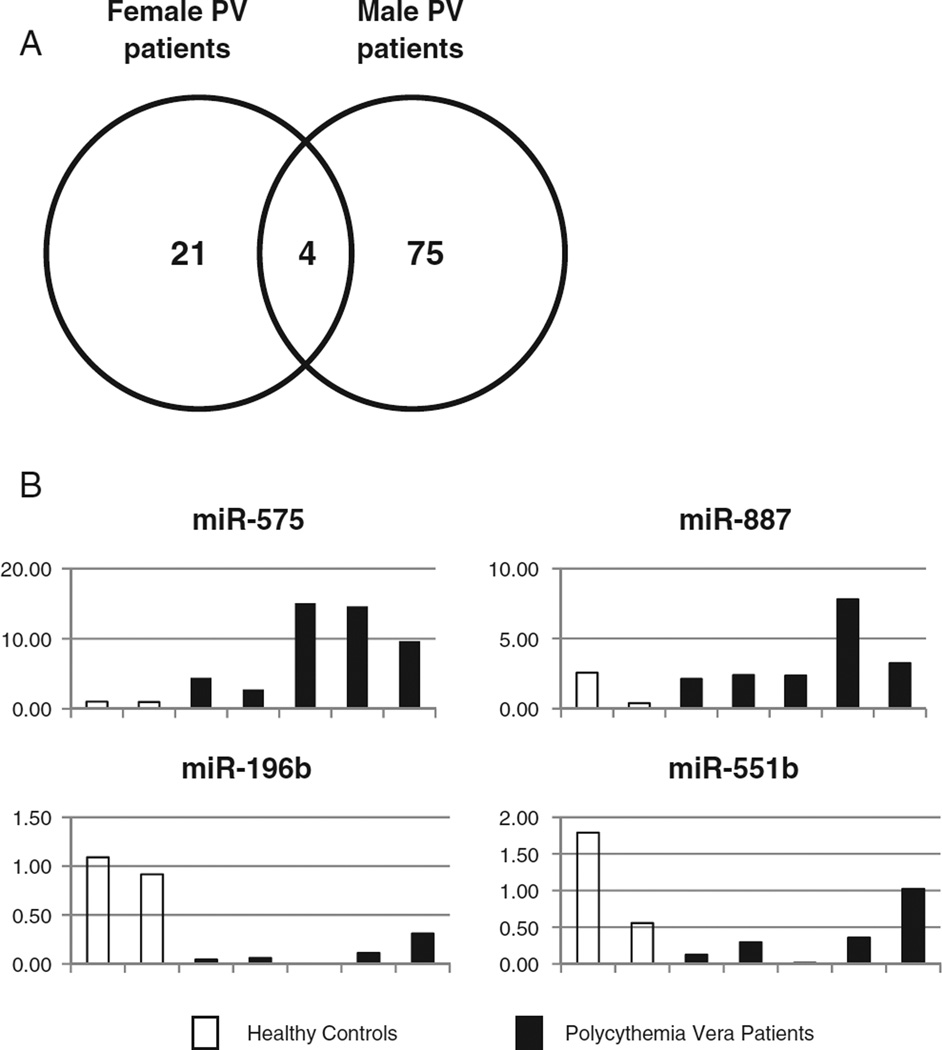
Given the phenotypic and genotypic differences between male and female PV patients [31–34], we analyzed differential miRNA expression separately by gender to identify gender-specific effects. Consistent with their different phenotypes, 79 miRNAs were differently regulated in the male PV patients (42 up and 37 down-regulated, p<0.05) compared to healthy male controls, while only 25 miRNAs were differently regulated in the female PV patients (9 up and 16 down-regulated, p<0.05) compared to healthy female controls. Only four miRNAs (miR-575 and miR-887, up; miR-196b and miR-551b, down) were significantly deregulated in both the male and female patients (p<0.05) suggesting that they may have a key role in disease pathogenesis (Fig. 1A). In a second group of five JAK2V617F-positive PV patients and two normal donors, quantitative RT-PCR confirmed the up-regulation of miR-575 (mean 9.3-fold) and miR-887 (mean 2.4-fold) and down-regulation of miR-196b (mean 9.1-fold) andmiR-551b (mean 3.3-fold) in PV patients (Fig. 1B). Using the PicTar, TargetScan, MicroCosm, and miRDB algorithms, we predicted the potential targets of these four core miRNAs (Table 3).
Fig. 1.
MicroRNA deregulation in both male and female polycythemia vera patients. (A) The Venn diagram shows the gender-specific differential miRNA expression in polycythemia vera (PV) patients. 79 miRNAs were differently regulated in the male PV patients compared to healthy male donors, while only 25 miRNAs were differently regulated in the female PV patients compared to healthy female donors. Only four miRNAs were significantly up- (miR-575 and miR-887) or down- (miR-196b and miR-551b) regulated in both the male and female patients (p<0.05). (B) Quantitative real-time polymerase chain reaction of miRNA-575, miR-887, miR-196b and miR-551b expression in healthy control (n = 2) and PV patient (n = 5) peripheral blood CD34+ cells. The individual sample miRNA expression was shown as the fold-change compared to the average healthy control expression which was set as “1”.
Table 3.
Putative target genes of the core deregulated miRNAs predicted using PicTar, TargetScan, MicroCosm, and miRDB prediction algorithms.
| miRNA ID | Chromosomal location |
Deregulation in PV CD34+ cells |
Putative targetsa |
|---|---|---|---|
| hsa-miR-575 | 4q21.22 | Up |
SFRS2, SFRS1, EPOR, HMGA2, TFPI |
| hsa-miR-887 | 5p15.1 | Up | GSK3A, BIM |
| hsa-miR-196b | 7p15.2 | Down |
HOXA5, HOXA7, HOXA9, HOXA10, HOXB6, HOXB7, HOXC8, HMGA2, ERG |
| hsa-miR-551b | 3q26.2 | Down | ERBB4 |
The gene names are available at http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene.
A unique miRNA expression signature for increased erythropoiesis in PV patients
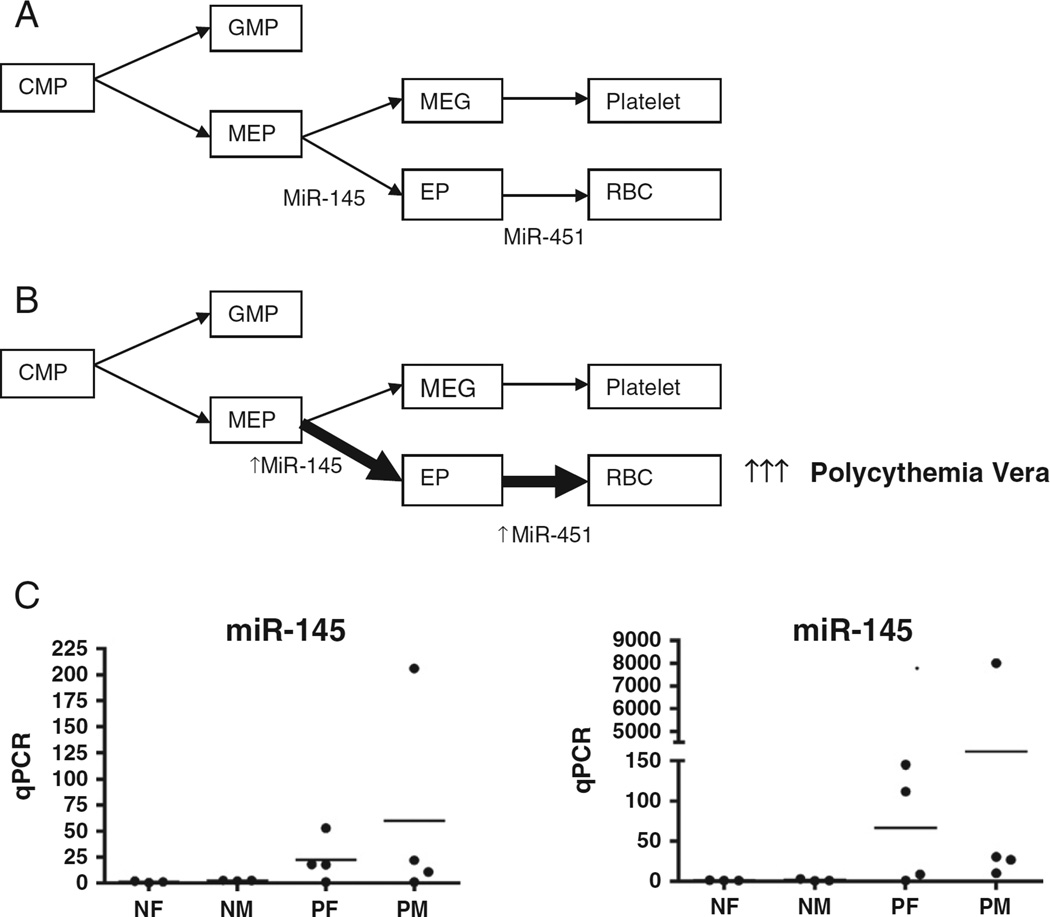
We looked for miRNAs that can possibly contribute to increased erythroid proliferation and differentiation, which is the principal feature of PV. As previously reported, miR-145 promotes erythrocyte differentiation of the megakaryocyte-erythroid progenitor (MEP) cell, the common progenitor of the erythroid and megakaryocytic lineages [36,37]. MiR-451 is an erythroid-specific miRNA that is significantly up-regulated during erythroid differentiation [26,38–40] (Fig. 2A). Among the differentially regulated miRNAs in our eight PV patient microarray analysis, there was up-regulation of miR-451 and miR-145 in PV patients compared to healthy controls, consistent with their roles in increased erythropoiesis (Fig. 2B). Quantitative RT-PCR confirmed the up-regulation of miR-451 (~900-fold, range 1–8003) and miR-145 (23-fold, range 1–206), although these changes were not statistically significant likely due to the small sample size and large sample-to-sample variation (Fig. 2C). A similar result was obtained in the second group of five JAK2V617F-positive PV patients (not shown).
Fig. 2.
A unique microRNA expression signature for increased erythropoiesis in polycythemia vera (PV). (A) In normal hematopoiesis, the developmental fate of MEP is regulated in part by miR-145, while miR-451 is associated with erythroid differentiation. (B) In microRNA microarray analysis of PV patients' peripheral blood CD34+ cells, we observed up-regulation of miR-145 and miR-451, which will promote increased erythropoiesis. (C) Point plot illustration of peripheral blood CD34+ cell expression of miRNA-145 and miRNA-451 in healthy controls (n = 6) and PV patients (n = 8) by quantitative real-time polymerase chain reaction. The individual sample miRNA expression is shown as the fold-change compared to the average normal female expression which was set as “1”. CMP, common myeloid progenitor; GMP, granulocyte macrophage progenitor; MEP, megakaryocyte-erythrocyte progenitor; EP, erythroid precursor; MEG, megakaryocyte precursor; RBC, red blood cell; NF, healthy female control; NM, healthy male control; PF, PV female patient; PM, PV male patient.
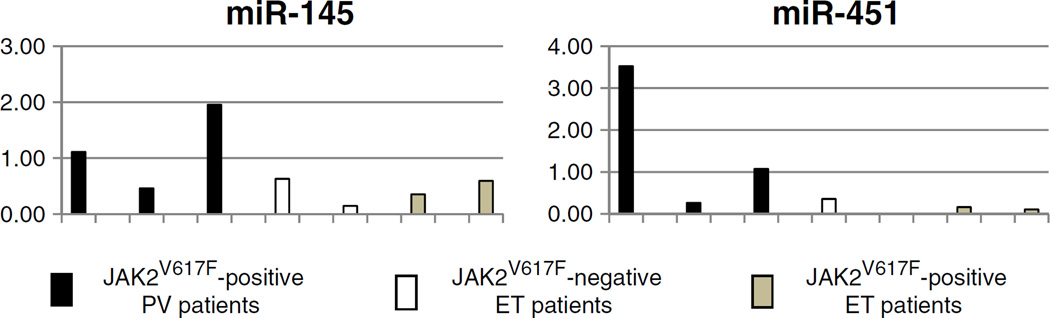
Since erythrocytosis is the only feature that distinguishes PV from ET, we then compared miR-145 and miR-451 expression in the nucleated erythroid cells directly descended from the BFU-E progenitor cells of three JAK2V617F-positive PV, two JAK2V617F-positive ET, and two JAK2V617F-negative ET patients. Briefly, patient peripheral blood MNCs were cultured using Methocult semisolid media with growth factors (Stem Cell Technologies, Vancouver, Canada) for 14 days and BFU-E colonies were plucked and pooled for total RNA extraction. Quantitative RT-PCR confirmed the up-regulation of miR-145 (mean 1.8-fold) and miR-451 (mean 10.6-fold) in PV BFU-E colony cells than in ET (both JAK2V617F-positive and JAK2V617F-negative) BFU-E colony cells (Fig. 3). These findings suggest that up-regulation of both miR-451 and miR-145 might be a potential “erythroid miRNA signature” of the PV phenotype.
Fig. 3.
Quantitative real-time polymerase chain reaction of miRNA-145 and miRNA-451 expression in burst forming unit-erythroid (BFU-e) colony cells derived from polycythemia vera (PV) and essential thrombocythemia (ET) patient peripheral blood mononuclear cells. The individual sample miRNA expression of 3 JAK2V617F(+) PV patients, 2 JAK2V617F(−) ET patients and 2 JAK2V617F(+) ET patients are shown here as the fold-change compared to the average PV BFU-e cell expression which was set as “1”.
Discussion
We report for the first time a unique CD34+ cell miRNA expression signature in JAK2V617F-positive PV patients compared to healthy controls. Consistent with the phenotypic and genotypic differences between male and female PV patients [31–34], there are different miRNA deregulations between male and female patients (Fig. 1). 79 miRNAs were differently regulated in the male PV patients compared to healthy male donors, while only 25 miRNAs were differently regulated in the female PV patients compared to healthy female donors. Only four miRNAs (“core miRNAs”) were significantly up- (miR-575 and miR-887) or down-regulated (miR-196b andmiR-551b) by both the male and female patients and they may have a key role in disease pathogenesis.
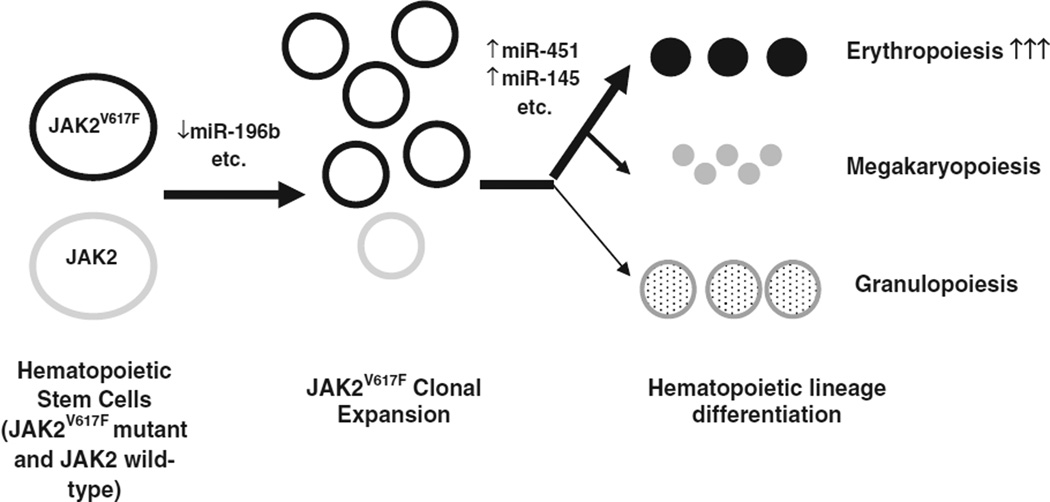
Using the PicTar, TargetScan, MicroCosm, and miRDB algorithms, we predicted the potential targets of these four core miRNAs (Table 3). MiR196b plays an important role in the control of hematopoietic stem cells (HSC) by regulating HSC self-renewal, proliferation and differentiation. MiR-196b is nearly undetectable in long-term HSCs (LT-HSCs) and is up-regulated during the transition from LT-HSCs to short-term HSCs (ST-HSCs); it is most abundant in ST-HSCs and is down-regulated in more differentiated cells. Overexpression of miR-196b causes both a differentiation block and a reduced HSC capacity in a bone marrow reconstitution assay [19,41,42]. The stem cell compartment in MPN is heterogeneous with the presence of both JAK2 wild-type clones and JAK2V617F mutant clones in most MPN patients. In PV, there is usually a gradual increase in the JAK2V617F mutant stem/progenitor cell population over time with a decrease in the normal stem cell population [32,43]. The effects of miR-196b down-regulation in JAK2V617F-positive clonal expansion and increased erythroid proliferation in PV are worth further exploration (Fig. 4). Potential targets of miR-575 are the serine/arginine-rich splicing factors SFRS2 (also known as SRSF2) and SFRS1 (also known as SRSF1), which are members of pre-mRNA splicing factors and constitute part of the spliceosome. An SRSF2 mutation is present in ~12% of patients with myelodysplastic syndromes [44–46] and frequently mutated in MDS/MPN patients (24%), particularly in CMML (28%) [47]. Although not common in the classic MPNs, SRSF2 mutations are more common in acute myeloid leukemia transformed from MPNs (18.9%) compared with leukemia transformation after myelodysplasia (4.8%) or de novo AML (5.6%) [48].
Fig. 4.
Hypothesis: deregulated miRNAs may contribute to JAK2V617F-positive clone expansion and erythroid lineage commitment in polycythemia vera.
As previously reported, miR-145 promotes erythrocyte differentiation of the megakaryocyte-erythroid progenitor cell while miR-451 is an erythroid-specific miRNA that is significantly up-regulated during erythroid differentiation [26,36–40]. Up-regulation of miR-451 and miR-145 was previously reported during in vitro erythroid differentiation from PV patient peripheral blood mononuclear cells and in PV patient peripheral blood mononuclear cells, respectively [26,27]. Enforced expression of miR-451 promoted erythroid differentiation of the K562 cells [49]. Our study shows for the first time that there is indeed up-regulation of miR-451 and miR-145 in PV CD34+ cells. This is consistent with a role for these miRNAs in erythropoiesis, which is the principal feature of PV. This is not surprising as PV CD34+ cells have been shown to be more lineage-committed than the normal CD34+ cells [50,51]. Our data provide the first microRNA basis for this lineage preference of PV hematopoietic stem/progenitor cells. We show further that in the nucleated erythroid cells of the BFU-E colonies there appears to be greater miR-145 and miR-451 expression in PV than in ET (both JAK2V617F-positive and JAK2V617F-negative) although our sample size is too small for statistical significance (Fig. 3). This suggests that an erythroid miRNA signature (i.e. up-regulation of miR-451 and miR-145) in PV may serve in part as the mechanism for promoting the differential phenotypes between PV and ET (Fig. 4). Further verification with larger sample size is needed.
Acknowledgments
Huichun Zhan is currently supported by the Veterans Affairs Career Development Award (grant 10959632) and Veterans Affairs Rehabilitation Research and Development Service grant B9212-C. We would like to thank Drs. Katia Basso, David Dominguez, and Riccardo Dalla-Favera from Columbia University for their assistance in microRNA analysis. We thank Drs. Min Lu and Ronald Hoffman from Mount Sinai School of Medicine for their assistance in the colony formation assay. We thank Drs. Yan Makeyev and Imitiaz Patel for providing precious patient samples. We thank Hai Xu from Johns Hopkins University for his assistance in microRNA microarray experiments. We thank Drs. Azra Raza and Naomi Galili from Columbia University Medical Center, Drs. Erik Langhoff, Mary Sano, and Elisa Sanchez-Valencia from James J. Peters VA Medical Center, and Dr. Robert Brodsky from Johns Hopkins University for their continuing support throughout this work.
Footnotes
Author contribution
HZ, CVD, and JLS designed the research study. HZ performed the research. HZ, CC, WY and AW analyzed the data. ARM provided CD34+ cell JAK2V617F allele burden data. HZ wrote the paper. JLS, CC, and CVD helped edit the paper. All authors approved the submitted version of the manuscript.
References
- 1.Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myelo-proliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J. Biol. Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamson JW, et al. Polycythemia vera: stem-cell and probable clonal origin of the disease. N. Engl. J. Med. 1976;295:913–916. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson RJ, Salo A, Fialkow PJ. Agnogenicmyeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosis. Blood. 1978;51:189–194. [PubMed] [Google Scholar]
- 8.Fialkow PJ, et al. Evidence that essential thrombocythemia is a clonal disorder with origin in a multipotent stem cell. Blood. 1981;58:916–919. [PubMed] [Google Scholar]
- 9.Tiedt R, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 10.Xing S, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernig G, et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacout C, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 13.Chen E, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–535. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambros A. The evolution of our thinking about microRNAs. Nat. Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 15.Ambros V, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 17.Guo S, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ooi AG, et al. MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21505–21510. doi: 10.1073/pnas.1016218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell RM, et al. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14235–14240. doi: 10.1073/pnas.1009798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, et al. MicroRNA-mediated control of cell fate in megakaryocyte–erythrocyte progenitors. Dev. Cell. 2008;14:843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar MS, et al. Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q- syndrome. Blood. 2011;118(17):4666–4673. doi: 10.1182/blood-2010-12-324715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelosi E, Labbaye C, Testa U. MicroRNAs in normal and malignant myelopoiesis. Leuk. Res. 2009;33:1584–1593. doi: 10.1016/j.leukres.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Edelstein LC, Bray PF. MicroRNAs in platelet production and activation. Blood. 2011;117:5289–5296. doi: 10.1182/blood-2011-01-292011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connell RM, Zhao JL, Rao DS. MicroRNA function in myeloid biology. Blood. 2011;118:2960–2969. doi: 10.1182/blood-2011-03-291971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byon JC, Papayannopoulou T. MicroRNAs: allies or foes in erythropoiesis? J. Cell. Physiol. 2012;227:7–13. doi: 10.1002/jcp.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruchova H, et al. Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp. Hematol. 2007;35:1657–1667. doi: 10.1016/j.exphem.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruchova H, Merkerova M, Prchal JT. Aberrant expression of microRNA in polycythemia vera. Haematologica. 2008;93:1009–1016. doi: 10.3324/haematol.12706. [DOI] [PubMed] [Google Scholar]
- 28.Guglielmelli P, et al. MicroRNA expression profile in granulocytes from primary myelofibrosis patients. Exp. Hematol. 2007;35:1708–1718. doi: 10.1016/j.exphem.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Guglielmelli P, et al. Overexpression of microRNA-16-2 contributes to the abnormal erythropoiesis in polycythemia vera. Blood. 2011;117:6923–6927. doi: 10.1182/blood-2010-09-306506. [DOI] [PubMed] [Google Scholar]
- 30.Lin X, et al. miR-433 is aberrantly expressed in myeloproliferative neoplasms and suppresses hematopoietic cell growth and differentiation. Leukemia. 2012 doi: 10.1038/leu.2012.224. [DOI] [PubMed] [Google Scholar]
- 31.Moliterno AR, et al. Phenotypic variability within the JAK2 V617F-positive MPD: roles of progenitor cell and neutrophil allele burdens. Exp. Hematol. 2008;36:1480–1486. doi: 10.1016/j.exphem.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein BL, et al. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica. 2010;95:1090–1097. doi: 10.3324/haematol.2009.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein BL, et al. Gender and vascular complications in the JAK2 V617F-positive myeloproliferative neoplasms. Thromb. 2011;2011:874146. doi: 10.1155/2011/874146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landolfi R, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med. 2012;7(6):509–515. doi: 10.1007/s11739-011-0634-3. [DOI] [PubMed] [Google Scholar]
- 35.Berk PD, et al. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin. Hematol. 1986;23:132–143. [PubMed] [Google Scholar]
- 36.Kumar MS, et al. Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q- syndrome. Blood. 2011;118:4666–4673. doi: 10.1182/blood-2010-12-324715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klimchenko O, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 38.Dore LC, et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3333–3338. doi: 10.1073/pnas.0712312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pase L, et al. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood. 2009;113:1794–1804. doi: 10.1182/blood-2008-05-155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan M, et al. MicroRNA expression dynamics during murine and human erythroid differentiation. Exp. Hematol. 2007;35:1015–1025. doi: 10.1016/j.exphem.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovic R, et al. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood. 2009;113:3314–3322. doi: 10.1182/blood-2008-04-154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold CP, et al. MicroRNA programs in normal and aberrant stem and progenitor cells. Genome Res. 2011;21:798–810. doi: 10.1101/gr.111385.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott LM, et al. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006;108:2435–2437. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida K, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 45.Thol F, et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood. 2012;119:3578–3584. doi: 10.1182/blood-2011-12-399337. [DOI] [PubMed] [Google Scholar]
- 46.Hirabayashi S, et al. Spliceosomal gene aberrations are rare, coexist with oncogenic mutations, and are unlikely to exert a driver effect in childhood MDS and JMML. Blood. 2012;119:e96–e99. doi: 10.1182/blood-2011-12-395087. [DOI] [PubMed] [Google Scholar]
- 47.Makishima H, et al. Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood. 2012;119:3203–3210. doi: 10.1182/blood-2011-12-399774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang SJ, et al. Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms reveals recurrent SRSF2 mutations which are associated with adverse outcome. Blood. 2012;119:4480–4485. doi: 10.1182/blood-2011-11-390252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruchova-Votavova H, Yoon D, Prchal JT. miR-451 enhances erythroid differentiation in K562 cells. Leuk. Lymphoma. 2010;51:686–693. doi: 10.3109/10428191003629362. [DOI] [PubMed] [Google Scholar]
- 50.Jamieson CH, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berkofsky-Fessler W, et al. Transcriptional profiling of polycythemia vera identifies gene expression patterns both dependent and independent from the action of JAK2V617F. Clin. Cancer Res. 2010;16:4339–4352. doi: 10.1158/1078-0432.CCR-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]