Abstract
Aim
The aim of the study was to evaluate whether the twinkle artefact is a valuable feature in the sonographic diagnosis of superficial epidermoid cysts.
Materials and methods
A retrospective search was undertaken of our institution’s Radiology Information System and pathology database to identify cases of superficial masses showing the twinkle artefact that proceeded to surgical excision.
Results
Eighteen superficial masses demonstrating the twinkle artefact were identified that were submitted for pathological analysis. Of these, 17 were confirmed to represent epidermoid cysts and only 1 case had an alternative diagnosis (proliferating trichilemmal cyst).
Conclusion
The presence of the twinkle artefact appears to be a specific and valuable ancillary sonographic feature for the diagnosis of superficial epidermoid cysts.
Keywords: Twinkle artefact, epidermoid cyst, sebaceous cyst, colour Doppler ultrasound
Introduction
The twinkle artefact (TA) is a colour Doppler artefact that describes rapidly fluctuating artefactual colour pixels behind strongly reflective static interfaces, particularly calcified lesions, which may simulate high velocity and disturbed blood flow.1,2 The artefact is well known to most ultrasound practitioners and can be employed as a valuable additional sonographic feature in detection of renal calculi, particularly in situations where the use of ionising radiation is undesirable.3 This artefact has also been described in a number of other abdominal and pelvic conditions.4
Colour and power Doppler are routinely used in the sonographic assessment of superficial masses to identify the presence of blood flow and are a valuable addition to grey scale ultrasound in differentiating solid from complex cystic lesions and also in assessing whether a mass has features of concern for malignancy.
Superficial visible or palpable masses are a common reason for patients to seek medical advice and ultrasound is now routinely requested in assessing these lesions. In addition to defining the position, borders and relationship to adjacent structures, ultrasound can assess for the presence of internal vascularity. Epidermoid cysts (often incorrectly referred to as sebaceous cysts) represent one of the most common superficial masses and will be regularly encountered by most ultrasound practitioners. Ultrasound has good accuracy in characterising epidermoid cysts based on their grey scale and Doppler appearances but there is a wide variety in their echogenicity, from cystic to ‘pseudo solid’, which may cause diagnostic uncertainty.5,6
Two of the authors of this study had independently observed the TA in patients with superficial skin lesions that had clinical and greyscale ultrasound features of epidermoid cysts in their routine clinical practice. No association between TA and epidermoid cysts was identified in a literature search and therefore a retrospective study was undertaken with the purpose of determining whether the TA was a specific sonographic feature of epidermoid cysts.
Materials and methods
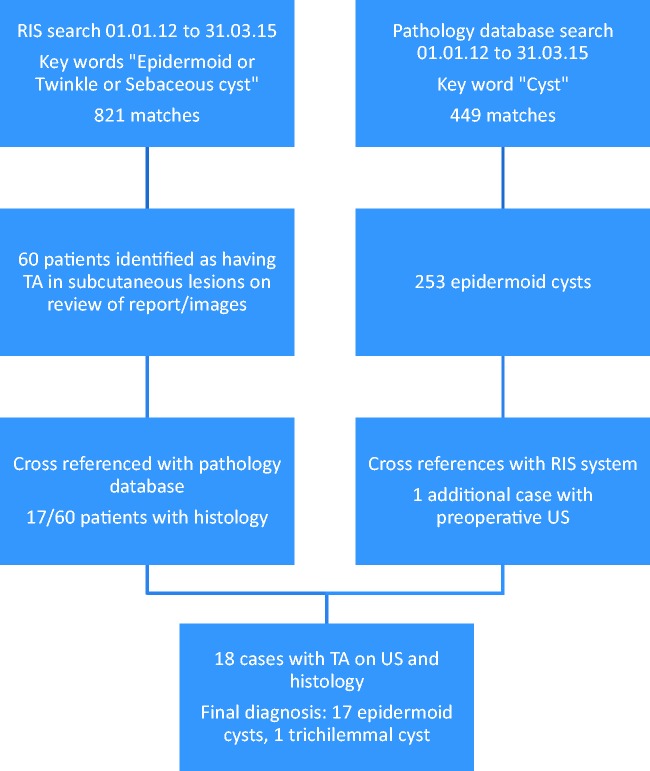
A word search of radiology reports on the hospital Radiology Information System (RIS) for the key words ‘epidermoid, twinkle and sebaceous cyst’ was undertaken covering all ultrasound studies between the dates 1 January 2012 and 31 March 2015. The search returned 821 reports which were then reviewed to identify cases of superficial lesions showing the TA; this resulted in 60 matches. These cases were cross-referenced with the hospital pathology database showing that 17 lesions had been excised and subjected to pathological analysis. The other 43 cases did not have pathology data and were eliminated from further analysis.
A further interrogation of the pathology database between the dates 1 Jan 2012 and 31 March 2015 using the key word ‘cyst’ identified 449 patients. Review of these cases revealed 253 cases of histologically proven epidermoid cysts. These cases were then cross-referenced with the RIS system and the reports and images were reviewed; this identified one additional patient not found in the initial radiology search with a superficial lesion showing the TA on a colour Doppler soft copy image where the key words had not been used in the subsequent report.
The study sample therefore consisted of 18 patients with superficial skin lesions demonstrating the TA with subsequent pathological analysis. The methodology is summarised in Figure 1.
Figure 1.
Summary of the study methodology
In one patient, with a clinical and sonographic diagnosis of an epidermoid cyst, we examined the excised surgical specimen sonographically in a water bath immediately after excision and before pathological analysis to determine whether the TA could be reproduced in vitro.
All ultrasound examinations were performed on Toshiba Aplio ultrasound platforms (Toshiba Medical Systems Corporation, Tochigi-ken, Japan) with a variety of high-frequency broadband linear array transducers using small parts and musculoskeletal specific manufacturer presets. There was a variety of levels of experience of the ultrasound practitioners undertaking the examinations, but the majority (15) were performed by consultant radiologists (sub-specialist interests: musculoskeletal radiology (4), ultrasound (10), and cross-sectional imaging (1)). The other scans were performed by a consultant sonographer (2) and experienced radiology SpR (1).
Institutional Review Board approval (and waiver of ethical approval) was granted for this retrospective study. The excised lesion that was studied in vitro was examined with written consent having been obtained from the patient concerned.
Results
The study consists of 18 patients with superficial lesions demonstrating the TA and with subsequent pathological analysis (see Figures 2 and 3). The 18 superficial masses included in the final analysis were located in the cranio-facial soft tissues (7), neck (6), axilla (2), anterior abdominal wall (1), ankle (1), and inguinal region (1); 17 of the 18 patients (94.4%) had a final histological diagnosis of epidermoid cyst. In only one case (5.6%) was there a different final diagnosis of a proliferating trichilemmal cyst.
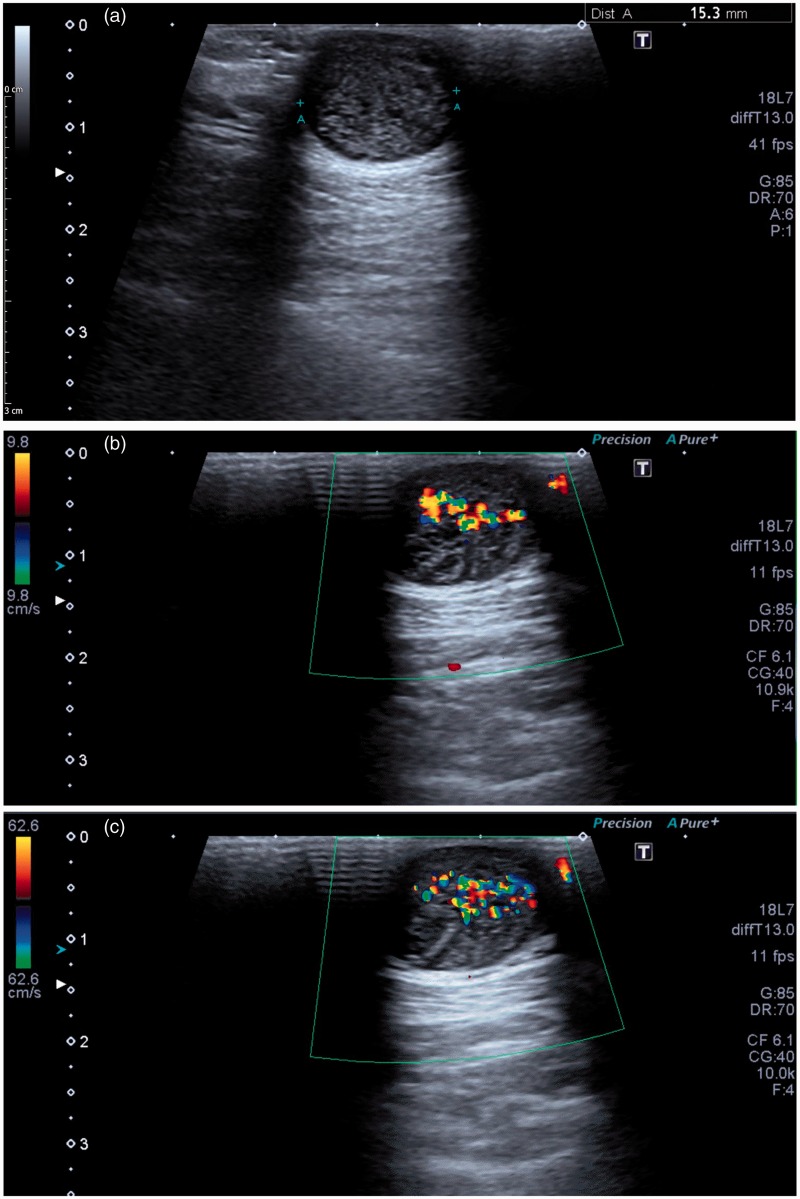
Figure 2.
Female patient referred for ultrasound with palpable mass in the posterior aspect of the neck. (a) Grey scale ultrasound demonstrates a spherical subcutaneous mass of mixed echogenicity with posterior acoustic enhancement. (b and c) The twinkle artefact is present on both low (9.8 cm/s) and high velocity (62.6 cm/s) flow settings showing that the artefact is independent of the velocity scale (pulse repetition frequency). Following surgical excision, histology confirmed an epidermoid cyst.
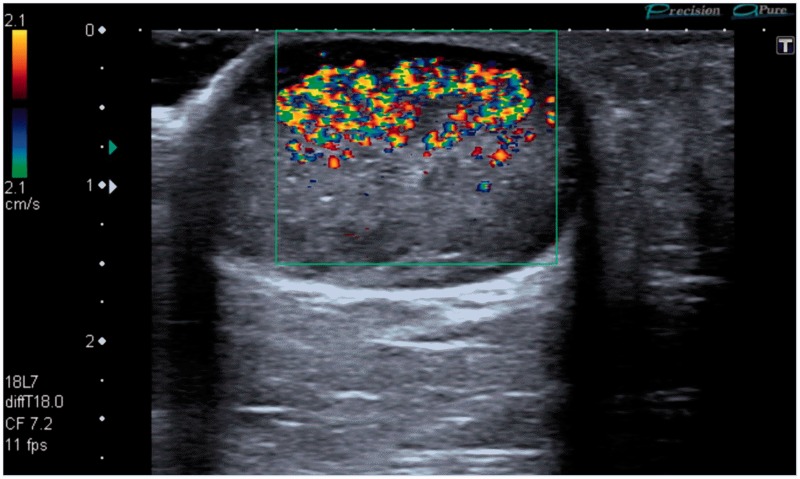
Figure 3.
A 49-year-old patient presenting for ultrasound examination of a palpable mass in the axilla. Note that TA is only seen at and above the focal zone.
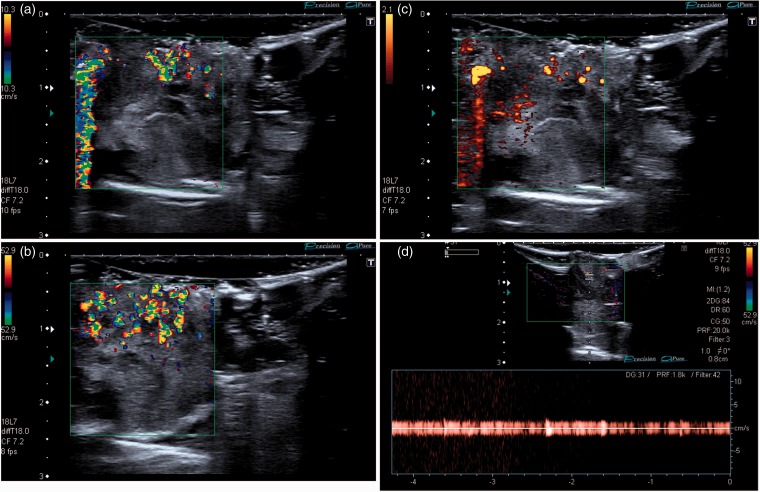
In one case, the excised lesion (subsequently histologically confirmed to be an epidermoid cyst) was scanned in a water bath within one hour of surgical excision. Ultrasound confirmed extensive TA in this specimen (Figure 4).
Figure 4.
Images of an excised epidermoid cyst scanned in a water bath. (a and b) The TA artefact is present and again seen to be independent of the velocity scale. (c) Colour artefact is also seen with power Doppler imaging. (d) Spectral Doppler analysis shows artefactual vertical bands without a discernible vascular waveform above background machine noise to the reader’s left of the spectral trace, abolished by reduction of Doppler gain to the right of the trace.
Discussion
TA is a poorly understood colour Doppler ultrasound artefact characterised by rapidly fluctuating colour Doppler pixels (Figures 2 to 4) and a similar artefact can also be seen with power Doppler applications (Figure 4(c)). TA is commonly seen behind highly reflective interfaces and is often associated with calcified lesions. Spectral Doppler analysis obtained from within the artefact typically shows closely aligned vertical bands without a discernible vascular waveform (Figure 4(d)),7 helping to confirm the artefactual nature of the colour signal. Strong TA signal is not usually significantly affected by the colour velocity scale (pulse repetition frequency),7 also confirming its artefactual nature (Figures 2 and 4). TA is influenced by other machine settings, particularly the focus (Figure 3) with the artefact occurring above the level of the focal zone. This characteristic can also be used to distinguish this artefact from true blood flow. Other machine settings including colour write priority, grey scale gain, and acoustic power4 may also affect the intensity of the artefact.
The cause of the TA is uncertain. One hypothesis suggests that it is due to the ultrasound beam impinging on a strongly reflecting surface with a rough interface, producing multiple complex reflections resulting in an increased pulse duration of the received ultrasound echo, interpreted as movement, and assigned colour pixels by the Doppler processing software within the ultrasound system.2 An alternative explanation is that the artefact is primarily dependent on narrow bandwidth noise introduced by phase (clock) jitter within the Doppler processing components of the ultrasound system, with roughness of the reflecting interface as a secondary cause.8 Other researchers believe that the twinkling artefact associated with renal calculi may be caused by tiny movements of the stones caused by the sound beam itself; this causes uncertainty as to the signal origin which is amplified by complex processing algorithms forming an image with TA.9 There is also evidence to suggest that the TA seen with renal calculi is caused by small gas bubbles trapped within imperfections on the stone surface.10
Regardless of its cause, the TA can be harnessed as a valuable artefact by ultrasound practitioners, particularly in the context of renal stone identification, but also in a variety of abdominal and pelvic pathologies including parenchymal calcifications, gallbladder adenomyomatosis, bile duct hamartomas, choledocholithiasis, and foreign body identification.4
Ultrasound has become a valuable and increasingly requested, imaging investigation to evaluate patients presenting with palpable superficial masses. It can determine whether the mass is solid, cystic, or mixed in consistency, evaluate the margins, define the relationship to adjacent structures, accurately measure size, and confirm that the mass is confined to the superficial skin layers. These features can help to categorise many masses as likely benign or more sinister in appearance and provide valuable information for patient management.5,11–13 Colour and power Doppler are routinely employed in the sonographic assessment of superficial masses to assess lesion vascularity and help to further characterise and define lesions as likely benign or potentially malignant.14 The addition of ultrasound has been shown to increase diagnostic accuracy for subcutaneous lesions over clinical palpation alone.15 In certain cases, ultrasound can also be used to guide percutaneous tissue biopsy.
Skin cysts are a common reason for patients to seek medical attention and represent one of the most frequently excised surgical specimens. Such cysts are frequently referred to as ‘sebaceous cyst’ but this is a misleading term as most of these cysts do not arise from sebaceous glands and are either epidermoid (inclusion) cysts or pilar (trichilemmal) cysts; true sebaceous cysts (containing sebum) are uncommon. Epidermoid cysts occur most frequently on the face, neck, and upper trunk and originate in the epidermis; they are thought to arise from the infundibulum or uppermost portion of the hair follicle and have a true epithelial lining. Pilar cysts are most commonly found on the scalp and originate from hair follicles. Both types of cyst contain keratin and they can only be differentiated by pathological examination following surgical excision.16 Surgical treatment for epidermoid and pilar cysts is not usually required unless the cyst becomes infected, interferes with everyday life, or is cosmetically unsightly. Treatment involves complete surgical excision to minimise the risk of recurrence. Biopsy should be avoided to prevent cyst rupture.16
Epidermiod cysts may have a variety of sonographic appearances. Typically, they are confined to the skin layers but may expand into the subcutaneous fat,17 have well-defined margins, and measure less than 5 cm in size. Usually, they demonstrate posterior acoustic enhancement, indicating their cystic nature (Figure 2(a)). They may vary from almost anechoic to echogenic, the more echogenic types having a ‘pseudo solid’ appearance.18 Frequently, the cyst will show an oval shape with homogeneous low to medium echogenicity simulating testicular parenchyma, an appearance that has been named the ‘pseudotestis sign’.6 Dermal attachment or dermal protrusion have also been described as features that help differentiate epidermal cysts from other superficial soft tissue masses.6,19 Epidermoid cysts do not demonstrate associated vascularity on colour Doppler examination unless they have ruptured, resulting in development of granulation tissue.20 Although the specificity of ultrasound in diagnosis of epidermoid cysts is high,15 they may be mistaken for other masses, particularly when showing the pseudo solid appearance.
In this retrospective study, we found the TA to be a highly specific feature of epidermoid cysts, being seen in 17 of the 18 superficial lesions where the TA was identified in the report or subsequent image review. In the one case with an alternative final histological diagnosis, the mass proved to be a trichilemmal (pilar) cyst, which is a closely related mass, and differentiation between these two entities is of no significance for patient management. No other types of superficial mass demonstrating the TA proceeded to excision and histology in this study. The artefactual nature of the colour Doppler TA was confirmed by scanning an excised epidermoid cyst in a water bath where the TA was strongly present (Figure 4). We suspect that the TA is explained by the strong reflecting interfaces arising from keratin layers in the epidermoid cyst, although further in vitro studies would be necessary to confirm this hypothesis. The location of the majority of the epidermoid cysts in this series was either in the cranio-facial soft tissues or the neck; this is a typical location for these lesions which are most commonly found on the face, neck, or upper body (chest and shoulders).15
This study has a number of significant limitations. The retrospective nature of data collection may have failed to identify cases where TA was present but not described in the radiological report and cases where colour Doppler analysis was not performed by the ultrasound operator. The study also assumes that all ultrasound practitioners in our institution are familiar with the TA and recognised it as an artefact rather than true vascular flow; we are unable to confirm that this is the case. Due to the retrospective methodology, it was not possible to standardise the machine settings, presets and transducers, all of which may have influenced TA. Finally, it is clear that in the majority of cases with a superficial mass showing TA, patients did not proceed to surgical excision and pathological analysis and that most of the surgically excised epidermoid cysts did not undergo preoperative ultrasound. Despite these limitations, this study suggests that the TA may be a specific feature of superficial epidermoid cysts and can be used as a valuable additional sonographic finding to differentiate epidermoid cysts from other superficial skin lesions. Further prospective studies would be desirable to confirm this preliminary observation and to establish the value of TA in the diagnosis of epidermoid cysts.
Acknowledgements
The authors would like to acknowledge the assistance of Mr R Morris, consultant plastic surgeon, who facilitated the ultrasound examination of the excised epidermoid cyst scanned in vitro (Figure 4).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The study was granted waiver of ethical approval by the institution’s Ethics and Research Committee. Written permission was obtained from the patient for the ex-vivo ultrasound study and use of images for the excised lesion reproduced in Figure 4.
Guarantor
SF.
Contributorship
SF and PS created the study concept and design. RC and RT collected the data and wrote the first manuscript draft. SF prepared the final manuscript which was edited and approved by all authors.
References
- 1.Dillman JR, Kappil M, Weadock WJ, et al. Sonographic twinkling artifact for renal calculus detection: correlation with CT. Radiology 2011; 259: 911–916. [DOI] [PubMed] [Google Scholar]
- 2.Rahmouni A, Bargoin R, Herment A, et al. Color Doppler twinkling artifact in hyperechoic regions. Radiology 1996; 199: 269–271. [DOI] [PubMed] [Google Scholar]
- 3.Winkel RR, Kalhauge A, Fredfeldt K-E. The usefulness of ultrasound colour-Doppler twinkling artefact for detecting urolithiasis compared with low dose nonenhanced computerized tomography. Ultrasound Med Biol 2012; 38: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 4.Kim HC, Yang DM, Jin W, et al. Color Doppler twinkling artifacts in various conditions during abdominal and pelvic sonography. J Ultrasound Med 2010; 29: 621–632. [DOI] [PubMed] [Google Scholar]
- 5.Hung EH, Griffith JF, Ng AW, et al. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. Amer J Roentgenol 2014; 202: W532–W540. [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Ko SF, Huang HY, et al. Epidermal cysts in the superficial soft tissue sonographic features with an emphasis on the pseudotestis pattern. J Ultrasound Med 2011; 30: 11–17. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Kim SH, Cho JY, et al. Color and power Doppler twinkling artifacts from urinary stones: clinical observations and phantom studies. Amer J Roentgenol 2001; 176: 1441–1445. [DOI] [PubMed] [Google Scholar]
- 8.Kamaya A, Tuthill T, Rubin JM. Twinkling artifact on color Doppler sonography: dependence on machine parameters and underlying cause. Amer J Roentgenol 2003; 180: 215–222. [DOI] [PubMed] [Google Scholar]
- 9.Shah A, Paun M, Kucewicz, et al. A “twinkling artifact” targets kidney stones for lithotripsy treatment, www.apl.washington.edu/projects/twinkling_artifact/experiments_modeling.html (accessed 15 February 2016).
- 10.Lu W, Sapozhnikov OA, Bailey MR, et al. Evidence for trapped surface bubbles as the cause for the twinkling artifact in ultrasound imaging. Ultrasound Med Biol 2013; 39: 1026–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiou HJ, Chou YH, Chiu SY, et al. Differentiation of benign and malignant superficial soft-tissue masses using grayscale and color Doppler ultrasonography. J Chinese Med Assoc 2009; 72: 307–315. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JM, Lee KS, Rosas H, et al. Accuracy of sonographic diagnosis of superficial masses. J Ultrasound Med 2013; 32: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 13.DiDomenico P, Middleton W. Sonographic evaluation of palpable superficial masses. Radiol Clin North Am 2014; 52: 1295–1305. [DOI] [PubMed] [Google Scholar]
- 14.Belli P, Costantini M, Mirk P, et al. Role of color Doppler sonography in the assessment of musculoskeletal soft tissue masses. J Ultrasound Med 2000; 19: 823–830. [DOI] [PubMed] [Google Scholar]
- 15.Kuwano Y, Ishizaki K, Wantanabe R, et al. Efficacy of diagnostic ultrasonography of lipomas, epidermal cysts, and ganglions. Arch Dermatol 2009; 145: 761–764. [DOI] [PubMed] [Google Scholar]
- 16.British Association of Dermatologists. Epidermoid and pilar cysts. www.bad.org.uk/for-the-public/patient-information-leaflets/cysts—epidermoid-and-pilar (accessed 15 February 2016).
- 17.Giess CS, Raza S, Birdwell RL. Distinguishing breast skin lesions from superficial breast parenchymal lesions: diagnostic criteria, imaging characteristics, and pitfalls. RadioGraphics 2011; 31: 1959–1972. [DOI] [PubMed] [Google Scholar]
- 18.Yasumoto M, Shibuya H, Gomi N, et al. Ultrasonographic appearance of dermoid and epidermoid cysts in the head and neck. J Clin Ultrasound 1991; 19: 455–461. [DOI] [PubMed] [Google Scholar]
- 19.Denison CM, Ward VL, Lester SC, et al. Epidermal inclusion cysts of the breast: three lesions with calcifications. Radiology 1997; 204: 493–496. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Joo KB, Song HT, et al. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J Clin Ultrasound 2001; 29: 374–383. [DOI] [PubMed] [Google Scholar]