Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder found to have widespread alterations in the function and synchrony of brain regions. These differences may underlie alterations in microstructural organization, such as in white matter pathways. To investigate the diffusion of major white matter tracts, the current study examined multiple indices of white matter diffusion in 42 children and adults with ASD and 44 typically developing (TD) age- and IQ-matched peers using diffusion tensor imaging. Diffusivity measures were compared between groups for the following tracts: bilateral cingulum bundle, corpus callosum, inferior longitudinal fasciculus, superior longitudinal fasciculus, and uncinate fasciculus. Results indicate a significant reduction in fractional anisotropy (FA) for the left superior longitudinal fasciculus (LSLF) in ASD children and adults compared with TD peers. A significant increase in radial diffusivity for ASD participants was also found in the same cluster along the LSLF. In addition, a significant positive correlation emerged for all subjects between FA for the LSLF and age, with FA increasing with age. These findings point to a significant alteration in long-distance white matter connectivity in children and adults with ASD, potentially underscoring the relationship between alterations in white matter diffusion and the ASD phenotype. These results also suggest that the white matter alterations in autism may be subtle and related to the developmental trajectory.
Keywords: : autism spectrum disorder, diffusion tensor imaging, fractional anisotropy, superior longitudinal fasciculus
Introduction
Converging findings from neuroimaging literature attribute the neuropathology of autism spectrum disorder (ASD) to widespread disruptions in brain connectivity (Just et al., 2012; Kana et al., 2011; Maximo et al., 2014). While most of these studies focus on functional connectivity (synchronization of brain activity across spatially distant regions), of late, alterations in white matter microstructure have also been reported in autism by diffusion tensor imaging (DTI) studies (see Travers et al. [2012] for a review). In addition, morphometric studies in ASD have uncovered alterations in the development of gray and white matter volumes (Ecker et al., 2015). Such volumetric differences seen in autism may arise from alterations in axonal density or organization or from changes in myelination, either of which could result in aberrant connectivity. Functional differences in connectivity and differences in white matter volume point to potential alterations in the development and organization of white matter tracts in ASD. Thus, a comprehensive account of connectivity in autism would warrant investigating the nature and organization of axons, which may be critical in brain functioning.
Structural limits on communication between brain regions may cause computational changes to evolve to deal with a pattern of reduced connectivity. DTI provides the opportunity to go beyond measurement of functional connections and volumetric measures to examine the microstructure of white matter tracts. Fractional anisotropy (FA), a measure derived from diffusion tensor data, is sensitive to developmental changes and pathological differences in axonal density, size, myelination, and the coherence of organization of fibers within a voxel and thus provides an index of the structural organization of the white matter (Basser, 1995; Basser and Pierpaoli, 1996; Beaulieu, 2002). The FA measurement represents the degree of anisotropic (directional) diffusion in a 3D ellipsoid distribution (Basser and Jones, 2002; Pfefferbaum et al., 2000). In addition to FA, DTI also provides measures of the average radius of diffusion as mean diffusivity (MD), diffusivity parallel to the white matter tract as axial diffusivity (AD), and diffusivity perpendicular to the tract as radial diffusivity (RD). These measures together provide multiple indices of diffusion along white matter tracts in the brain, enabling the investigation of potential alterations in the microstructure of white matter in disorders such as ASD.
DTI studies in ASD have found reduced FA in a number of white matter tracts, including corpus callosum, cingulum bundle, uncinate fasciculus, and tracts along the anterior–posterior direction in the brain, such as the inferior and superior longitudinal fasciculi (Alexander et al., 2007; Barnea-Goraly et al., 2004; Bloemen et al., 2010; Keller et al., 2007; Lee et al., 2007; see Travers et al. [2012] for a review). The arcuate fasciculus (AF) is a subdivision of the superior longitudinal fasciculus whose primary axons are thought to be involved in language processing (Geschwind, 1970) and FA of this tract has been found to predict reading skills in children (Deutsch et al., 2005; Feldman et al., 2012; Klingberg et al., 2000; Saygin et al., 2013; Yeatman et al., 2011). In individuals with ASD, tractography studies of the AF revealed atypical asymmetry (Fletcher et al., 2010; Joseph et al., 2014; Wan et al., 2012) and reduced volume (Moseley et al., 2016). While differences in FA have been found somewhat consistently in corpus callosum, cingulum, uncinate fasciculus, and inferior and superior longitudinal fasciculi (including AF) in studies of ASD, investigations reporting on multiple indices, such as MD, RD, and AD, have been sparse and inconsistent.
Only a few studies have pointed to the relationship between measures of diffusion and cognitive functioning in ASD. For example, reduced FA in ASD participants has been linked to increased ASD symptom severity and behaviors (Catani et al., 2008; Noriuchi et al., 2010; Poustka et al., 2012; Thakkar et al., 2008), as well as performance intellectual quotient (PIQ) (Alexander et al., 2007; Lange et al., 2010; Lee et al., 2009). In addition, alterations in FA in participants with ASD have been related to language abilities (Fletcher et al., 2010; Li et al., 2014; Mills et al., 2013), executive functions (Nair et al., 2015), and motor performance (Thompson et al., 2016). In developmental disorders such as ASD, DTI provides a unique opportunity to investigate the structural makeup and structural limits of functional communication in the brain. In addition, applying DTI to different age groups or following a cohort longitudinally can address the neurodevelopmental trajectory of the disorder and determine the best possible windows of time for effective treatment and intervention.
Despite the promises of diffusion techniques, DTI studies in general have focused on reporting differences in FA, without examining how changes in FA relate to ASD behavior. In addition, many studies use methods that may not be ideal for investigating the brain in a population such as ASD. For example, voxel-based methods, such as tract-based spatial statistics (TBSS), used in a large number of DTI studies on ASD, use a projected white matter skeleton to compute white matter properties for each subject. The size and shape of fiber tracts may vary from participant to participant when examining patient populations such as ASD. Thus, aligning the skeleton with actual white matter voxels in individual participants, or aligning the same tracts across participants, may not be accurate and may not be able to detect differences with the same accuracy across all tracts (Edden and Jones, 2011; Wassermann et al., 2011; Yeatman et al., 2011, 2012). In other words, tract features specific to individual subjects may get misaligned in TBSS, resulting in a skeleton that may not be best representing white matter tract measurements for the participant group. This is particularly troublesome for patient populations such as autism, which includes a subset of individuals with brain enlargement (Sacco et al., 2015), and thus could have misaligned white matter skeletons resulting in inaccurate diffusion properties for some subjects.
The current study examined the differences in white matter diffusion in children and adults with ASD in a set of major fiber tracts implicated in the disorder: cingulum bundle, corpus callosum, inferior and superior longitudinal fasciculi, and uncinate fasciculus (see Travers et al. [2012] for a review of findings related to these tracts in studies of ASD). To investigate diffusivity measures across the tracts, we used automated fiber quantification (AFQ), a relatively novel technique, which quantifies FA, MD, RD, and AD along the trajectories of major white matter tracts in the brain (Yeatman et al., 2012). This method facilitates the examination of diffusion along 100 points on a given fiber bundle instead of a single mean value for the entire tract. This is done by segmenting individual participants' white matter tracts, quantifying white matter tract properties, and evaluating differences in tract properties between groups. AFQ has been found to generate tract profiles that are not only consistent across subjects but are also precise and reliable in terms of quantifying the actual white matter tracts within each subject's brain (Yeatman et al., 2012). We used this technique to investigate potential differences in white matter diffusion in participants with ASD and the relationship between the resulting diffusion measures and behavior. It is hypothesized that participants with ASD, compared with their typically developing (TD) peers, will exhibit significant reduction in FA in the major tracts examined in this study. Findings from this study are significant, in that they provide information related to possible alterations in diffusion measures of several major white matter tracts in the brains of those with ASD. Differences in white matter diffusivity marked by DTI hold strong implications for explaining the alterations in function and synchrony of brain regions seen in individuals with ASD.
Materials and Methods
Participants
Forty-two high-functioning children, adolescents, and adults with ASD (36 males/6 females; mean age: 19.9 years) and 44 age- and intellectual quotient (IQ)-matched TD peers (37 males/7 females; mean age: 20.1 years) participated in this study (see Table 1 for demographic information and Supplementary Figure S1 [Supplementary Data are available online at www.liebertpub.com/brain] for the distribution of participant ages in each group). Verbal IQ (VIQ), PIQ, and Full-Scale IQ (FSIQ) were assessed using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) and handedness using the Edinburgh Handedness Inventory (Oldfield, 1971). Participants who were 18 years of age and younger were classified as children. Parents of children completed the autism-spectrum quotient child (AQ) about their child as a measure of symptom severity (Auyeung et al., 2008). Adult participants completed the Ritvo Autism–Asperger Diagnostic Scale-Revised (RAADS-R) to measure ASD symptoms (Ritvo et al., 2011). The RAADS-R has been found to be accurate in discriminating between individuals with ASD and those without ASD (sensitivity = 97%, specificity = 100%, test–retest reliability = 0.987, accuracy = 98.5%). Participants were recruited from the University of Alabama at Birmingham (UAB) Civitan Sparks Autism Spectrum Disorders Clinic, the UAB Medical Autism Clinic, the University of Alabama Autism Spectrum Disorders Clinic, from local community-based autism clinics, and from the greater Birmingham community. Participants with ASD had received a previous diagnosis of ASD based on Autism Diagnostic Interview-Revised symptoms and Autism Diagnostic Observation Schedule-Generic (Lord et al., 2000), conducted and confirmed by the licensed clinician at their respective ASD clinic. TD participants were screened through a self-report history questionnaire to rule out neurological disorders such as ASD, attention-deficit/hyperactivity disorder, or Tourette's Disorder that could potentially confound the results. The study was approved by our university's Institutional Review Board, and all participants and their guardians provided informed consent for their participation in the study.
Table 1.
Participant Demographic Information
| Autism (N = 42) | Control (N = 44) | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | t | p | |
| Age | 19.9 | 8–40 | 1.27 | 20.1 | 8–38 | 1.21 | 0.14 | 0.88 |
| Verbal IQ | 110.5 | 80–139 | 2.04 | 112.4 | 83–141 | 2.05 | 0.63 | 0.52 |
| Performance IQ | 112.6 | 85–145 | 2.36 | 113.7 | 94–137 | 1.86 | 0.37 | 0.70 |
| Full-scale IQ | 112.9 | 92–140 | 1.99 | 114.7 | 87–140 | 2.00 | 0.61 | 0.53 |
| AQ | 33.1 | 20–42 | 5.33 | 10.3 | 3–25 | 6.25 | 11.90 | <0.0001 |
| RAADS-R | 119.2 | 32–181 | 39.70 | 44.1 | 3–72 | 23.80 | 8.12 | <0.0001 |
AQ, autism-spectrum quotient child; IQ, intellectual quotient; RAADS-R, Ritvo Autism–Asperger Diagnostic Scale-Revised.
DTI data acquisition
Imaging was performed on a 3T head-only scanner (Siemens Allegra, Erlangen, Germany). Anatomical images were acquired using high-resolution T1-weighted scans using a 160 slice 3D magnetization-prepared rapid acquisition gradient-echo volume scan with a repetition time (TR) = 200 ms, echo time (TE) = 3.34 ms, flip angle = 120, field of view (FOV) = 25.6 cm, 256 × 256 matrix size, and 1 mm slice thickness. The diffusion-weighted images were collected using a single-shot, spin-echo eco-planar imaging (EPI) sequence. A diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence was used (TR = 7000 ms, TE = 90 ms, bandwidth = 2790 Hz/voxel, FOV = 220 mm, and matrix size = 128 × 128 × 27, resulting in an in-plane resolution of 1.7 × 1.7 × 3 mm3). Twenty-seven 3-mm-thick slices were imaged (no slice gap) with no diffusion-weighting (b = 0 s/mm2) and with diffusion-weighting (b = 1000 s/mm2) gradients applied in 46 orthogonal directions. Ninety-two images of each slice by gradient direction combination were acquired and averaged to produce the final diffusion imaging dataset for each participant.
Preprocessing and DTI tractography
Diffusion images were preprocessed using the mrDiffusion package (Stanford VISTA Lab). Through this pipeline, participant head motion and eddy current distortions were removed by a 14-parameter constrained nonlinear coregistration based on the expected pattern of distortions for each phase-encoded direction of the data (Rohde et al., 2004). Diffusion-weighted images were aligned to the unweighted (b = 0) images, and then rigid body aligned to each subject's anatomical T1 reference image. Data were resampled to 2 × 2 × 2 mm3 voxels with a 7th-order b-spline interpolation, taking into account head motion correction, eddy current distortion correction, and anatomical alignment transforms (Friston and Ashburner, 2004). The rotation matrices from the alignment steps were combined and applied to correctly orient the resampled data to their respective vectors. Finally, the tensor model was fit using a robust least-squares algorithm, and the resulting eigenvalues were used to compute FA, MD, RD, and AD (Basser and Pierpaoli, 1996).
The preprocessed data were analyzed using AFQ (Yeatman et al., 2012). The data for each participant were subjected to whole-brain tractography (using deterministic tractography). The data were then segmented into tracts for left cingulum bundle, right cingulum bundle, callosum forceps major, callosum forceps minor, left inferior longitudinal fasciculus (LILF), right inferior longitudinal fasciculus (RILF), left superior longitudinal fasciculus (LSLF), right superior longitudinal fasciculus (RSLF), left uncinate fasciculus (LUF), and right uncinate fasciculus (RUF). Next, stray fibers (statistical outliers) deviating substantially from the core of a fiber group were automatically removed, and tract properties (FA, RD, MD, and AD) were computed for 100 points along each tract for each participant. Norms for each tract were determined using control group data. For some participants, tract properties for specific tracts could not be computed (due to artifact, head motion, or quality [e.g., size or crossing fibers] of the tissue). Tracts with greater than 10% of participants' missing data (due to incomplete tractography) were removed from further analyses. These tracts included the left and right cingulum bundles (see Fig. 1 for a flow chart depicting analysis procedures).
FIG. 1.
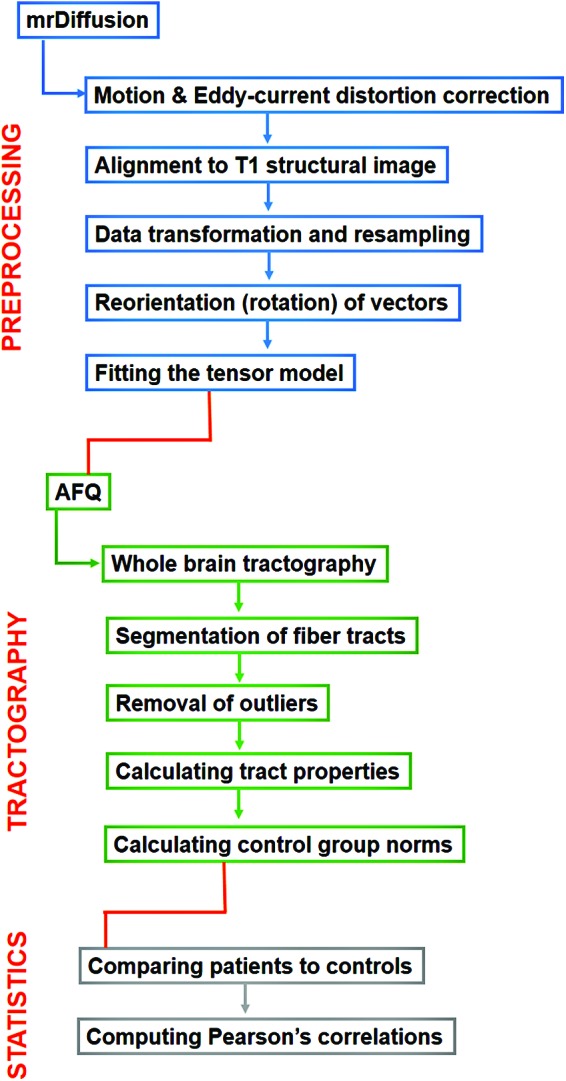
Flowchart depicting the main steps in the mrDiffusion preprocessing pipeline and automated fiber quantification (AFQ) tractography and statistical analysis steps. Color images available online at www.liebertpub.com/brain
Statistical analyses
To compare the ASD and TD groups on FA, RD, MD, and AD, t-tests were conducted pointwise along each fiber tract for 100 points. A permutation-based multiple comparison correction was applied to determine statistical significance (Nichols and Holmes, 2002), p < 0.05. For fiber tracts that showed significant differences between groups, Pearson's correlations were computed between mean FA values for the significant cluster and age, and ASD symptom measures (RAADS-R and AQ). Independent sample t-tests were used to compare groups on demographic information and neuropsychological assessment data.
As there may be differences in white matter properties in the brains of males and females, all analyses were conducted both with and without the female participants included. Results from the statistical analyses did not change with the removal of female participants. Therefore, to have increased statistical power, the results reported include all female participants within the ASD and TD groups. Although gender effects are an important area of investigation in ASD, the current study does not have an adequate sample size of females to include separate analyses that examine the effects of gender on white matter in ASD.
Results
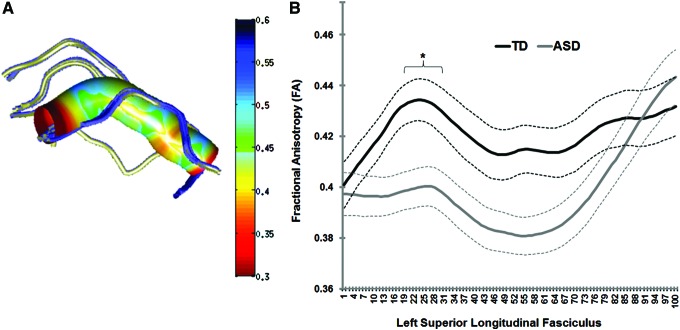
The ASD and TD groups did not significantly differ on age, VIQ, PIQ, or FSIQ. However, the children and adults with ASD had significantly greater ASD symptom severity compared with TD participants based on the AQ (children) and RAADS-R (adults; Table 1). The main finding of this study is a significant difference in white matter diffusion of the LSLF, with ASD participants showing significantly reduced FA compared with their TD peers (p < 0.05, corrected; Fig. 2). Differences in FA spanned most of the LSLF, with the cluster reaching corrected statistical significance appearing in the relatively anterior portion of the tract, and no differences in FA in the most posterior points (see Fig. 3 for a graph representing mean FA for both ASD and TD groups for the statistically significant cluster on the LSLF). At the same cluster along the LSLF, a significant increase in RD for the ASD group was also found compared with the TD group (p < 0.05, corrected). No significant differences in FA, RD, MD, or AD emerged between groups for the callosum forceps major, callosum forceps minor, LILF, RILF, RSLF, LUF, or RUF after correcting for multiple comparisons.
FIG. 2.
(A) A rendering of FA measurements for the LSLF for one subject as a visualization of the tract properties; (B) group means (solid lines) and standard deviations (dotted lines) for FA for the nodes along the LSLF for the TD (depicted in black) and ASD (depicted in gray) groups. The cluster with a significant reduction in FA in ASD participants (p < 0.05, corrected) is indicated with an asterisk. ASD, autism spectrum disorder; FA, fractional anisotropy; LSLF, left superior longitudinal fasciculus; TD, typically developing. Color images available online at www.liebertpub.com/brain
FIG. 3.
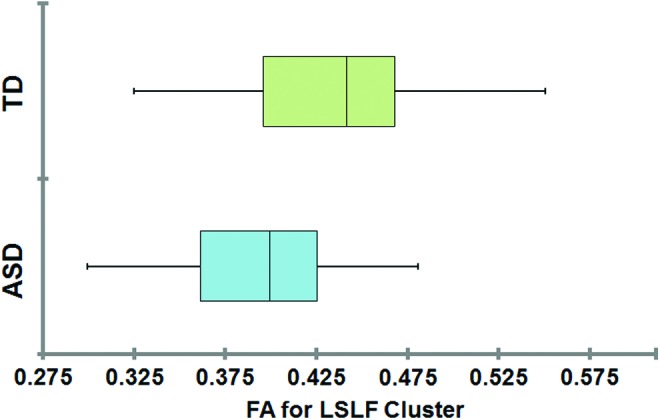
Mean subject FA values for the significant cluster on the LSLF for ASD and TD groups. Color images available online at www.liebertpub.com/brain
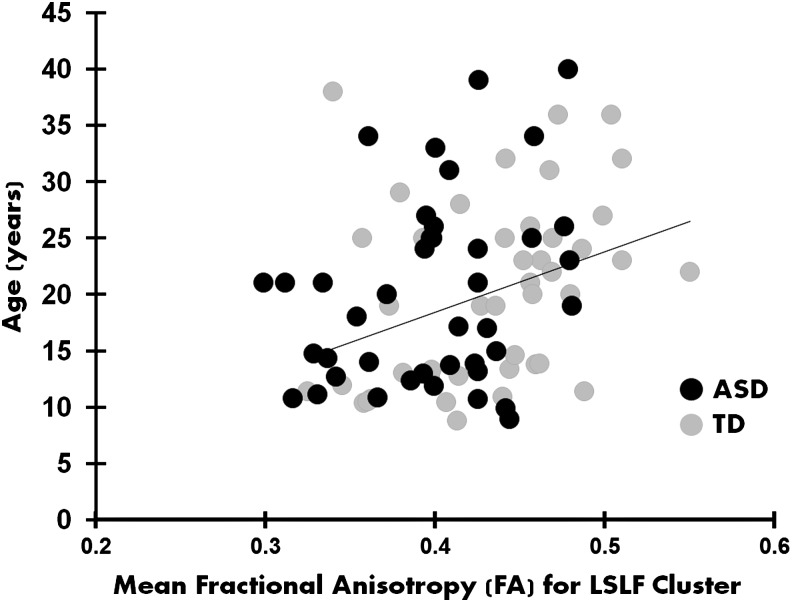
A significant positive correlation between FA for the LSLF cluster and age emerged (r = 0.304, p = 0.005) for all participants (Fig. 4). When this relationship was examined within each group separately, the TD participants had a significant positive correlation between FA for the LSLF cluster and age (r = 0.348, p = 0.021), whereas the correlation for the ASD group was only marginally significant (r = 0.286, p = 0.070). The two groups did not differ significantly in their correlation coefficients (Z = −0.308, p = 0.757). Thus, in all these correlations, white matter diffusion, indexed by FA, increased as a function of age.
FIG. 4.
Graph depicting the correlation between mean FA values for each participant for the significant cluster on the LSLF and age (r = 0.304, p = 0.005), indicating the development of the tract.
We also examined the relationship between diffusion measures and autism symptomatology. Within each group, the correlation between FA for the LSLF and RAADS-R scores in the adult participants was not significant (TD: r = −0.045, p = 0.83; ASD: r = 0.015, p = 0.945). In addition, the two groups did not significantly differ in their correlation coefficients (Z = 0.194, p = 0.845). For children in this study, within each group, the correlation between FA for LSLF and AQ (autism symptomatology as measured by the AQ) was not significant (TD: r = −0.073, p = 0.774; ASD: r = −0.184, p = 0.464), and the two groups did not significantly differ in their correlation coefficients (Z = 0.314, p = 0.753).
Discussion
The main finding of this study is a significant reduction in FA and a significant increase in RD in the LSLF in children and adults with autism relative to typical control participants. This reduction in FA is in line with the findings of several previous studies in ASD (Jeong et al., 2011; Jou et al., 2011a, 2011b; Noriuchi et al., 2010; Poustka et al., 2012; Shukla et al., 2011). Increased RD in the same tract in autism was also reported in a few previous studies (Jeong et al., 2011; Shukla et al., 2011).
The superior longitudinal fasciculus (SLF) is a fiber tract that spans from the inferior fontal gyrus to the superior temporal gyrus and temporoparietal junction, terminating close to Broca's Area, Wernicke's Area, precentral gyrus, and the supramarginal gyrus (Bernal and Altman, 2010; Catani and Jones, 2005; Makris et al., 2005; Wakana et al., 2004). It is a major association fiber pathway related to regulation of motor behavior, spatial attention, and language (Makris et al., 2005). In TD children, FA of the LSLF in particular has been found to be associated with attention, inhibitory control, and language skills (Urger et al., 2014), pointing to the role of this tract in many complex higher order behaviors. The SLF plays an important role in higher cortical functions, specifically in language and language disorders (Catani et al., 2003; Damasio and Damasio, 1980; Geldmacher et al., 2007; Tanabe et al., 1987; Wise, 2003). The SLF facilitates the formation of a bidirectional neural network that is necessary for core processes such as attention, memory, emotions, and language (Mesulam, 1998; Petrides and Pandya, 2002). In addition to being related to inattention (Skranes et al., 2007), the SLF connects cortical regions of the brain involved in reading (Frye et al., 2010; Pugh et al., 2000) and is also implicated in reading disability (Klingberg et al., 2000) and dyslexia (Hoeft et al., 2011; Steinbrink et al., 2008). In ASD, SLF alterations could thus impact a number of behaviors, including the social and communicative impairments that are hallmarks of the disorder.
The SLF is a particularly slow developing tract, not clearly visible in neonates, and its development has been associated with language use (Bengtsson et al., 2005; Hermoye et al., 2006; Huang et al., 2006; Paus et al., 1999; Thompson et al., 2000; Zhang et al., 2007). Previous studies have linked language impairments to diffusivity measures in the brain in ASD. For example, a recent study found increased RD in the left AF portion of the SLF in ASD to be related to poorer language index scores (Roberts et al., 2013), and another study found increased MD in the SLF to be related to increased language impairment in children with ASD (Nagae et al., 2012). For a disorder marked by impairment in language and communication, it comes as no surprise that the older children and adults included in this study would then have alterations in FA and RD in this fiber tract. However, considering that the SLF continues to develop slowly across infancy and childhood, and may develop in line with language skills, the alteration in LSLF in children and adults marks just how critical early intervention (especially for language) in young children with ASD is.
The LSLF in terms of our typical control norms was characterized by a peak in FA toward the anterior portion of the tract, with lower FA toward the center of the tract, and increasing FA again toward the posterior portion. While group differences were found across the majority of this tract, the cluster reaching statistical significance appeared at the relatively anterior peak in FA for the control subjects; meanwhile, FA levels for ASD participants normalized at the posterior end of the LSLF with no statistically significant differences at that portion of the tract. These findings suggest that alterations in diffusivity may not span the entire LSLF tract; instead, some parts of this tract (in this case, the anterior section) have more divergent diffusivity than others. This has important implications for both development and connectivity differences reported in autism. First, a difference in the diffusion of anterior and posterior aspects of this fiber bundle may be attributed to developmental alterations and/or differences in myelination across the tract. Thus, white matter connectivity differences in ASD may not be widespread, but rather may differ across different tracts as well as along different points on a given tract. Second, cortical connectivity theories of autism have postulated aberrations in long-distance connectivity in the brain in ASD, particularly with frontal brain regions holding most of the blame for alterations in function and connectivity (Kana et al., 2011). The anterior portion of the SLF projects to precentral gyrus (Bernal and Altman, 2010; Martino et al., 2013) and is delineated at superior, middle, and inferior frontal gyri (Makris et al., 2005). With terminations in frontal cortex, reduced FA in the anterior cluster of the LSLF supports the disrupted connectivity hypothesis of autism and suggests that the alterations in functional connectivity may be stemming from alterations in diffusion at anterior LSLF.
The current study also found a significant positive correlation between FA for the LSLF and age, with FA increasing with age for all participants. This is in line with previous findings of the same correlation in TD individuals (Ashtari et al., 2007; Lebel and Beaulieu, 2011; Lebel et al., 2008). It is perhaps telling that the LSLF continues to develop into adolescence and young adulthood, implying its plasticity even in older participants. This study did not find a significant relationship between FA in the LSLF and autism symptom scores in adults. A negative, but not significant, correlation for children was found between FA in the LSLF cluster and AQ scores, with lower FA in the LSLF related to greater ASD symptom severity again. These results may have been affected by the reliability of self-report and parent-report indices used to measure ASD symptomatology in the participants of this study. Future studies should use clinical diagnostic measurements of symptom severity across domains to better examine the relationship between white matter diffusion and behavior in ASD.
While a significant difference in FA and RD for the LSLF emerged between TD and ASD participants, no significant differences were found in any of the diffusion measurements for the remaining tracts studied (cingulum bundle, ILF, uncinate, and the forceps major and forceps minor of the corpus callosum). The tracts included in the current study have all been previously found to have significant FA reduction in ASD; however, studies still report inconsistencies when it comes to these white matter tracts in ASD (Travers et al., 2012). In addition, previous studies differ greatly in age, IQ, and symptom severity of their included participants. As there is variation in the development of different white matter fiber tracts, as well as links between tract diffusivity and ASD symptoms, subject characteristics may strongly impact findings. In addition, the tractography methodology used in previous studies varies. For example, while the preprocessing pipeline of the current study employs motion and eddy current distortion correction, many studies fail to report the use of any kind of correction. As diffusion imaging relies heavily on directional information, motion correction is of utmost importance if one wants reliable measures. It should also be noted that the current study could not compare groups for the cingulum bundle as tractography could not be computed for this tract for many subjects. The cingulum bundle is a small tract surrounded by gray matter tissue and the corpus callosum. Its size and location make it susceptible to motion artifacts and difficult to accurately calculate statistics for. While AFQ identified unreliable quantification for participants' tracts in the current study, many other automated techniques may not do so, rendering their findings potentially insensitive to noise and thus inaccurate.
Conclusion
Overall, this study found evidence for alterations in diffusion in the LSLF in children and adults with ASD and the relationship between diffusivity of this tract with age. Using a relatively novel method of DTI analysis, our study represents the first application of AFQ to DTI in the ASD population. This method provides the diffusion profile across an entire white matter fiber tract and avoids potential errors from misalignment of tracts and single subject white matter voxels (present in voxel-based analysis techniques). This method is also more informative than some others as diffusion information can be measured along the entire tract rather than relying on averaged measures across a given tract (which is unlikely to be sensitive enough to identify clinical differences in white matter microstructure). With this method, we were able to gather tractography measures of the major white matter tracts in the brain and compare the tract profiles of children and adults with ASD with their TD peers. This diffusion measure may be more accurate and reliable as this approach is not impacted by significant differences in brain sizes (which may be particularly relevant in a neurodevelopmental disorder such as ASD).
While a significant increase in RD was found at the same cluster on the LSLF with reduced FA in participants with ASD, this finding is difficult to interpret without further investigation. For example, studies in animals have linked increased RD to reductions in myelin (Harsan et al., 2006; Song et al., 2002; Tyszka et al., 2006). It is possible that reductions in myelin are driving the alterations in the white matter microstructure of the SLF in ASD; however, without using methods that specifically target myelination in our participants, we can only speculate. It is also possible that other alterations, such as number of crossing fibers, axonal density or diameter, and inflammation, could be driving a change in RD (Wheeler-Kingshott and Cercignani, 2009). Indeed, one study utilizing diffusional kurtosis imaging in young adults with ASD found reduced axonal water fraction in the SLF compared with controls, indicating altered intra-axonal volume and potentially lower axonal density (Lazar et al., 2014). Thus, evidence from the current study and from previous studies suggests that the LSLF may be an important fiber tract that shows significant alterations in the quality of white matter microstructure in autism.
One limitation of the current study includes that the data were collected at a relatively low b-value. Diffused weighted data sampled at a higher b-value (e.g., 3000 s/mm3) would enable nontensor-based reconstruction methods to be used. While tensor models are simple and easy to employ, they are not as effective when examining brain regions with complex white matter orientations (Alexander et al., 2001; Wiegell et al., 2000). These complex orientations include crossing fibers, bending or fanning fibers, and fiber bundles that are adjacent to each other, but within the same voxel (Tournier et al., 2011). Considering that the estimates of multiple white matter fiber orientations are found in over 90% of voxels (Jeurissen et al., 2010), it would be beneficial to use a model-free approach that can more accurately represent multiple fiber orientations (Tournier et al., 2011; Yeh et al., 2010, 2013; Yo et al., 2009).
Another limitation is that ASD diagnosis was not directly assessed in the current study, but was confirmed through the clinical reports provided by each participant's clinical provider. These reports confirmed the diagnosis of ASD through ADOS and ADI examinations. However, symptom severity scores were collected through self- and parent-report measures, which may have been influenced by factors such as individual and parent bias.
Future studies should also further examine the relationship between FA in the SLF and age. It would be interesting to study this relationship in older adults to investigate the neural development of this tract across the life span in ASD. In addition, in terms of early development and treatment, examining the development of SLF in very young children with ASD could potentially lead to more targeted treatment of the disorder (especially with regard to social skills and symptom severity).
Supplementary Material
Acknowledgments
This study was funded by the UAB Center for Clinical and Translational Sciences grant (5UL1RR025777). The authors would also like to thank Dr. Jason Yeatman, Dr. Brian Wandell, and the Stanford Vision and Imaging Science and Technology Laboratory for providing access to the mrDiffusion and AFQ software and for their assistance with these resources.
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. 2001. Analysis of partial volume effects in diffusion‐tensor MRI. Magn Reson Med 45:770–780 [DOI] [PubMed] [Google Scholar]
- Alexander AL, et al. 2007. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage 34:61–73 [DOI] [PubMed] [Google Scholar]
- Ashtari M, et al. 2007. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage 35:501–510 [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Wheelwright S, Allison C. 2008. The autism spectrum quotient: children's version (AQ-Child). J Autism Dev Disord 38:1230–1240 [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. 2004. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55:323–326 [DOI] [PubMed] [Google Scholar]
- Basser P, Jones DK. 2002. Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 15:456–467 [DOI] [PubMed] [Google Scholar]
- Basser PJ. 1995. Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed 8:333–344 [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. 1996. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219 [DOI] [PubMed] [Google Scholar]
- Beaulieu C. 2002. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15:435–455 [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. 2005. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150 [DOI] [PubMed] [Google Scholar]
- Bernal B, Altman N. 2010. The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging 28:217–225 [DOI] [PubMed] [Google Scholar]
- Bloemen OJ, et al. 2010. White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res 3:203–213 [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK. 2005. Perisylvian language networks of the human brain. Ann Neurol 57:8–16 [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R. 2003. Occipito‐temporal connections in the human brain. Brain 126:2093–2107 [DOI] [PubMed] [Google Scholar]
- Catani M, et al. 2008. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage 41:1184–1191 [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. 1980. The anatomical basis of conduction aphasia. Brain 103:337–350 [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. 2005. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 41:354–363 [DOI] [PubMed] [Google Scholar]
- Ecker C, Bookheimer SY, Murphy DG. 2015. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol 14:1121–1134 [DOI] [PubMed] [Google Scholar]
- Edden RA, Jones DK. 2011. Spatial and orientational heterogeneity in the statistical sensitivity of skeleton-based analyses of diffusion tensor MR imaging data. J Neurosci Methods 201:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Lee ES, Yeatman JD, Yeom KW. 2012. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia 50:3348–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PT, et al. 2010. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 51:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J. 2004. Generative and recognition models for neuroanatomy. Neuroimage 23:21–24 [DOI] [PubMed] [Google Scholar]
- Frye RE, et al. 2010. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Dev Med Child Neurol 52:760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher DS, Quigg M, Elias WJ. 2007. MR tractography depicting damage to the arcuate fasciculus in a patient with conduction aphasia. Neurology 69:321–322 [DOI] [PubMed] [Google Scholar]
- Geschwind N. 1970. The organization of language and the brain. Science 170:940–944 [DOI] [PubMed] [Google Scholar]
- Harsan LA, et al. 2006. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 83:392–402 [DOI] [PubMed] [Google Scholar]
- Hermoye L, et al. 2006. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29:493–504 [DOI] [PubMed] [Google Scholar]
- Hoeft F, et al. 2011. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci 108:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, et al. 2006. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage 33:27–38 [DOI] [PubMed] [Google Scholar]
- Jeong J-W, Kumar A, Sundaram SK, Chugani HT, Chugani DC. 2011. Sharp curvature of frontal lobe white matter pathways in children with autism spectrum disorders: tract-based morphometry analysis. Am J Neuroradiol 32:1600–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier J, Jones D, Sijbers J. Estimating the Number of Fiber Orientations in Diffusion MRI Voxels: A Constrained Spherical Deconvolution Study. Proceedings of the International Society for Magnetic Resonance in Medicine, Stockholm, Sweden, 2010, p. 573 [Google Scholar]
- Joseph RM, Fricker Z, Fenoglio A, Lindgren KA, Knaus TA, Tager-Flusberg H. 2014. Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav 8:60–72 [DOI] [PubMed] [Google Scholar]
- Jou R, Mateljevic N, Kaiser M, Sugrue D, Volkmar F, Pelphrey K. (2011a). Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. Am J Neuroradiol 32:1607–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Jackowski AP, Papademetris X, Rajeevan N, Staib LH, Volkmar FR. (2011b). Diffusion tensor imaging in autism spectrum disorders: preliminary evidence of abnormal neural connectivity. Aust N Z J Psychiatry 45:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. 2012. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev 36:1292–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Moore MS. 2011. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 8:410–437 [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. 2007. A developmental study of the structural integrity of white matter in autism. Neuroreport 18:23–27 [DOI] [PubMed] [Google Scholar]
- Klingberg T, et al. 2000. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron 25:493–500 [DOI] [PubMed] [Google Scholar]
- Lange N, et al. 2010. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res 3:350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M, Miles LM, Babb JS, Donaldson JB. 2014. Axonal deficits in young adults with High Functioning Autism and their impact on processing speed. NeuroImage Clin 4:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055 [DOI] [PubMed] [Google Scholar]
- Lee JE, et al. 2007. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett 424:127–132 [DOI] [PubMed] [Google Scholar]
- Lee JE, et al. 2009. A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. Neuroimage 44:870–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xue Z, Ellmore TM, Frye RE, Wong STC. 2014. Network-based analysis reveals stronger local diffusion-based connectivity and different correlations with oral language skills in brains of children with high functioning autism spectrum disorders. Hum Brain Mapp 35:396–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, et al. 2000. The Autism Diagnostic Observation Schedule—Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223 [PubMed] [Google Scholar]
- Makris N, et al. 2005. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15:854–869 [DOI] [PubMed] [Google Scholar]
- Martino J, et al. 2013. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct 218:105–121 [DOI] [PubMed] [Google Scholar]
- Maximo JO, Cadena EJ, Kana RK. 2014. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev 24:16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M. 1998. From sensation to cognition. Brain 121:1013–1052 [DOI] [PubMed] [Google Scholar]
- Mills BD, et al. 2013. White matter microstructure correlates of narrative production in typically developing children and children with high functioning autism. Neuropsychologia 51:1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RL, Correia MM, Baron-Cohen S, Shtyrov Y, Pulvermüller F, Mohr B. 2016. Reduced volume of the arcuate fasciculus in adults with high-functioning autism spectrum conditions. Front Hum Neurosci 10:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae L, et al. 2012. Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. Am J Neuroradiol 33:1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, et al. 2015. Regional specificity of aberrant thalamocortical connectivity in autism. Hum Brain Mapp 36:4497–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, et al. 2010. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res 1362:141–149 [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Paus T, et al. 1999. Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283:1908–1911 [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2002. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT. (eds.) Principles of Frontal Lobe Function 1. Oxford, UK: Oxford University Press; pp. 31–50 [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. 2000. Age‐related decline in brain white matter anisotropy measured with spatially corrected echo‐planar diffusion tensor imaging. Magn Reson Med 44:259–268 [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen-Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B. 2012. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry 13:269–280 [DOI] [PubMed] [Google Scholar]
- Pugh KR, et al. 2000. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 6:207–213 [DOI] [PubMed] [Google Scholar]
- Ritvo RA, et al. 2011. The Ritvo Autism asperger diagnostic scale-revised (RAADS-R): a scale to assist the diagnosis of Autism spectrum disorder in adults: an international validation study. J Autism Dev Disord 41:1076–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T, et al. 2013. Left hemisphere diffusivity of the arcuate fasciculus: influences of autism spectrum disorder and language impairment. Am J Neuroradiol 35:587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. 2004. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med 51:103–114 [DOI] [PubMed] [Google Scholar]
- Sacco R, Gabriele S, Persico AM. 2015. Head circumference and brain size in autism spectrum disorder: a systematic review and meta-analysis. Psychiatry Res 234:239–251 [DOI] [PubMed] [Google Scholar]
- Saygin ZM, et al. 2013. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J Neurosci 33:13251–13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller RA. 2011. Tract‐specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry 52:286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, et al. 2007. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130:654–666 [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. 2002. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436 [DOI] [PubMed] [Google Scholar]
- Steinbrink C, et al. 2008. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia 46:3170–3178 [DOI] [PubMed] [Google Scholar]
- Tanabe H, Sawada T, Inoue N, Ogawa M, Kuriyama Y, Shiraishi J. 1987. Conduction aphasia and arcuate fasciculus. Acta Neurol Scand 76:422–427 [DOI] [PubMed] [Google Scholar]
- Thakkar KN, et al. 2008. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain 131:2464–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, et al. 2016. Impaired communication between the motor and somatosensory homunculus is associated with poor manual dexterity in Autism Spectrum Disorder. Biol Psychiatry. DOI: 10.1016/j.biopsych.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. 2000. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature 404:190–193 [DOI] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. 2011. Diffusion tensor imaging and beyond. Magn Reson Med 65:1532–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, et al. 2012. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 5:289–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka JM, Readhead C, Bearer EL, Pautler RG, Jacobs RE. 2006. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage 29:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J. 2014. The superior longitudinal fasciculus in typically developing children and adolescents: diffusion tensor imaging and neuropsychological correlates. J Child Neurol 30:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. 2004. Fiber tract–based atlas of human white matter anatomy 1. Radiology 230:77–87 [DOI] [PubMed] [Google Scholar]
- Wan CY, Marchina S, Norton A, Schlaug G. 2012. Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann N Y Acad Sci 1252:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann D, et al. 2011. White matter bundle registration and population analysis based on Gaussian processes. Inf Process Med Imaging 22:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. 2009. About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260 [DOI] [PubMed] [Google Scholar]
- Wiegell MR, Larsson HB, Wedeen VJ. 2000. Fiber crossing in human brain depicted with diffusion tensor MR imaging 1. Radiology 217:897–903 [DOI] [PubMed] [Google Scholar]
- Wise RJ. 2003. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animal studies. Br Med Bull 65:95–119 [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. 2012. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One 7:e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, et al. 2011. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci 23:3304–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. 2013. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Wedeen VJ, Tseng W-Y. 2010. Generalized q-sampling imaging. IEEE Trans Med Imaging 29:1626–1635 [DOI] [PubMed] [Google Scholar]
- Yo T-S, Anwander A, Descoteaux M, Fillard P, Poupon C, Knösche TR. 2009. Quantifying brain connectivity: a comparative tractography study. Med Image Comput ComputAssist Interv 12(Pt 1):886–893 [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. 2007. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 38:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.