Abstract
Background
Malaria is one of the key research concerns in climate change-health relationships. Numerous risk assessments and modelling studies provide evidence that the transmission range of malaria will expand with rising temperatures, adversely impacting on vulnerable communities in the East African highlands. While there exist multiple lines of evidence for the influence of climate change on malaria transmission, there is insufficient understanding of the complex and interdependent factors that determine the risk and vulnerability of human populations at the community level. Moreover, existing studies have had limited focus on the nature of the impacts on vulnerable communities or how well they are prepared to cope. In order to address these gaps, a systems approach was used to present an integrated risk and vulnerability assessment framework for studies of community level risk and vulnerability to malaria due to climate change.
Results
Drawing upon published literature on existing frameworks, a systems approach was applied to characterize the factors influencing the interactions between climate change and malaria transmission. This involved structural analysis to determine influential, relay, dependent and autonomous variables in order to construct a detailed causal loop conceptual model that illustrates the relationships among key variables. An integrated assessment framework that considers indicators of both biophysical and social vulnerability was proposed based on the conceptual model.
Conclusions
A major conclusion was that this integrated assessment framework can be implemented using Bayesian Belief Networks, and applied at a community level using both quantitative and qualitative methods with stakeholder engagement. The approach enables a robust assessment of community level risk and vulnerability to malaria, along with contextually relevant and targeted adaptation strategies for dealing with malaria transmission that incorporate both scientific and community perspectives.
Keywords: Integrated risk and vulnerability assessment, Climate change impact on malaria transmission, Systems approach, Climate change and malaria risk, East Africa
Background
It is estimated that at least 3.3 billion people globally are at risk of malaria infection. The disease is responsible for over half a million deaths each year, mostly (90%) in sub-Saharan Africa. Current climate change projections estimate an increase in the population at risk of malaria by 1.6 million by 2030 and 1.8 million by 2050 [1, 2]. This risk is significant in East Africa whereby rising temperatures and changes in other climate conditions are projected to expand the transmission range of malaria into geographic areas where communities were previously unexposed to the disease [3]. Understanding the extent to which local communities are vulnerable to this risk and how well they cope, is necessary to inform policies and interventions for risk management.
Vulnerability is determined in part by changes in land use and associated socio-economic and cultural factors at the community level, which exacerbate climate change impacts on malaria transmission. Previous vulnerability assessments have largely overlooked the influence of these socio-economic and cultural factors, instead emphasizing the biophysical influences on malaria transmission. While the evidence is abundant on increased risk of malaria as a result of changing climate, more robust understanding is needed of environmental, cultural and socioeconomic factors that influence malaria transmission at the community and household levels. This requires an integrated approach, which considers climate along with the contribution of socio-economic and cultural factors in order to explore current and future risks and vulnerabilities to malaria transmission.
While there are general guidelines on conducting integrated risk and vulnerability assessments, there is not one accepted method or approach in use that reflects specific contexts and the availability of data. This paper will provide a review of literature in climate change and malaria transmission in East Africa, and use this previous research to identify key variables in malaria transmission in order to construct a systems conceptual model and an integrated risk and vulnerability assessment framework.
The threat of malaria in a warmer world: climate change and malaria research in East Africa
Warming over the African continent is faster than the global average [4]. Projections for the next century show that most areas of the continent will exceed the 2 °C threshold by the last two decades of this century under medium scenarios and that under high scenarios this will happen by mid-century and reach between 3 and 6 °C by the end of the century [3]. The malaria mosquito and parasite are both sensitive to changes in climate and climate variability and the projected rising temperatures and changes in rainfall patterns will create favourable conditions for mosquito breeding in many areas [3, 4]. In East Africa, climate scenarios suggest longer malaria transmission seasons and geographic expansion of the disease into highland areas [5–8]. According to published literature, the earliest malaria-climate connection in the East African highlands was identified in the 1980s when there was a series of malaria epidemics connected to increases and anomalies in mean monthly maximum temperatures and increase in rainfall in the highlands [9–12]. Since then, the frequency and size of epidemics increased with serious outbreaks in 1995, 1998 and 2002, corresponding to climate variations such as a significant increase (≥3 °C) in mean temperatures [9], high rainfall [10], drought and El Nino events [9, 13–17]. Concurrently, increasing human population and intensified agricultural activities in the highlands has led to land use changes that in turn have enhanced vector production. At local scales, these changes in land cover, along with differences in topography, result in micro-climatic variability, raise surface temperatures by up to 2 °C, may have more of an impact on malaria transmission than climate change alone [18–22] and therefore should be included in vulnerability and risk assessments.
Vulnerability assessments in climate change and malaria research in East Africa
Vulnerability studies, which have long been affiliated with the disaster risk reduction and climate change adaptation communities [23, 24] are now increasingly used to map and interpret current and future risks related to climate change. Vulnerability is determined in part by human activities or interventions at the local level, which may, if successful, counteract the negative impacts of climate change. Furthermore, studies focused on projected increases in malaria transmission as a result of changes in climate should take into account the global decline of the disease by 60% from 2000 to 2015 mainly as a result of aggressive human interventions and treatment [25–27]. Therefore, a robust vulnerability assessment should not only take into account the impact of the climate-induced hazard to the population, but also the heterogeneity of the population and for malaria transmission, the differences in topography and hydrological characteristics of the landscape and other biological and socio-economic influences of transmission [16, 28–36] in a holistic and integrated manner [4, 6, 7]. Such an approach can incorporate an understanding of how changes in climate will impact the current burden of the disease (biophysical vulnerability). Moreover, this approach is also critical in identifying vulnerable populations and their capacity to respond (social vulnerability), taking into account other factors that affect the current burden of malaria and the effectiveness of current policies and programmes to manage the disease [37, 38].
Very few vulnerability assessments on climate change and malaria in East Africa are in published literature. A conceptual and methodological framework for modelling of social vulnerability for the East African region in a spatially explicit manner and independent of current disease prevalence, in order to provide options for targeted interventions was presented by Kienberger and Hagenlocher [39]. Risk and vulnerability was framed within the recent Inter Governmental Panel on Climate Change (IPCC) definition [40] in a dynamic and holistic manner and a number of related factors influencing disease risk were considered. Analysis and results established links to risk governance, climate change adaptation and relevant intervention strategies to several water-related vector borne diseases, including malaria. In a related study, Bizimana et al. [41] applied a composite indicator approach to assess social vulnerability to malaria transmission in Rwanda at a district level. An adapted vulnerability assessment framework [39] was used to identify indicators of different components of vulnerability in terms of generic susceptibility (i.e., lacking capacity to anticipate) and biological susceptibility (i.e., lacking capacity to cope or recover). Both studies mapped the main indicators of social vulnerability to malaria at district [41] and East African regional [39] levels. While both of these approaches provide useful tools for decision-making, only social vulnerability to malaria was considered. Also, there is an assumption of homogeneity of the population and landscape, which suggests uniformity of indicators while in reality there are differences in population and factors such as topography that will have an impact on the weight of indicators. Both papers acknowledge these limitations by suggesting that interventions should take into account the relevance of specific indicators of malaria vulnerability for different regions [39] and that future research should focus on an integrated vulnerability assessment that combines both environmental and social drivers [41].
Further research by Hagenlocher and Castro [42] addressed some of these limitations by modelling multi-dimensional vulnerability in Tanzania in a holistic and spatially explicit manner, using estimates of entomological inoculation rate (EIR) i.e. risk of infective bite as a proxy for malaria hazard. Causes of malaria risk and vulnerability were demonstrated to vary considerably across the country and risk, hazard and vulnerability maps that allow prioritisation of areas for malaria control were produced. By integrating malaria risk, vulnerability, and contributing factors in a holistic framework, evidence of issues that needed to be addressed locally to reduce malaria risk while accounting for variability within districts was provided. A useful output was an easily adaptable modelling framework however; limitations included incompatibility of the model with data that were not available in a spatially disaggregated format. This means that key vulnerability indicators such as acquired immunity to malaria, availability of malaria drugs, migration patterns, quality of the healthcare system, personal beliefs, behaviours and social networks were not included in the final model [42].
More recently, Bizimana et al. [43] integrated a set of weighted vulnerability indicators to define homogenous regions of social vulnerability to malaria in Rwanda. Although a useful approach in determining targeted interventions to specific high-risk areas and focus on factors that influence vulnerability, the model limitations did not allow for inclusion of key vulnerability indicators that did not have quantitative measurements such as social networks, migration and behavioural change. While these studies provide suitable frameworks for assessment of social vulnerability to malaria, none of them considered climate change/variability and the associated biophysical and social vulnerability at the same time. Biophysical and socio-economic factors are interdependent and must be considered simultaneously within an integrated systems framework, which assesses risk and vulnerability of communities to malaria. Therefore, using a systems approach, this paper builds on previous research to develop a framework for conducting an integrated risk and vulnerability assessment of the interplay among biophysical (especially climate change) and socio-economic and cultural factors on malaria transmission in East Africa.
Methods
Building the systems model was an iterative process that involved problem definition and development of a conceptual model of the system under study. The model is then used to suggest the development and calibration of a Bayesian Belief Network (BBN) model. While there is not a standardized procedure for system’s modelling, there are some common steps as described by Sterman and Voinov [44, 45], which were adapted for our modelling process (Fig. 1). This approach captures contextual and expert knowledge of the system and then subjects the information to a structural analysis, which is used to formulate a conceptual model in the form of an influence diagram. This is the first step in developing a BBN model. BBN models are a useful method for undertaking scenario simulations because they can assimilate different kinds of data and information including qualitative social survey results, quantitative biophysical response functions, spatial environmental data, expert opinion and even missing data [43].
Fig. 1.
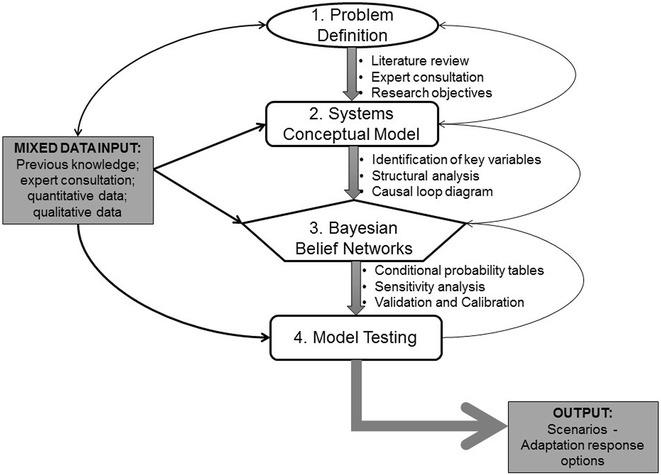
A flow chart showing the adapted systems modelling process
Problem definition
This step involved an extensive literature review and expert consultations on the key variables and relationships involved in the climate change and malaria transmission cycle. Three key academics well versed in climate change, malaria transmission and climate change-malaria research in East Africa were contacted and consulted. The experts were provided with contextual information regarding the research and were interviewed on their knowledge of the connections between climate change and malaria transmission. Comprehensive reviews of climate change and malaria transmission have been covered in other papers [21, 46–48]. Some of these studies have developed suitable environmental, socio-demographic and behavioural indicators of malaria risk at regional, community and household levels [15, 21, 33, 48–50]. This previous knowledge and expert consultations were used to capture relevant knowledge about the system and to identify the relationships between key variables influencing risk of malaria infection in East Africa.
Structural analysis
The malaria transmission cycle is a complex system with multiple non-linear and often interacting variables of climate change, environmental, biological and socio-economic influences thus, conceptualizing such a system is challenging. The cross-impact multiplication method (CIMM) [51] was used to undertake a structural analysis. The structural analysis method revealed key system components and interactions from a candidate set identified from the literature review and expert consultations and followed a four-step iterative process:
Compilation of a candidate set of key variables from the literature review and expert consultations;
Description of the relationships between variables based on contextual knowledge and expert opinion. The degree of influence between variables was rated 0 if there was no evidence of direct influence between two variables. Otherwise, the strength of the relationship was rated 1(low), 2 (medium), 3 (high) or 4 (potential);
Identification of key variables using the CIMM approach which calculates the intensity of influence and dependency between variables; and
The CIMM approach was also used to identify the relationships between the identified variables of the system through an analysis of the impact matrix by generating a map of direct influence, which separates the variables into four types according to degree of influence: (i) influential variables, which influence the system, but are not dependent on other variables; (ii) relay variables, which influence the system and are dependent on influential variables; (iii) dependent variables, which represent the system’s output variables and; (iv) autonomous variables, which are neither influential nor dependent and may or may not significantly affect the system depending on the strength of their relationships.
Visualization of the systems conceptual model
After the structural analysis phase, an influence diagram (also known as a causal loop diagram or CLD) was constructed to visualize the key variables and interactions of the system). In an influence diagram, variables represent a stock of something or a quality of some kind that can increase or decrease. The variables are connected or linked by arrows that indicate a causal relationship; typically, a flow of information, energy or materials that cause a shift in the stock or quality of the affected variable. The direction of the arrow indicates the direction of causality while the polarity sign at the tip of the arrow (+ or −) indicates whether the relationship between the two variables is positive (increasing the effect) or negative (dampening the effect). The influence diagram was visualized using the software Vensim DSS for Windows Version 6.3.
Developing the assessment framework
The systems conceptual model, represented by the influence diagram, complemented with existing risk and vulnerability assessment frameworks [21, 40] was used to develop an integrated assessment framework that considers both biophysical and social influences on malaria transmission. Multiple definitions exist for vulnerability, but for the development of our framework the definition of vulnerability in the context of vector-borne diseases as defined by [21] as “a combination of a change in exposure of humans to pathogens with environmental change and the sensitivity of the population to that change” was adopted. Also, the [21] definition of adaptive capacity as consisting of “…technologies, cultural tools and the public health infrastructure and resources that are available to implement appropriate management responses” was adopted. These definitions are consistent with those of IPCC Fifth Assessment Report, which defines vulnerability to disease and injury due to climate variability and climate change as “the propensity or predisposition to be adversely affected” dependent on generic (education, income, health status and responsiveness of government) and biological susceptibility (age, gender and immune status) [40, 52]. Finally, the risk of impacts from climate change was understood as “resulting from the overlap of hazards from the physical climate and the vulnerability and exposure of people, ecosystems, and assets” [40].
Results
Problem definition and identification of variables
The scope of this study was limited to the East African (Kenya, Tanzania, Uganda, Rwanda and Burundi) region based on researcher experience, existing networks and availability of previous extensive studies conducted in the region. The problem was therefore focused on determining how climate influences vulnerability to malaria transmission in East Africa. Based on the literature review and key insights from expert interviews, a candidate list of 36 variables were identified as important for understanding climatic impacts and malaria transmission cycle (Table 1) and grouped into two broad sets of biophysical and socio-economic indicators.
Table 1.
Variables in climate change and malaria transmission identified from literature review and expert consultation
| No | Variables | Description | Source |
|---|---|---|---|
| Biophysical variables | |||
| 1 | Air temperature | Air temperature suitable for malaria transmission i.e. between 16 and 34 °C | [17, 32, 48, 57–61] |
| 2 | Water temperature | Mosquito habitat temperature suitable for breeding | [60, 62–64] |
| 3 | El-Nino | Periods of extreme rainfall | [14–16, 48, 65] |
| 4 | Average rainfall/precipitation | Mean monthly rainfall of at least 150 mm; rainfall season | [9, 17, 21, 31, 48, 61, 66, 67] |
| 5 | Relative humidity | Amount of water vapour present in air | [68–71] |
| 6 | Altitude | Height/distance above sea level | [36, 48, 69, 70, 72] |
| 7 | Micro-habitat changes | Changes in mosquito habitat micro-climate due to loss of forest cover or other environmental controls such as clearing of bushes | [63, 68–70, 73–78] |
| 8 | Topography | Physical land surface including hills and valleys, elevation | [33, 48, 79, 80] |
| 9 | Topographic wetness index | Percentage of ground water saturation of at least 5% for suitable mosquito breeding site | [30, 31] |
| 10 | Wetlands and water bodies | Proximity to swamps and other stagnant water bodies | [33, 63, 68, 74, 76, 77, 81] |
| 11 | Bare areas | Land without forest cover or other vegetation | [33, 82, 83] |
| 12 | Forest edge | Human proximity to forest boundaries and potential exposure to exposed mosquito breeding sites due to deforestation | [33, 62] |
| 13 | Agriculture | Land clearance, planting, livestock and maize farming, swamp drainage and farming, and water management i.e. water conservation using shallow wells, small-scale irrigation and creation of water drainage channels | [31, 33, 48, 49, 76, 77, 84–87] |
| 14 | Vector abundance | Increase in numbers of malaria mosquitoes | [32, 60, 82, 88] |
| 15 | Vector biting | Likelihood of an infective bite from a mosquito | [48, 70, 82] |
| 16 | Vector infection rate | Efficiency of transmission and infection with the malaria parasite by the mosquito | [48, 73, 82] |
| 17 | Vector adaptive behaviour | Changes in mosquito vector behaviour such as early biting or indoor resting | Expert input |
| 18 | Population under 5 years | Number of individuals under 5 years old | [48, 49, 74] |
| 19 | Immune status | Lowered immunity to malaria due to pregnancy or inexposure; acquired immunity to malaria from long term exposure | [48, 49, 79, 89, 90] |
| 20 | Interactions | Co-infections with other diseases such as HIV increase likelihood and severity of infection | [15, 50] |
| 21 | Drug resistance | Resistance of the malaria parasite to drugs/parasite evolution | [15, 48, 50] |
| Socio-economic variables | |||
| 22 | Urbanisation | Expansion of urban areas and overcrowding in cities | [49] |
| 23 | Population migration/travel | Movement of people from low risk areas to malaria-endemic or epidemic-prone areas and vice versa | [48, 50] |
| 24 | Nutritional status | Poor health as a result of undernutrition or malnutrition | [48, 49] |
| 25 | Gender | Gender roles, expectations and cultural customs | [48, 49] |
| 26 | Poverty | Socio-economic conditions; household income, food and household assets | [15, 48, 49, 74] |
| 27 | Religious beliefs | Religion or superstitions in understanding or managing malaria and/or climate change impacts | [15, 49] |
| 28 | Perception | Knowledge and understanding of disease | [15, 33, 49] |
| 29 | Type of house | House with grass-thatched roof and mud walls (semi-permanent) or Bbrick house with tiled or aluminium roof (permanent); house with separate kitchen, house with ceiling and house with open eaves | [33, 48, 49] |
| 30 | Education level of household head | Education level of male or female head of household | [33] |
| 31 | Health-seeking behaviour | Willingness to seek treatment for malaria; households with malaria medicine in stock, self-medication, tradition/cultural norms and practices in malaria management | [48, 49] |
| 32 | Net use | Use of insecticide-treated bed nets to prevent malaria infection | [15, 33, 74] |
| 33 | Environmental controls | Keeping area around the houses cleared of shrubs and other overgrowth; safe disposal of plastics and other water-retaining containers | [15, 33] |
| 34 | Quality of health systems | Health services and policy; availability of health facilities; access to healthcare; quality of healthcare and capacity for malaria treatment | [15, 47, 48, 50] |
| 35 | Malaria vector control | Distribution and coverage of insecticide-treated bed nets by the government; coverage of households sprayed with malaria insecticide (indoor residual spraying) | [15, 48] |
| 36 | Quality of information | Reliable and easy to understand information systems for communicating weather and climate information or early warning systems for malaria epidemics | [15, 17, 50] |
Structural analysis
The direct influence-dependence map generated from the structural analysis provided the key influential, relay variables, dependent variables and autonomous variables as shown in Fig. 2.
Fig. 2.
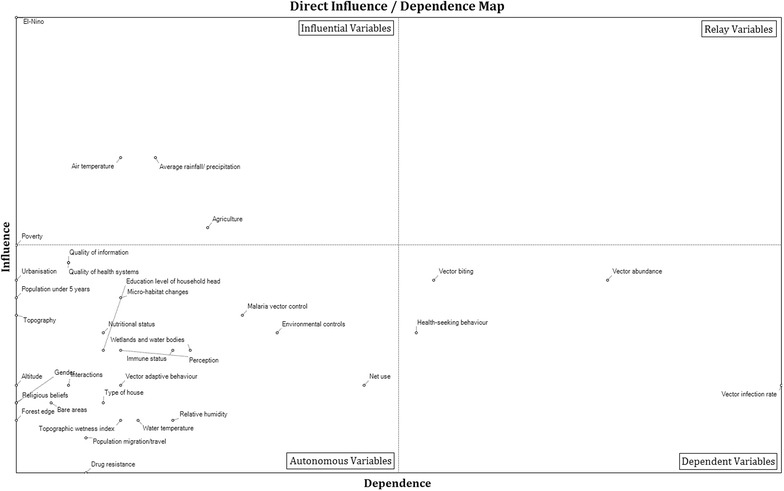
Direct influence-dependence map of variables of the climate change and malaria transmission system
Systems conceptual model of climate change and malaria transmission
The systems model was simplified by focusing mainly on the influential (El Nino, air temperature, average rainfall/precipitation, agriculture), dependent (vector biting, vector abundance, vector infection rate, health-seeking behaviour) and the autonomous variables with strong relationships within the system (water temperature, micro-habitat, topography, wetlands and water bodies, vector adaptive behaviour, population under five, immune status, poverty, education level of household head, nutritional status, perception, net use, malaria vector control, environmental controls, quality of information, quality of health systems).
The visualization of the system conceptual model is illustrated in Fig. 3: Hexagons represent influential variables; rectangles are dependent variables; and circles are autonomous variables. The colours represent major classes of variables: blue = climate, climate change and variability variables; green = land use and land use change variables; and pink = malaria vector attributes; Additional to these biophysical variables are the socio-economic variables that are colour coded orange. The strength of relationships between variables is represented by solid lines (stronger relationships) and dashed lines (weaker relationships). The red arrows represent a positive relationship (+) (i.e., the recipient variable’s state or quality increases) between variables while blue arrows represent negative relationships (−). Lack of a polarity sign or black arrow indicates that the relationship can be either positive or negative or that the relationship has not been determined.
Fig. 3.
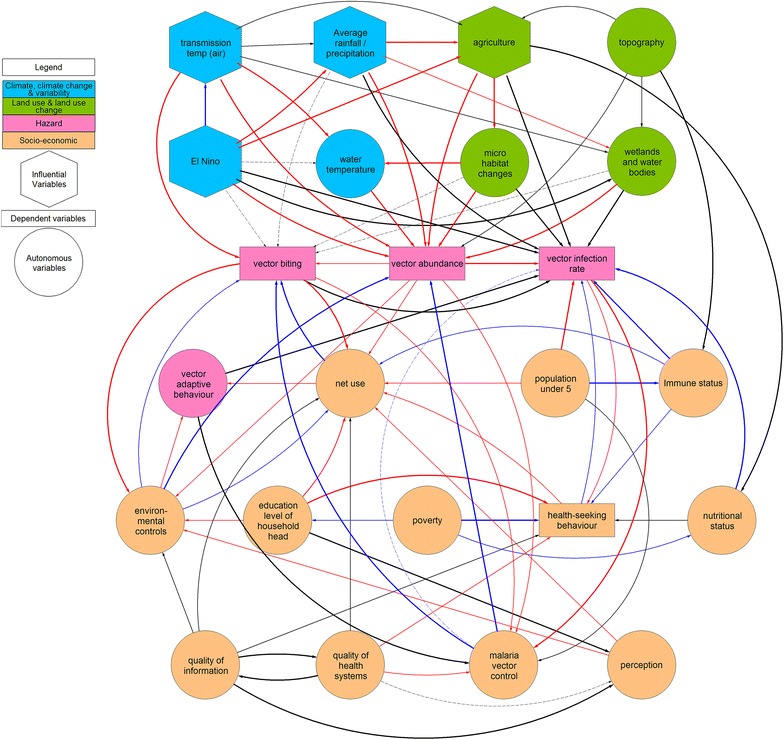
A systems conceptual model detailing the causal relationships between variables in the malaria transmission system
An integrated risk assessment framework
The risk assessment framework (Fig. 4) was constructed based on the conceptual systems model. Risk of malaria infection was identified as the climate-related hazard, which is influenced by exposure to changes in climate, climate variability, land use and land use change and malaria vector attributes. Vulnerability to risk of malaria infection is determined by biological susceptibility, generic susceptibility and coping strategies at community and institutional levels. This assessment framework can be operationalised using BBN models as these allow for sensitivity analysis and exploration of the efficacy of policy recommendations under different scenarios. Sensitivity analysis can reveal the relative significance or leverage of driving variables, providing an objective basis to identify a subset of variables for model formulation.
Fig. 4.

An integrated assessment framework to guide studies of climate change and malaria risk and vulnerability
Discussion
Conducting integrated vulnerability assessments in climate change and malaria research is a difficult process due to the scarcity of empirical infection data on malaria, the multiple interacting determinants, the often indirect and non-linear causal chains, variations in exposure within affected populations and the high degree of model uncertainty [21, 37]. Previous studies have suggested integrated assessment frameworks of climate change and malaria. A framework that illustrated the environmental, socio-economic and biological factors affecting malaria incidence in the African highlands was proposed by Lindsay and Martens [47]. They defined this as an eco-epidemiologic modelling approach and reiterated the need for integrated modelling built on systems-oriented analyses that consider the interactions and feedback mechanisms between different sub-systems rather than treating them in isolation.
Another integrated assessment framework for infectious diseases (including malaria) was proposed by Chan et al. [46]. This framework presented a means by which cross-disciplinary research could be used to integrate the biologic, epidemiologic, ecologic and sociologic knowledge of climate impact on disease transmission in order to provide a reliable estimate of the climate-induced impact on the disease. Although this framework takes into account feedback loops resulting from human interventions and disease prevention, it did not consider which variables carry more weight in influencing transmission. Further research by Sutherst [21] presented a comprehensive review of global change and vector borne diseases, highlighting the complexity of malaria transmission and the major challenges involved in vulnerability assessments of the same. An integrated assessment framework was proposed for the study of these diseases and suggestions for future research included more studies that focused not just on vulnerability, but on designing adaptation options to changes in transmission risk using a systems approach.
Application of integrated vulnerability assessments have been rare, but are gaining prominence in literature; Dickin and Schuster-Wallace [53], modelled vulnerability to dengue in North-eastern Brazil using a water associated disease index (WADI) while Lyth and Holbrook [54], utilized systems thinking to undertake a quantitative and qualitative assessment of the complex social-ecological factors contributing to occurrences of ross river virus and impacting human health vulnerability in Tasmania. Studies in East Africa however, have been limited. A review of the literature identified only one study, which attempted to address both the biophysical and social influences of malaria transmission in East Africa within the context of climate change.
This study by Wandiga et al. [15], aimed to assess the vulnerability and coping capacity of target populations as well as the excess risk to which they are exposed as a result of climate change using both quantitative and qualitative methods. A positive relationship was demonstrated between changes in malaria cases and variations in monthly minimum and maximum temperatures. Other socio-economic factors such as drug misuse, inadequate knowledge of disease control, myths and superstitions at the household level that could contribute to higher incidences of malaria morbidity and mortality, were also identified. High poverty levels and weak health-care systems were determined as factors that reduce the coping capacity of the community. However, quantitative and qualitative data analyses were conducted separately and no sensitivity analysis was done thus, there is no determination of the relative contribution of any of these factors in increasing or decreasing the risk of malaria transmission.
More studies using integrated assessments of risk of climate-related diseases such as malaria are needed in East Africa. In this paper, some of the limitations of previous research have been addressed by presenting a systems-based conceptual model and assessment framework as the basis for a more integrated analysis of the risk of communities to malaria infection. In the suggested framework, this risk is influenced by exposure to changes in climate, climate variability, land use and land use change and malaria vector attributes. The integrated risk assessment framework can be used with established indicators of malaria transmission at household and community level [48–50].
The framework can be operationalized using BBN models, a task for future research. This will require using data from a mix of quantitative and qualitative sources including community and expert stakeholder surveys. Expert stakeholders will provide the necessary contextual knowledge while empirical knowledge from local stakeholders and the general community will be important in identifying specific determinants of vulnerability. Factors that increase exposure such as local geography and climate, social, economic and other environmental factors must also be taken into account. Sensitivity on the other hand, requires measures of abundance of the vector and pathogen on one hand and intrinsic immunity of the population on the other. Specific human behaviours that may reduce, increase or generate differential exposure should also be considered as part of adaptive capacity.
While BBN models have been widely applied in a range of natural resource management and decision support contexts [55, 56], there has been limited application of the same in climate change and public health assessments. Combining information from a range of quantitative and qualitative sources and integrating them using BBNs should result in a more robust model, informed by community needs and capabilities, leading to further knowledge generation and identification of targeted response strategies for decision-making and policy.
The framework presented is novel in the following aspects: (a) utilizing systems thinking to frame the problem of climate change and malaria transmission; (b) drawing upon mixed methods to integrate knowledge from different fields with stakeholder participation at different geographical scales and levels of governance; (c) its applicability in data-poor regions as narratives from stakeholders can complement quantitative data; and; (d) being sufficiently generic so that it can be applied to study impacts of climate change on other kinds of transmissible diseases.
A major limitation to the approach used primarily relates to the subjective way in which the candidate set of variables was identified. However, this process can be iterative with the results of the structural analysis being used to provide feedback to experts and stakeholders for further refinement. Additionally, BBN models have constraints including a limited ability to capture feedback loops from the response variable back to the drivers [55], therefore, the full suite of variables and interactions identified in our systems conceptual model and operational framework cannot be represented within a single BBN model. This can be overcome by constructing multiple BBN models that capture feedbacks and enable more detailed inspection of system subcomponents.
Conclusions
It is important to remember that although climate change is the result of aggregate global emissions, climate change impacts vary regionally. Therefore, adaptation responses must always be tailored to local contexts and will be largely dependent on the risk and vulnerability profiles of communities. This vulnerability is context-specific and determined in part by human activities at the local level, which may far outweigh the influence of climate change. It follows that the same influencing factors for a developed country will not represent the same risks as those for rural communities in a developing country. Most vulnerability studies so far have been at global or regional levels and few have incorporated climate change, land use change and malaria transmission in an integrated manner at the community level.
The assessment framework presented here provides a first step approach for setting baselines against which risk can be assessed and for estimating the benefits of adaptation options for malaria risk management in East Africa. The approach is consistent with the current IPCC recommendations, which recognizes that risk of climate related hazards can occur due to many interacting influences and that focus should be on solutions for risk reduction. The framework can be applied at a community level using both quantitative and qualitative methods with stakeholder engagement and can be adapted to other data-poor regions with similar vulnerability profiles. The proposed use of BBN models with the framework would facilitate a robust assessment of contextually relevant and targeted adaptation strategies for dealing with malaria transmission, that incorporate both scientific and community perspectives.
Authors’ contributions
All authors contributed to the contextual knowledge and study design, development of the methodology and writing the manuscript. EAO contributed most of the background literature review and OS took the lead in the systems approach. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge the following experts for their contextual knowledge with regard to climate change and malaria research and vulnerability assessments: Dr. Andrew Githeko, Director, Climate Change and Vector Borne Disease Unit: Kenya Medical Research Institute; Prof. Shem Wandiga, Director, Institute for Climate Change and Adaptation: University of Nairobi, Kenya; Prof. Benson Estambale, Deputy Vice-Chancellor, Research, Innovation and Outreach: Jaramogi Oginga Odinga University of Science and Technology, Kenya.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
All data and supporting material used in the production of this manuscript is available to interested researchers upon written request to the Griffith University Human Research Ethics Committee.
Ethics approval and consent to participate
This research was approved by the Griffith University Human Research Ethics Committee, under Protocol No. ENV/21/14/HREC and by the Kenya Medical Research Institute (KEMRI), under Protocol No. KEMRI/RES/7/3/1. Informed consent to participate was obtained from all experts interviewed.
Funding
This research was supported in part by the Griffith Graduate School through the award of the Griffith University International Postgraduate Research Scholarship and the Griffith University Postgraduate Research Scholarship and through supplementary awards from the Griffith Climate Change Response Program and the Aga Khan University East African Institute.
Contributor Information
Esther Achieng Onyango, Email: esther.onyango@griffithuni.edu.au.
Oz Sahin, Email: o.sahin@griffith.edu.au.
Alex Awiti, Email: aawiti@gmail.com.
Cordia Chu, Email: c.chu@griffith.edu.au.
Brendan Mackey, Email: b.mackey@griffith.edu.au.
References
- 1.WHO . Quantitative risk assessment of the effects of climate change on selected causes of death, 2030s and 2050s. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.WHO . World malaria report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Niang I, Ruppel OC, Abdrabo MA, Essel A, Lennard C, Padgham J, et al. Africa. In: Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, et al editors. Climate change 2014: impacts, adaptation, and vulnerability. Cambridge: Cambridge University Press; 2014. p. 1199–265. http://www.ipcc.ch/pdf/assessment-report/ar5/wg2/WGIIAR5-Chap22_FINAL.pdf. Accessed 5 Dec 2015.
- 4.IPCC. Climate Change 2013. The physical science basis. In: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2013.
- 5.Peterson AT. Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect Dis. 2009;9:59. doi: 10.1186/1471-2334-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermert V, Fink AH, Paeth H. The potential effects of climate change on malaria transmission in Africa using bias-corrected regionalised climate projections and a simple malaria seasonality model. Clim Change. 2013;120:741–754. doi: 10.1007/s10584-013-0851-z. [DOI] [Google Scholar]
- 7.Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colón-González FJ, et al. Impact of climate change on global malaria distribution. Proc Natl Acad Sci USA. 2014;111:3286–3291. doi: 10.1073/pnas.1302089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonnang HEZ, Tchouassi DP, Juarez HS, Igweta LK, Djouaka RF. Zoom in at African country level: potential climate induced changes in areas of suitability for survival of malaria vectors. Int J Health Geogr. 2014;13:12. doi: 10.1186/1476-072X-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Githeko AK, Ndegwa W. Predicting malaria epidemics in the Kenyan highlands using climate data: a tool for decision makers. Glob Change Hum Health. 2001;2:54–63. doi: 10.1023/A:1011943131643. [DOI] [Google Scholar]
- 10.Pascual M, Cazelles B, Bouma MJ, Chaves LF, Koelle K. Shifting patterns: malaria dynamics and rainfall variability in an African highland. Proc Biol Sci. 2008;275:123–132. doi: 10.1098/rspb.2007.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omumbo JA, Lyon B, Waweru SM, Connor SJ, Thomson MC. Raised temperatures over the Kericho tea estates: revisiting the climate in the East African highlands malaria debate. Malar J. 2011;10:12. doi: 10.1186/1475-2875-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaves LF, Hashizume M, Satake A, Minakawa N. Regime shifts and heterogeneous trends in malaria time series from Western Kenya highlands. Parasitology. 2012;139:14–25. doi: 10.1017/S0031182011001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume M, Terao T, Minakawa N. The Indian Ocean dipole and malaria risk in the highlands of western Kenya. Proc Natl Acad Sci USA. 2009;106:1857–1862. doi: 10.1073/pnas.0806544106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wandiga SO, Opondo M, Olago D, Githeko A, Githui F, Marshall M, et al. Vulnerability to epidemic malaria in the highlands of Lake Victoria basin: the role of climate change/variability, hydrology and socio-economic factors. Clim Change. 2009;99:473–497. doi: 10.1007/s10584-009-9670-7. [DOI] [Google Scholar]
- 16.Zhou G, Minakawa N, Githeko AK, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci USA. 2004;101:2375–2380. doi: 10.1073/pnas.0308714100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Githeko AK, Ogallo L, Lemnge M, Okia M, Ototo EN. Development and validation of climate and ecosystem-based early malaria epidemic prediction models in East Africa. Malar J. 2014;13:329. doi: 10.1186/1475-2875-13-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patz JA, Olson SH, Uejio CK, Gibbs HK. Disease emergence from global climate and land use change. Med Clin N Am. 2008;92:1473–1491. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 20.Leemans R, Eickhout B. Another reason for concern: regional and global impacts on ecosystems for different levels of climate change. Glob Environ Change. 2004;14:219–228. doi: 10.1016/j.gloenvcha.2004.04.009. [DOI] [Google Scholar]
- 21.Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirtman B, Power SB, Adedoyin AJ, Boer GJ, Bojariu R, Camilloni I, et al. Near-term climate change: projections and predictability. In: Delecluse P, Palmer T, Shepherd T, Zwiers F, et al., editors. Climate change 2013: the physical science basis. Cambridge: Cambridge University Press; 2013. pp. 953–1028. [Google Scholar]
- 23.Adger WN. Vulnerability. Glob Environ Change. 2006;16:268–281. doi: 10.1016/j.gloenvcha.2006.02.006. [DOI] [Google Scholar]
- 24.Eriksen SH, Kelly PM. Developing credible vulnerability indicators for climate adaptation policy assessment. Mitig Adapt Strateg Glob Change. 2007;12:495–524. doi: 10.1007/s11027-006-3460-6. [DOI] [Google Scholar]
- 25.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. Climate change and the global malaria recession. Nature. 2010;465:342–345. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaria fact sheet. http://www.who.int/mediacentre/factsheets/fs094/en/. WHO; 2016. Accessed 20 June 2016.
- 28.Alonso D, Bouma MJ, Pascual M. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proc Biol Sci. 2011;278:1661–1669. doi: 10.1098/rspb.2010.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68:734–742. [PubMed] [Google Scholar]
- 30.Cohen JM, Ernst KC, Lindblade KA, Vulule JM, John CC, Wilson ML. Local topographic wetness indices predict household malaria risk better than land-use and land-cover in the western Kenya highlands. Malar J. 2010;9:328. doi: 10.1186/1475-2875-9-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen JM, Ernst KC, Lindblade KA, Vulule JM, John CC, Wilson ML. Topography-derived wetness indices are associated with household-level malaria risk in two communities in the western Kenyan highlands. Malar J. 2008;7:40. doi: 10.1186/1475-2875-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sewe M, Rocklöv J, Williamson J, Hamel M, Nyaguara A, Odhiambo F, et al. The association of weather variability and under five malaria mortality in KEMRI/CDC HDSS in western Kenya 2003 to 2008: a time series analysis. Int J Environ Res Public Health. 2015;12:1983–1997. doi: 10.3390/ijerph120201983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernst KC, Lindblade KA, Koech D, Sumba PO, Kuwuor DO, John CC, et al. Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands. A case-control study. Trop Med Int Health. 2009;14:1258–1265. doi: 10.1111/j.1365-3156.2009.02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson JC, Byrd BD, Omlin FX. Field assessments in western Kenya link malaria vectors to environmentally disturbed habitats during the dry season. BMC Public Health. 2004;4:33. doi: 10.1186/1471-2458-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paaijmans KP, Blanford JI, Crane RG, Mann ME, Ning L, Schreiber KV, et al. Downscaling reveals diverse effects of anthropogenic climate warming on the potential for local environments to support malaria transmission. Clim Change. 2014;125:479–488. doi: 10.1007/s10584-014-1172-6. [DOI] [Google Scholar]
- 36.Drakeley CJ, Carneiro I, Reyburn H, Malima R, Lusingu JP, Cox J, et al. Altitude-dependent and -independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J Infect Dis. 2005;191:1589–1598. doi: 10.1086/429669. [DOI] [PubMed] [Google Scholar]
- 37.Ebi KL. Healthy people 2100: modeling population health impacts of climate change. Clim Change. 2007;88:5–19. doi: 10.1007/s10584-006-9233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO . Protecting health from climate change: vulnerability and adaptation assessment. Geneva: World Health Organization; 2010. [Google Scholar]
- 39.Kienberger S, Hagenlocher M. Spatial-explicit modeling of social vulnerability to malaria in East Africa. Int J Health Geogr. 2014;13:29. doi: 10.1186/1476-072X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.IPCC. Climate change 2014: impacts, adaptation and vulnerability, part A: global and sectoral aspects. In: Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2014.
- 41.Bizimana J, Twarabamenye E, Kienberger S. Assessing the social vulnerability to malaria in Rwanda. Malar J. 2015;14:2. doi: 10.1186/1475-2875-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagenlocher M, Castro MC. Mapping malaria risk and vulnerability in the United Republic of Tanzania: a spatial explicit model. Popul Health Metr. 2015;13:1–14. doi: 10.1186/s12963-015-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bizimana JP, Kienberger S, Hagenlocher M, Twarabamenye E. Modelling homogeneous regions of social vulnerability to malaria in Rwanda. Geospat Health. 2016;11:129–146. doi: 10.4081/gh.2016.404. [DOI] [PubMed] [Google Scholar]
- 44.Sterman J. Business dynamics: systems thinking and modeling for a complex world. Boston: Irwin/McGraw-Hill; 2000. [Google Scholar]
- 45.Voinov AA. Systems science and modeling for ecological economics. London: Academic Press; 2010. [Google Scholar]
- 46.Chan NY, Ebi KL, Smith F, Wilson TF, Smith AE. An integrated assessment framework for climate change and infectious diseases. Environ Health Perspect. 1999;107:329–337. doi: 10.1289/ehp.99107329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindsay SW, Martens WJ. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998;76:33–45. [PMC free article] [PubMed] [Google Scholar]
- 48.Protopopoff N, Van Bortel W, Speybroeck N, Van Geertruyden JP, Baza D, D’Alessandro U, et al. Ranking malaria risk factors to guide malaria control efforts in African highlands. PLoS ONE. 2009;4:e8022. doi: 10.1371/journal.pone.0008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bates I, Fenton C, Gruber J, Lalloo D, Medina LA, Squire SB, et al. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease, Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004;4:267–277. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- 50.Bates I, Fenton C, Gruber J, Lalloo D, Lara AM, Squire SB, et al. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease, Part II: determinants operating at environmental and institutional level. Lancet Infect Dis. 2004;4:368–375. doi: 10.1016/S1473-3099(04)01047-3. [DOI] [PubMed] [Google Scholar]
- 51.Godet M. Creating futures: scenario planning as a strategic management tool. Paris: Economica; 2001.
- 52.IPCC. Climate change 2014: impacts, adaptation, and vulnerability, part B: regional aspects. In: Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2014.
- 53.Dickin SK, Schuster-Wallace CJ. Assessing changing vulnerability to dengue in northeastern Brazil using a water-associated disease index approach. Glob Environ Change. 2014;29:155–164. doi: 10.1016/j.gloenvcha.2014.09.007. [DOI] [Google Scholar]
- 54.Lyth A, Holbrook NJ. Assessing an indirect health implication of a changing climate: Ross river virus in a temperate island state. Clim Risk Manag. 2015;10:77–94. doi: 10.1016/j.crm.2015.06.004. [DOI] [Google Scholar]
- 55.Fenton N, Neil M. Risk assessment and decision analysis with Bayesian networks. Boca Raton: CRC Press; 2013. [Google Scholar]
- 56.Landuyt D, Broekx S, D’hondt R, Engelen G, Aertsens J, Goethals PLM. A review of Bayesian belief networks in ecosystem service modelling. Environ Model Softw. 2013;46:1–11. doi: 10.1016/j.envsoft.2013.03.011. [DOI] [Google Scholar]
- 57.Imbahale SS, Paaijmans KP, Mukabana WR, van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10:1. doi: 10.1186/1475-2875-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyons CL, Coetzee M, Terblanche JS, Chown SL. Thermal limits of wild and laboratory strains of two African malaria vector species, Anopheles arabiensis and Anopheles funestus. Malar J. 2012;11:226. doi: 10.1186/1475-2875-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE. 2013;8:e79276. doi: 10.1371/journal.pone.0079276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilioli G, Mariani L. Sensitivity of Anopheles gambiae population dynamics to meteo-hydrological variability: a mechanistic approach. Malar J. 2011;10:294. doi: 10.1186/1475-2875-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colón-gonzález FJ, Tompkins AM, Biondi R, Bizimana JP, Namanya DB. Assessing the effects of air temperature and rainfall on malaria incidence: an epidemiological study across Rwanda and Uganda. Geospat Health. 2016;11:379. doi: 10.4081/gh.2016.379. [DOI] [PubMed] [Google Scholar]
- 62.Tuno N, Okeka W, Minakawa N, Takagi M, Yan G. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in western Kenya highland forest. J Med Entomol. 2005;42:270–277. doi: 10.1603/0022-2585(2005)042[0270:SOAGSS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 63.Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack O-OJ, Githeko AK, et al. Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. Am J Trop Med Hyg. 2006;74:69–75. [PubMed] [Google Scholar]
- 64.Paaijmans K. Weather, water and malaria mosquito larvae. Wageningen: Wageningen Universiteit; 2008. [Google Scholar]
- 65.Chaves LF, Satake A, Hashizume M, Minakawa N. Indian Ocean dipole and rainfall drive a Moran effect in East Africa malaria transmission. J Infect Dis. 2012;205:1885–1891. doi: 10.1093/infdis/jis289. [DOI] [PubMed] [Google Scholar]
- 66.Paaijmans KP, Wandago MO, Githeko AK, Takken W. Unexpected high losses of Anopheles gambiae larvae due to rainfall. PLoS ONE. 2007;2(11):e1146. doi: 10.1371/journal.pone.0001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCann RS, Messina JP, MacFarlane DW, Bayoh MN, Vulule JM, Gimnig JE, et al. Modeling larval malaria vector habitat locations using landscape features and cumulative precipitation measures. Int J Health Geogr. 2014;13:17. doi: 10.1186/1476-072X-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, et al. Spatial distribution of anopheline larval habitats in western Kenyan highlands. Effects of land cover types and topography. Am J Trop Med Hyg. 2005;73:157–165. [PubMed] [Google Scholar]
- 69.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in western Kenya highlands. Effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77:660–666. [PubMed] [Google Scholar]
- 70.Afrane YA, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by land use and land cover on duration of gonotrophic cycles of Anopheles gambiae (Diptera: Culicidae) in western Kenya highlands. J Med Entomol. 2005;42:974–980. doi: 10.1603/0022-2585(2005)042[0974:EOMCCB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Omukunda E, Githeko A, Ndong’a MF, Mushinzimana E, Atieli H, Wamae P. Malaria vector population dynamics in highland and lowland regions of western Kenya. J Vector Borne Dis. 2013;50:85–92. [PubMed] [Google Scholar]
- 72.Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc Natl Acad Sci USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2006;74:772–778. [PubMed] [Google Scholar]
- 74.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerg Infect Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 76.Munga S, Yakob L, Mushinzimana E, Zhou G, Ouna T, Minakawa N, et al. Land use and land cover changes and spatiotemporal dynamics of anopheline larval habitats during a four-year period in a highland community of Africa. Am J Trop Med Hyg. 2009;81:1079–1084. doi: 10.4269/ajtmh.2009.09-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou G, Munga S, Minakawa N, Githeko AK, Yan G. Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg. 2007;77:29–35. [PubMed] [Google Scholar]
- 78.Githeko AK, Ototo EN, Guiyun Y. Progress towards understanding the ecology and epidemiology of malaria in the western Kenya highlands: opportunities and challenges for control under climate change risk. Acta Trop. 2012;121:19–25. doi: 10.1016/j.actatropica.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wanjala CL, Waitumbi J, Zhou G, Githeko AK. Identification of malaria transmission and epidemic hotspots in the western Kenya highlands: its application to malaria epidemic prediction. Parasites Vectors. 2011;4:1–13. doi: 10.1186/1756-3305-4-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tompkins AM, Caporaso L. Assessment of malaria transmission changes in Africa, due to the climate impact of land use change using coupled model intercomparison project phase 5 earth system models. Geospat Health. 2016;11:380. doi: 10.4081/gh.2016.380. [DOI] [PubMed] [Google Scholar]
- 81.Omukunda E, Githeko A, Ndong’a MF, Mushinzimana E, Yan G. Effect of swamp cultivation on distribution of anopheline larval habitats in Western Kenya. J Vector Borne Dis. 2012;49:61–71. [PMC free article] [PubMed] [Google Scholar]
- 82.Patz JA, Githeko AK, McCarty JP, Hussein S, Confalonieri UE, de Wet N, et al. Climate change and infectious diseases. In: Mcmichael AJ, Campbell-Lendrum DH, Corvalán CF, Ebi KL, Githeko AK, Scheraga JD, et al., editors. Climate change and human health: risks and responses. Geneva: WHO Press; 2003. pp. 103–132. [Google Scholar]
- 83.Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, et al. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye-Ebiyo Y, Pollack RJ, Spielman A. Enhanced development in nature of larval Anopheles arabiensis mosquitoes feeding on maize pollen. Am J Trop Med Hyg. 2000;63:90–93. doi: 10.4269/ajtmh.2000.63.90. [DOI] [PubMed] [Google Scholar]
- 85.Gleckler PJ, Santer BD, Domingues CM, Pierce DW, Barnett TP, Church JA, et al. Human-induced global ocean warming on multidecadal timescales. Nat Clim Change. 2012;2:524–529. [Google Scholar]
- 86.Himeidan YE, Zhou G, Yakob L, Afrane Y, Munga S, Atieli H, et al. Habitat stability and occurrences of malaria vector larvae in western Kenya highlands. Malar J. 2009;8:234. doi: 10.1186/1475-2875-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaves LF, Koenraadt CJM. Climate change and highland malaria: fresh air for a hot debate. Q Rev Biol. 2010;85:27–55. doi: 10.1086/650284. [DOI] [PubMed] [Google Scholar]
- 88.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 89.Bodker R, Kisinza W, Malima R, Msangeni H, Lindsay S. Resurgence of malaria in the Usambara mountains Tanzania-an epidemic of drug-resistant parasites. Glob Change Hum Health. 2000;1:134–150. doi: 10.1023/A:1010077105257. [DOI] [Google Scholar]
- 90.Ruiz D, Brun C, Connor SJ, Omumbo JA, Lyon B, Thomson MC. Testing a multi-malaria-model ensemble against 30 years of data in the Kenyan highlands. Malar J. 2014;13:206. doi: 10.1186/1475-2875-13-206. [DOI] [PMC free article] [PubMed] [Google Scholar]


