Abstract
Introduction
Familial hemiplegic migraine (FHM) is a rare autosomal dominant subtype of migraine with aura. The FHM3 subtype is caused by mutations in SCN1A, which is also the most frequent epilepsy gene encoding the voltage-gated Na+ channel NaV1.1. The aim of this study was to explore the clinical, genetic and pathogenetic features of a pure FHM3 family.
Methods
A three-generation family was enrolled in this study for genetic testing and assessment of clinical features. Whole cell patch-clamp was performed to determine the functions of identified mutant NaV1.1 channels, which were transiently expressed in human tsA201 cells together with β1 and β2 subunits.
Results and conclusions
We identified a novel SCN1A (p.Leu1624Pro) mutation in a pure FHM family with notably early-onset attacks at mean age of 7. L1624P locates in S3 of domain IV, the same domain as two of four known pure FHM3 mutations. Compared to WT channels, L1624P displayed an increased threshold-near persistent current in addition to other gain-of-function features such as: a slowing of fast inactivation, a positive shift in steady-state inactivation, a faster recovery and higher channel availability during repetitive stimulation. Similar to the known FHM3 mutations, this novel mutation predicts hyperexcitability of GABAergic inhibitory neurons.
Keywords: Familial hemiplegic migraine, early-onset, gain of function, FHM3, NaV1.1
Introduction
Familial hemiplegic migraine (FHM) is a rare autosomal dominant migraine subtype associated with aura including reversible hemiparesis (1,2). FHM has a remarkable phenotypic variability among patients even within the same family. Clinical onset is usually during youth. So far three causative genes have been identified, all encoding ion transport proteins: CACNA1A (FHM1) (3), ATP1A2 (FHM2) (4) and SCN1A (FHM3) (5). Recently PRRT2 has also been found to be associated with hemiplegic migraine (6). Mutations in these four genes are postulated to influence the initiation and propagation of cortical spreading depression (CSD), as evidenced by data from gene-targeted mouse models. CSD is a short-lasting propagating wave of cellular depolarization followed by a long-lasting suppression of the neuronal activity that is believed to be the basis for the migraine aura (7). The likelihood of CSD is increased by hyperexcitability of the neuronal network (8,9). In FHM1, CaV2.1 mutations induce a gain-of-function resulting in enhanced glutamate release and facilitated CSD. In FHM2, mutations of the sodium potassium ATPase cause a loss of function, leading to accumulation of extracellular K+ and the development of CSD (8,9). In FHM3, NaV1.1 mutations predict increased firing and a hyperexcitability of GABAergic neurons. This may also lead to an increase in extracellular K+ and thereby trigger CSD (10,11).
SCN1A, the gene encoding NaV1.1, is the most relevant gene in epilepsy with hundreds of mutations linked to a spectrum of epilepsy syndromes of varying severity (12). Previous studies of these epileptic mutations revealed mainly loss-of-function effects, particularly in mouse models. These studies predict network hyperexcitability due to specifically reduced action potential firing in inhibitory GABAergic interneurons, in which NaV1.1 is predominantly expressed (13–15). In vitro studies, also mainly loss-of-function effects were found, whereas a few studies also reported partial gain-of-function effects (16). Until now, only eight SCN1A missense mutations have been described to cause FHM3. Of those, some mutations (Q1489K, L1649Q, I1498M and F1661L) were identified in pure FHM families (5,17,18), whereas others were described to be linked to FHM associated with either epileptic seizures (L263Q, T1174S and Q1489H) or elicited repetitive daily blindness (Q1489H and F1499L) (19–21). Functional investigation of FHM3-causing SCN1A mutations revealed that most had gain-of-function effects such as increased non-inactivated depolarization-induced persistent sodium currents, delayed entry into inactivation, accelerated recovery from fast inactivation and rescue of folding defects by incubation at lower temperature or co-expression of interacting proteins (5,10,11,17,22). In contrast, the FHM3 mutation T1174S in NaV1.1, which incidentally caused a mixed FHM3 and epilepsy phenotype, showed both gain and loss of function (21).
In the present study, we identify a novel NaV1.1 L1624P mutation in a pure FHM family with early age at clinical onset. Electrophysiological studies show an overall gain of function of this mutation in agreement with the previously assumed pathomechanism of FHM3.
Materials and methods
Genetics
A three-generation family with five FHM patients was enrolled in this study, and the clinical features were analyzed. Their blood samples were collected for mutation screening after informed consent was obtained. All procedures were approved by the Ethics Committee of Ulm University and were in accordance with the Declaration of Helsinki. The mutation screening was performed by direct sequencing of all coding exons and exon-intron junctions of CACNA1A, ATP1A2 and SCN1A genes. SCN1A mutation nomenclature is based on reference transcript NM_001165963.1.
Functional studies
The shorter isoform (−11 amino acids) of the human NaV1.1 α subunit subcloned to the pCDM8 vector was used for site-directed mutagenesis of L1613P (equivalent mutation L1624P). SCN1A was transiently transfected in human tsA201 cells together with the auxiliary subunits β1 and β2 coupled to green fluorescent protein (GFP) and CD8 antigen, respectively. Whole-cell patch clamp recordings were performed 24 hours after transfection. Only cells expressing both reporters and producing voltage-dependent inward sodium currents as previously reported were used and analyzed (23). The pipette resistance was around 1.5 MΩ after filling with internal solution containing (in mM): NaF 10, CsF 110, CsCl 20, ethylene glycol tetraacetic acid (EGTA) 2, HEPES 10, NaATP 2 and NaGTP 0.2. The external solution contained (in mM) NaCl 145, KCl 4, CaCl2 1.8, MgCl2 1 and HEPES 10. pH was adjusted to 7.3 and osmolarity to ∼300 mOsm. Before data acquisition, cells were allowed to stabilize for 10 min after establishment of the whole-cell configuration. Sodium currents were recorded at room temperature (21–23℃) after partial series resistance compensation (∼85%) using an Axopatch 200B amplifier (Axon Instruments). The voltage errors were <5 mV. Data were filtered at 10 kHz and sampled at 50 kHz. Only amplitudes of peak sodium currents between 1.3 to 14 nA were used for analysis. All recordings were performed without online leak subtraction. The linear passive currents at various potentials were calculated from the value at the holding potential of −120 mV, and these currents were subtracted from the current recordings.
Data analysis
The steady-state activation was determined by fitting the peak current-voltage relation with the equation
with Vrev and gmax being the reversal potential and maximum conductance of channels, V0.5 the potential for half-maximal current, V the test potential and k the slope factor.
Time constants of fast inactivation were obtained by fitting a double exponential function to the current decay
with t being the time, Afast and Aslow the fraction of channels inactivating with time constant τfast and τslow, respectively, and C the asymptote.
Steady-state inactivation curves were evaluated by fitting the data to the equation
with V representing the pre-pulse potential, V0.5 the potential at which half of the channels are inactivated, C the fraction of non-inactivated channels and k is the slope factor.
The recovery from inactivation was analyzed by fitting the data with a double exponential function:
with τfast and τslow denoting a fast and a slow time constant, Afast and Aslow representing the two fractional amplitudes and C the level of non-inactivating sodium current.
Entry into slow inactivation was fitted by a single decay function
with t being the time, A the fraction of channels inactivating with time constant τ and C the asymptote.
Data were analyzed by a combination of pClamp (Axon Instruments), Excel (Microsoft), SPSS (IBM) and ORIGIN (MicroCal software). Data are presented as mean ± standard error of the mean (SEM). Student’s t-tests were applied for statistical evaluation with significance levels set to *p < 0.05, **p < 0.01 and ***p < 0.001.
Results
Phenotype and genotype
The three-generation German family contained five patients with migraine attacks fulfilling International Headache Society (IHS) criteria for FHM (Figure 1(a)). All patients, except the deceased grandfather of the index patient, underwent a detailed semi-structured individual personal interview or telephone interview, putting particular emphasis on the occurrence and severity of motor aura symptoms. Detailed clinical characteristics are depicted in Table 1.
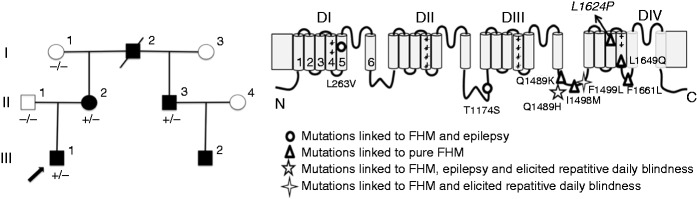
Figure 1.
FHM family with a novel SCN1A-L1624P mutation.
(a) Family pedigree. The proband is indicated by an arrow. III.2 did not attend the genetic test. Squares show men and circles women. Black inlays show affected and white healthy individuals. +/– refers to presence or absence of mutated SCN1A allele. (b) Scheme of the NaV1.1 α subunit showing the location of the novel mutation L1624P indicated by arrow and known FHM3 mutations. FHM: familial hemiplegic migraine.
Table 1.
Clinical features of the FHM3 family.
| Aura symptoms during hemiplegic attacks |
Hemiplegic attacks |
Headache characteristics |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age (years) | HM onset (years) | Triggering factors for hemiplegic attacks | H | S | V | A | Others | Duration | Frequency | Side | Duration | Side | Character | Temporal relation to hemiplegic attacks | Anatomical relation to hemiplegic attacks | Nausea/ vomiting/ photophobia/ phonophobia | Other types of attacks |
| I.2 | a | NK | − | + | + | NK | NK | − | NK | NK | NK | NK | NK | NK | NK | NK | NK | NK |
| II.2 | 58 | 8 | Emotional stress | + | + | + | − | Panic | 30 minutes to three hours | 2–3/year | R > L | Up to six hours | Unilateral | Piercing | HP > HA | Ipsilateral | −/−/−/− | MO |
| II.3 | 48 | 8 | − | + | + | + | + | Dysarthria | 30 minutes | 3–4/year | R = L | Up to 24 hours | Uni- and bilateral | Pressing | HP > HA | Ipsilateral | −/−/−/+ | Isolated visual aura |
| III.1 | 27 | 5 | Nutrients: walnuts, aspartame, cheese | + | + | + | + | − | 15 minutes to 12 hours | 1–2/month | R < L | Up to 12 hours | Unilateral | Stabbing, throbbing | HP > HA | Ipsi- and contralateral | +/+/+/+ | Isolated hemiplegic aura |
| III.2 | 10 | 6 | − | + | + | + | + | − | 20 minutes | 3/year | R = L | Up to 24 hours | Bilateral | Pressing | HP > HA | NA | −/−/−/+ | − |
Deceased. ID: identification; FHM3: familial hemiplegic migraine 3; NK: not known; NA: not applicable; H: hemiplegic; S: sensory disturbance; V: visual disturbance; A: aphasia; MO: migraine without aura; HP > HA: hemiplegic attack precedes headache; R: right side; L: left side.
III.1 The index patient, currently 27 years of age, had hemiplegic migraine since the age of 5. Attacks occurred once or twice per month. Hemiplegia started usually in the upper limb with spreading to the lower limb causing inability to walk or hold objects. Scintillating scotoma, unilateral numbness and moderate aphasia often preceded hemiplegia. The hemiplegic attacks usually lasted for several hours, overlapping with the occurrence of ipsilateral or contralateral migrainous headache. After puberty attacks became less frequent (1–2×/year) and less severe. Cerebral magnetic resonance imaging (MRI) with contrast enhancement was normal and repeated electroencephalograms (EEGs), including 24-hour EEG and sleep-deprivation EEG, showed no epileptic features.
II.2 The index patient’s mother, currently 58 years of age, had hemiplegic migraine since the age of 8. She reported attacks two to three times/year. Hemiplegia occurred simultaneously in the upper and lower limb with clear preponderance of the right side. Scintillating scotoma and paresthesia could regularly precede hemiplegia. Aura symptoms usually lasted for several hours, followed by ipsilateral migrainous headache. After puberty the attacks became milder and less frequent. Migraine without aura occurred once a month. Cerebral MRI and repeated EEGs were normal.
II.3 The index patient’s uncle, currently 48 years of age, had hemiplegic migraine since the age of 8 years. He reported hemiplegic attacks several times per year. Hemiplegia was preceded by spreading visual and sensory aura as well as dysarthria. Aura symptoms persisted for 30 minutes, and after dissolution unilateral or bilateral headache followed. On rare occasions isolated visual aura occurred. Cerebral MRI and repetitive EEGs were normal.
III.2 The son of II.3 started having similar attacks at the age of 6 years. He is now 10 years old. Hemiplegia was preceded by visual and sensory aura and dysarthria and persisted for 20 to 30 minutes. Headache occurred after migraine dissolution.
I.2 The grandfather was deceased at the time of the interview. According to the family members, he suffered from hemiplegic migraine attacks that were reported as very similar to the phenotype of II.2.
All four tested affected family members (I.2, II.2, II.3, III.1) harbor a heterozygous T to C substitution at nucleotide 4871, corresponding to an L1624P mutation in segment S3 of domain IV of NaV1.1 (Figure 1(b)). This variant has not been reported in >120,000 alleles included in the ExAC browser. No mutations were detected in CACNA1A and ATP1A2 genes. III.2 did not attend the genetic test.
Electrophysiology
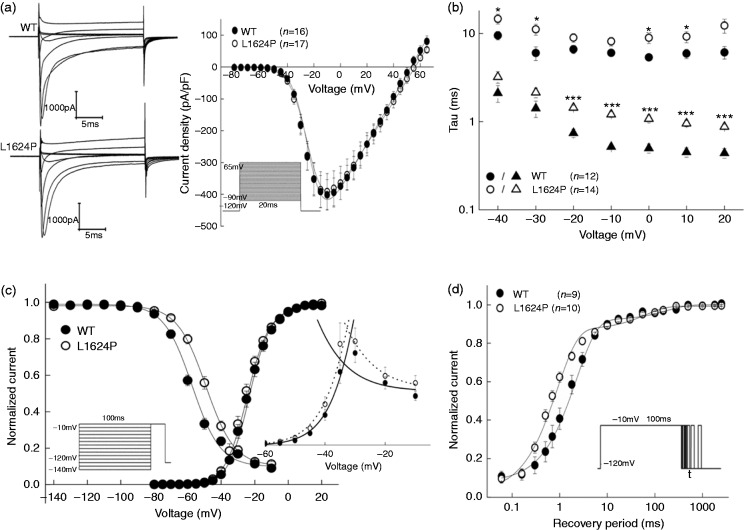
Representative whole-cell currents recorded from tsA201 cells expressing wild-type (WT) or mutant channels were elicited by various test potentials from a holding potential of −120 mV (Figure 2(a)). Peak current density of the mutant channels was similar to that of the WT channels (L1624P: −391.11 ± 56.46 pA/pF vs WT: −401.88 ± 47.93 pA/pF, Figure 2(a)). Likewise, voltage dependence of activation revealed no significant difference between WT (V0.5 = −23.93 ± 1.18 mV) and mutant L1624P channels (V0.5 = −25.36 ± 1.04 mV). The time course of fast inactivation was fitted to a second-order exponential function with currents being elicited by test pulses between −40 and 20 mV. There was a significant slowing of mutant channels, especially of the fast component (Figure 2(b)); i.e. at a test potential of −10 mV eliciting the maximum peak current, τfast of the mutant L1624P (1.21 ± 0.09 ms) was around 2.4 times slower than that of WT channels (0.52 ± 0.02 ms). This finding suggests a destabilization of the inactivated state of NaV1.1 channels. In agreement with this hypothesis, mutant channels showed a significant depolarizing shift of the steady-state fast inactivation curve (V0.5 = −49.10 ± 1.18 mV) compared with WT NaV1.1 channels (V0.5 = −58.29 ± 1.17 mV) (Table 2).
Figure 2.
Functional characteristics of WT (filled symbols) and mutant L1624P (open symbols) channels expressed in tsA201 cells.
(a) Averaged whole-cell currents of WT and mutant channels elicited from a holding potential of −120 mV by voltage steps between −80 and +65 mV in 5 mV intervals for 20 ms. For clarification, the currents were shown every 20 mV. (b) Voltage-dependence of both fast and slow time constants of fast inactivation of WT and mutant channels. (c) Steady-state fast inactivation curves (WT: n = 16 vs L1624P: n = 22) and conductance – voltage curves. Inactivation curves were determined from a holding potential of −120 mV using a series of 100 ms prepulses to potentials between −140 and −10 mV in 10 mV steps followed by a −10 mV test pulse. An overlay of WT and L1624P window currents are shown in inset. (d) Recovery from inactivation with a two-pulse protocol: a 100 ms-lasting depolarization prepulse to −10 mV was used to inactivate sodium channels, a second −10 mV test pulse followed after an increasing interval from 0.06 to 2500 ms from a holding potential of −120 mV. Protocols are shown in insets. Data are shown as means ± SEM. Lines are fits to corresponding equations given in the text. Fitting parameters are listed in Table 2. Significance levels were set to *p < 0.05, ***p < 0.001. WT: wild-type.
Table 2.
Gating parameters of WT and mutant channels.
| Parameters | WT | L1624P |
|---|---|---|
| Parameters for fast kinetics | ||
| Voltage dependence of activation | n = 16 | n = 17 |
| Peak current density (pA) | −401.88 ± 47.93 | −391.11 ± 56.46 |
| Capacitance (pF) | 10.73 ± 0.64 | 9.95 ± 0.68 |
| Vrev (mV) | 53.81 ± 1.64 | 54.77 ± 1.53 |
| V0.5 (mV) | −23.93 ± 1.18 | −25.36 ± 1.04 |
| k | 5.84 ± 0.27 | 5.95 ± 0.31 |
| Voltage dependence of inactivation | n = 16 | n = 22 |
| V0.5 (mV) | −58.29 ± 1.17 | −49.10 ± 1.18a |
| k | −8.28 ± 0.89 | −7.98 ± 0.47 |
| Recovery from fast inactivation | n = 9 | n = 10 |
| τfast (ms) | 1.86 ± 0.38 | 0.79 ± 0.07* |
| τslow (ms) | 55.0 ± 21 | 36.7 ± 8.01 |
| Parameters for slow kinetics | ||
| Entry into slow inactivation | n = 6 | n = 5 |
| τ | 3768.9 ± 729.1 | 2458.3 ± 237.9 |
| Steady-state slow inactivation | n = 9 | n = 7 |
| V0.5 (mV) | −51.68 ± 2.02 | −51.57 ± 2.38 |
| k | −7.42 ± 0.32 | −5.30 ± 0.09a |
| Recovery from slow inactivation | n = 4 | n = 5 |
| τfast (ms) | 76.48 ± 39.95 | 114.19 ± 19.60 |
| τslow (ms) | 982.73 ± 403.86 | 1855.32 ± 538.69 |
Significance levels were set to *p<0.05, ap < 0.001.
WT: wild-type.
To investigate the recovery from inactivation states, cells were depolarized to −10 mV for 100 ms to inactivate Na+ channels and then repolarized to −120 mV recovery potential for increasing duration (Figure 2(d), inset). Its time course was well fit to a second-order exponential function, yielding two time constants, τfast and τslow (Figure 2(d)). The faster time constant τfast was significantly decreased for mutant compared with WT channels, whereas the slower component τslow was similar between WT and mutant channels. For the fast component, the mutant channels recover two times faster than WT channels. All gating parameters are listed in Table 2.
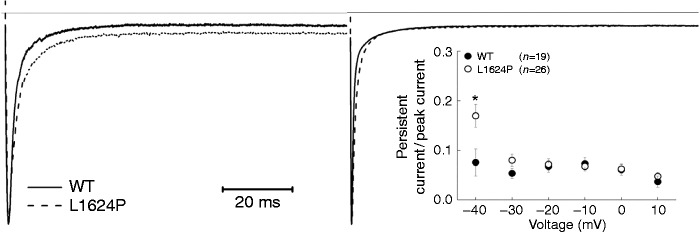
As a result of the depolarizing shift of steady-state inactivation, the window currents of mutant L1624P channels were increased by 27.73% (Figure 2(c) inset). Therefore, we expected an increase of persistent sodium currents. To test this hypothesis, we examined currents that were elicited by 100 ms depolarizing pulses between −40 and 10 mV (in 10 mV increments) and calculated the ratio of the non-inactivating current, which could be blocked by tetrodotoxin (TTX) (data not shown), and the peak transient sodium current at various potentials (Figure 3 inset). We found a significant increase of this ratio for test potentials near threshold of an action potential in cells expressing mutant L1624P channels (0.17 ± 0.02) compared with WT-expressing cells (0.08 ± 0.03) (Figure 3). Altogether, these results of an increased window and persistent current as well as an accelerated recovery from fast inactivation indicate a gain of function and predict an increase in neuronal excitability via depolarization of the membrane and a shortening of the refractory period after an action potential.
Figure 3.
Persistent sodium currents of WT and mutant L1624P channels.
Sodium currents were elicited by a 100 ms-long depolarizing pulse to −40 (left traces) or −10 mV (right traces) from a holding potential of −120 mV. The upper line indicates the zero line. Each trace was normalized to the peak of the transient sodium current. Percentage of persistent current to peak current responding to various depolarization pulses (from −40 to −10 mV) is given in the inset. Data are shown as means ± SEM. Significance levels were set to *p < 0.05.
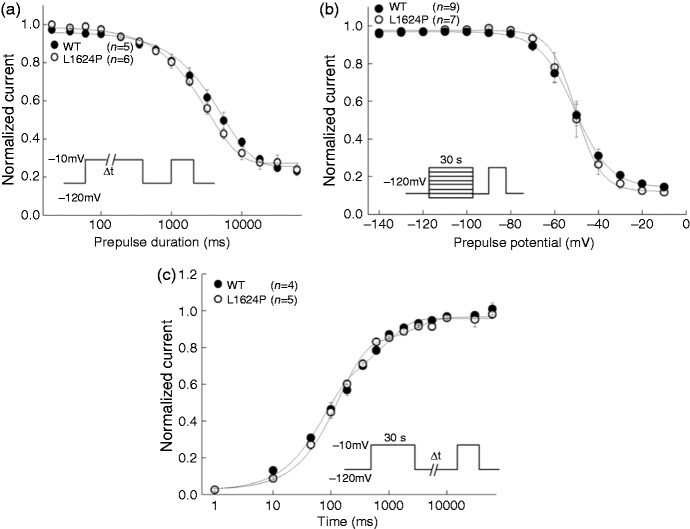
We also tested the slow inactivation properties both for WT and mutant channels (Figure 4). Entry into slow inactivation was monoexponential, with a time constant around 3000 ms for both WT and L1624P channels without significant difference (Table 2). There was no voltage shift of steady-state slow inactivation for L1624P compared to WT, while the slow inactivation voltage sensitivity was significantly different (Table 2). Both WT and L1624P recover in a biexponential way from slow inactivation without significant differences (Table 2). Compared to WT, the slow inactivation properties of L1624P almost remained unchanged.
Figure 4.
Slow inactivation properties of WT (filled circles) and mutant L1624P (open circles).
(a) Entry into slow inactivation was elicited from a holding potential of −120 mV by a depolarizating pulse to −10 mV for an increasing time period (0.02−60 s). An interval of 50 ms at −120 mV followed to recover the fast inactivated but not slow inactivated channels. Then the fraction of slow inactivated channels was determined by the following 20 ms test pulse to −10 mV. Cells were held at −120 mV between trials for 30 s to allow the recovery from slow inactivation. (b) Steady-state slow inactivation was determined by a 30 s conditioning pulse between −140 to −10 mV, followed by a 50 ms recovery period at −120 mV prior to the −10 mV test pulse. The cumulative steady-state slow inactivation was performed with no recovery from slow inactivation between two trials. (c) Recovery from slow inactivation was measured by a 30 s depolarizing pulse to −10 mV followed by an increasing recovery duration (from 0.001 to 60 s) from holding potential of −120 mV. The test pulse to −10 mV was then employed to record the recovered sodium currents. Cells were held at −120 mV between trials for 30 s to allow the recovery from slow inactivation. Protocols are shown in insets. Data are shown as means ± SEM. Lines are fits to corresponding equations given in the text. Fitting parameters are listed in Table 2.
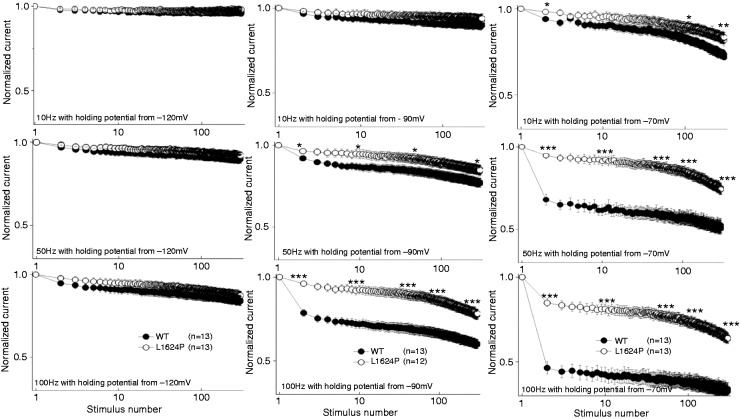
To simulate the channel availability during neuronal firing, we recorded the so-called use-dependence, i.e. whole-cell peak currents during 300 repetitive depolarizing voltage steps to −10 mV at different frequencies and from different holding potentials (Figure 5). At the holding potential of −120 mV, there was no significant difference between the availability of WT and L1624P channels, with both maintaining high channel availability. However, at holding potentials in the physiological range (i.e. more depolarized), mutant channels exhibited a significantly higher availability compared with WT channels. Especially around the neuronal resting membrane potential of −70 mV and a stimulation frequency of 100 Hz (Figure 5), the residual channel availability of mutant L1624P channels was 0.64 ± 0.03, almost twofold higher than for WT channels (0.33 ± 0.03), indicating a marked gain of function in the physiologically relevant range (Table 3). Altogether, our functional studies show a clear gain-of-function effect for mutant NaV1.1 in comparison to WT channels.
Figure 5.
Use dependence of WT and mutant L1624P channels.
Currents were elicited by 300 consecutive trains of 2 ms-long depolarizing pulses to −10 mV from different holding potentials (−120 mV (left), −90 mV (middle) and −70 mV (right graphs)) at different frequencies (10 Hz (top graphs), 50 Hz (middle graphs) and 100 Hz (bottom graphs)). Each trace was normalized to the initial transient peak sodium current. Data are shown as means ± SEM. Significance tested for each fiftieth pulse were set to *p < 0.05, **p < 0.01, ***p < 0.001. WT: wild-type.
Table 3.
Parameters of use dependence.
| Parameter | WT | L1624P |
|---|---|---|
| Holding potential of −120 mV | ||
| 10 Hz | 0.96 ± 0.01 | 0.98 ± 0.01 |
| 50 Hz | 0.90 ± 0.02 | 0.92 ± 0.02 |
| 100 Hz | 0.84 ± 0.03 | 0.87 ± 0.02 |
| Holding potential of −90 mV | ||
| 10 Hz | 0.90 ± 0.02 | 0.94 ± 0.03 |
| 50 Hz | 0.77 ± 0.02 | 0.85 ± 0.03a |
| 100 Hz | 0.60 ± 0.02 | 0.78 ± 0.03c |
| Holding potential of −70 mV | ||
| 10 Hz | 0.72 ± 0.02 | 0.83 ± 0.03b |
| 50 Hz | 0.52 ± 0.04 | 0.74 ± 0.03c |
| 100 Hz | 0.33 ± 0.03 | 0.64 ± 0.03c |
Significance levels were set to ap < 0.05, bp < 0.01, cp < 0.001. WT: wild-type.
Discussion
Here we report a German family with pure FHM. In detail, there is a high intra-individual clinical variability with respect to attack duration, severity and frequency. Maybe the most notable clinical feature is that all patients were found to have an early onset of the attacks before puberty (mean age 7 (range 5 to 8) compared to a mean age of 13 in other reported FHM3 families (range 6 to 24) (24)). Permanent neurological disturbances that have been previously described for FHM1 and 2 families such as cognitive deficits (FHM1 and 2) or cerebellar dysfunction (FHM1) (25,26) were not observed. In addition, we did not observe elicited repetitive daily blindness, for which a partial cosegregation with FHM had been described in two French FHM3 pedigrees (19). The identified novel mutation, expanding the spectrum of FHM3 mutations by increasing their number to nine, is located in the S3 segment of domain IV, in the same domain as two of four known mutations causing pure FHM (F1661L and L1649Q). Until now, mutations causing pure FHM3 have been reported to affect this domain as well as the DIII/IV linker, whereas the others are located in different regions of the channel (see also Figure 1), therefore the novel L1624P mutation expands the spectrum of FHM3 mutations by increasing their number to nine. Other missense mutations in IV/S3 have been described causing different clinical phenotypes such as severe myoclonic epilepsy of infancy (SMEI), borderline SMEI (SMEB) or intractable childhood epilepsies with frequent generalized tonic-clonic seizures (27–30).
Functional studies of L1624P revealed a severe disturbance of fast inactivation with a slower inactivation time course, an 11-mV depolarizing shift of the steady-state inactivation curve, and a two times accelerated recovery from fast inactivation. These effects suggest a destabilization of the inactivated state. L1624P substitutes a proline residue with a high turn propensity (31) for the hydrophobic, relatively long side-chain of leucine; L1624P therefore is likely to introduce a hinge in the S3 segment of domain IV distorting the regular structure of the segment (32). Segment IV/S3 has been shown to be involved in fast inactivation before (as, for example, suggested for the muscle sodium channel NaV1.4; (33)). It is therefore conceivable that L1642P affects the channel’s fast inactivation.
Also mutant L1624P channels displayed an abnormal threshold-near persistent current that might be due to the increased window current. This is different from the persistent current detected in other FHM3 mutations, which appear even at more depolarized potential ranges (11,21,22). This unusual persistent current near the threshold might also contribute to more sodium influx during the repolarizing phase of action potentials and facilitate the longer duration of action potentials leading to increased neuron excitability (21). Investigation of slow inactivation properties showed L1624P mostly unchanged, which indicates slow inactivation might not be involved in the pathogenesis of FHM.
All these results indicate a clear gain of function of mutant NaV1.1 channels predicting increased availability and enhanced sodium influx. Furthermore, the higher availability maintained by mutant L1624P in use-dependence experiments suggests that neurons expressing L1624P mutant channels can sustain high-frequency firing better than cells expressing WT channels, or that cells with mutant channels can generate higher firing frequencies than neurons with only WT channels. This is in agreement with most previous results of functional studies of FHM3 mutations (5,11,17,22) and strengthens the main hypothesis, that gain-of-function mutations in the SCN1A gene cause FHM3. As NaV1.1 is the predominant channel in GABAergic interneurons (13,14), our results are compatible with increased firing of inhibitory neurons. And increased firing of inhibitory GABAergic neurons may lead to higher extracellular potassium concentrations, which could trigger CSD as the neurophysiological correlate of the migraine aura (11).
In summary, we add the ninth mutation L1624P to the spectrum of FHM3 mutations. The unusual earlier onset might at least partly be related to this novel mutation. The gain-of-function defects, including the unique persistent current appearing near the threshold, are consistent with the pathomechanisms underlying FHM3. Here, we provide a detailed description of the novel L1624P mutation from the clinical, genetic and molecular points of view that will contribute to the understanding of the pathogenesis of the underlying migraine.
Article highlights
-
Our studies identify a novel L1624P mutation in NaV1.1 linked to pure familial hemiplegic migraine 3 (FHM3) with a remarkably early age of onset.
Our gain-of-function results, including the unique threshold-near persistent current, are consistent with hyperexcitability of inhibitory interneurons, which, by means of increasing extracellular potassium, may facilitate cortical spreading depression (CSD).
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Frank Lehmann-Horn and Karin Jurkat-Rott are fellows of the non-profit Hertie Foundation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the German Federal Ministry of Research, BMBF and the IonNeurONet (grant number 01GM1105B).
References
- 1.Thomsen LL, Eriksen MK, Roemer SF, et al. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125: 1379–1391. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 3.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996; 87: 543–552. [DOI] [PubMed] [Google Scholar]
- 4.De Fusco M, Marconi R, Silvestri L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet 2003; 33: 192–196. [DOI] [PubMed] [Google Scholar]
- 5.Dichgans M, Freilinger T, Eckstein G, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005; 366: 371–377. [DOI] [PubMed] [Google Scholar]
- 6.Riant F, Roze E, Barbance C, et al. PRRT2 mutations cause hemiplegic migraine. Neurology 2012; 79: 2122–2124. [DOI] [PubMed] [Google Scholar]
- 7.Lauritzen M. Pathophysiology of the migraine aura. Brain 1994; 117: 199–210. [DOI] [PubMed] [Google Scholar]
- 8.De Vries B, Frants RR, Ferrari MD, et al. Molecular genetics of migraine. Hum Genet 2009; 126: 115–132. [DOI] [PubMed] [Google Scholar]
- 9.Silberstein SD, Dodick DW. Migraine genetics: Part II. Headache 2013; 53: 1218–1229. [DOI] [PubMed] [Google Scholar]
- 10.Cestèle S, Scalmani P, Rusconi R, et al. Self-limited hyperexcitability: Functional effect of a familial hemiplegic migraine mutation of the NaV1.1 (SCN1A) Na+ channel. J Neurosci 2008; 28: 7273–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cestèle S, Schiavon E, Rusconi R, et al. Nonfunctional NaV1.1 familial hemiplegic migraine mutant transformed into gain of function by partial rescue of folding defects. Proc Natl Acad Sci U S A 2013; 110: 17546–17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H, Xu HQ, Yu L, et al. The SCN1A Mutation Database: Updating information and analysis of the relationships among genotype, functional alteration, and phenotype. Hum Mutat 2015; 36: 573–580. [DOI] [PubMed] [Google Scholar]
- 13.Yu FH, Mantegazza M, Westenbroek RE, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 2006; 9: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 14.Ogiwara I, Miyamoto H, Morita N, et al. NaV1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 2007; 27: 5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedrich UB, Liautard C, Kirschenbaum D, et al. Impaired action potential initiation in GABAergic interneurons causes hyperexcitable networks in an epileptic mouse model carrying a human Na(V)1.1 mutation. J Neurosci 2014; 34: 14874–14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliva M, Berkovic SF, Petrou S. Sodium channels and the neurobiology of epilepsy. Epilepsia 2012; 53: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 17.Vanmolkot KR, Babini E, de Vries B, et al. The novel p.L1649Q mutation in the SCN1A epilepsy gene is associated with familial hemiplegic migraine: Genetic and functional studies. Hum Mutat 2007; 28: 522–522. [DOI] [PubMed] [Google Scholar]
- 18.Weller CM, Pelzer N, de Vries B, et al. Two novel SCN1A mutations identified in families with familial hemiplegic migraine. Cephalalgia 2014; 34: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 19.Vahedi K, Depienne C, Le Fort D, et al. Elicited repetitive daily blindness: A new phenotype associated with hemiplegic migraine and SCN1A mutations. Neurology 2009; 72: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 20.Castro MJ, Stam AH, Lemos C, et al. First mutation in the voltage-gated NaV1.1 subunit gene SCN1A with co-occurring familial hemiplegic migraine and epilepsy. Cephalalgia 2009; 29: 308–313. [DOI] [PubMed] [Google Scholar]
- 21.Cestèle S, Labate A, Rusconi R, et al. Divergent effects of the T1174S SCN1A mutation associated with seizures and hemiplegic migraine. Epilepsia 2013; 54: 927–935. [DOI] [PubMed] [Google Scholar]
- 22.Kahlig KM, Rhodes TH, Pusch M, et al. Divergent sodium channel defects in familial hemiplegic migraine. Proc Natl Acad Sci U S A 2008; 105: 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lossin C, Wang DW, Rhodes TH, et al. Molecular basis of an inherited epilepsy. Neuron 2002; 34: 877–884. [DOI] [PubMed] [Google Scholar]
- 24.Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: Pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol 2011; 10: 457–470. [DOI] [PubMed] [Google Scholar]
- 25.Freilinger T, Bohe M, Wegener B, et al. Expansion of the phenotypic spectrum of the CACNA1A T666M mutation: A family with familial hemiplegic migraine type 1, cerebellar atrophy and mental retardation. Cephalalgia 2008; 28: 403–407. [DOI] [PubMed] [Google Scholar]
- 26.Jurkat-Rott K, Freilinger T, Dreier JP, et al. Variability of familial hemiplegic migraine with novel A1A2 Na+/K+-ATPase variants. Neurology 2004; 62: 1857–1861. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara T, Sugawara T, Mazaki-Miyazaki E, et al. Mutations of sodium channel α subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain 2003; 126: 531–546. [DOI] [PubMed] [Google Scholar]
- 28.Herini ES, Gunadi F, Van Kempen MJ, et al. Novel SCN1A mutations in Indonesian patients with severe myoclonic epilepsy in infancy. Pediatr Int 2010; 52: 234–239. [DOI] [PubMed] [Google Scholar]
- 29.Marini C, Mei D, Temudo T, et al. Idiopathic epilepsies with seizures precipitated by fever and SCN1A abnormalities. Epilepsia 2007; 48: 1678–1685. [DOI] [PubMed] [Google Scholar]
- 30.Mulley JC, Scheffer IE, Petrou S, et al. SCN1A mutations and epilepsy. Hum Mutat 2005; 25: 535–542. [DOI] [PubMed] [Google Scholar]
- 31.Monné M, Nilsson I, Elofsson A, et al. Turns in transmembrane helices: Determination of the minimal length of a “helical hairpin” and derivation of a fine-grained turn propensity scale. J Mol Biol 1999; 293: 807–814. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Scheuer T, Catterall WA. Reversed voltage-dependent gating of a bacterial sodium channel with proline substitutions in the S6 transmembrane segment. Proc Natl Acad Sci U S A 2004; 101: 17873–17878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon SC, Hayward LJ, Beech J, et al. Sodium channel inactivation is impaired in equine hyperkalemic periodic paralysis. J Neurophysiol 1995; 73: 1892–1899. [DOI] [PubMed] [Google Scholar]