Abstract
Background: The vast majority of thyroid cancers, in particular the non-anaplastic follicular cell–derived thyroid carcinomas (non-ANA FCDC), are considered indolent tumors with very low mortality. Hence, it is crucial to analyze the subgroup of these patients who die of disease (DOD) in order to identify clinicopathologic features predictive of disease-specific mortality.
Methods: All non-ANA FCDC operated at a tertiary cancer center between 1985 and 2010 who were DOD were identified and submitted to a meticulous clinicopathologic analysis.
Results: Out of 3750 non-ANA FCDC, 58 (1.5%) DOD cases were identified. The DOD group was composed of 33 (57%) poorly differentiated carcinomas (PDTC), 14 (24%) tall-cell variant papillary thyroid carcinomas (TCVPTC), four (7%) Hürthle cell carcinomas, three (5%) papillary microcarcinomas, two (3%) classical variant PTC, and two (3%) follicular variant PTC. Twenty-seven (47%) patients presented with distant metastases (DM), 28 (48%) developed DM during follow-up, while the remaining three (5%) had locally advanced non-resectable recurrence. Gross extension beyond the thyroid (GET) was present in 36 (62%) and extensive vascular invasion (VI) in 21 (36%) of cases. All microcarcinomas had PDTC in their clinically apparent cervical lymph nodes at presentation. Encapsulated thyroid carcinomas were responsible for 17% of DOD cases, and all had extensive VI and/or DM at presentation. All patients had at least one of these high-risk features at diagnosis: DM at presentation, PDTC, GET, and/or extensive VI. The majority of patients died from DM (n = 51; 88%), three (5%) from locoregional disease, three (5%) from both, and one (2%) from unknown cause.
Conclusions: PDTC and TCVPTC are responsible for the vast majority of deaths in differentiated thyroid carcinomas, while the few fatal classical, follicular variant PTC and microcarcinomas all harbor a PDTC component, DM, or GET. Encapsulated differentiated thyroid carcinoma with focal capsular and/or VI without DM at presentation does not seem to cause death. Lack of DM at presentation, PDTC, GET, and extensive VI identify thyroid carcinomas that are at almost no risk of DOD. The vast majority of patients die of DM rather than locoregional invasion, prompting the need for effective systemic treatment.
Keywords: : thyroid, carcinomas, fatal, poorly differentiated, tall cell, follicular
Introduction
The incidence of thyroid cancer has been steadily increasing while mortality from thyroid cancer has gradually declined over the past few decades worldwide (1). Although reports from the 1980s and 1990s have documented a mortality rate of 3.3–11.1% in differentiated thyroid carcinomas (2–7), only 1–3% of these have been fatal in recent years (1,8). Nowadays, the vast majority of thyroid cancers, in particular the non-anaplastic follicular cell–derived thyroid carcinomas (non-ANA FCDC), are considered indolent tumors with very low mortality. Several well-recognized organizations, including the American Thyroid Association (ATA) (9) and the National Comprehensive Cancer Network (10), have published clinical management guidelines advocating for risk stratification using a variety of clinical and pathologic parameters. In order to identify subgroups at risk for poor outcome and assess the predictive power of these risk stratification schemes, it is crucial to analyze the subgroup of patients who die of disease (DOD). Toward that purpose, a meticulous clinicopathologic examination was performed of all DOD cases treated in a single tertiary cancer center with the hope that it will help better guide patient stratification and therapy.
Material and Methods
Inclusion criteria
The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY). The institutional surgical database was searched for cases meeting the following inclusion criteria: (i) patients operated between 1985 and 2010 and subsequently followed at MSKCC; (ii) a pathologic diagnosis of non-ANA FCDC, which included poorly differentiated thyroid carcinoma (PDTC), follicular carcinoma (FC), Hürthle cell carcinoma (HCC), and papillary carcinoma (PTC); and (iii) a confirmed fatal clinical outcome attributed to the thyroid carcinoma. All fatal cases were subjected to a meticulous clinicopathologic analysis under the supervision of a head and neck pathologist with special interests in thyroid neoplasia (R.G.).
Pathology review
All hematoxylin and eosin slides sampled in the specimen of the primary resection (i.e., thyroid and neck lymph nodes) were subjected to the pathologic review. Tumor size was measured as the maximum diameter of the resected tumor specimen. Mitotic rate was determined by counting 10 high-power fields (HPFs; 400×, field size 0.24 mm2) with an Olympus microscope (U-DO model BX41; Olympus America, Inc., Center Valley, PA) in the areas of greatest concentration of mitotic figures. Capsular invasion was defined as complete penetration of the capsule by tumor, and the number of these foci was recorded. The presence of vascular invasion (VI) was noted only when such foci were present within or beyond the capsule in accordance with criteria outlined by the Armed Forces Institute of Pathology (AFIP) fascicle (11). Briefly, only when the invasive focus protruded into the lumen of the vessel in a polypoid manner covered by endothelial cells, or when it was attached to the vessel wall or associated with thrombus formation, was it considered true VI. Areas of VI that were closely adjacent to one another were counted as separate foci. The capsular and VI was subdivided into two categories: focal (<4 invasive foci) and extensive (≥4 foci). The presence or absence of microscopic extrathyroidal tumor extension (ETE) into the perithyroid soft-tissue stroma was documented. ETE was subdivided into (i) absent; (ii) focal (presence of one or two microscopic foci of ETE, each measuring ≤1 mm); and (iii) extensive (presence of more than two microscopic foci of ETE, each measuring ≤1 mm, or any foci >1 mm in size). Microscopic resection margins were categorized as positive (tumor at the inked margin) or negative (no tumor at the inked margin). Finally, when regional lymph node sampling was performed during the initial surgery, the number of lymph nodes, metastatic status, size, presence of extranodal extension (ENE), and histological type of the metastasis were also recorded.
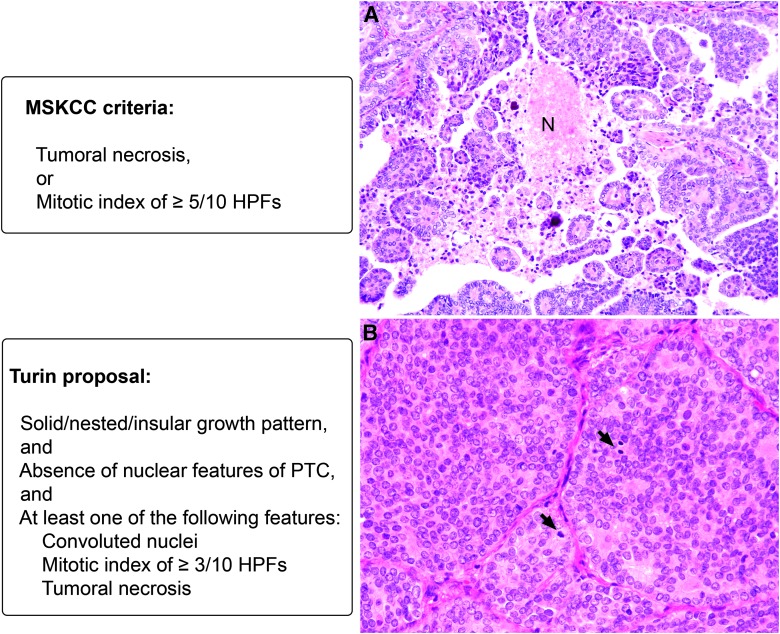
Poorly differentiated carcinoma was defined using the MSKCC criteria (12) and the Turin proposal (13) (Fig. 1). In brief, the MSKCC criteria defined PDTC as a non-ANA FCDC exhibiting tumor necrosis or an elevated mitotic index of ≥3/10 HPFs (400×), regardless of the growth pattern and nuclear features (12). On the other hand, the Turin proposal classified PDTC based on the presence of a solid/nested/insular growth pattern, the absence of nuclear features of PTC, and at least one of the following three features: convoluted nuclei, mitotic index of ≥3/10 HPFs, and/or tumoral necrosis (Fig. 1) (13).
FIG. 1.
Criteria to diagnose poorly differentiated thyroid carcinoma (PDTC). The Memorial Sloan Kettering Cancer Center (MSKCC) criteria require tumor necrosis or a mitotic index of ≥5/10 high power fields (HPFs). The Turin proposal requires solid/nested/insular growth pattern, and absence of nuclear features of papillary thyroid carcinoma (PTC), and at least one of the following three features: convoluted nuclei, mitotic index of ≥3/10 HPFs and/or tumoral necrosis. (A) Thyroid carcinoma displaying the nuclear features of PTC, as well as papillary architecture and tumor necrosis (N). This tumor will be classified as PDTC by MSKCC criteria and as PTC by the Turin proposal. (B) A thyroid carcinoma without the nuclear features of PTC demonstrating a solid growth pattern and a high mitotic index of 7/10 HPFs (arrows). This tumor will be classified as MSKCC-PDTC and Turin-PDTC.
Clinical review
Each patient's charts were reviewed to confirm thyroid cancer–related mortality and to document the following clinical parameters: age at diagnosis, sex, type of surgery, development of locoregional recurrence and/or distant metastasis (DM), site(s) of DM, presenting symptoms at the time of initial clinical visit, time interval from initial surgery to death, and mode of demise (i.e., death related to locoregional recurrence and/or DM). Additionally, gross extension beyond the thyroid (GET), defined as any appreciable gross adhesion and/or frank invasion of the primary thyroid carcinoma or metastatic carcinoma from a neck lymph node to the adjacent structures observed during operation, was documented. Because only 15/58 (25%) of cases had post-thyroidectomy serum thyroglobulin (Tg) and thyrotropin (TSH) available in the appropriate time frame (six weeks to three months), the postoperative Tg and TSH data were not analyzed. The lack of appropriate Tg data was related to the fact that many of these cases were treated prior to 2000 when routine Tg measurements (in the appropriate time frame) were introduced.
Statistics
Statistical analyses were performed using IBM SPSS Statistics for Windows v22.0 (IBM Corporation, Armonk, NY). A two-tailed Student's t-test was performed to compare time to death between patients with DM at presentation and those without. p-Values of <0.05 were considered to be statistically significant.
Results
Clinicopathologic characteristics of the study cohort
Fifty-eight (1.5%) fatal cases were retrieved from the institutional database of 3750 non-ANA FCDC. The clinical features of this cohort are summarized in Table 1. The median age at diagnosis was 65 years (range 28–89 years). There was a roughly equal sex distribution, with a male-to-female ratio of 1:1.07. The median tumor size was 4.05 cm (M = 4.4 cm; range 0.4–12.0 cm).
Table 1.
Clinicopathologic Features of 58 Patients Harboring Fatal Non-Anaplastic Follicular Cell–Derived Thyroid Carcinomas
| N (column %) | |
|---|---|
| N | 58 |
| Age (years), median (range) | 65 (28–89) |
| Tumor size (cm), median (range) | 4.05 (0.4–12.0) |
| Sex | |
| Female | 30 (52%) |
| Male | 28 (48%) |
| Classification of primary thyroid carcinoma using the MSKCC criteria for PDTC | |
| PDTC | 33 (57%) |
| Hürthle cell carcinoma | 4 (7%) |
| Tall-cell variant PTC | 14 (24%) |
| Classical variant PTC | 2 (3%) |
| Follicular variant PTC | 2 (3%) |
| PTC microcarcinoma | 3 (5%) |
| Classification of primary thyroid carcinoma using the Turin proposal criteria for PDTC | |
| PDTC | 15 (26%) |
| Follicular carcinoma | 1 (2%) |
| Hürthle cell carcinoma | 6 (10%) |
| Tall-cell variant PTC | 20 (34%) |
| Columnar variant PTC | 4 (7%) |
| Solid variant PTC | 1 (2%) |
| Classical variant PTC | 5 (9%) |
| Follicular variant PTC | 3 (5%) |
| PTC microcarcinoma | 3 (5%) |
| Carcinoma of the highest histologic grade in the primary resection (thyroid and neck lymph nodes) using the MSKCC criteria for PDTCa | |
| PDTC | 36 (62%) |
| Hürthle cell carcinoma | 4 (7%) |
| Tall-cell variant PTC | 14 (24%) |
| Classical variant PTC | 1 (2%) |
| Follicular variant PTC | 2 (3%) |
| Solid variant PTC | 1 (2%) |
| Mitotic index (10 HPFs, 400×) | |
| ≥5/10 HPFs | 25 (43%) |
| 0–4/10 HPFs | 33 (57%) |
| Tumor necrosis | |
| Extensive | 10 (17%) |
| Focal | 11 (19%) |
| None | 37 (64%) |
| Tumor encapsulation | |
| Partially encapsulated or non-encapsulated | 47 (81%) |
| Encapsulated | 10 (17%) |
| NA | 1 (2%) |
| Capsular invasion in encapsulated carcinomas (n = 10) | |
| Extensive | 6 (60%) |
| Focal | 4 (40%) |
| Vascular invasion | |
| Extensive | 21 (36%) |
| Focal | 10 (17%) |
| None | 25 (43%) |
| NA | 2 (3%) |
| Margin status | |
| Positive | 29 (50%) |
| Negative | 27 (47%) |
| N/A | 2 (3%) |
| Gross extension beyond the thyroid | |
| Presentb | 36 (62%) |
| Absent | 22 (38%) |
| Microscopic extrathyroidal extension of the dominant carcinoma | |
| Extensive | 40 (69%) |
| Focal | 6 (11%) |
| None | 10 (18%) |
| N/A | 2 (4%) |
| Metastasis to neck lymph nodes in patients who underwent lymph node sampling at initial surgery (n = 44) | |
| Present | 35 (80%) |
| Absent | 9 (20%) |
| Metastasis to ≥5 neck lymph nodes (n = 44) | |
| Present | 19 (43%) |
| Absent | 25 (57%) |
| ENE in 35 patients with positive lymph nodes at the initial surgery | |
| Present | 23 (66%) |
| Absent | 12 (34%) |
| Distant metastasis | |
| Present at initial presentation | 27 (47%) |
| Developed during clinical follow-up | 28 (48%) |
| Absentc | 3 (5%) |
| Mode of death | |
| Distant metastasis | 51 (88%) |
| Distant metastasis and locoregional recurrence | 3 (5%) |
| Unknown | 1 (2%) |
| Locoregional recurrence | 3 (5%) |
The carcinoma of the higher histological grade was documented when the histology of the carcinoma from the thyroid and from the neck lymph nodes were not identical.
One patient with tall-cell variant PTC had gross ENE. The remaining patients had gross ETE.
All three patients were diagnosed with tall-cell variant PTC and died due to unresectable locoregional recurrence.
PTC, papillary thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; HPFs, high power fields; ENE, extranodal extension; NA, not available/not applicable; ETE, extrathyroidal extension.
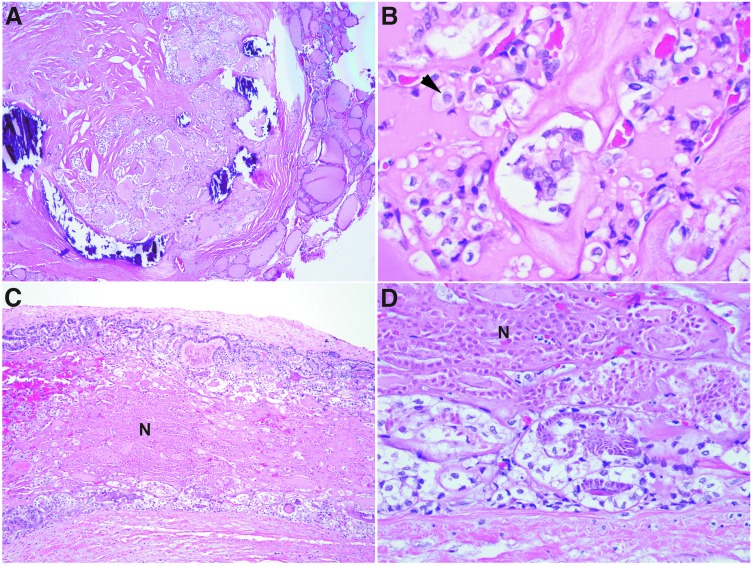
When MSKCC criteria of PDTC were applied, 33/58 (57%) tumors were classified as PDTC (MSKCC-PDTC). In descending order of frequency, the remaining 25 patients harbored tall-cell variant PTC (TCVPTC; n = 14; 24%), HCC (n = 4; 7%), PTC microcarcinoma variant (n = 3; 5%), classical variant PTC (CVPTC; n = 2; 3%), and follicular variant PTC (FVPTC; n = 2; 3%). Forty-four patients had lymph node sampling from the central compartment and/or lateral neck at the time of initial surgery. Metastatic carcinoma to neck lymph node(s) was detected in 35/44 (80%) patients. None of the nine patients that were pN0 (the number of lymph nodes sampled ranged from 1 to 17) at the initial surgery developed lymph node recurrence prior to their demise. Among patients with nodal metastasis, 19 patients had metastases involving at least five lymph nodes. The histologic type of the metastases was the same as the primary tumors with four exceptions. All three patients with papillary microcarcinomas harbored sizable lymph node metastasis (ranging from 1.0 to 4.7 cm in greatest diameter) in the form of metastatic MSKCC-PDTC in the lateral neck compartments (Fig. 2). Additionally, one patient with FVPTC in the thyroid harbored metastatic PTC solid variant in the neck lymph nodes. The distribution of histology in the primary resection (thyroid tumor and lymph node metastasis) was as follows: MSKCC-PDTC (n = 36; 62%), HCC (n = 4; 7%), TCVPTC (n = 14; 24%), FVPTC (n = 2; 3%), PTC classical variant (n = 1; 2%), and PTC solid variant (n = 1; 2%; Table 1).
FIG. 2.
A patient from the current lethal cohort with a 0.4 cm papillary microcarcinoma of the thyroid and concurrent metastatic PDTC in a 3.2 cm ipsilateral neck level III lymph node. (A) The primary thyroid microcarcinoma was a partially encapsulated papillary microcarcinoma composed exclusively of follicles. Nuclear features of papillary carcinoma, such as nuclear membrane irregularity, chromatin clearing, and nuclear grooves, were readily identified (B). (C) and (D) The metastatic MSKCC-PDTC in the lymph node exhibited follicular growth pattern and tumor necrosis (N) while retaining the nuclear features of PTC (D).
The vast majority of the primary tumors from the study cohort demonstrated aggressive histological features, including: elevated mitotic activity (n = 25; 43%), extensive or focal tumor necrosis (n = 21; 36%), infiltrative growth pattern with no or partial tumor capsule (n = 47; 81%), extensive VI (n = 21, 36%), and extensive microscopic ETE (n = 40; 69%; Table 1). Additionally, GET causing adhesion/invasion of adjacent structure was a common finding, affecting 36/58 (62%) patients. Not surprisingly, the rate of positive surgical margins was relatively high (50%) in the cohort, possibly related to the difficulty in achieving complete resection for tumors with GET.
Fatal cases of encapsulated carcinomas and non-PDTC non-TCVPTC carcinomas
A total of 11 (19%) patients in the cohort were diagnosed with carcinomas other than PDTC and TCVPTC (Table 2), including four HCC, three papillary microcarcinomas, two CVPTC, and two FVPTC. Among them, six patients presented with DM, including one with HCC, one with papillary microcarcinoma, two with FVPTC, and two with CVPTC. The remaining five patients developed DM during clinical follow-up. Extensive VI was present in three encapsulated HCC and FVPTC. GET was documented intraoperatively in one CVPTC and in one 0.8 cm papillary microcarcinoma. Three patients were diagnosed with papillary microcarcinomas in the thyroid. However, all three patients had sizable lymph node metastasis (≥1 cm) with a pathology diagnosis of metastatic PDTC (Fig. 2). Taken together, all 11 patients with non-PDTC non-TCVPTC in the thyroid had aggressive clinicopathologic features (e.g., extensive VI, metastatic PDTC in the lymph nodes, GET, and/or DM) at presentation.
Table 2.
Clinicopathologic Characteristics of the 11 Patients Harboring Non-Poorly Differentiated, Non-Tall-Cell Variant Thyroid Carcinomas
| # | Age | Sex | Histology of primary tumor | Tumor size (cm) | Encapsulation | Capsular invasion | Vascular invasion | Margin status | Highest grade carcinoma of LN metastasis | ETE | DM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 44 | M | Hürthle cell carcinoma | 7.2 | Complete | Extensive | Extensive | Negative | PTC, classical | Microscopic | During FU |
| 2 | 75 | F | Hürthle cell carcinoma | 5.0 | Complete | Extensive | Extensive | Negative | NA | None | During FU |
| 3 | 44 | M | Hürthle cell carcinoma | 4.0 | Complete | Focal | Extensive | Negative | NA | None | During FU |
| 4 | 70 | M | Hürthle cell carcinoma | 5.5 | Complete | Focal | None | Negative | NA | None | At presentation |
| 5 | 66 | M | FVPTC | 5.0 | Complete | Extensive | Extensive | Negative | NA | None | At presentation |
| 6 | 28 | F | FVPTC | 2.0 | None | NA | None | Positive | FVPTC | Microscopic | At presentation |
| 7 | 78 | F | PTC, classical | NA | None | NA | None | Positive | NA | GET | At presentation |
| 8 | 76 | M | PTC, classical | 2.0 | Partial | NA | Focal | Negative | PTC solid | Microscopic | At presentation |
| 9 | 58 | M | PMC | 0.4 | None | NA | Focal | Negative | PDTC | None | During FU |
| 10 | 51 | F | PMC | 0.4 | None | NA | None | Negative | PDTC | None | At presentation |
| 11 | 77 | M | PMC | 0.8 | Partial | NA | None | Negative | PDTC | GET | During FU |
This patient harbored multifocal thyroid carcinomas, including an encapsulated thyroid-confined 7.2 cm Hürthle cell carcinoma with extensive vascular and capsular invasion, an encapsulated Hürthle cell carcinoma with two foci of capsular invasion, and two PTC (one of which had microscopic extrathyroidal extension into the perithyroidal adipose tissue). The histological classification of the lymph node metastases was papillary carcinoma, classical variant.
LN, lymph node; M, male; F, female; FVPTC, follicular variant papillary thyroid carcinoma; PMC, papillary microcarcinoma; PDTC, poorly differentiated carcinoma according to MSKCC criteria; NA, not applicable; FU, follow-up; DM, distant metastasis.
The cohort contained 10 (17%) fatal cases of encapsulated thyroid carcinomas, including four HCC, one FVPTC, and five PDTC. All 10 cases had extensive VI (n = 8) and/or DM at presentation (n = 6; Table 3). There were two patients who did not have extensive VI, despite extensive sampling (2.0 and 2.4 tumor blocks per centimeter of tumor).
Table 3.
Clinicopathologic Features of the 10 Patients with Encapsulated Thyroid Carcinomas
| # | Age | Sex | Histology of primary tumor | Tumor size (cm) | Capsular invasion | Vascular invasion | Highest grade carcinoma in LN | ETE | DM | Time to death (years) | Mode of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 44 | M | HCC | 7.2 | Extensive | Extensive | PTC, classical | Microscopic | During FU | 5.8 | DM |
| 2 | 75 | F | HCC | 5.0 | Extensive | Extensive | NA | None | During FU | 8.2 | DM |
| 3 | 44 | M | HCC | 4.0 | Focal | Extensive | NA | None | During FU | 8.4 | DM |
| 4 | 70 | M | HCC | 5.5 | Focal | None | NA | None | At presentation | 0.2 | DM |
| 5 | 66 | M | FVPTC | 5.0 | Extensive | Extensive | NA | None | At presentation | 2.9 | DM |
| 6 | 77 | M | PDTC | 4.4 | Focal | Focal | NA | None | At presentation | 4.9 | DM |
| 7 | 74 | F | PDTC | 5 | Extensive | Extensive | NA | GET | At presentation | 4.8 | DM |
| 8 | 61 | M | PDTC | 8.7 | Focal | Extensive | NA | GET | At presentation | 4.2 | DM |
| 9 | 59 | M | PDTC | 5.3 | Extensive | Extensive | NA | Microscopic | At presentation | 2.5 | DM |
| 10 | 51 | F | PDTC | 4.0 | Extensive | Extensive | NA | None | During FU | 8.9 | DM |
Patients #1–5 correspond to patients #1–5 in Table 2.
HCC, Hürthle cell carcinoma; GET, gross extension beyond the thyroid.
DM and mode of death
The majority of patients died from DM (n = 51; 88%), while only three (5%) died from locoregional disease and three (5%) from both (Table 1). The mode of death was unclear in one patient with advanced locoregional recurrence and extensive lung metastases. Twenty-seven (47%) patients presented with DM at the initial clinic visits, 28 (48%) developed DM during follow-up, while the remaining three (5%) had GET and positive margin at the initial resection. The median time to death was 4.1 years (range 0.2–13 years) and was significantly shorter in patients with DM at presentation (median 3.3 years; range 0.2–10.2 years) compared with those without DM (median 6.2 years; range 1.4–13 years; p = 0.001). The most common sites of DM were the lung (44/55 patients; 80%), bone (26/55 patients; 47%), and the brain (9/55 patients; 16%). Other documented sites of metastases included the liver (n = 3), adrenal gland (n = 2), pericardium (n = 1), chest wall (n = 1), pleura (n = 1), spleen (n = 1), and retroperitoneum (n = 1).
Among the 27 patients who were diagnosed with DM at initial presentation, 13 patients sought clinical attentions as a result of symptoms related to DM such as hip pain (n = 4), back pain (n = 3), chest pain (n = 2), dyspnea (n = 2), palpable chest wall mass (n = 1), and cognitive alteration (n = 1). Thirteen patients presented with palpable or symptomatic locoregional disease and were found to have DM during the initial radiological investigations. The remaining patient was asymptomatic and had incidental bilateral pulmonary nodules detected during routine workups for long-standing chronic obstructive pulmonary disease. These lung deposits were subsequently proven to be metastatic thyroid carcinoma on biopsies. The histologic diagnoses of the primary thyroid carcinomas for the 27 patients with DM at presentation were as follows: PDTC (n = 17; 63%); TCVPTC (n = 4; 15%), CVPTC (n = 2; 7%), FVPTC (n = 2; 7%), PTC microcarcinoma (n = 1; 4%), and HCC (n = 1; 4%).
Correlation between MSKCC criteria and Turin proposal in diagnosing PDTC
All patients who were diagnosed as PDTC using the Turin proposal criteria (Turin-PDTC) were also classified as PDTC using the MSKCC criteria (MSKCC-PDTC). Conversely, only 45% of PDTC classified using the MSKCC criteria fulfilled the Turin proposal definition of PDTC (Table 4). If the Turin proposal was used to define PDTC, the remaining cases would be classified as variants of PTC (n = 15; 45%), as these tumors retained some nuclear features of PTC, or less commonly as FC/HCC (n = 3; 9%), as they lacked a solid/nested growth pattern. Those 15 PTC cases would be regarded as high-grade PTC by many endocrine pathologists.
Table 4.
Correlation Between MSKCC Classification and Turin Proposal for PDTC
| Patients with a diagnosis of PDTC using MSKCC criteria (n = 33) | |
| PDTC using the Turin proposal | 15 (45%) |
| Well-differentiated carcinoma using Turin proposal | |
| Follicular carcinoma | 1 (3%) |
| Hürthle cell carcinoma | 2 (6%) |
| Tall-cell variant PTC | 6 (18%) |
| Columnar variant PTC | 4 (12%) |
| Classical variant PTC | 3 (9%) |
| Solid variant PTC | 1 (3%) |
| Follicular variant PTC | 1 (3%) |
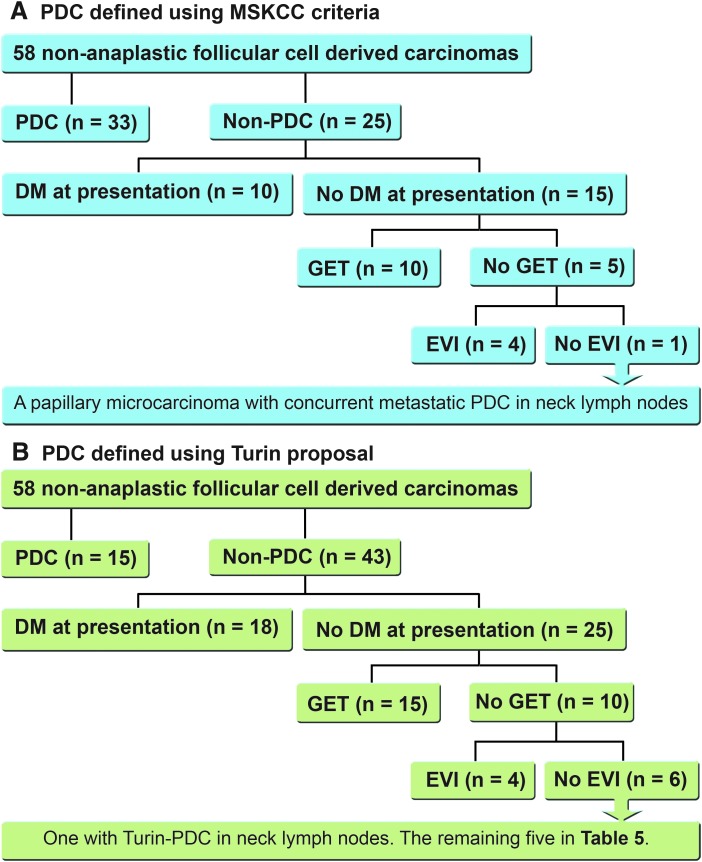
When using MKSCC criteria to define PDTC, all patients in the cohort had at least one of the four high-risk features at diagnosis: DM at presentation, GET, a pathologic diagnosis of MSKCC-PDTC in the initial resection (thyroid primary tumor and/or lymph node metastasis), or extensive VI (Fig. 3A). On the other hand, if one was to apply the Turin proposal for PDTC, 5/58 patients would be labeled as PTC without extensive VI, DM at presentation, and GET (Fig. 3B). The characteristics of these five patients are summarized in Table 5. Using the risk stratification system proposed by the ATA 2015 guidelines for differentiated thyroid carcinoma (9), one patient would be categorized as high risk because of pathologic N1 disease, with the largest metastatic lymph node measuring 3.2 cm in the greatest dimension (patient #1); three would be classified as intermediate risk, with TCVPTC (patient #2 and #3) and columnar variant PTC (patient #4); and one would be classified as low risk (patient #5). All five patients harbored tumors without lymphovascular invasion. Although one of these low-/intermediate-risk carcinomas (patient #5) would be deemed not to require radioactive (RAI) treatment using the 2015 ATA guidelines (9), all five patients underwent total thyroidectomy and received RAI treatment at the initial presentation. Additionally, if one was to use dynamic ongoing response to therapy reclassification during the first two years of follow-up (14), two of the four low-/intermediate-risk PTC would be categorized as having a structural incomplete response (patients #2 and #4), while the remainder would be classed as having an excellent response (patient #3 and #5).
FIG. 3.
Characteristics of the fatal non-anaplastic follicular cell–derived carcinomas based on four clinicopathologic features: PDTC, distant metastasis at presentation, gross extension beyond the thyroid, and extensive vascular invasion using MSKCC criteria (A) and the Turin proposal (B).
Table 5.
Clinicopathologic Characteristics, Initial Risk Stratification, and Response to Therapy Reclassification in the Five Patients Classified as MSKCC-PDTC but Non-PDTC by Turin Proposal, and Lacking DM at Presentation, EVI, and GET
| Classification by Turin proposal | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Age | Sex | Primary tumor | Lymph node metastasis | Tumor size (cm) | Margin status | Total number of lymph nodes with metastasis | Size of the largest lymph node (cm) | Mode of death | Time to death (years) | Site of DM | ATA 2015 risk stratification | Response to treatment |
| 1 | 58 | M | PTC, microcarcinomaa | Follicular variant PTC | 0.4 | Negative | 2 | 3.2 | Distant | 8.4 | Lung, bone, retroperitoneum | High risk | Biochemical incomplete response |
| 2 | 82 | F | Tall-cell variant PTC | Tall-cell variant PTC | 4.1 | Positive | 4 | 1.2 | Distant | 1.4 | Lung | Intermediate risk | Structural incomplete response |
| 3 | 70 | F | Tall-cell variant PTC | Tall-cell variant PTC | 1.8 | Positive | 8 | 0.6 | Distant | 11.3 | Lung | Intermediate risk | Excellent response |
| 4 | 78 | F | Columnar variant PTC | NA | 2.5 | Negative | NA | NA | Distant | 3.1 | Lung | Intermediate risk | Structural incomplete response |
| 5 | 72 | F | Classical variant PTC | Classical variant PTC | 2 | Negative | 3 | 0.6 | Unknown | 11.9 | Lung | Low risk | Excellent response |
This is the patient shown in Figure 2.
ATA, American Thyroid Association.
Discussion
Clinical features of fatal non-ANA FCDC
Differentiated thyroid carcinoma is considered an indolent cancer that rarely causes death. To date, only a handful of studies have been published focusing exclusively on lethal non-ANA FCDC, and most of the data came from earlier studies between 1964 and 1999 (2–6,15,16). The reported mortality is 4.2–5.4% in differentiated FCDC overall (4–6), 3.3–6.5% in PTC (2,3,5), and 11.1% in FC (2). The present study reports a considerably lower mortality rate of 1.5% from a large cohort of 3750 patients of non-ANA FCDC treated in a single tertiary cancer center. It is compatible with the 1–3% population-based mortality of thyroid cancer reported worldwide in recent years (1,8), reflecting the steady decline in mortality from thyroid cancer over the past few decades.
Compared with PTC and FC, which usually affects patients in their 40s (1,17), fatal non-ANA FCDC tends to occur in older patients, with a mean age at presentation of 52–59 years in previous studies (3,4,6,15) and 65 years in the present cohort. In the current study, lethal non-ANA FCDC affected females and males equally, similar to the sex ratio reported by Smith et al. (3), suggesting a higher mortality in male patients with non-ANA FCDC.
Patients who die of differentiated thyroid carcinoma commonly develop DM during their clinical course. The reported rate of DM ranges from 74% to 82.8% (3,4,6). In the present cohort, the rate was 95%. The most common sites of DM in a descending rate of involvement are lung, bone, and brain (3,4), which is confirmed by the current study. Smith et al. (3) reported that a shorter interval between initial surgery and the discovery of DM correlated with decreased survival. Similarly, in the present cohort, the median time from diagnosis to death was significantly shorter in patients with DM at initial presentation (3.3 years) compared with those without DM (6.2 years). In two studies from Japan published in the 1990s, the immediate cause of death was attributed solely to uncontrollable locoregional disease in 29% and 35.4% of patients, respectively (4,6). In contrast, in the present study, locoregional disease was the immediate cause of death in <10% of non-ANA FCDC. Such a difference could be explained in part by the advances in locoregional control in recent years (18).
Pathologic features that may identify potentially fatal non-ANA FCDC
The histologic composition of the lethal cohort included 57% MSKCC-PDTC, 7% HCC, and 36% PTC (including 24% TCVPTC). Alternatively, if the Turin proposal was applied to define PDTC, the cohort consisted of 26% Turin-PDTC, 2% FC, 10% HCC, and 64% PTC. Piana et al. (2010) studied 43 lethal cases using the Turin proposal for PDTC and reported similar results. In their study, 67% of fatal cases were PTC, 21% PDTC, 7% widely invasive FC, and 5% HCC (5).
All lethal carcinomas in the present cohort demonstrated at least one of four aggressive clinical or pathologic features, including DM at presentation in 47% of patients, GET appreciated during the initial surgery in 62%, a diagnosis of primary MSKCC-PDTC in 57%, and presence of extensive VI in 36%. Hence, these four features may be useful tools at the time of initial surgery to recognize potentially fatal tumors and assist subsequent clinical decision making. Importantly, the absence of these four features can be used to identify patients at an extremely low risk of death, so they can be reassured and not overtreated. Due to the study design, it is not possible to provide the frequency of these four features in non-fatal differentiated carcinomas. However, unpublished data from our group analyzing 1072 patients with differentiated thyroid carcinoma have shown that extensive VI and MSKCC-PDTC were relatively infrequent, accounting for 5% and 9% of cases, respectively.
Controversy surrounding PDTC
In the early 1980s, PDTC was first described as a group of tumors intermediate between the indolent differentiated thyroid carcinomas and the nearly always fatal anaplastic carcinoma (17). Since then, there has been continuous controversy with regard to the very definition of this entity. The MSKCC criteria (12) define PDTC based exclusively on elevated mitotic index (≥5/10 HPFs) and/or tumor necrosis, regardless of tumor growth pattern and nuclear features. On the other hand, the Turin proposal for the diagnosis of PDTC is more restrictive (13). It requires the presence of insular/solid/trabecular growth pattern, the absence of nuclear features of PTC, and one or more of the following three features: convoluted nuclei, mitotic index ≥3/10 HPFs, and/or tumor necrosis. The reported mortality rates in PDTC using the MSKCC criteria and the Turin proposal have been 38% and 41%, respectively (12,13). Because the MSKCC criteria for PDTC encompasses tumors with a FC, HCC, and PTC phenotype, the rate of PDTC (57% in this cohort using the MSKCC definition) is higher than when the Turin proposal is used to classify tumors (26%). Under the Turin approach, carcinomas with high-grade features (necrosis, high mitotic rate) and a PTC nuclear phenotype are not labeled as PDTC but rather as PTC. This is an important subset, since it comprises 26% (15/58) of the cohort of fatal patients. The latter terminology may misguide the clinician into thinking he/she is dealing with an indolent PTC. Indeed, in the current cohort of fatal cases, four carcinomas would have been categorized as low to intermediate risk according to ATA stratification if the Turin proposal was applied, as they lacked high-risk features (e.g. GET, extensive VI, DM at presentation, or pN1 disease involving lymph nodes ≥3 cm in size). Furthermore, one patient may have been treated conservatively without RAI therapy. A dynamic ongoing response to therapy reclassification would have signaled a more aggressive behavior to the clinician in some (2/4) but not all of these patients classified as low/intermediate risk by the Turin proposal. Hence, if a pathologist uses the Turin proposal to define PDTC, the term “high grade” should be used in designating these PTC in order to communicate to the clinician their potential for a fatal outcome. This will prevent false reassurance and prompt more aggressive management.
The fact that the rare PDTC (5–7% of all thyroid carcinomas) are overrepresented in fatal non-anaplastic FCDC could be explained by their genotype. In addition to the presence of a significant amount of RAS (mainly found in Turin PDTC) and BRAF mutations (mainly detected in PDTC diagnosed solely by MSKCC criteria), these PDTC display additional genetic events (18). When compared with PTC, PDTC are associated with a higher mutation burden and a higher rate (40%) of telomerase reverse transcriptase (TERT) promoter mutations (19). The TERT promoter mutation is a molecular signature associated with aggressive clinical behavior, including a propensity for DM and disease-specific death (19,20).
Fatal papillary microcarcinoma and encapsulated thyroid carcinoma
The overall mortality of papillary microcarcinoma is extremely low, reported at 0% for incidental and 0.1% for non-incidental papillary microcarcinomas (21). However, rare fatal cases of papillary microcarcinomas have been documented in the literature (22). The ATA 2015 management guidelines have acknowledged an alternative conservative approach of active surveillance management for papillary microcarcinomas without clinical evident metastases, local invasion, and no convincing cytological or molecular evidence of aggressive disease (9). Hence, it is critical to identify papillary microcarcinomas that could be potentially fatal at the time of initial presentation in order to avoid undertreatment. Piana et al. (2013) reported three such cases of papillary microcarcinomas (22). All three patients had metastases to cervical lymph nodes at the time of initial surgery: one patient had a 6 cm lymph node containing TCVPTC, one had a 3 cm lymph node with metastatic PDTC, and one patient presented with metastatic TCVPTC with elevated mitotic index (5/10 HPFs) and ENE involving all five lymph nodes examined. These patients subsequently developed DM, which eventually led to the patients’ demise. Similarly, in the present cohort, three (5%) patients were diagnosed with papillary microcarcinomas. All three had metastatic PDTC in multiple lymph nodes measuring at least 1.0 cm in greatest dimension, and all three developed DM either at the time of presentation or subsequently. The current data and that of Piana et al. clearly show that all reported fatal papillary microcarcinomas harbor certain aggressive clinico-histological features at initial presentation (e.g., PDTC transformation in the lymph node metastases, multiple/large lymph node metastases, and/or DM at presentation). These aggressive features would disqualify these patients from all known active surveillance protocols.
Ten (17%) patients of the present fatal cohort harbored encapsulated carcinomas, including five cases of encapsulated PDTC, four encapsulated HCC, and one encapsulated FVPTC. All 10 cases inevitably exhibited extensive VI or DM at presentation. These findings are in line with what has been previously reported by Piana et al. (5) and Xu et al. (23). Encapsulated differentiated FCDC without extensive VI or DM at presentation appear to be highly indolent lesions and do not seem to cause death.
In conclusion, this study demonstrates the power of meticulous clinical, operative, and histopathologic examination in stratifying differentiated thyroid carcinoma. Based on the presence of one of four aggressive features (PDTC in the primary resection, extensive VI, GET, and/or DM at presentation), one can recognize patients at risk of death from thyroid carcinomas. The lack of any of these aggressive clinicopathologic findings identifies those harboring almost no risk of dying, thus prompting reassurance and avoiding overtreatment. The vast majority of patients die of DM rather than locoregional invasion, prompting the need for molecular analysis of these cases in order to administer and develop effective systemic treatment.
Acknowledgments
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist for any contributory authors.
References
- 1.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. 2015. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136:2187–2195 [DOI] [PubMed] [Google Scholar]
- 2.Simpson WJ, McKinney SE, Carruthers JS, Gospodarowicz MK, Sutcliffe SB, Panzarella T. 1987. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med 83:479–488 [DOI] [PubMed] [Google Scholar]
- 3.Smith SA, Hay ID, Goellner JR, Ryan JJ, McConahey WM. 1988. Mortality from papillary thyroid carcinoma. A case-control study of 56 lethal cases. Cancer 62:1381–1388 [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Asakawa H, Tamaki Y, Umeshita K, Monden M. 1996. Fatal differentiated thyroid cancer. J Surg Oncol 62:123–127 [DOI] [PubMed] [Google Scholar]
- 5.Piana S, Frasoldati A, Di Felice E, Gardini G, Tallini G, Rosai J. 2010. Encapsulated well-differentiated follicular-patterned thyroid carcinomas do not play a significant role in the fatality rates from thyroid carcinoma. Am J Surg Pathol 34:868–872 [DOI] [PubMed] [Google Scholar]
- 6.Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, Ito K, Ito K, Tanaka S. 1999. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab 84:4043–4049 [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Torotti A. 2010. Thyroid cancer staging. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Torotti A. (eds) AJCC Cancer Staging Manual. Seventh edition. Springer-Verlag, New York, NY, pp 59–64 [Google Scholar]
- 8.Siegel R, Ma J, Zou Z, Jemal A. 2014. Cancer statistics, 2014. CA Cancer J Clin 64:9–29 [DOI] [PubMed] [Google Scholar]
- 9.Haugen BRM, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RMM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuttle RM. NCCN clinical practice guidelines in oncology: thyroid cancer. Available at: www.nccn.org/professionals/physician_gls/PDF/thyroid.pdf (accessed February2016)
- 11.Rosai J CM, Delellis RA. 1992. Tumors of the Thyroid Gland. Armed Forces Institute of Pathology, Washington, DC [Google Scholar]
- 12.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. 2006. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 106:1286–1295 [DOI] [PubMed] [Google Scholar]
- 13.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M, Sobrinho-Simoes M, Bussolati G, Rosai J. 2007. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 31:1256–1264 [DOI] [PubMed] [Google Scholar]
- 14.Momesso DP, Tuttle RM. 2014. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am 43:401–421 [DOI] [PubMed] [Google Scholar]
- 15.Tollefsen HR, Decosse JJ, Hutter RV. 1964. Papillary carcinoma of the thyroid. A clinical and pathological study of 70 fatal cases. Cancer 17:1035–1044 [DOI] [PubMed] [Google Scholar]
- 16.Silverberg SG, Hutter RV, Foote FW., Jr 1970. Fatal carcinoma of the thyroid: histology, metastases, and causes of death. Cancer 25:792–802 [DOI] [PubMed] [Google Scholar]
- 17.Delellis RA, Lloyd RV, Heitz RU, Eng C. 2004. Pathology and Genetics of Tumours of Endocrine Organs. International Agency for Research on Cancer (IARC) Press, Lyon, France [Google Scholar]
- 18.Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, Patel SG, Tuttle RM, Shah JP, Ganly I. 2014. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab 99:1245–1252 [DOI] [PubMed] [Google Scholar]
- 19.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. 2016. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, Castro P, Prazeres H, Lima J, Amaro T, Lobo C, Martins MJ, Moura M, Cavaco B, Leite V, Cameselle-Teijeiro JM, Carrilho F, Carvalheiro M, Maximo V, Sobrinho-Simoes M, Soares P. 2014. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 99:E754–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, McCabe C, Boelaert K, Franklyn JA. 2014. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21,329 person-years of follow-up. J Clin Endocrinol Metab 99:2834–2843 [DOI] [PubMed] [Google Scholar]
- 22.Piana S, Ragazzi M, Tallini G, de Biase D, Ciarrocchi A, Frasoldati A, Rosai J. 2013. Papillary thyroid microcarcinoma with fatal outcome: evidence of tumor progression in lymph node metastases: report of 3 cases, with morphological and molecular analysis. Hum Pathol 44:556–565 [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. 2015. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell–derived thyroid carcinomas: a clinicopathologic study of 276 cases. Hum Pathol 46:1789–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]