Abstract
Background
Antecedent hypertension is associated with poor outcome in patients with ST-elevation myocardial infarction (STEMI). Whether differences in myocardial salvage, infarct size and microvascular injury contribute to the adverse outcome is unknown. We investigated the association between antecedent hypertension and cardiovascular magnetic resonance (CMR) parameters of myocardial salvage and damage in a multicenter CMR substudy of the AIDA-STEMI trial (Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction).
Methods
We analyzed 792 consecutive STEMI patients reperfused within 12 h after symptom onset. Patients underwent CMR imaging for assessment of myocardial salvage, infarct size and microvascular obstruction within 10 days after infarction. Major adverse cardiac events (MACE) were recorded at 12-month follow-up.
Results
Antecedent hypertension was present in 540 patients (68 %) and was associated with a significantly increased baseline risk profile (advanced age, higher body mass index, higher incidence of diabetes, hypercholesterolemia, previous angioplasty and multivessel disease, p < 0.001 for all). MACE were more frequent in patients with hypertension as compared to patients without hypertension (45 [8 %] vs. 8 [3 %], p < 0.01). Antecedent hypertension remained an independent predictor of MACE after multivariate adjustment (hazard ratio 3.42 [confidence interval 1.45–8.08], p < 0.01). There was, however, no significant difference in the area at risk, infarct size, myocardial salvage index, extent of microvascular obstruction, and left ventricular ejection fraction between the groups (all p > 0.05).
Conclusion
Despite a higher rate of MACE in contemporary reperfused STEMI patients with antecedent hypertension, there was no difference in reperfusion efficacy, infarct size and reperfusion injury as visualized by CMR.
Trial registration
Keywords: Hypertension, ST-elevation myocardial infarction, Cardiovascular magnetic resonance, Major adverse cardiac events
Background
Arterial hypertension (HTN) is a major cardiovascular risk factor affecting approximately 30–45 % of the general population [1, 2]. HTN is not only associated with an increased incidence of other risk factors, but also directly contributes to the development and progression of atherosclerotic disease [3]. Accordingly, patients with myocardial infarction (MI) and a history of HTN are known to be older and to have a higher rate of comorbidities and more extensive atherosclerotic disease than patients with MI but no HTN [4]. Several studies demonstrated that patients with MI and antecedent HTN are at increased risk for adverse cardiovascular events, such as heart failure, recurrent MI or death [4–11]. However, whether differences in myocardial salvage, infarct size or microvascular injury contribute to the adverse outcome in contemporary reperfused MI patients is unknown. Cardiovascular magnetic resonance (CMR) is the reference technique for the determination of these infarct characteristics [12], which are known to be strongly correlated with clinical prognosis in patients with ST-elevation MI (STEMI) reperfused by primary percutaneous coronary intervention (PPCI) [13–16].
The aim of this study was therefore to assess the association of antecedent HTN with myocardial salvage and damage determined by CMR as well as to evaluate its prognostic significance on adverse clinical events in a large multicenter cohort of consecutive patients treated with PPCI for STEMI.
Methods
Study design and population
This was an analysis of data from the multicenter CMR substudy of the AIDA STEMI trial (Abciximab Intracoronary versus intravenously Drug Application in ST-Elevation Myocardial Infarction; NCT00712101). The rationale, design and main results of AIDA STEMI as well as its CMR substudy have been reported elsewhere [15, 17–20]. Briefly, AIDA STEMI randomized 2065 consecutive STEMI patients treated within 12 h after symptom onset to either intracoronary or intravenous abciximab bolus (0.25 mg/kg) during PPCI with a subsequent 12 h dose-adjusted intravenous infusion. The CMR substudy enrolled 795 patients at 8 sites with proven expertise in CMR imaging, which was performed within 10 days after infarction. Importantly, there was no difference in CMR parameters of myocardial salvage and damage between both treatment groups [20]. The presence of antecedent arterial HTN was prospectively questioned and hypertension was diagnosed if patients were on antihypertensive treatment or had ≥3 systolic blood pressure values >140 mmHg on at least two different days. Patients were categorized as having antecedent HTN or not. Major adverse cardiac events (MACE), defined as a composite of all-cause death, nonfatal re-MI and new congestive heart failure, were recorded at 12 months after STEMI. Detailed endpoint definitions have been published previously [17]. The local ethics committees approved the study and all patients gave a written informed consent.
Cardiovascular magnetic resonance
Patients were examined at rest in the supine position on 1.5 or 3.0 Tesla scanners at day 1 to 10 after STEMI. Standardized imaging sequences were used as described previously [17, 20, 21]. Blinded readers performed image analysis at the CMR core laboratory (University of Leipzig - Heart Center, Leipzig, Germany). Commercially available software was used for post-processing (cmr42, Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada). The core laboratory has proven excellent reproducibility and low inter- as well as intra-observer variability in patients with acute STEMI [22]. Measurements of the area-at-risk, infarct size, and microvascular obstruction were expressed as the percentage of left ventricular (LV) volume (%LV). Myocardial salvage index was determined from area at risk and infarct size as previously described [13]. As described previously [20], LV function was available for 792 patients. Late gadolinium enhancement (LGE) for the determination of infarct size and microvascular obstruction was available in 774 patients. T2-weighted imaging for the assessment of the area-at-risk was available for 695 patients. The prevalence of incomplete CMR scans was not significantly different between patients with or without antecedent HTN.
Statistical analysis
Since most of the continuous variables were non-normally distributed, all continuous data were expressed as median with interquartile range for reasons of uniformity. Categorical data were depicted as counts and percentage. Distribution of data was tested using the Shapiro-Wilk test. Testing for differences between groups was performed with Mann–Whitney-U test or Kruskal-Wallis test for continuous variables and x 2-test for categorical variables. To identify predictors of infarct size, microvascular obstruction, myocardial salvage index, and left ventricular ejection fraction, multiple linear regression analysis was performed. Outcome functions were assessed using Kaplan-Meier graphs with log-rank comparison between groups. In addition, univariate and multivariate Cox regression analysis was performed to identify predictors for time to MACE. Multivariate analysis was performed using variables with a p < 0.05 in univariate analysis. All variables listed in Table 1 were assessed in univariate analysis (except the TIMI risk score). Tests were two-tailed, and a probability value of <0.05 was considered statistically significant. All analyses were performed using SPSS version 22.0.0 (IBM, Armonk, NY, USA).
Table 1.
Baseline characteristics
| All patients (n = 792) | Hypertension (n = 540) | No hypertension (n = 252) | p value | |
|---|---|---|---|---|
| Age, years | 62 [51–71] | 66 [55–73] | 54 [48–63] | <0.001 |
| Male sex, n (%) | 600 (76) | 389 (72) | 211 (84) | <0.001 |
| Body mass index, kg/m2 | 27 [25–30] | 28 [25–31] | 26 [24–29] | <0.001 |
| Cardiovascular risk factors | ||||
| Current smoking, n (%) | 339/724 (43) | 194/482 (36) | 145/242 (58) | <0.001 |
| Diabetes mellitus, n (%) | 160/789 (20) | 142/539 (26) | 18/250 (7) | <0.001 |
| Hypercholesterolemia, n (%) | 303/784 (38) | 250/536 (46) | 53/250 (21) | <0.001 |
| Family history for CAD, n (%) | 268/611 (34) | 177/407 (33) | 91/204 (36) | 0.79 |
| Systolic blood pressure, mmHg | 130 [117–147] | 135 [119–150] | 126 [114–140] | <0.001 |
| Diastolic blood pressure, mmHg | 80 [70–88] | 80 [70–90] | 80 [70–85] | 0.07 |
| Heart rate, beats/min | 76 [67–87] | 76 [68–88] | 76 [65–85] | 0.23 |
| Time from symptom onset to PCI hospital admission, min | 180 [109–310] | 183 [110–311] | 166 [104–306] | 0.23 |
| Door-to-balloon time, min | 30 [22–42] | 30 [23–42] | 29 [20–42] | 0.56 |
| Previous infarction, n (%) | 48/791 (6) | 42/539 (8) | 6/631 (2) | <0.01 |
| Previous PPCI, n (%) | 67 (9) | 61 (11) | 6 (2) | <0.001 |
| Previous CABG, n (%) | 11 (1) | 11 (2) | 0 (0) | 0.02 |
| Anterior infarction, n (%) | 395/755 (50) | 262/516 (49) | 133/239 (53) | 0.21 |
| Killip-class on admission | 0.52 | |||
| 1, n (%) | 696 (88) | 472 (87) | 224 (89) | |
| 2, n (%) | 59 (7) | 42 (8) | 17 (7) | |
| 3, n (%) | 20 (3) | 16 (3) | 4 (2) | |
| 4, n (%) | 17 (2) | 10 (2) | 7 (3) | |
| Number of diseased vessels | <0.01 | |||
| 1, n (%) | 419 (53) | 261 (48) | 158 (63) | |
| 2, n (%) | 225 (28) | 165 (31) | 60 (24) | |
| 3, n (%) | 148 (19) | 114 (21) | 34 (14) | |
| Infarct related artery | 0.59 | |||
| Left anterior descending, n (%) | 344 (43) | 240 (44) | 104 (41) | |
| Right coronary artery, n (%) | 344 (43) | 226 (42) | 118 (47) | |
| Left circumflex, n (%) | 97 (12) | 68 (13) | 29 (12) | |
| Left main, n (%) | 5 (1) | 4 (1) | 1 (0) | |
| Bypass graft, n (%) | 2 (0) | 2 (0) | 0 (0) | |
| TIMI risk score | 3 [2–5] | 4 [2–5] | 2 [2–4] | <0.001 |
| TIMI-flow before PPCI | 0.64 | |||
| TIMI-flow 0, n (%) | 444 (56) | 298 (55) | 146 (58) | |
| TIMI-flow 1, n (%) | 103 (13) | 73 (14) | 30 (12) | |
| TIMI-flow 2, n (%) | 128 (16) | 92 (17) | 36 (14) | |
| TIMI-flow 3, n (%) | 117 (15) | 77 (14) | 40 (16) | |
| TIMI-flow after PPCI | 0.38 | |||
| TIMI-flow 0, n (%) | 12 (2) | 9 (2) | 3 (1) | |
| TIMI-flow 1, n (%) | 19 (2) | 14 (3) | 5 (2) | |
| TIMI-flow 2, n (%) | 61 (8) | 47 (9) | 14 (6) | |
| TIMI-flow 3, n (%) | 699 (88) | 469 (87) | 230 (91) | |
| Stent implanted, n (%) | 774 (98) | 527 (98) | 247 (98) | 0.60 |
| Thrombectomy, n (%) | 190 (24) | 124 (23) | 66 (26) | 0.32 |
| Concomitant medications | ||||
| Aspirin, n (%) | 790 (100) | 538 (100) | 252 (100) | 1.0 |
| ß-blockers, n (%) | 757 (96) | 519 (96) | 238 (94) | 0.19 |
| ACE-I/ARB, n (%) | 751 (95) | 515 (95) | 236 (94) | 0.21 |
| Statin, n (%) | 749 (95) | 508 (94) | 241 (96) | 0.47 |
| Aldosterone antagonist, n (%) | 80 (10) | 55 (12) | 25 (10) | 0.34 |
Data are given as median plus interquartile range or number and percentage
Abbreviations: CAD coronary artery disease, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIMI thrombolysis in myocardial infarction, ACE-I angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker
Results
Study population
Between July 2008 and April 2011 795 STEMI patients were enrolled in the CMR substudy of the AIDA STEMI trial. Information on antecedent HTN was available in 792 (99.6 %) patients, representing the final study cohort. Characteristics of the overall cohort as well as stratified by history of HTN are listed in Table 1. Antecedent HTN was associated with more advanced age, a higher body mass index, a higher prevalence of female sex, diabetes mellitus, hypercholesterolemia (p < 0.001 respectively), multivessel disease (p < 0.01), and previous myocardial infarction (p < 0.01), but a lower prevalence of current smoking (p < 0.001). HTN was also associated with a higher systolic blood pressure on admission (p < 0.001) and a trend for a higher diastolic blood pressure (p = 0.07) with no significant difference in heart rate (p = 0.23). Pre- and post-PPCI TIMI-flow were equal in both groups (p = 0.64 and p = 0.38, respectively).
Antecedent hypertension and clinical outcome
During the follow-up period of 12 months, 53 patients (7 %) experienced MACE. Of these 22 (3 %) died. Patients with antecedent HTN were at higher risk for MACE (45 [8 %] versus 8 [3 %], p < 0.01; hazard ratio: 2.70 [1.27–5.72], p = 0.01) and mortality (20 [4 %] versus 2 [1 %], p = 0.02; hazard ratio: 4.83 [1.13–20.67], p = 0.03) as compared to patients without antecedent HTN (Table 2). Consequently, antecedent HTN showed a significant association with MACE- and mortality-free survival (p < 0.01 and p = 0.02, respectively, Fig. 1). In multivariate Cox regression analysis, antecedent HTN was independently associated with the occurrence of MACE (Table 3). In a second Cox regression model including established CMR prognosis markers (left ventricular ejection fraction, infarct size, microvascular obstruction), antecedent HTN was also independently associated with MACE after 12 months (hazard ratio: 2.55 [1.20–5.43], p = 0.02). 339 (43 %) of patients had HTN at the time of the acute event (defined as systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥ 90 mmHg). In contrast with antecedent HTN, HTN at the time of the acute event was not significantly associated with the rate of MACE (log-rank p = 0.18).
Table 2.
Event rates at 12 months after infarction
| All patients (n = 792) | Hypertension (n = 540) | No hypertension (n = 252) | p value | |
|---|---|---|---|---|
| MACE, n (%) | 53 (7) | 45 (8) | 8 (3) | <0.01 |
| Death, n (%) | 22 (3) | 20 (4) | 2 (1) | 0.02 |
| Re-infarction, n (%) | 21 (3) | 16 (3) | 5 (2) | 0.42 |
| Heart failure event, n (%) | 25 (3) | 21 (4) | 4 (2) | 0.08 |
Data are given as number and percentage
Abbreviations: MACE major adverse cardiac events
Fig. 1.
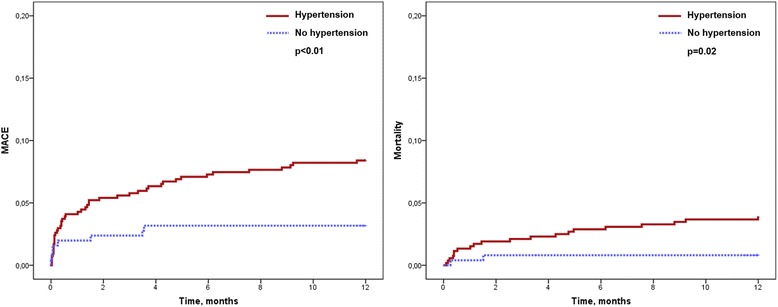
Kaplan-Meier curves for the occurrence of major adverse cardiac events and death according to antecedent hypertension
Table 3.
Predictors of MACE in uni- and multivariate Cox regression analysis
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Age, >median | 2.49 [1.38–4.47] | <0.01 | - | - |
| Gender, 1 female | 1.95 [1.12–3.40] | 0.02 | - | - |
| Smoking status, 1 yes | 0.46 [0.24–0.87] | 0.02 | - | - |
| Diabetes mellitus, 1 yes | 1.91 [1.07–3.41] | 0.03 | - | - |
| Hypertension, 1 yes | 2.70 [1.27–5.72] | 0.01 | 3.42 [1.45–8.08] | <0.01 |
| Heart rate, >median | 1.96 [1.12–3.43] | 0.02 | - | - |
| Killip-class, I-IV | 1.90 [1.46–2.48] | <0.001 | 1.91 [1.40–2.60] | <0.001 |
| Vessel Disease, I-III | 1.34 [1.01–1.78] | 0.04 | - | - |
Uni- and multivariate Cox regression analysis for the prediction of MACE at 12 months after infarction. Only significant variables in univariate analysis are reported
Abbreviations: MACE major adverse cardiac events, HR hazard ratio, CI confidence interval
Antecedent hypertension and cardiovascular magnetic resonance findings
The median time between the index event and CMR scan was 3 [2–4] days. CMR findings and their relation with antecedent HTN are shown in Table 4. Patients with antecedent HTN had a similar extent of the area at risk (p = 0.18), infarct size (p = 0.74), microvascular obstruction (p = 0.93), and a comparable myocardial salvage index (0.20). There was also no significant difference in left ventricular ejection fraction between groups (p = 0.47). Antecedent HTN was not predictive for infarct size, microvascular obstruction, myocardial salvage index or left ventricular ejection fraction at linear regression analyses (all p > 0.05). The power of this study to exclude a difference in infarct size or LV ejection fraction of 3 % between groups is 94.9 and 98.5 %, respectively.
Table 4.
Cardiovascular magnetic resonance findings
| All patients (n = 792) | Hypertension (n = 540) | No hypertension (n = 252) | p value | |
|---|---|---|---|---|
| Area at risk, % LV | 35 [25–48] | 34 [25–47] | 37 [26–49] | 0.18 |
| Infarct size, % LV | 16.7 [8.5–24.9] | 16.9 [8.8–24.8] | 16.6 [8.3–25.0] | 0.74 |
| Presence of microvascular obstruction, n (%) | 400 (51) | 274 (51) | 126 (50) | 0.85 |
| Extent of microvascular obstruction, % LV | 0 [0–1.8] | 0 [0.0–1.6] | 0 [0.0–2.0] | 0.93 |
| Myocardial salvage index, % area at risk | 51 [33–69] | 50 [33–67] | 54 [32–72] | 0.20 |
| LV ejection fraction, % | 50 [43–58] | 50 [43–58] | 51 [44–58] | 0.47 |
| LV myocardial mass, g | 132 [109–156] | 134 [111–158] | 131 [109–155] | 0.38 |
Data are given as median plus interquartile range or number and percentage
Abbreviations: CMR cardiovascular magnetic resonance, LV left ventricular
Discussion
This is the first study evaluating the impact of antecedent HTN on CMR parameters of myocardial salvage as well as myocardial and microvascular damage in patients undergoing PPCI for acute STEMI. There are two main findings: (1) antecedent HTN was independently associated with a higher rate of MACE and mortality at 12-month follow-up; (2) however, there were no differences in CMR-determined myocardial salvage, infarct size or extent of microvascular obstruction between patients with or without antecedent HTN. Together, these findings highlight the importance of a history of HTN as risk factor of adverse outcome in contemporary reperfused STEMI patients, which is however not attributed to differences in PPCI efficacy (myocardial salvage), extent of myocardial necrosis or microvascular injury as visualized by CMR.
Antecedent hypertension, baseline risk profile and clinical outcome
As a consequence of its well established role as a major cardiovascular risk factor [1], antecedent HTN is a frequent finding in MI patients. In our analysis, history of HTN was present in 68 % of STEMI patients. These results fit well with recent prospective STEMI registry studies which reported a prevalence between 60 and 70 % [23, 24]. Antecedent HTN has been acknowledged as significant risk factor for adverse outcome in survivors of acute MI for many years [4–6]. For instance, Thune et al. found that antecedent HTN at the time of MI was strongly associated with an increased risk of future heart failure, stroke, cardiovascular death and a composite endpoint consisting of death, myocardial infarction, heart failure, stroke, or cardiac arrest in more than 14.000 patients included in the VALIANT trial (Valsartan in Myocardial Infarction Trial) [4]. Nevertheless, over the last years, reperfusion strategies and pharmacological interventions were significantly refined and consequently improved the outcome of patients presenting with acute MI [25]. These advances might have modified the relationship between HTN and the clinical course after STEMI [26]. Indeed, in a meta-analysis performed by Chen et al. the strength of the association between antecedent HTN and poor clinical outcome has decreased over time [11]. However, antecedent HTN was still associated with increased MACE and mortality rates at 12-month follow-up in our study. This was also observed after adjustment for confounding variables. Therefore, our findings highlight that even in the contemporary era of PPCI the simple information of HTN history can be used to identify patients at risk for adverse prognosis post-STEMI. This high risk group might also benefit from more intensified medical treatment strategies including aggressive antihypertensive treatment. Contrary to the information on antecedent HTN, HTN at the time of the acute event was not related with MACE in our study. Consequently, antecedent HTN seems of greater importance as compared with HTN at the time of STEMI.
It is well established that MI patients with a history of HTN show a distinctive baseline risk profile, irrespective of STEMI or non-STEMI presentation. Studies consistently observed that hypertensive patients with MI were older, more often female, had a higher body mass index, and higher rates of other cardiovascular risk factors including diabetes or hypercholesterolemia [6–10]. Our data confirm these previous findings and it is likely that these factors might at least partly explain the higher risk of hard clinical events of hypertensive STEMI patients. Further observations from our and other studies are the higher incidence of a previous cardiac disease and the greater extent of coronary artery disease [4, 7, 9, 10]. Since both have been proved to be associated with adverse clinical outcome in STEMI patients [27], these factors might also contribute to the increased risk of infarction patients with antecedent HTN. Recent studies could also disclose that diffuse myocardial fibrosis, measured by T1 mapping, is increased in patients with hypertension and previous infarction [28].
Antecedent hypertension and infarct characteristics
It has been speculated that coronary occlusion in patients with pre-existing HTN might be associated with more extensive myocardial damage, greater myocardial dysfunction, and consequently adverse clinical outcome [11, 26]. Contrary, one might argue that a higher diastolic pressure could enhance coronary perfusion and collateral circulation with subsequently improved efficacy of reperfusion and limited expansion of myocardial necrosis. Studies investigating the impact of antecedent HTN on myocardial damage are very limited so far [29]. In particular, there is no study answering these questions by using the current reference standard technique, which is CMR [12]. CMR not only enables exact infarct sizing but also detailed tissue characterization of the jeopardized and infarcted myocardium [12]. These additional assessed parameters, primarily microvascular obstruction, but also myocardial salvage index provide strong prognostic information that is incremental to clinical, biomarker, electrocardiographic, and angiographic risk markers [15, 30]. To the best of our knowledge, the study by De Luca et al. [29]. is the first and only study that evaluated the impact of HTN on infarct size in 830 STEMI patients undergoing PPCI. The authors found that HTN was not associated with larger infarcts as determined by scintigraphy at 1 month after infarction. Nevertheless, some important limitations of this study have to be mentioned. The authors used a scintigraphic technique to assess infarct size, which is less accurate compared to CMR, especially for the detection of subendocardial infarcts [31]. Secondly, De Luca et al. were also not able to assess the association of antecedent HTN with myocardial salvage and microvascular dysfunction. Our study therefore complements and extends the previous literature by showing for the first time that the adverse outcome of reperfused STEMI patients with antecedent HTN is not associated with differences in reperfusion success or more pronounced myocardial injury.
Limitations
Our study has some important limitations. Based on the in- and exclusion criteria of this multicenter clinical trial as well as the treatment of patients in specialized high-volume centers, our results might not be generalizable to all STEMI populations. Although AIDA STEMI was designed as all-comers trial to represent a “real-world” STEMI population [18, 20], further confirmation of our findings in other studies would be important. It is, however, important to note that the power of our study to exclude a 3 % difference in infarct size or LV ejection fraction between groups was approximately 95 %. Information on HTN was based on patient history and data on the duration of HTN as well as patients adherence to medication and effectiveness of HTN treatment, which could have prognostic implications as well, was not available. Nevertheless, our study shows that the simple assessment of antecedent HTN by anamnesis or during hospitalization has strong prognostic implications in STEMI patients treated with PPCI. Forty-eight patients (6 %) had a previous myocardial infarction. Chronic infarcts were not included in infarct size measurements and thus the “true volume” of the infarcted myocardium is not represented in the reported values. On the other hand, omitting those patients from statistical analysis did not significantly change the results.
Conclusion
This study found higher rates of MACE and mortality in contemporary reperfused STEMI patients with antecedent HTN despite no difference in reperfusion success and extent of myocardial and microvascular damage as determined by CMR.
Acknowledgments
None.
Funding
None.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
HT, IE, SD, GF, and SDW coordinated and performed the AIDA-STEMI trial. SJR, SD, and IE participated in the analysis of CMR. SJR, TS, CE, HT, and IE conceived the study. SJR, TS, CE, MS, BM, GF and IE helped to interpret the data and draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- AIDA-STEMI
Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction
- CMR
Cardiovascular magnetic resonance
- HTN
Arterial hypertension
- LV
Left ventricular
- MACE
Major adverse cardiac events
- MI
Myocardial infarction
- PPCI
Primary percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction
- VALIANT
Valsartan in Myocardial Infarction Trial
Contributor Information
Sebastian J. Reinstadler, Email: sebastian.reinstadler@tirol-kliniken.at
Thomas Stiermaier, Email: thomas.stiermaier@uksh.de.
Charlotte Eitel, Email: charlotte.eitel@uksh.de.
Mohammed Saad, Email: mohammed.saad@uksh.de.
Bernhard Metzler, Email: bernhard.metzler@tirol-kliniken.at.
Suzanne de Waha, Email: suzanne.dewaha@uksh.de.
Georg Fuernau, Email: georg.fuernau@uksh.de.
Steffen Desch, Email: steffen.desch@uksh.de.
Holger Thiele, Email: holger.thiele@uksh.de.
Ingo Eitel, Phone: +49 451 500 2291, Email: ingoeitel@gmx.de.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective SC. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 3.Rakugi H, Yu H, Kamitani A, Nakamura Y, Ohishi M, Kamide K, et al. Links between hypertension and myocardial infarction. Am Heart J. 1996;132:213–21. doi: 10.1016/S0002-8703(05)80002-X. [DOI] [PubMed] [Google Scholar]
- 4.Thune JJ, Signorovitch J, Kober L, Velazquez EJ, McMurray JJ, Califf RM, et al. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension. 2008;51:48–54. doi: 10.1161/HYPERTENSIONAHA.107.093682. [DOI] [PubMed] [Google Scholar]
- 5.Fresco C, Avanzini F, Bosi S, Franzosi MG, Maggioni AP, Santoro L, et al. Prognostic value of a history of hypertension in 11,483 patients with acute myocardial infarction treated with thrombolysis. GISSI-2 Investigators. Gruppo Italiano per lo Studio della, Sopravvivena nell’Infarto Miocardico. J Hypertens. 1996;14:743–50. doi: 10.1097/00004872-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Richards AM, Nicholls MG, Troughton RW, Lainchbury JG, Elliott J, Frampton C, et al. Antecedent hypertension and heart failure after myocardial infarction. J Am Coll Cardiol. 2002;39:1182–8. doi: 10.1016/S0735-1097(02)01737-0. [DOI] [PubMed] [Google Scholar]
- 7.Rembek M, Goch A, Goch J. The clinical course of acute ST-elevation myocardial infarction in patients with hypertension. Kardiol Pol. 2010;68:157–63. [PubMed] [Google Scholar]
- 8.Lee MG, Jeong MH, Lee KH, Park KH, Sim DS, Yoon HJ, et al. Prognostic impact of diabetes mellitus and hypertension for mid-term outcome of patients with acute myocardial infarction who underwent percutaneous coronary intervention. J Cardiol. 2012;60:257–63. doi: 10.1016/j.jjcc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 9.De Luca G, van’t Hof AW, Huber K, Gibson CM, Bellandi F, Arntz HR, et al. Impact of hypertension on distal embolization, myocardial perfusion, and mortality in patients with ST segment elevation myocardial infarction undergoing primary angioplasty. Am J Cardiol. 2013;112:1083–6. doi: 10.1016/j.amjcard.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 10.De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, et al. Impact of hypertension on clinical outcome in STEMI patients undergoing primary angioplasty with BMS or DES: insights from the DESERT cooperation. Int J Cardiol. 2014;175:50–4. doi: 10.1016/j.ijcard.2014.04.180. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Hemmelgarn B, Alhaider S, Quan H, Campbell N, Rabi D. Meta-analysis of adverse cardiovascular outcomes associated with antecedent hypertension after myocardial infarction. Am J Cardiol. 2009;104:141–7. doi: 10.1016/j.amjcard.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Klug G, Metzler B. Assessing myocardial recovery following ST-segment elevation myocardial infarction: short- and long-term perspectives using cardiovascular magnetic resonance. Expert Rev Cardiovasc Ther. 2013;11:203–19. doi: 10.1586/erc.12.173. [DOI] [PubMed] [Google Scholar]
- 13.Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, et al. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart. 2011;97:2038–45. doi: 10.1136/heartjnl-2011-300098. [DOI] [PubMed] [Google Scholar]
- 14.Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, et al. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:46. doi: 10.1186/1532-429X-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217–26. doi: 10.1016/j.jacc.2014.06.1194. [DOI] [PubMed] [Google Scholar]
- 16.Eitel I, Poss J, Jobs A, Eitel C, de Waha S, Barkhausen J, et al. Left ventricular global function index assessed by cardiovascular magnetic resonance for the prediction of cardiovascular events in ST-elevation myocardial infarction. J Cardiovasc Magn Reson. 2015;17:62. doi: 10.1186/s12968-015-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiele H, Wohrle J, Neuhaus P, Brosteanu O, Sick P, Prondzinsky R, et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention: design and rationale of the Abciximab Intracoronary versus intravenously Drug Application in ST-Elevation Myocardial Infarction (AIDA STEMI) trial. Am Heart J. 2010;159:547–54. doi: 10.1016/j.ahj.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Thiele H, Wohrle J, Hambrecht R, Rittger H, Birkemeyer R, Lauer B, et al. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: a randomised trial. Lancet. 2012;379:923–31. doi: 10.1016/S0140-6736(11)61872-2. [DOI] [PubMed] [Google Scholar]
- 19.Desch S, Wohrle J, Hambrecht R, Rittger H, Birkemeyer R, Lauer B, et al. Intracoronary versus intravenous abciximab bolus in patients with ST-segment elevation myocardial infarction: 1-year results of the randomized AIDA STEMI trial. J Am Coll Cardiol. 2013;62:1214–5. doi: 10.1016/j.jacc.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Eitel I, Wohrle J, Suenkel H, Meissner J, Kerber S, Lauer B, et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J Am Coll Cardiol. 2013;61:1447–54. doi: 10.1016/j.jacc.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 21.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desch S, Engelhardt H, Meissner J, Eitel I, Sareban M, Fuernau G, et al. Reliability of myocardial salvage assessment by cardiac magnetic resonance imaging in acute reperfused myocardial infarction. Int J Cardiovasc Imaging. 2012;28:263–72. doi: 10.1007/s10554-011-9802-9. [DOI] [PubMed] [Google Scholar]
- 23.Bauer T, Hochadel M, Brachmann J, Schachinger V, Boekstegers P, Zrenner B, et al. Use and outcome of radial versus femoral approach for primary PCI in patients with acute ST elevation myocardial infarction without cardiogenic shock: Results from the ALKK PCI registry. Catheter Cardiovasc Interv. 2015;86:S8–14. doi: 10.1002/ccd.25987. [DOI] [PubMed] [Google Scholar]
- 24.Kontos MC, Scirica BM, Chen AY, Thomas L, Anderson ML, Diercks DB, et al. Cardiac arrest and clinical characteristics, treatments and outcomes among patients hospitalized with ST-elevation myocardial infarction in contemporary practice: A report from the National Cardiovascular Data Registry. Am Heart J. 2015;169:515–22. doi: 10.1016/j.ahj.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. doi: 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedrinelli R, Ballo P, Fiorentini C, Denti S, Galderisi M, Ganau A, et al. Hypertension and acute myocardial infarction: an overview. J Cardiovasc Med. 2012;13:194–202. doi: 10.2459/JCM.0b013e3283511ee2. [DOI] [PubMed] [Google Scholar]
- 27.Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28:1709–16. doi: 10.1093/eurheartj/ehm184. [DOI] [PubMed] [Google Scholar]
- 28.Treibel TA, Zemrak F, Sado DM, Banypersad SM, White SK, Maestrini V, et al. Extracellular volume quantification in isolated hypertension - changes at the detectable limits? J Cardiovasc Magn Reson. 2015;17:74. doi: 10.1186/s12968-015-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Luca G, Parodi G, Sciagra R, Bellandi B, Comito V, Vergara R, et al. Impact of hypertension on infarct size in ST elevation myocardial infarction patients undergoing primary angioplasty. J Hypertens. 2013;31:2433–7. doi: 10.1097/HJH.0b013e328364cbee. [DOI] [PubMed] [Google Scholar]
- 30.de Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Stiermaier T, et al. Prognosis after ST-elevation myocardial infarction: a study on cardiac magnetic resonance imaging versus clinical routine. Trials. 2014;15:249. doi: 10.1186/1745-6215-15-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–9. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


