Abstract
Resting-state functional connectivity (rsFC) is widely used to examine cerebral functional organization. The imaging literature has described lateralization of insula activations during cognitive and affective processing. Evidence appears to support a role of the right-hemispheric insula in attentional orientation to salient stimulus, interoception, and physiological arousal, and a role of the left-hemispheric insula in cognitive and affective control, as well as perspective taking. In this study, in a large data set of healthy adults, we examined lateralization of the rsFC of the anterior insula (AI) by computing a laterality index (LI) of connectivity with 54 regions from the Automated Anatomic Labeling atlas. At a corrected threshold (p < 0.001), the AI is left lateralized in connectivity with the dorsomedial prefrontal cortex, superior frontal gyrus, inferior frontal cortex, and posterior orbital gyrus and right lateralized in connectivity with the postcentral gyrus, supramarginal gyrus, and superior parietal lobule. In gender differences, women, but not men, showed right-lateralized connectivity to the thalamus. Furthermore, in a subgroup of participants assessed by the tridimensional personality questionnaire, novelty seeking is correlated with the extent of left lateralization of AI connectivity to the pallidum and putamen in men and with the extent of right lateralization of AI connectivity to the parahippocampal gyrus in women. These findings support hemispheric functional differentiation of the AI.
Keywords: : aging, hemisphericity, insula, laterality, rsFC, sex difference
Introduction
The anterior insula (AI) integrates inputs from cortical and subcortical structures to support emotional and cognitive processes (Craig, 2002, 2009). Functional lateralization of the AI has been observed for interoception in association with the autonomic activity. The right AI increases activation to internal focus on physical and emotional states (Critchley et al., 2004; Napadow et al., 2013; Zaki et al., 2012). In contrast, the left AI is implicated in affiliative behaviors in macaques in response to electrical stimulation (Caruana et al., 2011). Sympathetic and parasympathetic projections from the ventromedial nucleus of the thalamus are lateralized to the right and left AI, respectively (Craig, 2005). These lateralized projections result in differential autonomic control by the AI, such that direct stimulation of the right and left insula, each produces sympathetic and parasympathetic cardiac effects (Oppenheimer et al., 1992). In humans, left AI increases activation to perception of others experiencing an emotion more so than the right AI (Caria et al., 2010; Singer et al., 2004; Wicker et al., 2003), with the level of activation varying with individual rating of empathy (Singer et al., 2004). The left AI also responds both when participants smell a disgusting odor and when they observe others smelling the odor (Wicker et al., 2003), with activity increasing to negative valence ratings of stimuli (Caria et al., 2010). A recent review reported hemispheric dominance of the left insula in emotion perception (Duerden et al., 2013), providing support for cerebral lateralization in affective processing, but also noting inconsistencies due to task designs and highlighting the limitations of exploring functional laterality within a single paradigm.
Other studies support gender differences in emotional processing that involve lateralized insula responses (Duerden et al., 2013; Wager et al., 2003). During exposure to emotional stimuli, the left AI was activated more than the right AI in men, whereas both hemispheres activated to similar levels in women (Duerden et al., 2013). In an earlier meta-analysis, men had greater activation in the left AI, compared to women, in response to emotional stimuli (Wager et al., 2003). However, other studies reported left insula activation to aversive stimuli following negative mood induction by olfaction in women, but not in men (Koch et al., 2007) and covariance of women's, but no men's, subjective rating of a cartoon's funniness with the left insula activity (Kohn et al., 2011). Thus, gender differences in functional lateralization of the insula may be task specific.
The AI shows functional lateralization in cognitive processes. The AI, along with medial prefrontal cortex and subcortical structures, including the thalamus and midbrain, forms the salience network (SN) and responds to infrequent and behaviorally relevant stimuli (Ham et al., 2013; Seeley et al., 2007). Dynamic causal modeling suggests that the right AI is the most likely input to the SN during error processing (Ham et al., 2013). The SN is functionally connected to the default mode network (DMN) as well as the central executive network (CEN). Right AI connectivity modulates DMN activity during cognitive performance (Bonnelle et al., 2012; Sridharan et al., 2008). In patients with traumatic brain injury, the extent of damage to the white matter tracts between the medial prefrontal cortex and right AI predicts less deactivation of the DMN during response inhibition in a stop signal task, with the patient group as a whole showing less DMN deactivation than healthy controls (Bonnelle et al., 2012). Granger causality analyses support a causal role of the right AI in switching between the CEN and DMN in an auditory segmentation task, a visual oddball task, and resting state (Sridharan et al., 2008). Together, these results suggest that the right AI may serve as a “neural hub,” channeling inputs into the SN upon error detection and regulating activity within the CEN and DMN to optimize performance. This line of research supports a right-lateralized attention network (Schotten et al., 2011; Sturm et al., 1999) with the AI playing a central role in the orientation of attention to behaviorally salient targets.
In contrast, the left AI appears to be more involved in top-down behavioral modulation (Ham et al., 2013; Späti et al., 2014). Effective connectivity between the left AI and dorsal anterior cingulate cortex correlates with posterror slowing (Ham et al., 2013), and the left AI activity is significantly higher during self than externally attributed trials in a monetary reward task (Späti et al., 2014). The left, but not right, AI responds to preresponse conflicts (Ullsperger et al., 2010). These findings demonstrate the left AI's role in planning and behavioral adjustment. Thus, on a network level, functional lateralization of the AI embodies right hemispheric dominance in attention (Schotten et al., 2011; Sturm et al., 1999) and left hemispheric dominance in cognitive motor control (Barber et al., 2012; Gotts et al., 2013).
Nonetheless, cognitive and affective functions frequently require both top-down and bottom-up processes and engage the insula bilaterally. For instance, bilateral insula increases activation to prolonged RT following anticipation of a stop signal in the stop signal task (Hu et al., 2015), likely reflecting slower accumulation of sensory information through trial by trial learning (Hu et al., 2014). The bilateral AI responds to emotional interference resolution (Levens and Phelps, 2010), risky decisions during gambling (Xue et al., 2010), and cognitive control (Cole and Schneider, 2007; Dosenbach et al., 2007; Yeung et al., 2006). As with affective responses, functional lateralization of the AI during cognitive processes warrants further investigation.
Functional lateralization of the AI may be elucidated by assessing its resting-state functional connectivity (rsFC) to the whole brain. RsFC characterizes how low-frequency blood oxygenation-level dependent (BOLD) signal fluctuations are coordinated between functionally related regions (Biswal et al., 1995; Dosenbach et al., 2007; Fox and Raichle, 2007). Using rsFC, we have previously characterized whole-brain connectivity and the effects of age and medications on many cortical and subcortical areas (Farr et al., 2014; Li et al., 2014; Manza et al., 2015; Zhang and Li, 2012, 2014; Zhang et al., 2012; Zhang et al., 2015). Other investigators have used rsFC to examine functional architecture of the insula in healthy adults (Cauda et al., 2011), and explore its role in attention-deficit hyperactivity disorder (Tian et al., 2006), as well as depression (Liu et al., 2010) and anxiety (Baur et al., 2013).
In this study, we assessed the rsFC of the AI in 250 healthy adults, focusing on lateralization and the effects of age and gender on the extent of lateralization, on the basis of a literature of age-related changes (Mather, 2012) and gender differences in insula functions. Following previous studies, we computed the Laterality Index (LI) for individual brain regions as defined by the automated anatomical labeling (AAL) atlas (Di et al., 2014; Tzourio-Mazoyer et al., 2002). The insula has also been related to novelty seeking (NS) (Rn Enzi et al., 2009; Song et al., 2015) and harm avoidance (HA) (Ma et al., 2014; Markett et al., 2013). In a subgroup of 57 adults, we explored how personality traits, as evaluated by the tridimensional personality questionnaire (TPQ), are related to lateralized rsFC of the AI (Cloninger, 1987; Sher et al., 1995).
Materials and Methods
Data set
Resting-state fMRI scans were pooled from three data sets (Leiden_2180/Leiden_2200, Newark, and Beijing_Zang, n = 144) from the 1000 Functional Connectomes Project (Biswal et al., 2010) and our own data (n = 106). In selecting the data, we tried to include as many subjects as possible to have more stable findings, as in our earlier work (Zhang and Li, 2014; Zhang et al., 2012). We used only datasets acquired under conditions identical to our own (e.g., similar TR, all under 3T, participants scanned with eye closed). Individual subjects' images were viewed one by one to ensure that the whole brain was covered. A total of 250 healthy subjects' resting-state data (18–49 years of age; 104 men; one scan per participant; duration: 4.5–10 min) were analyzed. Table 1 summarizes these data sets. Men and women did not differ in age (25.4 ± 7.2 vs. 24.0 ± 5.8 years; p = 0.096).
Table 1.
Demographic Information and Imaging Parameters of the Resting-State Functional MRI Data Obtained from the Image Repository for the 1000 Functional Connectomes Project and Our Laboratory
| Dataset | Subjects | Ages (years) | Time points | TR (s) | Slice acquisition order |
|---|---|---|---|---|---|
| Beijing_Zang | 31 M/66 F | 18–26 | 225 | 2 | Interleaved ascending |
| Leiden_2180 | 10 M/0 F | 20–27 | 215 | 2.18 | Sequential descending |
| Leiden_2200 | 11 M/8 F | 18–28 | 215 | 2.2 | Sequential descending |
| Newark | 9 M/9 F | 21–39 | 135 | 2 | Interleaved ascending |
| Our own | 63 M/43 F | 19–49 | 295 | 2 | Interleaved ascending |
M, males; F, females; TR, repetition time.
In a subgroup of 57 individuals (20–47 years of age, 18 men) were assessed with the TPQ-short; (Sher et al., 1995). Derived from the 100-item long form of the TPQ (Cloninger, 1987), the TPQ-Short demonstrated reliability and validity. It consists of 44 yes/no questions, which cover the three dimensions: NS (13 items), HA (22 items), and reward dependence (RD; 9 items). Each personality subscale score was calculated by summing the item scores, reverse scored where necessary. A higher subscore each represents a higher level of NS, HA, and RD. We explored whether these personality traits are related to lateralized AI connectivity to each of the 54 brain regions of the AAL atlas (see below).
Imaging data processing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, United Kingdom). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Standard image preprocessing was performed. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were coregistered with the high resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999; Friston et al., 1995).
Additional preprocessing was applied to reduce spurious BOLD variances that are unlikely to reflect the neuronal activity (Fair et al., 2007; Fox and Raichle, 2007; Fox et al., 2005; Rombouts et al., 2003). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular system, and white matter signals were also included in the regression. Cordes et al. suggested that BOLD fluctuations below a frequency of 0.1 Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). Thus, we applied a temporal band-pass filter (0.009 Hz <f <0.08 Hz) to the time course to obtain low-frequency fluctuations, as in previous studies (Fox and Raichle, 2007; Fox et al., 2005; Lowe et al., 1998).
Head motion
As extensively investigated in Van Dijk et al. (2012), micro-head motion (>0.1 mm) is an important source of spurious correlations in rsFC analysis (Van Dijk et al., 2012). Therefore, we applied a “scrubbing” method proposed by Power et al. (2012) and successfully applied in previous studies (Power et al., 2012; Smyser et al., 2010; Tomasi and Volkow, 2014) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by 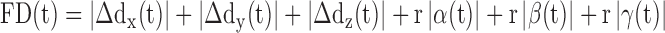 , where
, where  and
and  are the translational and rotational movements, respectively, and r (=50 mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transforms rotations into displacements (Powers et al., 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in% BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows:
are the translational and rotational movements, respectively, and r (=50 mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transforms rotations into displacements (Powers et al., 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in% BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: 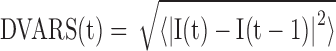 , where the brackets indicate the mean across brain voxels. Finally, to compute each subject's correlation map, we removed every time point that exceeded the head motion limit FD(t) >0.5 mm or DVARS(t) >0.5% (Power et al., 2012; Tomasi and Volkow, 2012). On average, 1% of the time points were removed across subjects.
, where the brackets indicate the mean across brain voxels. Finally, to compute each subject's correlation map, we removed every time point that exceeded the head motion limit FD(t) >0.5 mm or DVARS(t) >0.5% (Power et al., 2012; Tomasi and Volkow, 2012). On average, 1% of the time points were removed across subjects.
Seed based correlation and group analyses
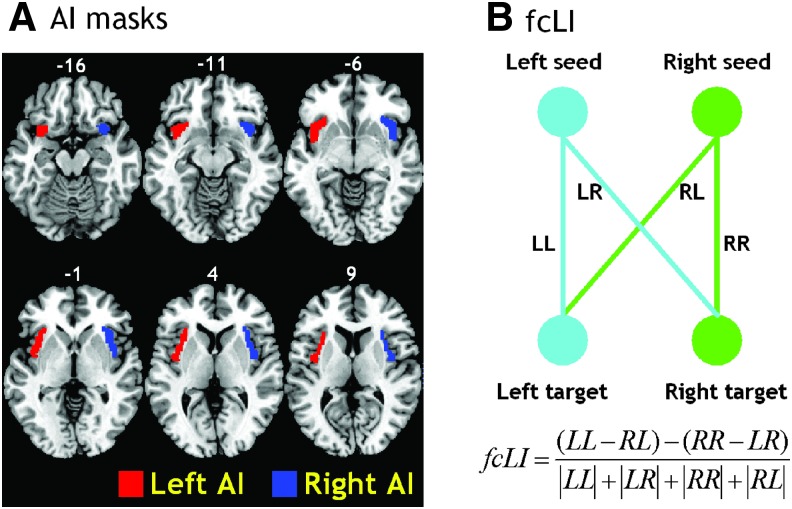
The left and right AI masks were generated using both cytoarchitectonic and topographical criteria based on Neuromorphometrics labels (www.neuromorphometrics.com) included in the SPM 12 software package (Fig. 1A). The BOLD time courses were averaged spatially each over the left and right AI seed. For individual subjects, we computed the correlation coefficient between the averaged time course of each seed region and the time courses of all other brain voxels. To assess and compare the rsFC, we converted these image maps, which were not normally distributed, to z-score maps by Fisher's z-transform (Berry and Mielke, 2000; Jenkins and Watts, 1968). The Z maps were used in group random-effect analyses. We performed one-sample t-test each on the Z maps of left and right AI and paired-sample t test comparing the two Z maps.
FIG. 1.
(A) Masks of the right and left anterior insula; (B) Laterality Index (LI). The value of LI ranges from −1 (R lateralization) to +1 (L lateralization), with a larger absolute value indicating greater lateralization in the connectivity between the seed and target. Color images available online at www.liebertpub.com/brain
Functional connectivity laterality index
A few considerations distinguished the computation of functional connectivity laterality index (fcLI) from the LI employed conventionally to characterize lateralization of cerebral activations to cognitive challenges: (L−R)/(L+R). First, negative connectivity of a brain region to the L (or R) seed cannot be distinguished from positive connectivity to the R (or L) seed. Second, target regions in the same hemisphere of the seed region will always have stronger functional connectivity than their counterparts in the other hemisphere (please see Results below). To manage these issues, therefore, we followed previous studies (Liu et al., 2009) to compute the fcLI based on connectivities of paired seed and target regions between the hemispheres. Briefly, the fcLI was computed as follows:
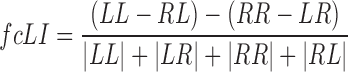 |
where LL is the functional connectivity between the L seed and L target region; RR is the functional connectivity between the R seed and R target region; RL is the functional connectivity between the R seed and L target region; and LR is the functional connectivity between the L seed and R target region (Fig. 1B). As computed, positive fcLI indicates left lateralization; that is, the target region, irrespective of its hemisphericity, has more connectivity to the L than R seed region. By contrast, a negative fcLI indicates right lateralization. The value of fcLI ranges from −1 (R lateralization) to +1 (L lateralization), with a larger value indicating greater lateralization in the connectivity between the seed and target.
In the sample assessed for TPQ, we examined whether NS and HA traits are related to lateralized AI connectivity to each of the 54 brain regions of the AAL atlas. Because of multiple tests, an alpha of 0.05/(54 × 2)–0.0005 would be required to guard against Type I error. However, we considered that not all of the 54 brain regions should be considered independent from one another, and used a p < 0.001 to examine the pair-wise regressions.
Results
Differences in whole-brain connectivity between right and left insula
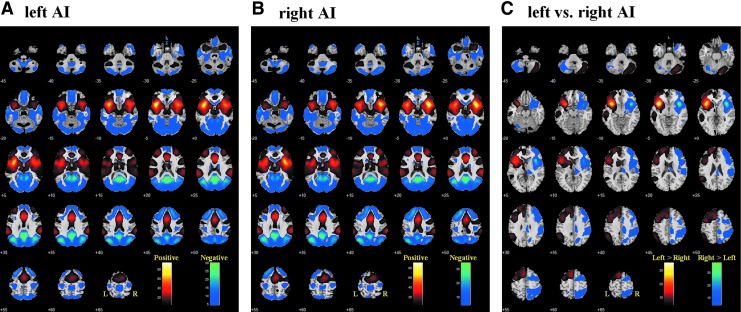
Figure 2A, B each shows the whole-bran rsFC of the left and right AI at p < 0.05 corrected for family-wise error of multiple comparisons. A direct contrast between these two maps demonstrated the left and right AI each with greater connectivity (greater positive or less negative connectivity) to regions in the same hemisphere (Fig. 2C).
FIG. 2.
(A) Whole-brain functional connectivity of the (A) left and (B) right AI; (C) differences in whole-brain functional connectivity of the left versus right AI. p < 0.05, FWE corrected. AI, anterior insula.
Lateralized regional connectivities to the AI
To examine whether the AI shows lateralized cerebral connectivity, one needs to go beyond this intrinsic, “biased” pattern of connectivity. An important question to ask is whether a given brain region is more connected to the left or right AI irrespective of the regions' hemisphericity. To this end, we followed previous studies (Liu et al., 2009) to derive a lateralization index (LI) of connectivity between each of the 54 brain regions with both L and R hemispheric masks from the AAL atlas.
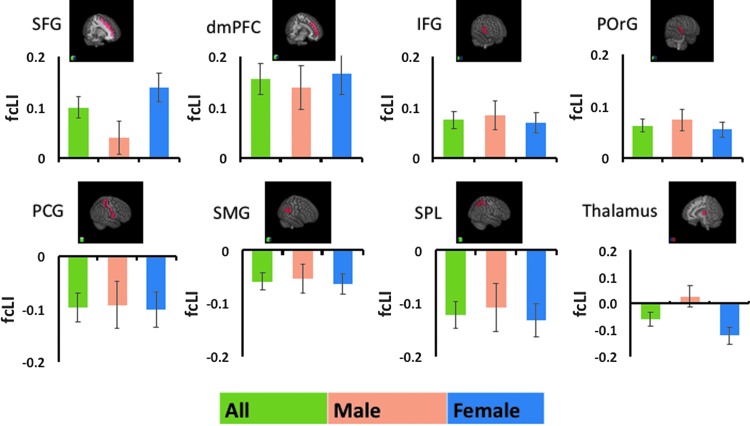
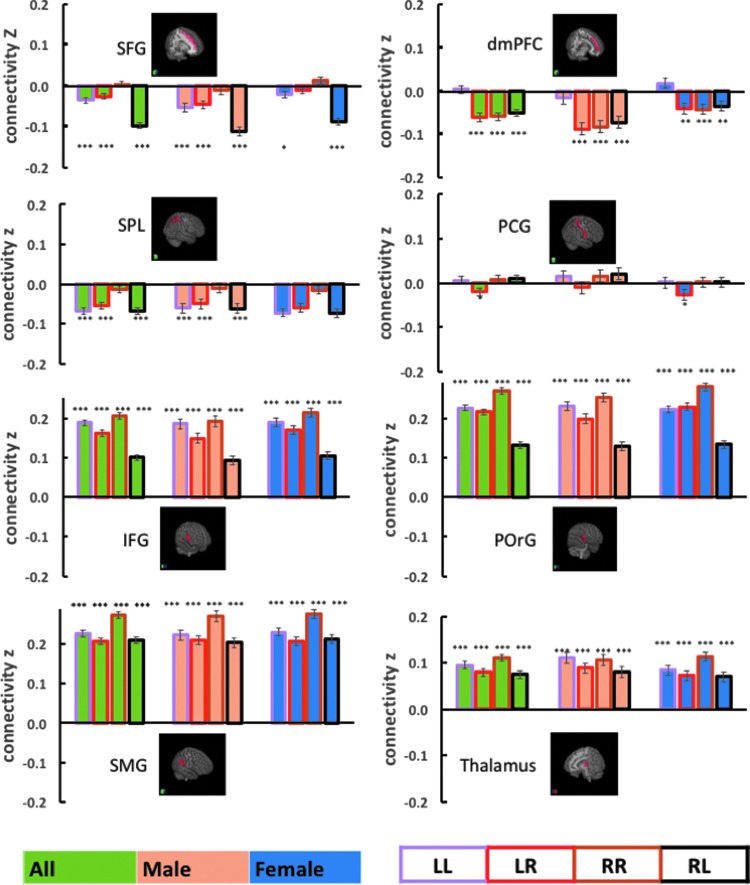
The results showed that, at a corrected threshold (p < 0.05/54–0.001), the superior frontal gyrus (SFG), inferior frontal gyrus (IFG), posterior orbital gyrus (POrG), and dorsal medial prefrontal cortex (dmPFC) showed left lateralization. The postcentral gyrus (PCG), superior parietal lobule (SPL), and supramarginal gyrus (SMG) showed right lateralization (Fig. 3). A two-sample t-test showed that the left lateralization of SFG connectivity is significantly greater in women than in men (p < 0.019). Of note, although the thalamus did not show significant lateralization within the general group analysis, women showed a significant right lateralization (p < 0.001), whereas men did not (p > 0.050). This gender difference was confirmed in a two-sample t-test of the LI (p < 0.005). In Figure 4, we showed the connectivity measures broken down for L and R seed and target regions.
FIG. 3.
Brain regions with a significant LI of functional connectivity (fcLI) to the AI. Bar plots show mean ± standard error of fcLI for men and women combined (green), and men (orange) and women (blue) separately. The top row shows regions with positive LI or left lateralization of AI connectivity. The bottom row shows regions with negative LI or right lateralization of AI connectivity.
FIG. 4.
Mean ± standard error for the connectivity z value for the eight target regions with lateralization in AI connectivity, broken down according to gender (men vs. women), AI (L vs. R), and target region (L vs. R) hemisphericity. Significance of the connectivity was further examined by one-sample t-test against zero for z values and marked with ***p < 0.001, **p < 0.01, and *p < 0.05.
The effect of age on lateralized functional connectivity of the AI
We assessed whether age influences lateralization of the rsFC of the AI. The extent of left lateralization of AI connectivity to the SFG was negatively correlated with age in men (r = −0.33, p < 0.001), but not in women (r = 0.03, p = 0.67), while the correlation for the group as a whole was not significant at a corrected threshold (r = −0.15, p = 0.02). Men and women showed a significant difference in slope of the linear regressions (p = 0.001). It should be noted that the SFG showed significantly left lateralization for women, but not for men, with a significant gender difference (see above). It thus appears that as men age, AI connectivity to the SFG becomes significantly less left lateralized, while women continue to maintain left lateralization in connectivity to the AI.
The effect of an NS trait on lateralized functional connectivity of the AI
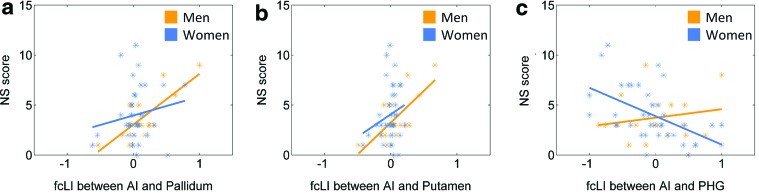
In a smaller cohort of subjects evaluated with the TPQ, we examined whether HA, RD, and NS subscore correlate with the LI at a corrected threshold (p < 0.001/3–0.00033). The results showed that NS subscore is positively correlated with the LI of the pallidum in men (p < 0.0002, r = 0.76), but not in women (p = 0.3134, r = 0.17). At a less stringent threshold of p < 0.001, NS subscore is also positively correlated with the LI of the putamen in men (p < 0.0009, r = 0.71), but not in women (p = 0.2007, r = − 0.21), and negatively correlated with the LI of the parahippocampal gyrus (PHG) in women (p < 0.0006, r = −0.53), but not in men (p = 0.4329, r = 0.20). That is, NS is associated with left lateralization of AI connectivity to the pallidum and putamen in men and with right lateralization of AI connectivity to the PHG in women. Figure 5 shows scatter plots and linear regressions of these correlations. However, a test of slope difference in regression between men and women was significant for the PHG (p < 0.01), but not the palladium or putamen (p's > 0.05). Of note, men and women did not differ in age (28.1 ± 7.2 vs. 26.1 ± 5.3 years; p = 0.23) or in NS subscore (3.7 ± 2.2 vs. 4.0 ± 2.7; p = 0.68) in this cohort.
FIG. 5.
Linear regression of the LI of (A) pallidum, (B) putamen, and (C) parahippocampal gyrus against novelty seeking (NS) subscore each for men (orange) and women (blue). Color images available online at www.liebertpub.com/brain
Discussion
rsFC of the AI
Our findings broadly corroborated those of previous studies that assessed rsFC of the insula, with the anterior network connected with the medial prefrontal regions (including the anterior cingulate cortex), middle and inferior frontal gyri, as well as the SMG (Cauda et al., 2011). The discussion below focused on lateralization of functional connectivity of the AI.
Hemispheric lateralization of the AI rsFC
The AI is right lateralized in connectivity to the PCG, SMG, and SPL. These findings are consistent with the correlated right-hemispheric dorsal AI and SPL/SMG activity in relation to attention performance (Touroutoglou et al., 2012). As part of the ventral attention system, the SMG responds to both auditory (Opitz et al., 1999) and visual (Ardekani et al., 2002) “odd-ball” stimuli. Critical for sensorimotor integration, the SPL responds to attention to a moving stimulus (Molenberghs et al., 2007; Vandenberghe et al., 2001), and SPL lesions compromise visuospatial attention in humans (Vandenberghe et al., 2012). The PCG includes the somatosensory cortex and differentiates salient from frequent sensory inputs in conjunction with the insula (Chen et al., 2010). Neuronal recordings from the primary somatosensory cortex in monkeys related the synchrony of neuronal firings to modulations in attention and task switching (Steinmetz et al., 2000). Human neuroimaging linked prestimulus alpha oscillations and mu rhythm in the somatosensory cortex to attentional demands (Anderson and Ding, 2011; Haegens et al., 2012). Thus, right lateralization of functional connectivity of the AI to these structures supports responses to salient stimuli and attention reorientation for actions.
The dorsomedial prefrontal cortex (dmPFC) and inferior frontal cortex (IFC) were significantly left lateralized in AI connectivity in both women and men. The mPFC includes multiple regions that have been implicated in proactive control (Hu et al., 2015) and error monitoring (Gehring and Willoughby, 2002; Ide et al., 2013; Ridderinkhof and Ullsperger, 2004; Zhang et al., 2012). Likewise, the IFC has shown distinct roles in attention orientation and response inhibition (Cai et al., 2014; Chao et al., 2009; Duann et al., 2009; Leung and Cai, 2007; Swick et al., 2008). Left lateralized connectivity to dmPFC and IFC accords with previous findings linking the left AI to “moment to moment adjustments in behavioral control” (Ham et al., 2013).
The dmPFC also plays a critical role in self-referencing and perspective taking (Northoff and Bermpohl, 2004; Northoff et al., 2006). Positron emission tomography (PET) imaging showed a higher activity in the dmPFC in medical students answering questions from the perspective of a lay person versus a medical professional (Ruby and Decety, 2003). The dmPFC increased activation in participants rating how pleased a person is to have their photo taken, compared to rating the symmetry of facial features (Mitchell et al., 2005). A meta-analysis of 65 imaging studies of emotion found greater activity in the mPFC in approach compared to withdrawal emotions (Wager et al., 2003). Likewise, the IFG and POrG have each been implicated in processing others' emotions (Schulte-Rüther et al., 2007; Wrase et al., 2003) and perspective taking (Hodzic et al., 2009). Also, in support of left lateralized connectivity of the AI to these regions, are previous findings of the left insula responding to perception of others' emotional state (Caria et al., 2010; Singer et al., 2004; Wicker et al., 2003). Taken together, evidence accumulates to support the left AI in empathetic affiliative behaviors (Craig, 2005), which involves an externalized awareness of the self in relation to the other, in concert with parasympathetic autonomic control (Caruana et al., 2011).
Age-related changes and gender differences in functional lateralization of the AI
The SFG significantly decreased in left-lateralized connectivity to the AI in men as a function of age, in contrast to women who maintained significant left lateralization in rsFC. The SFG is part of the dorsal lateral prefrontal cortex (DLPFC), a functional region implicated in age-related cognitive changes (Harlé Katia and Sanfey, 2012; Langner et al., 2015; MacPherson et al., 2002; Zhu et al., 2014). Compared to young adults, older adults show impaired performance in resolving stimulus–response conflict and decreased connectivity of bilateral AI to dorsomedial PFC and DLPFC (Langner et al., 2015). Older adults, compared to younger adults, showed higher activation in the right DLPFC and right AI within a task-switching paradigm (Zhu et al., 2014). Within unfair offer trials of an economic decision task, older adults exhibited higher activation in the DLPFC and lower activation in bilateral AI relative to younger adults (Harlé Katia. and Sanfey, 2012). Last, a study found a positive relationship between a measure of BOLD variability (SDBOLD) of the SFG and insula during resting state and performance on a memory task in older adults (Burzynska et al., 2015). These findings, however, do not address why the age-related changes transpire in men, but not in women.
Thalamic connectivity to the AI was significantly right lateralized in women, but not in men, with the LI being significantly different between genders. The insula receives input from the ventromedial thalamus (Barbaresi et al., 1992.; Cechetto and Saper, 1987; Friedman et al., 1986), a region that encodes nociceptive stimulus intensity (Hutchison et al., 1994). Women had a significantly higher activity, as measured by PET imaging, in the AI and thalamus when experiencing heat stimuli and rated heated stimuli as more painful than men (Paulson et al., 1998). In studies of micturition control, the right AI and midbrain periaqueductal gray were more active at higher than at lower bladder volumes, and responses of the right thalamus and several other right hemispherical regions were stronger in women than in men (Kuhtz-Buschbeck et al., 2009). Along with these studies, the finding of right-lateralized AI thalamic connectivity supports higher sensitivity to salient somatosensory and interoceptive stimuli in women. Multiple studies have demonstrated gender differences in behavior on tasks involving both pain (for review see Fillingim and Maixner, 1995) and emotional sensitivity (Dimberg and Lundquist, 1990; Kring and Gordon, 1998), with insula responding differently between men and women in various emotional tasks (for review see Duerden et al., 2013). Right-lateralized connectivity between the AI and thalamus in women, but not men, appears to support gender differences in pain and affective processing.
NS and functional lateralization of the AI
The personality trait NS describes a tendency to react to novel stimuli intensely and has been associated with reward-seeking behavior, including substance misuse (Cloninger, 1987; Cotto et al., 2010; Sher et al., 1995). Within our sample, men, but not women, showed a positive relationship between NS and left-lateralized connectivity of the AI to the pallidum and putamen. The striatum and insula are heavily connected anatomically (Chikama et al., 1997) and both structures are associated with reward predictions (Tachibana and Hikosaka, 2012; Tanaka et al., 2004; Wittmann et al., 2011). An earlier work linked NS to differential activities of these regions, with novelty response of the left striatum correlated with individual NS score (Wittmann et al., 2008). In another study, NS scores correlated negatively with activity during risk prediction in the left AI and right striatum (Wang et al., 2015). Broadly consistent with these earlier studies, this finding may be further explored for a link to gender differences in neural mechanisms of substance misuse and other addictive behaviors (Cohen et al., 1993; Cotto et al., 2010; Kampov-Polevoy et al., 2004).
The human hippocampal formation is involved in processing novelty signals (Daselaar et al., 2006; Köhler et al., 2005; Kumaran and Maguire, 2009; Schroeder et al., 2004). Unlike the hippocampus, which responds to changes in the relationship between objects and background, the PHG is engaged only by scene novelty, in participants performing an incidental target-detection task (Howard et al., 2011). Our finding of right-lateralized connectivity of AI to PHG supports a mechanism of concerted attention orientation in women. How functional connectivity between the AI and PHG subserves novelty detection remains to be investigated.
Conclusion
This study assessed lateralized functional connectivity between the AI and cortical and subcortical areas. The results showed a distinct pattern of right lateralization with regions implicated in attention orientation and arousal and left lateralization with regions implicated in cognitive motor control and perspective taking. The pattern of functional lateralization appears to accord with the role of the right and left AI each in sympathetic and parasympathetic autonomic responses (Craig, 2005) as well as hemispheric lateralization of neural networks to support bottom-up and top-down processing (Gotts et al., 2013). Our findings also suggest that functional lateralization of the AI may vary with age, gender, and personality traits.
A few limitations need to be considered. First, we could not study the functional implications of the patterns of lateralized connectivity because participants were not assessed for neurocognitive performance. Second, although we reported age-related effects, this sample included only young and middle-aged adults and the findings should be considered specific to this age range. Third, in discussing these findings, we sometimes referred to an imaging literature that does not always distinguish between the anterior and posterior insula. These important issues need to be addressed in future work.
Acknowledgments
Supported by NIH grants DA023248, DA026990, AA021449, and K25DA040032, and the Peter McManus Charitable Trust. The funding agencies otherwise have no role in the conceptualization of the study, data collection and analysis, or the decision to publish these results. We do not have financial interest in this work.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson KL, Ding M. 2011. Attentional modulation of the somatosensory mu rhythm. Neuroscience 180:165–180 [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Choi SJ, Hossein-Zadeh G-A, Porjesz B, Tanabe JL, Lim KO, et al. . 2002. Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Res Cogn Brain Res 14:347–356 [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 1999. Nonlinear spatial normalizatioon using basis functions. Hum Brain Mapp 7:254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi P, Minellia A, Manzoni T. 1992. Thalamic connections of the second somatic sensory area in cats studied with anterograde and retrograde tract-tracing techniques. Neuroscience 46:149–163 [DOI] [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. 2012. Motor “dexterity”: evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cerebral Cortex 22:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Langer N, Jäncke L. 2013. Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92 [DOI] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW. 2000. A monte carlos investigation of the fisher Z transformation for normal and nonnormal distributions. Psychol Rep 87:1101–1114 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, et al. . 2010. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. 2012. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci 109:4690–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Wong CN, Voss MW, Cooke GE, McAuley E, Kramer AF. 2015. White matter integrity supports BOLD signal variability and cognitive performance in the aging human brain. PLoS One 10:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li C-SR, Menon V. 2014. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci 34:14652–14667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. 2010. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry 68:425–432 [DOI] [PubMed] [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. 2011. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol 21:195–199 [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. 2011. Functional connectivity of the insula in the resting brain. NeuroImage 55:8–23 [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. 1987. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262:27–45 [DOI] [PubMed] [Google Scholar]
- Chao HHA, Luo X, Chang JLK, Li C-SR. 2009. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—an intra-subject analysis. BMC Neurosci 10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TL, Babiloni C, Ferretti A, Perrucci MG, Romani GL, Rossini PM, et al. . 2010. Effects of somatosensory stimulation and attention on human somatosensory cortex: An fMRI study. NeuroImage 53:181–188 [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. 1997. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci 17:9686–9705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger R. 1987. A systematic method for clinical description and classification of personality variants. Arch Gen Psychiatry 44:573–588 [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, et al. . 1993. An epidemiological study of disorders in late childhood and adolescence—I. Age- and gender-specific prevalence. J Child Psychol Psychiatry 34:851–867 [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. 2007. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage 37:343–360 [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. . 2001. Frequencies contributing to functional connectivity in the cerebral cortex in resting-state data. Am J Neuroradiol 22:1326–1333 [PMC free article] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SR. 2010. Gender effects on drug use, abuse, and dependence: a special analysis of results from the national survey on drug use and health. Gender Med 7:402–413 [DOI] [PubMed] [Google Scholar]
- Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666 [DOI] [PubMed] [Google Scholar]
- Craig AD. 2005. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci 9:566–571 [DOI] [PubMed] [Google Scholar]
- Craig DB. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195 [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. 2006. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol 96:1902–1911 [DOI] [PubMed] [Google Scholar]
- Di X, Kim EH, Chen P, Biswal BB. 2014. Lateralized resting-state functional connectivity in the task-positive and task-negative networks. Brain Connect 4:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U, Lundquist LO. 1990. Gender differences in facial reactions to facial expressions. Biol Psychol 30:151–159 [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. . 2007. Distinct brain networks for adaptive and stable task control in humans. Proce Natl Acad Sci U S A 104:11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, Li CSR. 2009. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29:10171–10179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Arsalidou M, Lee M, Taylor MJ. 2013. Lateralization of affective processing in the insula. NeuroImage 78, 159–175 [DOI] [PubMed] [Google Scholar]
- Fair D, Schlaggar B, Cohen A. 2007. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage 35:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Zhang S, Hu S, Matuskey D, Abdelghany O, Malison RT, Li C-SR. 2014. The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int J Neuropsychopharmacol 17:1177–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W. 1995. Gender differences in the responses to noxious stimuli. Pain Forum 4:209–221 [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O'Neill JB, Mishkin M. 1986. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolumbic pathway for touch. J Comp Neurol 252:323–347 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. 1995. Spatial registration and normalization of images. Hum Brain Mapp 3:165–189 [Google Scholar]
- Gehring WJ, Willoughby AR. 2002. The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295:2279–2282 [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A. 2013. Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci U S A 110:E3435–E3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O. 2012. Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cogn Neurosci 24:677–685 [DOI] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. 2013. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci 33:7091–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlé Katia MKM, Sanfey AG. 2012. Social economic decision-making across the lifespan: an fMRI investigation. Neuropsychologia 50:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic A, Kaas A, Muckli L, Stirn A, Singer W. 2009. Distinct cortical networks for the detection and identification of human body. NeuroImage 45:1264–1271 [DOI] [PubMed] [Google Scholar]
- Howard LR, Kumaran D, Ólafsdóttir HF, Spiers HJ. 2011. Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. J Neurosci 31:5253–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Li C, Shan R. 2015. Anticipating conflict: neural correlates of a Bayesian belief and its motor consequence. NeuroImage 119:286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Tseng YC, Winkler AD, Li CSR. 2014. Neural bases of individual variation in decision time. Hum Brain Mapp 35:2531–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Luhn MAB, Schmidt RF. 1994. Responses of lateral thalamic neurons to algesic chemical stimulation of the cat knee joint. Exp Brain Res 101:452–464 [DOI] [PubMed] [Google Scholar]
- Ide JS, Shenoy P, Yu AJ, Li CR. 2013. Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci 33:2039–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. 1968. Spectral Analysis and Its Applications. San Francisco, CA: Holden-Day [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. 2004. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res 28:1291–1298 [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, et al. . 2007. Gender differences in the cognitive control of emotion: an fMRI study. Neuropsychologia 45:2744–2754 [DOI] [PubMed] [Google Scholar]
- Köhler S, Danckert S, Gati JS, Menon RS. 2005. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus 15:763–774 [DOI] [PubMed] [Google Scholar]
- Kohn N, Kellermann T, Gur RC, Schneider F, Habel U. 2011. Gender differences in the neural correlates of humor processing: implications for different processing modes. Neuropsychologia 49:888–897 [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. 1998. Sex differences in emotion: expression, experience, and physiology. J Pers Soc Psychol 74:686–703 [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Gilster R, Van Der Horst C, Hamann M, Wolff S, Jansen O. 2009. Control of bladder sensations: an fMRI study of brain activity and effective connectivity. NeuroImage 47:18–27 [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. 2009. Novelty signals: a window into hippocampal information processing. Trends Cogn Sci 13:47–54 [DOI] [PubMed] [Google Scholar]
- Langner R, Cieslik EC, Behrwind SD, Roski C, Caspers S, Amunts K, Eickhoff SB. 2015. Aging and response conflict solution: behavioural and functional connectivity changes. Brain Struct Funct 220:1739–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H-C, Cai W. 2007. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci 27:9893–9900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. 2010. Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. J Cogn Neurosci 22:2790–2803 [DOI] [PubMed] [Google Scholar]
- Li CSR, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. 2014. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. NeuroImage 97:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. 2009. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci U S A 106:20499–20503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y, et al. . 2010. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res 182:211–215 [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. 1998. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage 7:119–132 [DOI] [PubMed] [Google Scholar]
- Ma Y, Li B, Wang C, Shi Z, Sun Y, Sheng F, et al. . 2014. 5-HTTLPR polymorphism modulates neural mechanisms of negative self-reflection. Cerebral Cortex 24:2421–2429 [DOI] [PubMed] [Google Scholar]
- MacPherson S, Phillips L, Della Sala S. 2002. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychol Aging 17:598–609 [PubMed] [Google Scholar]
- Manza P, Zhang S, Hu S, Chao HH, Leung H-C, Li CR. 2015. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. NeuroImage 107:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markett S, Weber B, Voigt G, Montag C, Felten A, Elger C, Reuter M. 2013. Intrinsic connectivity networks and personality: the temperament dimension harm avoidance moderates functional connectivity in the resting brain. Neuroscience 240:98–105 [DOI] [PubMed] [Google Scholar]
- Mather M. 2012. The emotion paradox in the aging brain. Ann NY Acad Sci 1251:33–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. 2005. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci 17:1306–1315 [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RRC. 2007. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cerebral Cortex 17:2703–2712 [DOI] [PubMed] [Google Scholar]
- Napadow V, Sheehan JD, Kim J, Lacount LT, Park K, Kaptchuk TJ, et al. . 2013. The brain circuitry underlying the temporal evolution of nausea in humans. Cerebral Cortex 23:806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. 2004. Cortical midline structures and the self. Trends Cogn Sci 8:102–107 [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. 2006. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage 31:440–457 [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Von Cramon DY, Kruggel F. 1999. Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology 36:142–147 [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. 1992. Cardiovascular effects of human insular cortex stimulation. Neurology 42:1727–1732 [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL. 1998. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M. 2004. The role of the medial frontal cortex in cognitive control. Science 306:443–447 [DOI] [PubMed] [Google Scholar]
- Rn Enzi B, De Greck M, Prö Sch U, Tempelmann C, Northoff G. 2009. Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS One 4:e8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SARB, Stam CJ, Kuijer JPA, Scheltens P, Barkhof F. 2003. Identifying confounds to increase specificity during a “no task condition”: evidence for hippocampal connectivity using fMRI. NeuroImage 20:1236–1245 [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. 2003. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur J Neurosci 17:2475–2480 [DOI] [PubMed] [Google Scholar]
- Schotten MT. De, Acqua FD, Forkel S, Vergani F, Murphy DGM, Catani M, et al. . 2011. A lateralized brain network for visuospatial attention. Nat Neurosci 14:1245–1246 [DOI] [PubMed] [Google Scholar]
- Schroeder U, Hennenlotter A, Erhard P, Haslinger B, Stahl R, Lange KW, Ceballos-Baumann AO. 2004. Functional neuroanatomy of perceiving surprised faces. Hum Brain Mapp 23:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. 2007. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci 19:1354–1372 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. . 2007. Behavioral/systems/cognitive dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Crews TM, Vandiver PA. 1995. The tridimensional personality questionnaire: reliability and validity studies and derivation of a short form. Psychol Assessment 7:195–208 [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan R, Frith C. 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–1162 [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. 2010. Longitudinal analysis of neural network development in preterm infants. Cerebral Cortex 20:2852–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhu Q, Li J, Wang X, Liu J. 2015. Typical and atypical development of functional connectivity in the face network. J Neurosci 35:14624–14635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späti J, Chumbley J, Brakowski J, Dörig N, Grosse Holtforth M, Seifritz E, Spinelli S. 2014. Functional lateralization of the anterior insula during feedback processing. Hum Brain Mapp 35:4428–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. 2000. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404:187–190 [DOI] [PubMed] [Google Scholar]
- Sturm W, De Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, et al. . 1999. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia 37:797–805 [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. 2008. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. 2012. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron 76:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. 2004. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci 7:887–893 [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, et al. . 2006. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 400:39–43 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Laterality patterns of brain functional connectivity: gender effects. Cerebral Cortex 22:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2014. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cerebral Cortex 24:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. 2012. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage 60:1947–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. . 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. 2010. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct 214:629–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk KRA, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. 2001. Functional specificity of superior parietal mediation of spatial shifting. NeuroImage 14:661–673 [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Molenberghs P, Gillebert CR. 2012. Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia 50:1092–1103 [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. 2003. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage 19:513–531 [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Yang L, Gu F, Li X, Zha R, et al. . 2015. Novelty seeking is related to individual risk preference and brain activation associated with risk prediction during decision making. Sci Rep 5:10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. 2003. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 40:655–664 [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Daw ND, Seymour B, Dolan RJ. 2008. Striatal activity underlies novelty-based choice in humans. Neuron 58:967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Lovero KL, Lane SD, Paulus MP. 2011. Now or later? Striatum and insula activation to immediate versus delated rewards. J Neurosci Psychol Econ 3:15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, et al. . 2003. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci Lett 348:41–45 [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. 2010. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. NeuroImage 50:709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Nystrom L, Aronson J, Cohen J. 2006. Between-task competition and cognitive control in task switching. J Neurosci 26:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. 2012. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62:493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li C-SR. 2012. Resting-state functional connectivity of the medial superior frontal cortex. Cerebral Cortex 22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR. 2012. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage 59:3548–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR. 2014. Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect 4:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Tsai S-J, Hu S, Xu J, Chao HH, Calhoun VD, Li C-SR. 2015. Independent component analysis of functional networks for response inhibition: inter-subject variation in stop signal reaction time. Hum Brain Mapp 36:3289–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Hakun JG, Johnson NF, Gold BT. 2014. Age-related increases in right frontal activation during task switching are mediated by reaction time and white matter microstructure. Neuroscience 278:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]