Abstract
This study examined within- and across-session consistency of visual analog scale (VAS) pain intensity and unpleasantness ratings of contact heat stimuli in 64 subjects (32 male). Subjects participated in four sessions over 14 days, with three stimulus series per session. Two levels of painful heat (pain-lo: rated 40, and pain-hi: rated 70 on a 0–100 VAS) were delivered in randomized order during each series, with temperatures selected on an individual subject basis to equalize pain perception across subjects. Across-session ratings declined by the fourth session for both pain levels (p=0.01). Within-session ratings declined by the third series for both pain levels (p<0.001). While significant, changes in across- and within-session ratings were of small magnitude. Comparison of coefficients of variation (CV) for across- and within-session ratings revealed that pain-lo ratings were more variable than pain-hi ratings (p<0.001). Across- and within-session CVs were highly correlated for each pain level (pain-lo p<0.001; pain-hi p=0.001), suggesting that variability of VAS ratings is a characteristic of individual subjects over both short and long time scales. Across- and within-session CVs were significantly negatively correlated with individual ratings of the stimuli, but were not correlated with demographic or psychosocial factors. Furthermore, sex did not impact consistency of ratings, demonstrating that neither sex is more variable in ratings than the other over time. Taken together, these findings suggest that VAS ratings of painful contact heat are relatively stable over time but the variability of these ratings is significantly impacted by the perceived intensity of the stimulus.
Keywords: pain, nociception, heat, visual analog scale, repeatability, sex
INTRODUCTION
Contact heat stimuli delivered with a Peltier-type thermode are commonly used in experimental and clinical studies of pain because they can be precisely controlled and are well-tolerated. Increasingly, phasic contact heat stimuli at temperatures above pain threshold (suprathreshold) are used to evaluate the efficacy of pain-reducing manipulations (such as placebo, distraction tasks, painful conditioning stimuli, and drugs) and associated changes in brain activation patterns measured by functional neuroimaging (Hoffman et al. 2004; Kong et al. 2006a). Such studies typically involve serial application of multiple suprathreshold stimuli to achieve sufficient statistical power to detect changes in pain perception or brain activity, often with modulatory manipulations either intervening between series of heat stimuli within a session or applied during sessions on separate days. Despite this practice, few studies have examined whether the perception of suprathreshold contact heat stimuli remains stable over time. Therefore, though visual analog scale (VAS) ratings have been shown to be valid ratio scale measures of pain (Price et al. 1983; Price et al. 1994) that change significantly in response to modulatory manipulations (Price et al. 1986; Lautenbacher et al. 2002; Edwards et al. 2003), their suitability for use in studies requiring long series of repetitive heat stimuli within a session or across multiple sessions has not been definitively demonstrated. Furthermore, the effects of factors known to influence pain perception (such as sex, sleep habits, anxiety, depression, and mood; Berkley 1997; Fillingim 2000; Lautenbacher et al. 2006) on stability of VAS ratings have not been examined.
Previous reports indicate that VAS ratings of painful heat are not highly repeatable measures of pain on an individual subject level (Yarnitsky et al. 1996) but exhibit relatively stable mean values for groups of individuals within a single session (Granot et al. 2003) and across multiple sessions over a 4-week period (Rosier et al. 2002). However, a recent study reported that group ratings of painful heat stimuli declined significantly across an 8-day period, with associated reductions in pain-related brain activity (Bingel et al. 2007). Replication and extension of these findings is desirable because these studies tested small numbers of subjects or applied stimuli at a frequency not consistent with typical pain investigations (4 stimuli over an hour-long session by Granot et al., 60 stimuli per session by Bingel et al.).
The purposes of this report are to (1) evaluate within- and across-session consistency of group VAS ratings for two levels of perceptually-equalized painful contact heat stimuli; (2) characterize the effects of sex on pain rating stability; and (3) investigate whether other factors influence individual variability in heat pain ratings, such as stimulus attributes, demographic variables, and psychosocial measures.
MATERIALS AND METHODS
Subjects
Male (n=32) and female (n=32) subjects of comparable age and race participated in the study (Table 1). All subjects were healthy, with no major medical, neurological, or chronic pain disorders. No subjects used prescription medication during the study period, with the exception of hormonal contraception (13 females) and hormone replacement therapy (1 female). Female subjects were tested during the follicular phase of their menstrual cycle (days 3 through 11) to control for potential effects of gonadal hormone fluctuations on pain perception (Riley et al. 1999). Three female subjects were in a persistent follicular phase due to continuous use of hormonal contraception or hormone replacement therapy without breaks for menstruation. Informed consent was obtained from all subjects prior to experimentation. The protocol for this study was approved by the University of Maryland Institutional Review Board for the Protection of Human Subjects.
Table 1.
Subject demographics, stimulation parameters, and psychosocial measures
| Variable | Female (n=32) |
Male (n=32) |
Statistic | p-value |
|---|---|---|---|---|
| Age | 26.2 (2.5) | 26.0 (2.7) | MWU Z=−0.3 | 0.7 |
| Race | ||||
| White | 62% | 59% | ||
| Asian | 13% | 13% | ||
| Hispanic | 13% | 13% | ||
| S. Asian Indian | 6% | 6% | ||
| Black | 3% | 6% | ||
| American Indian | 3% | 3% | ||
| Temperature for pain-hi | 48.6 (1.1) | 49.1 (1.1) | MWU Z=−1.9 | 0.05* |
| Temperature for pain-lo | 47.5 (1.2) | 48.1 (1.1) | MWU Z=−2.2 | 0.03* |
| Pain intensity ratings for pain-hi | 71.0 (12) | 68.2 (10) | F=0.06 | 0.8 |
| Pain intensity ratings for pain-lo | 41.4 (16) | 42.9 (14) | F=0.06 | 0.8 |
| Pain intensity ratings of 48C stimulus | 62.2 (23) | 52.2 (22) | t=1.8 | 0.08 |
| Unpleasantness ratings for pain-hi | 72.2 (13) | 67.5 (12) | F=0.4 | p=0.5 |
| Unpleasantness ratings for pain-lo | 40.5 (17) | 41.6 (17) | F=0.4 | p=0.5 |
| Trait anxiety | 37 (7) | 32 (6) | t=2.8 | 0.006* |
| Trait positive affect | 35.2 (6) | 35 (5) | t=0.1 | 0.9 |
| Trait negative affect | 17 (5) | 16 (6) | MWU Z=−1.1 | 0.3 |
| Depression | 3.9 (3.7) | 3.1 (2.9) | MWU Z=−0.5 | 0.6 |
| Emotional reactivity | 79 (55) | 63 (10) | MWU Z=−2.4 | 0.02* |
| Insomnia | 4.7 (4.3) | 4.3 (3.2) | MWU Z=−0.2 | 0.9 |
| Average sleep (hours/night) | 7.2 (0.9) | 7.3 (0.7) | MWU Z=−0.5 | 0.6 |
Values are means (SD)
Statistically significant result
MWU Mann-Whitney U test
Stimulation
Painful heat stimuli were delivered to the medial surface of the right lower leg using a Peltier thermal probe with a 2.6 cm2 contact surface (TSA-II, Medoc Ltd., Israel). The probe was held in place during testing with a Velcro strap. Two temperatures of painful heat were delivered to each subject: (1) a subject-specific temperature perceived as strongly painful (pain-hi), which was defined as the temperature the subject rated between 70 and 80 on a 100-point computerized VAS for pain intensity and (2) a subject-specific temperature perceived as moderately painful (pain-lo), which was defined as the temperature the subject rated between 40 and 50 on the VAS. Mean stimulus temperatures required to evoke these perceptions are listed in Table 1.
Experimental Protocol
Subjects participated in a training session and four test sessions held on separate days.
Training Session
During the training session, subjects were first presented with an ascending series of thermal stimuli ranging from 43 to 50.5°C. Each temperature was presented for 15 seconds (including ramp up and down time using a 5°C/sec ramp rate), followed by a 30-second interstimulus period of nonpainful warmth (37°C). Subjects were then presented with a series of heat stimuli expected to be in the painful range (45 to 50.5°C), with temperatures presented twice each in a randomized order. After each stimulus, subjects used a trackball to rate peak pain intensity on a computerized VAS, which consisted of a vertical scale labeled “no pain” at the bottom and “most intense pain imaginable” at the top (DAPSYS, Brian Turnquist, Johns Hopkins University, www.dapsys.net). VAS ratings were converted to numerical values ranging from 0 to 100. Individual subject ratings were used to determine subject-specific temperatures that evoked perceptions of strong pain (70–80 VAS rating) and moderate pain (40–50 VAS rating). These stimulus levels are referred to as pain-hi (strong pain) and pain-lo (moderate pain) in this paper. Subjects were presented with a third series of randomized heat stimuli and rated unpleasantness of the stimuli using the computerized VAS. The unpleasantness VAS was labeled “not unpleasant” at the bottom and “most unpleasant sensation imaginable” at the top. Subjects were instructed to distinguish the pain intensity and unpleasantness scales using the “radio analogy” protocol (Price et al. 1983).
Test Sessions
Each subject participated in four test sessions conducted on separate days. Test sessions were conducted between 12 and 7 pm, and at the same time of day for each individual subject to control for circadian fluctuations in pain sensitivity (Strian et al. 1989). The interval between test sessions ranged from 1 to 8 days, with a mean of 2.4 days (SD 1.5). All test sessions were completed within a 14-day period with the exception of one female subject (17 days) and two male subjects (19 days and 25 days).
In each test session, subjects were presented with three series of painful heat stimuli separated by 10-minute intervals. Across-session repeatability of heat pain ratings was evaluated using data from the first series of each test session. A series consisted of the two subject-specific temperatures delivered four times each in a randomized order. After each stimulus, the computerized VAS was presented to the subject and the subject rated peak pain intensity or unpleasantness of the stimulus using the trackball. The order in which the two pain dimensions were rated was counterbalanced across subjects. Half the subjects rated pain intensity of the first four stimuli in each series and unpleasantness of the last four stimuli in the series; the order was reversed for the remaining subjects.
During the second heat series in each test session, electrical stimulation was applied concurrently with the heat stimuli using a transcutaneous electrical nerve stimulation unit (Mettler Trio-Stim, Anaheim, CA). Electrical stimuli were delivered to the left forearm over the median nerve in 0.2 msec square pulse waves at a frequency of 4 Hz. Electrical stimuli were applied at different intensities in each session in order to evaluate their ability to modulate the heat pain ratings (data to be reported separately). One of the sessions (order counterbalanced across subjects) was a control session in which mild, nonpainful electrical stimulation was applied. Within-session repeatability of heat pain ratings was evaluated using data from this control session for each subject.
Psychosocial Measures
Subjects completed several questionnaires to assess psychosocial variables that may influence pain responses. These questionnaires included (1) Speilberger’s Trait Anxiety Inventory, which quantifies generalized (trait) anxiety (Spielberger et al. 1970); (2) the trait version of the Positive and Negative Affect Scale, which measures generalized positive affect and negative affect (Watson et. al. 1988); (3) the Beck Depression Inventory, which measures attitudes and symptoms of depression (Beck et al. 1961); (4) the Kohn Reactivity Scale, which assesses a subject’s reactivity and arousability to external stimuli (Kohn 1985); (5) the Insomnia Questionnaire, which diagnoses the presence and quantifies the severity of clinical insomnia (Morin 1993); and (6) the Coping Strategies Questionnaire, which evaluates the degree to which an individual utilizes various cognitive strategies for coping with pain, including diverting attention, reinterpreting pain sensation, making coping self-statements, ignoring pain, praying/hoping, and catastrophizing (Rosenstiel and Keefe 1983).
Statistical Analysis
To test for across-session effects on group heat pain perception, pain ratings obtained for the first heat series of each session were evaluated using a 3-way mixed-effects analysis of variance (ANOVA), with sex as the between-subjects factor and session and pain level as within-subjects factors. To test for within-session effects on group heat pain perception, pain ratings obtained during the three heat series of the control session were evaluated using a 3-way mixed effects ANOVA, with sex as the between-subjects factor and series number and pain level as within-subjects factors. Post-hoc analyses for simple effects consisted of 1-way ANOVAs and followup t-tests, with Bonferroni adjustments to correct for multiple comparisons. Pain intensity and unpleasantness ratings were evaluated separately using this approach. All ratings data met the requirements for parametric statistical analysis.
To obtain quantitative measures of across-session and within-session variability, coefficients of variation (CV) (SD/mean) were calculated for each subject for each stimulus level. CVs were calculated separately for pain intensity and unpleasantness ratings. CVs were log-transformed to achieve a normal distribution (as confirmed by the Shapiro-Wilk test). Across-session CVs (log-transformed) were evaluated using a 2-way mixed-effects ANOVA, with sex as the between-subjects factor and stimulus level as the within-subjects factor. Within-session CVs calculated for each subject (log-transformed) were evaluated using a similar approach. Direct comparison of variability in pain intensity and unpleasantness ratings was made using a 2-way repeated-measures ANOVA on the log-transformed CVs, with pain dimension and pain level as factors.
Associations between heat pain rating variability (CVs) and factors such as parameters of the heat stimuli, individual subject pain sensitivity, and psychosocial measures were examined with Spearman’s rho correlations. This nonparametric test was selected because most of the evaluated variables were not normally distributed. Sex differences in demographic factors, stimulus temperatures, heat pain ratings, pain sensitivity, and psychosocial measures were tested for using independent sample t-tests (two-tailed) for normally-distributed variables and the Mann-Whitney U test (two-tailed) for variables that were not normally distributed. Significance was set at 0.05 for all statistical tests.
RESULTS
Subject Characteristics
The male and female groups were comparable with respect to age, race, and numerous psychosocial variables, including trait positive affect, trait negative affect, depression, insomnia, and average sleep (Table 1). Females had significantly higher trait anxiety and emotional reactivity scores than males (Table 1).
Heat Stimulus Parameters
Mean pain intensity ratings for the 2 stimulus levels were within the target ranges for both female and male subjects, with relatively low standard deviations (Table 1), providing evidence that the protocol used to achieve equal pain perceptions across subjects was successful. Pain intensity and unpleasantness ratings of each pain level did not differ significantly between the female and male groups (Table 1). Mean pain intensity ratings for pain-hi and pain-lo differed significantly (t=−14.0, p<0.001), indicating that the two temperatures used in the study represent distinct intensities of pain, with pain-lo evoking a perception in the lower half of the pain range and pain-hi evoking a perception in the upper half of the pain range. Comparable differences were found for unpleasantness ratings (pain level, F(1,62)=190, p<0.001). Though pain perception did not differ between the female and male groups, females required significantly lower temperatures than males to evoke both targeted levels of perceived pain (Table 1). Pain-lo temperatures ranged from 45–49.5°C for women and 46–49.5°C for men. Pain-hi temperatures ranged from 46–50.5°C for women and 47–50.5°C for men. A direct comparison of pain intensity and unpleasantness ratings revealed no significant differences between ratings of the two dimensions of pain (F(1,63)=0.2, p=0.7).
Heat Pain Ratings Across Sessions: Group Effects
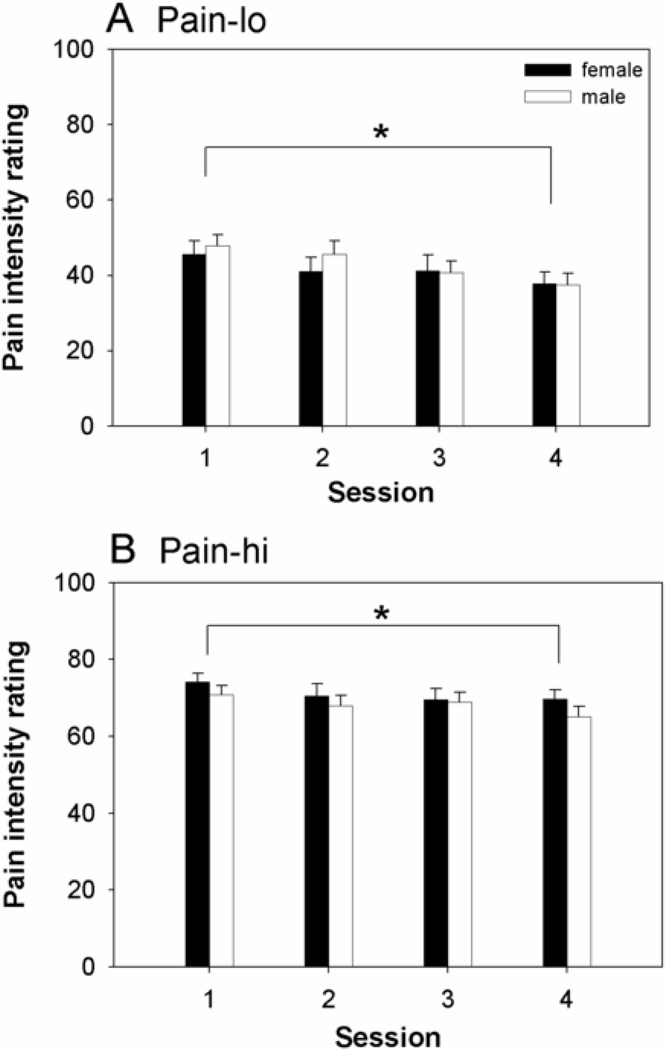
An omnibus 3-way mixed effects ANOVA (sex, session, pain level) detected significant main effects of session (F(3,62)=4.3, p=0.006) and pain level (F(1,62)=210, p<0.001), but no significant main effect of sex (F(1,62)=0.06, p=0.8) or significant interactions among the various factors (F-values ranging from 0.3 to 1.5, all p-values p>0.2). Post-hoc analyses revealed that pain intensity ratings trended downward across the four sessions (Figure 1, Table 2), with a significant difference detected between sessions 1 and 4 (p=0.01, corrected).
Figure 1.
Female and male group average (+SE) pain intensity ratings across four sessions for (A) moderately painful heat (pain-lo) and (B) strongly painful heat (pain-hi). * denotes significant differences in post-hoc pairwise comparisons (p<0.05, corrected for multiple comparisons).
Table 2.
Pain ratings across sessions
| Pain intensity ratings | Session 1 | Session 2 | Session 3 | Session 4 |
|---|---|---|---|---|
| Pain-lo | ||||
| group (n=64) | 46.7 (19) | 43.3 (21) | 41.0 (21) | 37.6 (17)* |
| female (n=32) | 45.5 (21) | 41.1 (22) | 41.3 (24) | 37.7 (18) |
| male (n=32) | 47.8 (17) | 45.6 (20) | 40.7 (17) | 37.5 (17) |
| Pain-hi | ||||
| group (n=64) | 72.5 (13) | 69.1 (17) | 69.3 (15) | 67.5 (14)* |
| female (n=32) | 74.2 (13) | 70.5 (18) | 69.5 (17) | 71.0 (12) |
| male (n=32) | 70.8 (14) | 67.9 (16) | 68.9 (14) | 68.2 (10) |
| Unpleasantness ratings | Session 1 | Session 2 | Session 3 | Session 4 |
|---|---|---|---|---|
| Pain-lo | ||||
| group (n=64) | 47.1 (20) | 41.9 (23) | 38.5 (21)* | 36.8 (20)* |
| female (n=32) | 47.5 (21) | 41.5 (25) | 37.6 (24) | 35.5 (19) |
| male (n=32) | 46.6 (19) | 42.2 (21) | 39.4 (19) | 38.2 (21) |
| Pain-hi | ||||
| group (n=64) | 73.7 (15) | 68.7 (18) | 68.1 (18)* | 68.9 (15)* |
| female (n=32) | 75.9 (15) | 69.8 (20) | 71.2 (20) | 72.2 (14) |
| male (n=32) | 71.5 (15) | 67.6 (16) | 65.0 (16) | 65.7 (16) |
Values are means (SD)
Significantly different from session 1
Comparable results were found for unpleasantness ratings (Table 2), with significant main effects of session (F(3,62)=5.2, p=0.002) and pain level (F(1,62)=190, p<0.001), but no significant main effect of sex (F(1,62)=0.4, p=0.5) or significant interactions among the various factors (all p-values >0.05). Post-hoc analyses revealed that unpleasantness ratings trended downward across the four sessions (Table 2), with ratings significantly higher in session 1 than in session 3 (p=0.02, corrected) and session 4 (p=0.006, corrected).
Heat Pain Ratings Within a Session: Group Effects
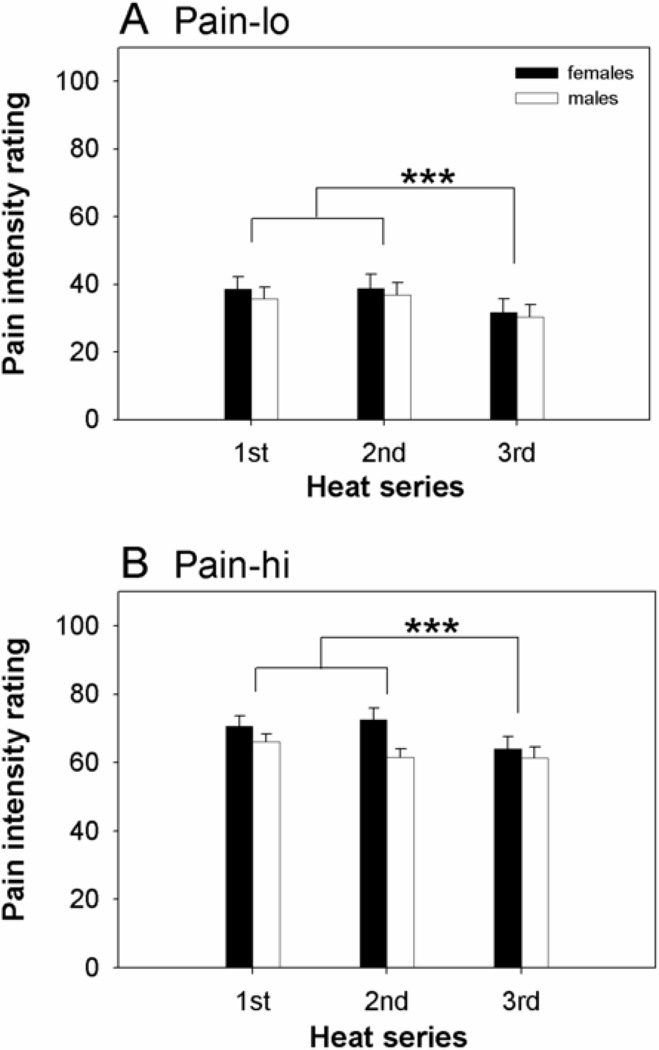
An omnibus 3-way mixed effects ANOVA (sex, series, pain level) detected significant main effects of series (F(2,62)=11.2, p=0.004) and pain level (F(1,62)=170, p<0.001), but no significant main effect of sex (F(1,62)=1.2, p=0.3) or significant interactions among the various factors (F-values ranging from 0.7 to 1.9, all p-values >0.2). Post-hoc analyses revealed that pain intensity ratings trended downward across the session (Figure 2, Table 3), with the ratings for the third series significantly lower than ratings for the first (p<0.001, corrected) or second (p<0.001, corrected) series.
Figure 2.
Female and male group average (+SE) pain intensity ratings across three series (separated by 10 minute intervals) delivered in a single session. Each series included (A) moderately painful heat stimuli (pain-lo) and (B) strongly painful heat (pain-hi). *** denotes significant differences in post-hoc pairwise comparisons at the p<0.001 level, corrected for multiple comparisons.
Table 3.
Pain ratings across series in the control session
| Pain intensity ratings | Series 1 | Series 2 | Series 3 | |
|---|---|---|---|---|
| Pain-lo | ||||
| group (n=64) | 36.3 (20) | 37.8 (22) | 31.0 (21)* | |
| female (n=32) | 38.5 (21) | 38.8 (24) | 31.6 (23) | |
| male (n=32) | 35.8 (19) | 36.8 (21) | 30.4 (20) | |
| Pain-hi | ||||
| group (n=64) | 68.4 (15) | 67.1 (17) | 62.7 (19)* | |
| female (n=32) | 70.7 (17) | 72.6 (19) | 64.1 (20) | |
| male (n=32) | 66.2 (13) | 61.5 (14) | 61.4 (18) | |
| Unpleasantness ratings | Series 1 | Series 2 | Series 3 | |
|---|---|---|---|---|
| Pain-lo | ||||
| group (n=64) | 38.4 (22) | 34.1 (20) | 31.7 (21)* | |
| female (n=32) | 38.9 (25) | 35.8 (22) | 33.9 (22) | |
| male (n=32) | 38.0 (19) | 32.4 (17) | 29.5 (20) | |
| Pain-hi | ||||
| group (n=64) | 68.2 (19) | 66.6 (19) | 62.4 (21)* | |
| female (n=32) | 72.3 (20) | 71.7 (19) | 63.6 (24) | |
| male (n=32) | 64.1 (17) | 61.6 (18) | 61.2 (18) | |
Values are means (SD)
Statistically different from series 1
Comparable results were found for unpleasantness ratings (Table 3), with significant main effects of series (F(2,62)=7.0, p=0.001) and pain level (F(1,62)=150, p<0.001), but no significant main effect of sex (F(1,62)=1.7, p=0.2) or significant interactions among the various factors (all p-values >0.2). Post-hoc analyses revealed that unpleasantness ratings trended downward across the session (Table 3), with ratings for the third series significantly lower than ratings for the first series (p=0.002, corrected).
Across-Session Variability of Heat Pain Ratings
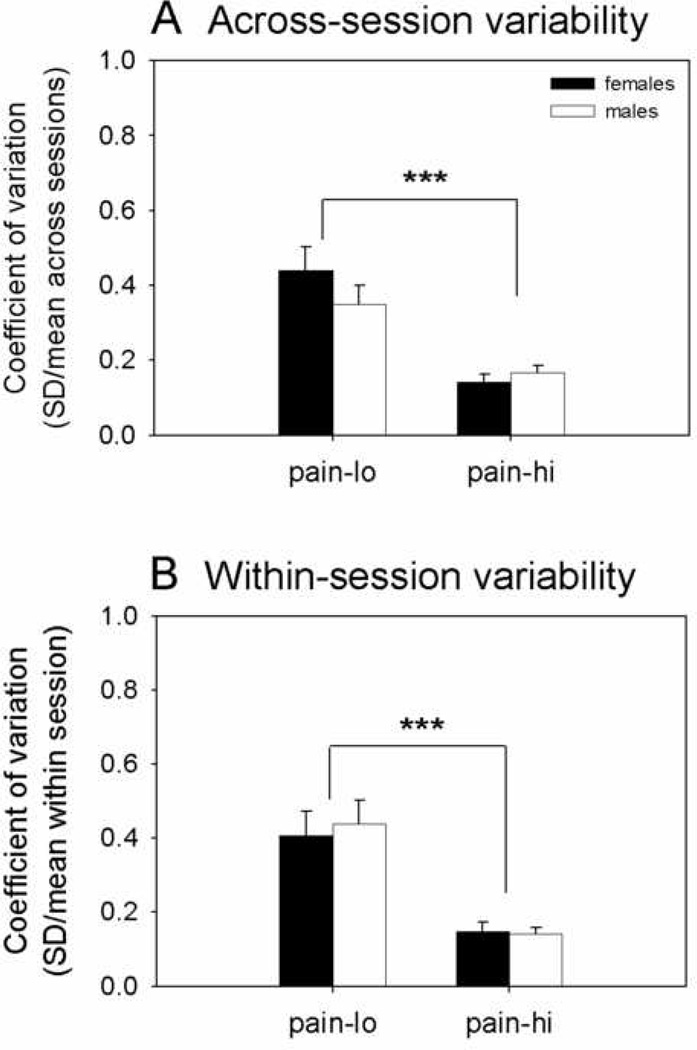
Across-session variability of pain intensity ratings for each pain level was quantified by calculating CVs (standard deviation of pain intensity ratings across sessions divided by mean rating across sessions) for each individual subject (Figure 3A, Table 4). The effects of sex and pain level on across-session CVs (log-transformed) were evaluated with a 2-way mixed effects ANOVA. Across-session CVs were found to be significantly higher for pain-lo than pain-hi (F(1,62)=88, p<0.001), independent of sex (F(1,62)=0.02, p=0.9). The interaction between sex and pain level was not significant (F(1,62)=2.7, p=0.1). CVs did not correlate significantly with inter-session interval (pain-hi rho=0.02, p=0.9, pain-lo rho=−0.1, p=0.4), indicating that variations in testing interval did not influence across-session variability in pain intensity ratings.
Figure 3.
Female and male group average CV (+SE) for heat pain intensity ratings (A) across four sessions and (B) within a single session for moderately painful heat stimuli (pain-lo) and strongly painful heat (pain-hi). CVs were log-transformed for statistical analysis. *** denotes significant differences between variability in pain-lo and pain-hi ratings at the p<0.001 level, corrected for multiple comparisons.
Table 4.
Coefficients of variation for across- and within-session ratings
| Pain intensity ratings | Across-session CV pain-lo |
Across-session CV pain-hi |
Within-session CV pain-lo |
Within-session CV pain-hi |
|---|---|---|---|---|
| Group (n=64) | 0.40 (0.3) | 0.15 (0.1) | 0.42 (0.4) | 0.14 (0.1) |
| female (n=32) | 0.44 (0.4) | 0.14 (0.1) | 0.40 (0.4) | 0.14 (0.1) |
| male (n=32) | 0.35 (0.3) | 0.16 (0.1) | 0.44 (0.4) | 0.14 (0.1) |
| Unpleasantness ratings | Across-session CV pain-lo |
Across-session CV pain-hi |
Within-session CV pain-lo |
Within-session CV pain-hi |
|---|---|---|---|---|
| Group (n=64) | 0.41 (0.3) | 0.17 (0.1) | 0.35 (0.3) | 0.15 (0.1) |
| female (n=32) | 0.49 (0.3) | 0.17 (0.1) | 0.35 (0.3) | 0.16 (0.2) |
| male (n=32) | 0.34 (0.3) | 0.18 (0.1) | 0.35 (0.2) | 0.13 (0.1) |
Values are means (SD)
Comparable effects were seen for unpleasantness ratings (Table 4), with across-session CVs significantly higher for pain-lo than pain-hi (F(1,62)=50, p<0.001). A significant interaction was detected between sex and pain level (F(1,62)=5.6, p=0.02), reflecting that female CVs were higher than male CVs for pain-lo but not for pain-hi. CVs did not correlate significantly with inter-session interval (pain-hi rho=−0.09, p=0.5, pain-lo rho=−0.2, p=0.2), indicating that variations in testing interval did not influence across-session variability in unpleasantness ratings.
Within-Session Variability of Heat Pain Ratings
Within-session variability of pain intensity ratings for each pain level (Figure 3B, Table 4) was assessed in a similar manner, with CVs calculated for each individual subject, log-transformed, and evaluated with a 2-way mixed effects ANOVA (sex, pain level). As was the case for across-session CVs, within-session CVs were found to be significantly higher for pain-lo than pain-hi (F(1,62)=90, p<0.001), independent of sex (F(1,62)=0.8, p=0.4). The interaction between sex and pain level was not significant (F(1,62)=0.2, p=0.7). CVs did not correlate significantly with inter-session interval (pain-hi rho=−0.01, p=0.9, pain-lo rho=−0.09, p=0.5), indicating that variations in testing interval did not influence within-session variability in pain intensity ratings.
Comparable effects were seen for unpleasantness ratings (Table 4), with within-session CVs significantly higher for pain-lo than pain-hi (F(1,62)=44, p<0.001), independent of sex (F(1,62)=0.2, p=0.6). The interaction between sex and pain level was not significant (F(1,62)=0.1, p=0.7). CVs did not correlate significantly with inter-session interval (pain-hi rho=0.07, p=0.6, pain-lo rho=−0.1, p=0.3), indicating that variations in testing interval did not influence within-session variability in unpleasantness ratings.
Correlation of Across- and Within-Session Variability of Heat Pain Ratings
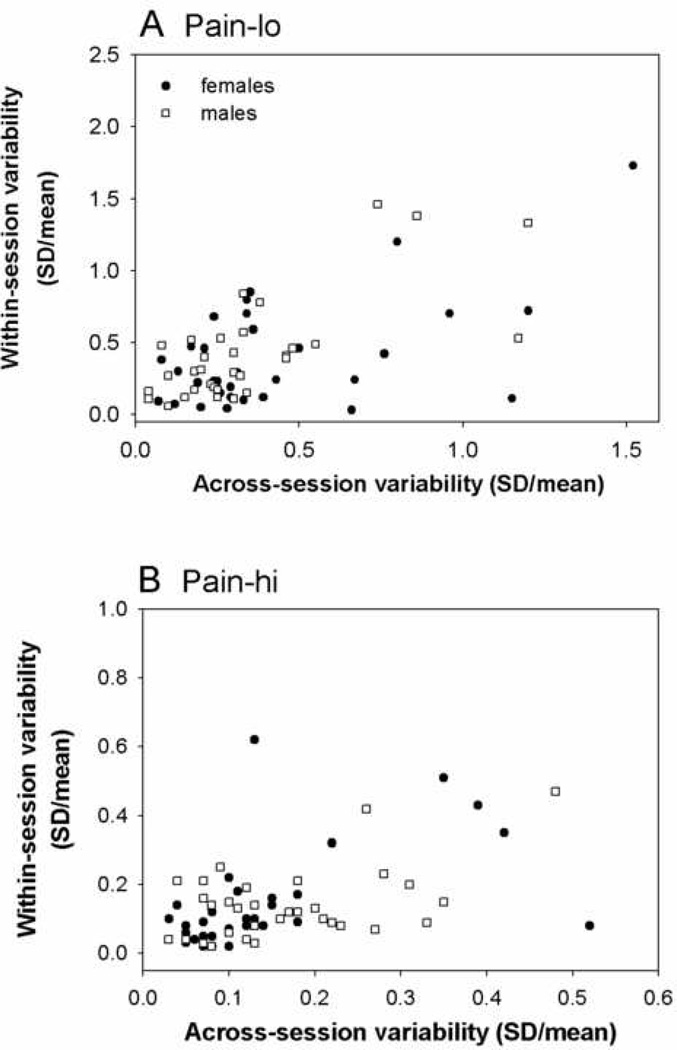
The relationship between across- and within-session variability was evaluated by correlating across-session CVs with within-session CVs, separately for each pain level and each dimension of pain. For pain intensity CVs, significant correlations were found for both pain levels, independent of sex. For pain-lo (Figure 4A), significant correlations between across- and within-session CVs were found for the pooled data (rho=0.46, p<0.001), and for each group separately (female rho=0.36, p=0.045; male rho=0.64, p<0.001). For pain-hi (Figure 4B), significant correlations between across- and within-session CVs were found for the pooled data (rho=0.43, p<0.001), and for the female group (rho=0.58, p<0.001) but not the male group (rho=0.22, p=0.2).
Figure 4.
Scatter plots of across- and within-session variability in heat pain intensity ratings for (A) moderately painful heat stimuli (pain-lo) and (B) strongly painful heat (pain-hi). Correlations (Spearman’s rho) were significant for each pain level for the group of males and females combined (p<0.001).
Comparable results were found for unpleasantness CVs, with significant correlations detected for both pain levels, independent of sex. For pain-lo, significant correlations between across- and within-session CVs were found for the pooled data (rho=0.50, p<0.001) and for the female group (rho=0.65, p<0.001), with the correlation for males nearing but not achieving statistical significance (rho=0.33, p=0.06). For pain-hi, significant correlations between across- and within-session CVs were found for the pooled data (rho=0.54, p<0.001), and for each group separately (female rho=0.56, p=0.001; male rho=0.51, p=0.003).
Comparison of Variability in Pain Intensity Ratings with Variability in Unpleasantness Ratings
To assess differences in variability between the two pain dimensions, log-transformed CVs were evaluated using 2-way repeated measures ANOVA, with pain dimension and pain level as factors. No differences between the two pain dimensions was detected for either across-session or within-session variability. The ANOVA for across-session CVs (log-transformed) detected a significant main effect of pain level as expected (F(1,63)=82, p<0.001), but no significant main effect of pain dimension (F(1,63)=1.2, p=0.28) or interaction between the factors (F(1,63)=0.4, p=0.5). Similarly, the ANOVA for within-session CVs (log-transformed) detected a significant main effect of pain level (F(1,63)=82, p<0.001), with pain-lo CVs significantly higher than pain-hi CVs, but no significant main effect of pain dimension (F(1,63)=1.2, p=0.27) or interaction between the factors (F(1,63)=1.8, p=0.2).
Factors Associated with Individual Differences in Heat Pain Rating Variability
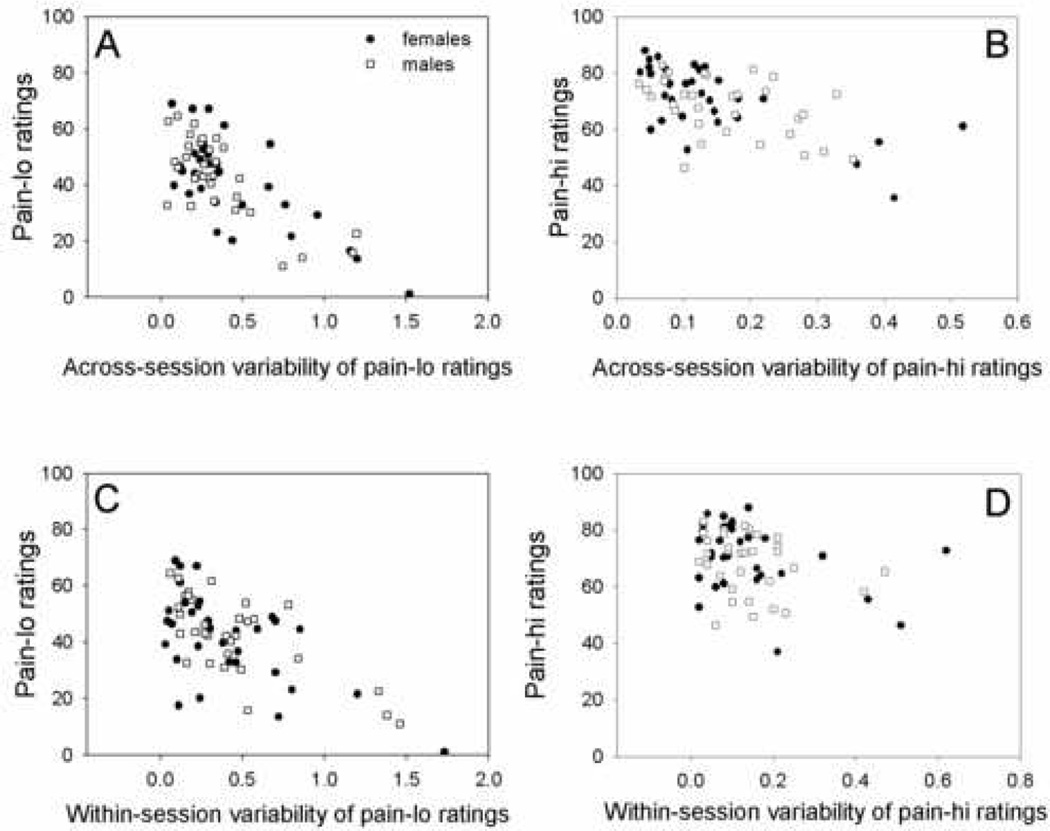
Exploratory correlations were performed to assess factors potentially contributing to individual variability in heat pain ratings. Across- and within-session pain intensity CVs for each pain level were significantly negatively correlated with mean pain intensity ratings for each pain level (Table 5, Figure 5), indicating that the lower an individual subject rated a painful heat stimulus, the greater the variability in their ratings both across and within sessions. The correlations between these measures were statistically significant for the group of men and women combined (Table 5). The correlations were also statistically significant in the male and female groups separately, except for the correlation of within-session CVs for pain-hi with pain-hi ratings, which showed a nonsignificant trend in the same direction for both females (p=0.2) and males (p=0.2). Pain intensity rating variability significantly correlated with a few other variables, but not in a systematic or consistent manner (Table 5). No significant correlations with pain rating variability were found in either the combined group or separate male and female groups for numerous variables, including age, stimulus temperature, pain sensitivity (rating of 48°C stimulus), average sleep, positive affect, negative affect, depression, or any pain coping strategies except catastrophizing (p>0.05).
Table 5.
Correlation of pain intensity rating variability with pain ratings and psychosocial measures.
| Variable+ | CV across- session, pain-lo |
CV across- session, pain-hi |
CV within- session, pain-lo |
CV within- session, pain-hi |
|---|---|---|---|---|
| All subjects (n=64) | ||||
| Pain intensity rating |
rho=−0.58 p<0.001* |
rho=−0.53 p<0.001* |
rho=−0.52 p<0.001* |
rho=−0.29 p=0.02* |
| Trait anxiety |
rho=0.27 p=0.04* |
rho=0.15 p=0.24 |
rho=−0.03 p=0.83 |
rho=0.07 p=0.60 |
| Emotional reactivity | rho=0.04 p=0.75 |
rho=−0.12 p=0.36 |
rho=−0.25 p=0.045* |
rho=−0.03 p=0.84 |
| Insomnia | rho=0.23 p=0.07 |
rho=0.11 p=0.40 |
rho=0.14 p=0.28 |
rho=0.11 p=0.39 |
| Catastrophizing |
rho=0.27 p=0.03* |
rho=0.05 p=0.71 |
rho=0.02 p=0.89 |
rho=0.02 p=0.86 |
| Females (n=32) | ||||
| Pain intensity rating |
rho=−0.57 p=0.001* |
rho=−0.57 p=0.001* |
rho=−0.49 p=0.004* |
rho=−0.24 p=0.2 |
| Trait anxiety | rho=0.23 p=0.21 |
rho=0.19 p=0.29 |
rho=0.001 p=1.0 |
rho=0.17 p=0.35 |
| Emotional reactivity | rho=0.08 p=0.67 |
rho=−0.08 p=0.65 |
rho=−0.31 p=0.08 |
rho=0.09 p=0.63 |
| Insomnia | rho=0.12 p=0.52 |
rho=0.14 p=0.45 |
rho=0.02 p=0.91 |
rho=−0.04 p=0.84 |
| Catastrophizing | rho=0.26 p=0.15 |
rho=−0.09 p=0.63 |
rho=−0.09 p=0.62 |
rho=0.07 p=0.72 |
| Males (n=32) | ||||
| Pain intensity rating |
rho=−0.6 p<0.001* |
rho=−0.43 p=0.01* |
rho=−0.59 p<0.001* |
rho=−0.26 p=0.2 |
| Trait anxiety | rho=0.28 p=0.13 |
rho=0.17 p=0.37 |
rho=0.06 p=0.74 |
rho=−0.02 p=0.90 |
| Emotional reactivity | rho=−0.05 p=0.79 |
rho=−0.03 p=0.86 |
rho=−0.15 p=0.43 |
rho=−0.10 p=0.58 |
| Insomnia |
rho=0.39 p=0.03* |
rho=−0.01 p=0.96 |
rho=0.36 p=0.047* |
rho=0.35 p=0.06 |
| Catastrophizing | rho=0.27 p=0.15 |
rho=0.14 p=0.45 |
rho=0.14 p=0.45 |
rho=−0.04 p=0.83 |
Statistically significant result
Other variables were assessed but no significant correlations were found in any group (p>0.05). These variables included age, stimulus temperature, pain sensitivity, average sleep, positive affect, negative affect, depression, and various pain coping strategies.
Figure 5.
Scatter plots of across- and within-session variability in heat pain intensity ratings for moderately painful heat stimuli (pain-lo) and strongly painful heat stimuli (pain-hi). Plots show (A) across-session variability of pain-lo ratings with pain-lo ratings, (B) across-session variability of pain-hi ratings with pain-hi ratings, (C) within-session variability of pain-lo ratings with pain-lo ratings, and (D) within-session variability of pain-hi ratings with pain-hi ratings. All correlations (Spearman’s rho) were significant for the group of males and females combined (p<0.05).
Similar to the findings for pain intensity ratings, across- and within-session unpleasantness CVs for each pain level were significantly negatively correlated with mean unpleasantness ratings for each pain level (Table 6), indicating that the less unpleasant an individual subject reported a painful heat stimulus, the greater the variability in their ratings both across and within sessions. The correlations between these measures were statistically significant for the group of men and women combined (Table 6). The correlations were also statistically significant in the male and female groups separately, except for the correlations in males of within-session CVs with pain-lo unpleasantness ratings (p=0.4) and pain-hi unpleasantness ratings (p=0.2).
Table 6.
Correlation of unpleasantness rating variability with unpleasantness ratings
| Variable+ | CV across- session, pain-lo |
CV across- session, pain-hi |
CV within- session, pain-lo |
CV within- session, pain-hi |
|---|---|---|---|---|
| Combined group (n=64) | ||||
| Unpleasantness rating |
rho=−0.69 p<0.001* |
rho=−0.55 p<0.001* |
rho=−0.37 p=0.003* |
rho=−0.32 p=0.009* |
| Females | ||||
| Unpleasantness rating |
rho=−0.83 p<0.001* |
rho=−0.58 p<0.001* |
rho=−0.53 p=0.002* |
rho=−0.43 p=0.01* |
| Males | ||||
| Unpleasantness rating |
rho=−0.53 p=0.002* |
rho=−0.38 p=0.03* |
rho=−0.15 p=0.4 |
rho=−0.23 p=0.2 |
Statistically significant result
Other experimental, demographic, and psychosocial variables were assessed but no significant correlations were found in any group (p>0.05).
DISCUSSION
Across-session stability of pain ratings
Group pain ratings trended downward across the test sessions, with a significant decline detected between the first and fourth sessions. These results are consistent with reports of increased heat pain thresholds across repeated sessions (Yarnitsky et al. 1996), regardless of body site tested (Taylor et al. 1993), and with a study reporting significant habituation of suprathreshold heat pain ratings over 8 daily sessions (Bingel et al. 2007). Though the present findings are not consistent with Rosier et al. (2002), who did not detect statistically significant changes in suprathreshold heat pain ratings across four weekly test sessions, the inconsistency is likely due to the small number of subjects used in that study (n=15). Rosier et al.’s data show a downward trend in group VAS ratings that may have become statistically significant with a larger number of subjects.
Within-session stability of pain ratings
Group pain ratings were stable across two stimulus series separated by 10 minutes, but were significantly reduced thereafter. The basis for this habituation is unclear but may be related to activation of endogenous analgesic systems, changes in attentional state, or fatigue. Changes in primary afferent responsiveness may also contribute, given that repetitive heat stimuli have been found to reduce C-fiber responses in monkeys (La Motte and Campbell 1978). However, La Motte and Campbell reported complete recovery of C-fiber responsiveness after a 10-minute stimulus-free interval, suggesting that desensitization of primary afferent nociceptors is unlikely to account for reduced pain ratings over the time scale used in this study. Simultaneous application of mild electrical stimulation during the second heat series may have contributed to this reduction, a possibility that cannot be ruled out because none of the sessions were conducted without electrical stimulation. However, this possibility seems unlikely given the report that mild transcutaneous electrical nerve stimulation did not change pain ratings at heterotopic body sites (Chesterton et al. 2003) and reports that other modalities of nonpainful conditioning stimulation did not alter pain perception (Price and McHaffie 1988; Lautenbacher et al. 2002; Edwards et al. 2003).
The results of the present study are not consistent with the findings of Granot et al. (2003), who examined across-session variability by applying 4 stimuli of a single temperature over a 1-hour period and found no within-session changes in pain ratings. Inconsistencies between the studies are likely due to methodological differences such as interstimulus interval and number of stimuli. The present study used a protocol more consistent with typical pain investigations and may therefore provide more applicable information to be considered during the design of future studies.
Comparison of variability across pain dimensions
This study provided novel data demonstrating that ratings of pain intensity and unpleasantness habituated comparably with repeated testing and were comparably variable, suggesting that the neural mechanisms underlying habituation are common to both pain dimensions. Given that the relationship between pain intensity and unpleasantness can be profoundly affected by stimulus modality and the context in which pain is experienced (Rainville et al. 1992; Price et al. 1987), these findings may be generalizable only to experimental paradigms that use phasic painful stimuli in a controlled laboratory environment.
Lack of sex effects on pain rating stability
Small but significant sex differences in responses to painful experimental stimuli have consistently been reported (Fillingim and Maixner 1995; Holdcroft and Berkley 2005), with women more sensitive than men. This study found that women required significantly lower temperatures to evoke comparable perceptions of pain as men, with the small magnitude of the difference (0.5°C) consistent with the literature (Riley et al. 1998). Despite well-established sex differences in pain, no studies to date have examined whether sex impacts the consistency or variability of psychophysical pain ratings. The present study addressed this issue using a large group of subjects and found no sex differences in any measure of heat pain intensity ratings stability or variability. Thus, this study demonstrates that for both men and women (1) contact heat stimuli can be perceptually equalized at targeted pain levels, (2) VAS ratings habituate comparably with repeated testing, and (3) VAS ratings are more variable for lower pain levels.
Effect of pain level on consistency of pain intensity ratings
Pain intensity and unpleasantness ratings of strongly painful stimuli were about 50% less variable (relative to the mean) than ratings of moderately painful stimuli, both across multiple sessions and within a single session. Lower variability of strong pain ratings cannot be attributed to a ceiling effect, as none of the subjects rated the stimuli higher than 90 on the VAS. These findings are consistent with Kong et al. (2006b), who found greater variability of subjective ratings of a perceptually-equalized mid-range painful heat stimulus compared to a stimulus in the upper portion of the pain range. The reasons for the relationship between perceived pain and variability in pain ratings are unclear but one possibility is that subjects may have a more precise concept of the meaning of the upper versus lower portion of the VAS, resulting in more consistent ratings. Kong et al. (2006b) provide two lines of evidence to support this hypothesis. First, their subjects reported that mid-range stimuli were more difficult to rate than stimuli in the upper portion of the pain range. Second, mid-range painful stimuli produced significantly greater fMRI activation than more strongly painful stimuli in cortical regions involved in cognitive evaluation of pain intensity, possibly due to increased difficulty of the rating task (Kong et al. 2006b). Taken together, these findings have implications for studies evaluating group differences in pain or modulation of pain by an experimental manipulation or drug. Such studies strive to reduce variability in order to detect small group differences. Based on the results of this study, it appears that use of strongly painful stimuli (rated in the upper portion of the VAS) can significantly reduce ratings variability and enhance the sensitivity to detect group differences in pain and pain modulation.
Individual variability in heat pain intensity ratings
The strong correlation between across- and within-subject variability for each pain level suggests that variability of VAS pain ratings is an intrinsic characteristic of individual subjects. Exploratory correlations performed to investigate factors that may contribute to this individual variability did not reveal significant associations with age, sleep, stimulus temperature, or psychosocial factors such as anxiety, depression, mood, emotional reactivity, or use of various pain coping strategies. While these findings suggest that individual variability in pain rating is not related to such demographic measures, stimulus attributes, or psychosocial factors, it should be recognized that the numerical range of many of these factors was relatively narrow in the healthy young population participating in the study. Thus, these factors are not likely to affect variability of pain ratings in studies using healthy populations, but may contribute to pain rating variability in other groups, such as the elderly or chronic pain patients. Furthermore, it is possible that pain rating variability may be associated with higher order interactions among combinations of variables, rather than individual variables alone. The present study did not have sufficient statistical power to address this possibility.
Interestingly, the one factor that was significantly correlated with pain rating variability was the perceived painfulness of the stimuli. While stimuli were chosen to be perceptually equalized across subjects, some variability in the actual ratings of those stimuli by individual subjects was inevitable. Correlating these individual subject ratings with across- and within-session CVs revealed that, for both pain levels, individuals who tended to rate the stimuli as less painful than other individuals made more variable pain ratings. Thus, even on the individual level, perceived pain intensity of a stimulus relates to variability in pain ratings, paralleling the group finding that across- and within-session CVs were significantly higher for the lower pain level than the higher pain level.
Conclusions
Rosier et al. (2002) provided important design recommendations for minimizing across-session variability in heat pain ratings, including use of a training session separate from the experimental sessions, a VAS rather than the visual descriptor scale, and multiple, short duration stimuli. The present study extends those findings, demonstrating that across- and within-session heat pain rating variability is influenced by the level of perceived pain. This study also demonstrates specific circumstances under which heat pain ratings habituate both within a test session and across multiple test sessions. Based on these findings, studies of pain modulation would ideally include: (1) a separate control session to quantify and account for within-session pain rating habituation; (2) counterbalancing of the control session with sessions in which experimental manipulations are applied to account for across-session pain rating habituation; and (3) the use of two randomly-presented stimulus levels, one in the upper portion of the pain range and one in the lower portion, to reduce expectation effects and ceiling/floor effects as well as to address the effect of pain level on ratings variability and the potential for differential modulation depending on pain level.
Acknowledgments
The authors gratefully acknowledge NIH support: F31-NS049731 (RLQ) and P50-AR49555 (JDG). This work constituted part of a PhD dissertation submitted to the University of Maryland, Baltimore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beck AT, Ward CH, Mendelson M, Mock JE, Erbaugh JK. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Bingel U, Schoell E, Herken W, Buchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007 doi: 10.1016/j.pain.2006.12.005. in press, corrected proof online ( http://www.sciencedirect.com/science/article/B6T0K-4MX54SN-1/2/4ff2a0f00caef8e53d92059d757a101c) [DOI] [PubMed]
- Chesterton LS, Foster NE, Wright CC, Baxter GD, Barlas P. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain. 2003;106:73–80. doi: 10.1016/s0304-3959(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Fillingim RB. Sex, gender, and pain: A biopsychosocial framework. In: Fillingim RB, editor. Sex, Gender, and Pain. Seattle: IASP Press; 2000. pp. 1–6. [Google Scholar]
- Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4(4):209–221. [Google Scholar]
- Granot M, Sprecher E, Yarnitsky D. Psychophysics of phasic and tonic heat pain stimuli by quantitative sensory testing in healthy subjects. Eur J Pain. 2003;7(2):139–43. doi: 10.1016/S1090-3801(02)00087-3. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Richards TL, Coda B, Bills AR, Blough D, Richards AL, Sharar SR. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport. 2004;15(8):1245–8. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- Holdcroft A, Berkley KJ. Sex and gender differences in pain and its relief. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5th. Oxford, UK: Elsevier Science Publishers; 2005. pp. 1180–1198. [Google Scholar]
- Kohn PM. Sensation-seeking, augmenting-reducing, and strength of the nervous system. In: Spence JT, Izard CE, editors. Motivation, emotion, and personality. Amsterdam: Elsevier; 1985. [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006a;26(2):381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006b;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Motte RH, Campbell JN. Comparsion of the responses of warmth and nociceptive C fiber afferents in monkey with human judgments of thermal pain. J Neurophys. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Roscher S, Strian F. Inhibitory effects do not depend on the subjective experience of pain during heterotopic noxious conditioning stimulation (HNCS): A contribution to the psychophysics of pain inhibition. Eur J Pain. 2002;6:365–374. doi: 10.1016/s1090-3801(02)00030-7. [DOI] [PubMed] [Google Scholar]
- Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical and visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- Price DD, Harkins SW, Rafii A, Price C. A simultaneous comparison of fentanyl’s analgesic effects on experimental and clinical pain. Pain. 1986;24:197–203. doi: 10.1016/0304-3959(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analog scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Price DD, McHaffie JG. Effects of heterotopic conditioning stimuli on first and second pain: a psychophysical evaluation in humans. Pain. 1988;34:245–252. doi: 10.1016/0304-3959(88)90119-4. [DOI] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res. 1992;9(4):265–277. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–216. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R. The state-trait anxiety inventory manual. Palo Alto, CA: Consulting Psychology Press; 1970. [Google Scholar]
- Strian F, Lautenbacher S, Galfe G, Holzl R. Diurnal variations in pain perception and thermal sensitivity. Pain. 1989;36:125–131. doi: 10.1016/0304-3959(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, McGillis SLB, Greenspan JD. Body site variation of heat pain sensitivity. Somatosens Mot Res. 1993;10(4):455–465. doi: 10.3109/08990229309028850. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. J Pers Soc Psychol. 1988;47:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA. Multiple session experimental pain measurement. Pain. 1996;67:327–333. doi: 10.1016/0304-3959(96)03110-7. [DOI] [PubMed] [Google Scholar]