Abstract
Background: Fine-needle aspiration (FNA) of thyroid nodules is commonly performed, and despite the use of ultrasound (US) guidance, the rate of non-diagnostic FNAs is still significant. The risk of malignancy of thyroid nodules with a non-diagnostic FNA is not clearly defined. However, most studies exclude the majority of patients without a repeat biopsy or surgery, thus increasing the likelihood of selection bias. The aims of this study were to determine the malignancy risk in nodules with an initial non-diagnostic FNA, and to identify the factors associated with malignancy.
Methods: This was a retrospective cohort study of patients with thyroid nodules who underwent US-guided FNA between 2004 and 2010 and had a non-diagnostic result. Patients were followed until confirmatory diagnosis of the nature of the nodule was made. The outcome of malignant or benign disease was based on one of the following: (i) final surgical pathology following thyroidectomy; (ii) repeat biopsy; (iii) clinically, based on repeat ultrasound performed at least three years following biopsy; or (iv) report of thyroid status for patients without follow-up visits contacted by mail.
Results: There were 699 nodules from 665 patients included. The mean age was 59 ± 15 years, and 71.7% were women. There was complete follow-up of 495 nodules. After a median follow-up of 2.7 years, thyroid cancer was found in 15 nodules. The prevalence of malignancy was 3% (15/495). The presence of nodular calcifications was the strongest predictor of thyroid malignancy (odds ratio 5.03 [confidence interval 1.8–14.7]). Initial nodule size was inversely associated with malignancy (odds ratio 0.55 [confidence interval 0.28–0.93]). However, the 193 patients without follow-up had smaller nodules compared with those included in the analysis. None of the patients with repeatedly non-diagnostic results were diagnosed with thyroid cancer at follow-up.
Conclusion: The prevalence of thyroid cancer in nodules with non-diagnostic results is lower than the malignancy rate in thyroid nodules in general, but not negligible. They should be followed as per guidelines with heightened suspicion for nodules containing calcifications. Nodules with repeatedly non-diagnostic FNA results especially in the absence of calcifications have a low risk of malignancy and may be observed.
Keywords: : thyroid nodule, FNA, thyroid cancer, diagnostic
Introduction
Thyroid nodules are present in a significant percent of the population. In a study including 635 volunteers who presented for a preventive health checkup, thyroid nodules were found by ultrasonography in 68% (1). In another study of 253 volunteers selected from a random sample of the population in Finland, thyroid nodules were found by ultrasound in 21% (2). Moreover, the prevalence of thyroid nodules in autopsy studies of patients without known thyroid disease varies between 30% and 50% (3). The evaluation of thyroid nodules to exclude thyroid cancer includes fine-needle aspiration (FNA) biopsy, depending on clinical and ultrasound characteristics. The American Thyroid Association (ATA) guidelines for management of patients with thyroid nodules and differentiated thyroid cancer recommend that non-diagnostic thyroid FNA results be followed by a repeat FNA biopsy (4). The non-diagnostic rate for ultrasound (US)-guided FNAs varies widely among studies, with rates as high as 47% and as low as 0.6% in studies with on-site cytology (5–7). More commonly, however, the non-diagnostic rate usually ranges between 8% and 20% (8–10). The risk of malignancy for non-diagnostic thyroid nodules is not clearly defined and varies among studies between 2% and 15% (11–13). The predictors of malignancy in nodules with non-diagnostic cytology are unknown. Understanding the risk of malignancy in nodules with non-diagnostic cytology will help to refine the diagnostic/therapeutic strategies, an aspect that is particularly important for nodules with repeatedly non-diagnostic FNAs.
The aims of this study were to evaluate the prevalence of thyroid cancer in nodules of patients with an initial non-diagnostic FNA, and to identify clinical, US, and cytological factors associated with a higher risk of malignancy in these nodules.
Material and Methods
Patients with thyroid nodules with an initial non-diagnostic US-guided FNA performed between 2004 and 2010 at the Mayo Clinic (Rochester, MN) were included in this retrospective cohort study. The initial nodule was the index nodule that was followed in all subsequent assessments. Thyroid nodules with non-diagnostic FNAs were identified from a tissue registry that captures all pathology samples from the inpatient and outpatient setting. The exclusion criteria included: FNA not guided by US, patients with previous history of thyroid cancer, a non-diagnostic FNA with a previously benign biopsy of the same nodule, and purely cystic nodules. This study was approved by the Mayo Clinic Institutional Review Board.
Fine-needle aspirates of thyroid nodules were classified as diagnostic (including all Bethesda categories except non-diagnostic) or non-diagnostic (10). At the Mayo Clinic, an FNA is considered non-diagnostic when there are fewer than the required six cell groups with ≥15 cells/group.
The US-guided FNA was performed by experienced radiologists and endocrinologists, each performing >100 FNAs/year. A 25–27 gauge needle was used under US guidance, and four to six passes were made into each nodule. The sample was obtained mainly by the capillary method (without suction). However, aspiration with suction was performed if deemed necessary by the operator. The slides were fixed in 95% ethyl alcohol and stained using the Papanicolau method. The specimens were not reviewed on-site for adequacy.
Patients were followed to assess the outcome of the index nodule. The final diagnosis of benign versus malignant disease in the index nodule was based on one of the following: (i) final surgical pathology following thyroidectomy; (ii) a repeat FNA or core biopsy; (iii) clinically, based on repeat US performed at least three years following biopsy; or (iv) patients without available follow-up information were contacted by mail to ascertain their thyroid nodule outcome. Nodules were considered benign at follow-up if they had at least one of the following: (i) benign histology following surgery, (ii) a benign repeat FNA or core biopsy, (iii) <20% increase in diameter size on an US performed at least three years after initial biopsy and absence of cervical adenopathy (for those without definite diagnosis after biopsy or without a repeat biopsy), or (iv) no report of thyroid cancer diagnosis when contacted by mail after at least three years from non-diagnostic FNA (for those with no follow-up information in the record). Patients with non-diagnostic thyroid nodules who died from non-thyroid cancer- related causes were included in the analysis and were considered to have benign thyroid nodules if no indication for malignancy existed based on the above criteria.
The main outcome of interest was diagnosis of thyroid cancer in the index nodule. Several factors were analyzed for the association with thyroid cancer diagnosis. The clinical factors evaluated were age, sex, family history of thyroid cancer, previous radiation to the neck, and serum thyrotropin (TSH) levels. The US factors (based on the US report) included initial nodule size (maximal dimension), nodule echogenicity (for solid nodules), type of nodule (complex or solid), presence of calcifications, and nodule vascularity. The cytological factors according to the FNA report included presence of colloid, presence of macrophages, absence of cells, and bloody aspirate.
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD) when normally distributed, and as median with interquartile range (IQR) when not normally distributed. Categorical variables were expressed as percentages. Because a patient could have one or more non-diagnostic nodules, demographic and patient-related characteristics (age, sex, family history of thyroid cancer, and TSH values) were reported using patients as the unit of analysis. For nodule-specific characteristics (US features, nodule size, and cytological factors), the unit of analysis was non-diagnostic nodules. To evaluate the clinical, US, or cytological factors associated with thyroid cancer, logistic regression was performed using diagnosis of thyroid cancer as the outcome (dependent variable). Odds ratios were used to determine the magnitude of the association between the factors and malignancy.
There was inconsistent reporting of vascularity. Therefore, the vascularity of the nodules was reviewed by US in all the thyroid cancer cases. A set of patients was then identified who were matched by age and sex to the thyroid cancer patients at a 2:1 ratio, and vascularity was assessed in these nodules as well. Conditional logistic regression was used for the matched analysis of the association between vascularity of the nodule and malignancy. The statistical software JMP c10.0.0 was used for all analyses.
Results
There were 8430 FNAs performed in the study period between 2004 and 2010, of which 994 (11.7%) were non-diagnostic. Of the non-diagnostic procedures, 295 were excluded. The reasons for exclusion were previous history of thyroid cancer, lack of US guidance, previous diagnostic FNA, or purely cystic nodules. Therefore, a total 699 nodules from 665 patients were included in the study.
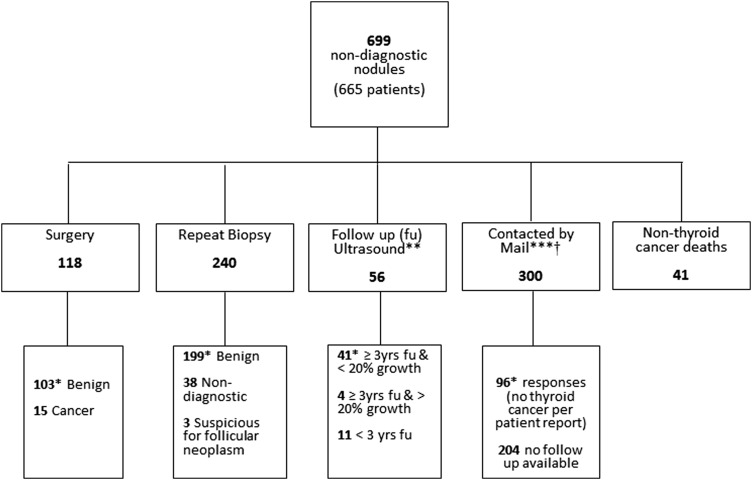
The mean age at the time of non-diagnostic FNA was 59 ± 15 years (range 12–93 years), and 72% were women (Table 1). The proportion of solid nodules was 44.6%, and the mean nodule size was 2.1 ± 1.3 cm (range 0.3–10 cm). The main pathway employed to identify the final pathology is depicted in Figure 1. Approximately half (51%) of the nodules had either a repeat biopsy or surgery, and a definitive diagnosis was reached in 88.5% of these nodules. Forty-one participants had died, and it was possible to ascertain that they died from non-thyroid cancer–related causes. Therefore, 472 patients with 495 nodules (454 with follow-up information and 41 nodules of patients with non-thyroid cancer related deaths) were included in the analysis.
Table 1.
Baseline Characteristics of 665 Participants with 699 Non-Diagnostic Thyroid Nodules
| Clinical characteristics in 665 participants | |
| Age (years), mean ± SD | 58.84 ± 15 |
| Female, % (n) | 71.7% (472/665) |
| TSH median, IQR (mIU/L) | 1.5 (0.9–2.3) |
| Family history of thyroid cancer, % (n) | 3.4% (20/590) |
| Previous neck radiation, % (n) | 7.4% (30/403) |
| Multiple nodules, % (n) | 79.8% (507/635) |
| Ultrasound characteristics of 699 nodules | |
| Solid, % (n) | 44.6 (311) |
| Size (cm), mean ± SD | 2.1 ± 1.3 |
| Hypoechogenicity, % (n) | 67.5 (335/496)a |
| Calcifications, % (n) | 18 (128) |
Echogenicity was assessed for fully solid nodules.
SD, standard deviation; TSH, thyrotropin; IQR, interquartile range.
FIG. 1.
Follow-up of 699 non-diagnostic nodules. *Considered benign at follow-up. **If surgery or a repeat diagnostic biopsy was available, not included in the ultrasound category. ***Patients with thyroid surgery, a repeat diagnostic biopsy or ≤20% growth by ultrasound (US) ≥3 years were not contacted by mail. †Forty-one patients with a repeat biopsy that was non-diagnostic or suspicious for follicular neoplasm and 15 patients with a follow-up US <3 years or with >20% growth were contacted by mail.
There were 193 patients (204 nodules) without follow-up information available, and they had a similar sex distribution, family history of thyroid cancer, previous history of neck radiation, and nodule type distribution (complex vs. solid) as the rest of the cohort (data not shown). However, patients without follow-up were on average three years younger (Mage = 56.7 years [confidence interval (CI) 54.3–58.9]) compared with 59.9 years [CI 58.6–61.1] in those with follow-up (p = 0.01)], and they had a lower serum TSH level at baseline (median 1.25 IU/L, IQR 0.7–2, compared with 1.5 IU/L, IQR 0.9–2.4 in those with follow-up; p = 0.002). Patients without follow-up had smaller nodules compared with those with available follow-up (M = 1.8 cm [CI 1.6–1.9] vs. 2.2 cm [CI 2.1–2.3]; p < 0.0001).
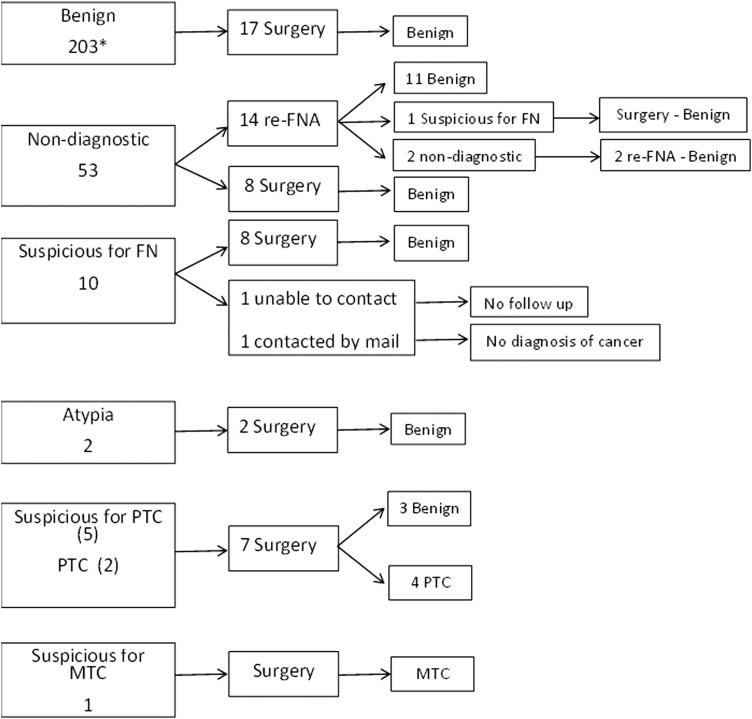
A repeat FNA was performed in 276 nodules, and their outcomes are described in Figure 2. There were 53 patients with 53 nodules with a repeat non-diagnostic FNA at follow-up. None of these 53 patients were diagnosed with thyroid cancer: 22/53 (41.5%) were considered benign after a second or third repeat biopsy or surgery, 5/53 had nodules stable in size by US at least three years after presentation, 15/53 had no diagnosis of cancer when contacted by mail, and 2/53 had died from a non-thyroid cancer–related cause. Only 9/53 (17%) had no follow-up information available.
FIG. 2.
Outcomes of repeated FNA of 276 initially non-diagnostic biopsies. Re-FNA, repeat fine-needle aspiration; FN, follicular neoplasm; PTC, papillary thyroid cancer; MTC, medullary thyroid cancer. *179 were FNA and 24 were core biopsies.
Thyroid cancer
After a median follow-up of 2.7 years (IQR 0.09–5.9 years), 15/495 (35) thyroid nodules with an initial non-diagnostic FNA were found to be malignant. The diagnosis of thyroid cancer was confirmed by histology in all; 13/15 were papillary thyroid cancer (PTC), 1/15 had follicular thyroid cancer, and 1/15 had medullary thyroid cancer. In the PTC group, 5/13 (38%) were microcarcinomas (<1 cm), and 3/13 (23%) were cystic PTC (Fig. 3). All five microcarcinomas were identified in the index nodule (median nodule size 0.7 cm; range 0.5–0.9 cm). Eight (61.5%) PTC cases were classified as Stage I, and the other five (38.5%) were Stage III at time of diagnosis. The follicular thyroid cancer was a 2.8 cm solid nodule, also Stage I at diagnosis. The patient with a non-diagnostic FNA later diagnosed with medullary thyroid cancer after thyroidectomy presented with a 4.3 cm nodule found incidentally by magnetic resonance imaging. The patient also had an abnormal-appearing 1.4 cm lymph node ipsilateral to the thyroid nodule. While the FNA of the thyroid nodule was non-diagnostic, the FNA of the lymph node obtained during the same procedure showed neoplastic cells. After a mean ± SD follow-up of 6 ± 2.2 years since thyroid cancer diagnosis, none of these 15 patients had evidence of recurrence.
FIG. 3.
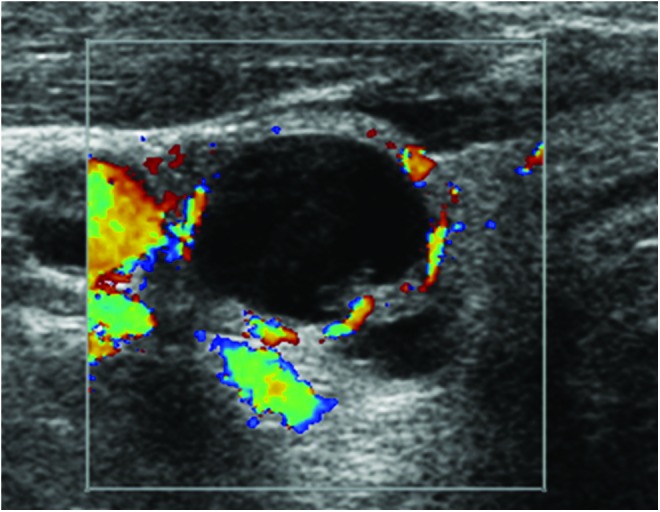
Ultrasound image of a cystic papillary thyroid cancer with an initial non-diagnostic fine-needle aspiration.
Factors associated with thyroid cancer
In univariate analysis, the clinical and US factors associated with thyroid cancer were initial nodule size and the presence of calcifications (Table 2). When age was analyzed, it was found to be significantly different between patients with benign and malignant nodules. Patients with thyroid cancer were a median of 12 years younger than patients with benign nodules when each nodule (n = 495) was considered independently (p = 0.04). When the analysis focused on patients (n = 472), independent of the number of nodules per patient, there was still a strong trend for younger age predicting malignancy, but the difference was not statistically significant (p = 0.07). Other clinical factors such as sex (p = 0.5), family history of thyroid cancer (p = 0.52), and serum TSH level (p = 0.69) were not associated with a diagnosis of thyroid cancer, and the results were similar, irrespective of the analysis modality employed.
Table 2.
Clinical and Radiological Features in Patients with Non-Diagnostic Thyroid Nodulesa
| Factors | Malignant nodules (n = 15) | Benign nodules (n = 480) | OR [CI] | p |
|---|---|---|---|---|
| Clinical factors based on 472 patients | ||||
| Age (years) | 49 (39–67) | 61 (52.3–70.2) | 0.97 [0.94–1] | 0.07 |
| Sex (male) | 35.7% (5/14) | 27.5% (126/458) | 0.5 [0.03–3.15] | 0.50 |
| Family history of thyroid cancer | 16.7% (2/12) | 3.4% (14/416) | 4.79 [0.7–19.9] | 0.52 |
| History of neck radiation | 0 | 7.4% (21/281) | 0.19 | |
| TSH (mIU/L) | 1.4 (0.6–2.3) | 1.5 (0.9–2.4) | 0.89 [0.39–1.27] | 0.69 |
| Ultrasound and pathology factors based on 495 nodules | ||||
| Initial nodule size (cm) | 1.2 (0.8–2.3) | 1.95 (1.2–2.9) | 0.55 [0.28–0.93] | 0.02 |
| Solid nodule | 73.3% (11/15) | 50.7% (221/436) | 2.67 [0.9–9.77] | 0.08 |
| Hypoechogenicityb | 27.3% (3/11) | 33.8% (75/222) | 1.81 [0.56–8.0] | 0.34 |
| Presence of calcifications | 53.3% (8/15) | 18.5% (80/432) | 5.03 [1.76–14.73] | <0.01 |
| Increased vascularityc | 53.3% (8/15) | 66.7% (20/30) | 1.33 [0.71–1.40] | 0.69 |
| Presence of colloid | 0 (0/15) | 11.3% (54/479) | 0.39 | |
| Presence of macrophages | 26.7% (4/15) | 19% (91/479) | 1.55 [0.42–4.65] | 0.47 |
| Acellular aspirate | 6.7% (1/15) | 2.5% (12/478) | 2.8 [0.15–15.7] | 0.40 |
| Bloody aspirate | 0 (0/15) | 7% (34/479) | 0.61 | |
Continuous variables are presented as median with IQR, and categorical variables as percentage (n). Statistically significant values are shown in bold.
Only 472 patients with 495 nodules included in the analysis.
Echogenicity was assessed for fully solid nodules.
Vascularity was assessed in all 15 cancers and 30 controls matched by age and sex at a 1:2 ratio.
OR, odds ratio; CI, confidence interval.
Larger nodules were associated with a lower risk of thyroid cancer diagnosis (OR 0.55 [CI 0.28–0.93]), and the presence of calcifications in the non-diagnostic nodule was associated with a higher risk of thyroid cancer (OR 5.03 [CI 1.76–14.73]). However, nodule type (complex vs. solid) and echogenicity were not associated with thyroid malignancy risk.
In the matched analysis for vascularity, each thyroid cancer patient was matched by age and sex (matched ratio 1:2), with a “control” nodule considered benign at follow-up. Increased vascularity was not associated with higher risk of thyroid cancer (p = 0.69).
None of the cytological factors evaluated—presence of colloid, acellular aspirate (p = 0.4), bloody aspirate (p = 0.6), and presence of macrophages (p = 0.47)—was associated with a thyroid cancer diagnosis. None of the nodules with confirmed malignancy was reported to have significant colloid in the initial non-diagnostic sample, whereas 11.3% (54/479) of the nodules considered benign at follow-up had abundant colloid in the initial FNA sample. However, this difference was not statistically significant (p = 0.39).
Discussion
This study evaluated the risk of malignancy in thyroid nodules with an initial non-diagnostic FNA and the factors associated with cancer. The prevalence of thyroid cancer was 3% (15/495) for nodules with an initial non-diagnostic FNA. Smaller nodule size and presence of calcifications were associated with a higher risk of thyroid cancer.
Several studies report rates of malignancy in non-diagnostic nodules between 10% and 15% (13–15), a rate significantly higher than what would be expected for thyroid nodules in general. However, most of these studies are at high risk of selection bias. Some of them estimate the risk of malignancy based only on results of patients who undergo surgery (10,15). In a meta-analysis to assess the risk of malignancy of thyroid nodules classified according to the Bethesda categories based on histology follow-up, only 16% of patients with a non-diagnostic FNA had histological follow-up to assess malignancy risk (10). Other studies estimate the malignancy risk based on patients with a repeat diagnostic FNA or histology (13,14). Close to 50% or more patients with non-diagnostic FNAs included in the aforementioned studies (13,14) did not have a repeat FNA or surgery, and were not included in the assessment of malignancy risk. Therefore, it is likely that the risk of thyroid cancer in thyroid nodules with a non-diagnostic FNA is overestimated.
We tried to overcome this selection bias by including patients with clinical follow-up, even if they did not have pathology confirmation of a benign nodule. By doing this, certain nodules might be misclassified as “benign” when in fact they might harbor indolent thyroid cancer. However, given the lack of growth and/or clinical diagnosis of thyroid cancer over three or more years, those lesions were considered to be very unlikely to represent “clinically significant cancers.” A prevalence rate of 3% was found for thyroid cancer, which is lower than the estimated prevalence of malignancy in thyroid nodules in general (between 4% and 6.5%) (16–18). Hryhorczuk et al. reported a similar prevalence of thyroid malignancy: 2% (5/256) in nodules with a non-diagnostic cytology (12). In this study, patients with repeat FNA and those without a repeat biopsy but with clinical and imaging follow-up were included. Therefore, the results are comparable to those in the present study.
The 2015 ATA management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer recommend a repeat FNA for all thyroid nodules with non-diagnostic cytology (4). This is a reasonable approach, although based on the present results, observation without the need for repeat FNA could be considered in selected patients without clinical or sonographic risk factors. However, the clinical or US factors that increase or decrease the risk for malignancy in thyroid nodules with non-diagnostic cytology have not been clearly defined. It can be presumed that the US features associated with high risk for malignancy in thyroid nodules in general (taller than wide, presence of microcalcifications, increased vascularity, hypoechogenicity, and irregular margins) are also associated with malignancy in non-diagnostic nodules.
This study found the presence of calcifications to be the strongest predictor for malignancy. Additionally, a smaller nodule size was statistically significantly associated with a higher risk of malignancy. This might be explained by differential loss to follow-up. No follow-up information was available for 30% (204/699) of the nodules identified. It is known that these patients had smaller nodules compared with those included in the analysis. Very likely, these individuals had no evidence of thyroid cancer over the years, this being the reason for the lack of re-evaluation of their thyroid. If that is correct, nodule size may not have been found to be a significant risk factor for malignancy if their data had been available for analysis. Furthermore, there is conflicting evidence on the association between nodule size and cancer risk. Larger nodule size has been associated with malignancy in some studies (19,20). A study of 7348 nodules found that risk of malignancy was higher in nodules ≥2 cm compared with nodules 1–1.9 cm. However, there was no graded increase in malignancy risk for nodules >2 cm in size (20). In contrast, other studies have not found an association between nodule size and cancer risk (18,21), including a study of 3013 patients with thyroid nodules that ranged in size between 0.5 and >4 cm (21). Moreover, none of these studies found smaller nodules to be at higher risk of malignancy. Therefore, it is hypothesized that the smaller nodule size association with thyroid cancer in the present cohort was related to the differential loss to follow-up.
Some studies suggest that younger age is a risk factor for thyroid cancer in thyroid nodules in general, and the risk seems to be higher for individuals <30 years of age (22,23). The present study found that patients with thyroid cancer were, on average, 12 years younger than those without cancer. However, this trend did not reach statistical significance. Moreover, only 4% of the patients included were <30 years of age, which may explain the lack of statistical association between age and thyroid cancer in this study.
There is uncertainty about the best diagnostic-therapeutic approach of nodules with repeatedly non-diagnostic FNAs. There are discrepant reports on the risk of thyroid malignancy (15,24). Moon et al. (24) found that suspicious US features, if present, were associated with a higher malignancy rate in nodules with two consecutive non-diagnostic FNAs. In the current study, it was not possible to compare US features and malignancy rates among patients with multiple non-diagnostic FNAs, given that none of the repeatedly non-diagnostic nodules were found to be malignant at follow-up. The results imply that the risk of malignancy on these nodules may not be as high as previously reported (24), and surgery might not be necessary in most patients.
Strengths and limitations
One of the strengths of this study is the large sample size of non-diagnostic nodules identified. Additionally, follow-up information was available on patients who did not have a repeat diagnostic FNA or surgery. Therefore, more than two-thirds of the initial sample was included in the analysis. With this approach, the risk of selection bias was reduced, and estimates of malignancy risk are provided that can be applied in clinical practice.
However, it is important to consider that this is not a population-based study, and the results of the study need to be interpreted and applied in the appropriate context. The Mayo Clinic is a tertiary referral center, the FNAs are performed by experienced radiologists and endocrinologists, and the cytology slides are interpreted by experienced cytopathologists.
Given the large sample size and the retrospective nature of this study, interobserver variability of reported US features and variability on adequacy determination of FNA specimens was not assessed. Furthermore, the number of events was low (15 thyroid malignancies at follow-up). Because of this, it was only possible to do univariate analysis, and no adjustment was made for potential confounders to avoid overfitting the model. Given the low number of events, it is possible that other predictors of malignancy such as increased vascularity or echogenicity were not found due to lack of statistical power.
The 2015 ATA management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer consider growth of a nodule to be significant if there is >20% increase in diameter size in addition to at least a 2 mm increase in size in at least two dimensions. This study used a similar cutoff for nodule growth. However, because the nodules size was taken from US reports and all three nodule dimensions are inconsistently reported, it was only possible to confirm that the nodules that increased in size by >20% in diameter had also increased in size by at least 2 mm in one dimension. Therefore, some nodules could potentially have been misclassified as having significant growth instead of a stable size if the complete definition of nodule growth according to ATA guidelines had been used.
In conclusion, the rate of thyroid malignancy in thyroid nodules with a non-diagnostic cytology is low. Moreover, the rate of malignancy in thyroid nodules with repeatedly non-diagnostic cytology seems to be even lower. However, the presence of calcifications in the nodule by US indicates a higher risk of malignancy and should prompt the clinician to evaluate the nodule further with repeat FNA.
Acknowledgments
This publication was supported by CTSA Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. 2009. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 39:699–706 [DOI] [PubMed] [Google Scholar]
- 2.Brander A, Viikinkoski P, Nickels J, Kivisaari L. 1991. Thyroid gland: US screening in a random adult population. Radiology 181:683–687 [DOI] [PubMed] [Google Scholar]
- 3.Tan GH, Gharib H. 1997. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 126:226–231 [DOI] [PubMed] [Google Scholar]
- 4.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceresini G, Corcione L, Morganti S, Milli B, Bertone L, Prampolini R, Petrazzoli S, Saccani M, Ceda GP, Valenti G. 2004. Ultrasound-guided fine-needle capillary biopsy of thyroid nodules, coupled with on-site cytologic review, improves results. Thyroid 14:385–389 [DOI] [PubMed] [Google Scholar]
- 6.Al Maqbali T, Tedla M, Weickert MO, Mehanna H. 2012. Malignancy risk analysis in patients with inadequate fine needle aspiration cytology (FNAC) of the thyroid. PLoS One 7:e49078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, Marqusee E. 2002. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab 87:4924–4927 [DOI] [PubMed] [Google Scholar]
- 8.Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. 2009. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid 19:1215–1223 [DOI] [PubMed] [Google Scholar]
- 9.Luu MH, Fischer AH, Pisharodi L, Owens CL. 2011. Improved preoperative definitive diagnosis of papillary thyroid carcinoma in FNAs prepared with both ThinPrep and conventional smears compared with FNAs prepared with ThinPrep alone. Cancer Cytopathol 119:68–73 [DOI] [PubMed] [Google Scholar]
- 10.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. 2012. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 56:333–339 [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Pascual L, Barahona MJ, Balsells M, del Pozo C, Anglada-Barcelo J, Casalots-Casado J, Veloso E, Torres J. 2011. Complex thyroid nodules with nondiagnostic fine needle aspiration cytology: histopathologic outcomes and comparison of the cytologic variants (cystic vs. acellular). Endocrine 39:33–40 [DOI] [PubMed] [Google Scholar]
- 12.Hryhorczuk AL, Stephens T, Bude RO, Rubin JM, Bailey JE, Higgins EJ, Fox GA, Klein KA. 2012. Prevalence of malignancy in thyroid nodules with an initial nondiagnostic result after ultrasound guided fine needle aspiration. Ultrasound Med Biol 38:561–567 [DOI] [PubMed] [Google Scholar]
- 13.Coorough N, Hudak K, Jaume JC, Buehler D, Selvaggi S, Rivas J, Sippel R, Chen H. 2013. Nondiagnostic fine-needle aspirations of the thyroid: is the risk of malignancy higher? J Surg Res 184:746–750 [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Youk JH, Kim JA, Kwak JY, Kim EK, Ryu YH, Son EJ. 2012. Initially non-diagnostic ultrasound-guided fine needle aspiration cytology of thyroid nodules: value and management. Acta Radiol 53:168–173 [DOI] [PubMed] [Google Scholar]
- 15.Richards ML, Bohnenblust E, Sirinek K, Bingener J. 2008. Nondiagnostic thyroid fine-needle aspiration biopsies are no longer a dilemma. Am J Surg 196:398–402 [DOI] [PubMed] [Google Scholar]
- 16.Werk EE, Jr, Vernon BM, Gonzalez JJ, Ungaro PC, McCoy RC. 1984. Cancer in thyroid nodules. A community hospital survey. Arch Intern Med 144:474–476 [PubMed] [Google Scholar]
- 17.Lin JD, Chao TC, Huang BY, Chen ST, Chang HY, Hsueh C. 2005. Thyroid cancer in the thyroid nodules evaluated by ultrasonography and fine-needle aspiration cytology. Thyroid 15:708–717 [DOI] [PubMed] [Google Scholar]
- 18.Hagag P, Strauss S, Weiss M. 1998. Role of ultrasound-guided fine-needle aspiration biopsy in evaluation of nonpalpable thyroid nodules. Thyroid 8:989–995 [DOI] [PubMed] [Google Scholar]
- 19.Raparia K, Min SK, Mody DR, Anton R, Amrikachi M. 2009. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: patient's sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med 133:787–790 [DOI] [PubMed] [Google Scholar]
- 20.Kamran SC, Marqusee E, Kim MI, Frates MC, Ritner J, Peters H, Benson CB, Doubilet PM, Cibas ES, Barletta J, Cho N, Gawande A, Ruan D, Moore FD, Jr, Pou K, Larsen PR, Alexander EK. 2013. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab 98:564–570 [DOI] [PubMed] [Google Scholar]
- 21.Shrestha M, Crothers BA, Burch HB. 2012. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine-needle aspiration: a 10-year study from a single institution. Thyroid 22:1251–1256 [DOI] [PubMed] [Google Scholar]
- 22.Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C, Vigneri R. 1992. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med 93:363–369 [DOI] [PubMed] [Google Scholar]
- 23.Hegedus L. 2004. Clinical practice. The thyroid nodule. N Engl J Med 351:1764–1771 [DOI] [PubMed] [Google Scholar]
- 24.Moon HJ, Kwak JY, Choi YS, Kim EK. 2012. How to manage thyroid nodules with two consecutive non-diagnostic results on ultrasonography-guided fine-needle aspiration. World J Surg 36:586–592 [DOI] [PubMed] [Google Scholar]