Abstract
Background:
Traumatic brain injury (TBI) is common in young soldiers of armed forces leading to significant morbidity and mortality. We studied the prevalence of hypopituitarism following TBI and its association with trauma severity.
Materials and Methods:
We conducted a 12-month prospective study of 56 TBI patients for the presence of hormonal dysfunction. Hormonal parameters were estimated during the early phase (0–10 days posttraumatically) and after 6 and 12 months. Dynamic testing was done when required, and the results were analyzed by appropriate statistical methods.
Results:
Hormonal dysfunction was seen in 39 of the 56 (70%) patients at initial assessment. Persisting pituitary deficiencies are seen in 7 and 8 patients at the end of 6 months and 12 months, respectively. Hypogonadotropic hypogonadism, hypothyroidism, and growth hormone deficiency are the most common diagnoses. Initial severe TBI and plurihormonal involvement predicted the long-term hypopituitarism.
Conclusion:
Early hypopituitarism was common in severe TBI, but recovers in majority. Evaluation for the occult pituitary dysfunction is required during the rehabilitation of TBI patients.
Key words: Central hypothyroidism, head injury, hypogonadism, hypopituitarism, traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is assuming epidemic proportions and affects the young population. In India, about 35,000 patients die each year, 125,000 acquire a disability every year secondary to TBI, and over a crore persons among population live with the chronic disability.[1] Survivors of TBI frequently suffer multiple physical, endocrine, and neuropsychiatric complications requiring assistance with activities of daily living following head injury. Hypopituitarism is reported to the tune of 27%–47% in cases of TBI.[2] Most of them have growth hormone (GH) deficiency and some of them have other associated anterior pituitary hormonal deficiency.[3] There is also considerable overlap between the chronic sequelae of TBI and clinical features of hypopituitarism. The pathophysiological mechanisms leading to long-term consequences after TBI include changes to the blood-brain barrier, free radical-induced damage, increased apoptosis, and deposition of β-amyloid plaque.[4]
Armed forces are also exposed to the problem of TBI leading to morbidity and mortality in young soldiers. The admission rate due to head injury is in excess of 15–20 per month at most of the tertiary centers and many of them are being followed up without any formal evaluation for hypopituitarism. TBI affects mostly young soldiers in productive years of life. The previous reports suggest that moderate to severe TBI is associated with neurocognitive deficits, dementia of Alzheimer type, Parkinson disease, and endocrine dysfunction.[5] The clinical features of hypopituitarism are subtle and overlap with the neurological and psychiatric sequelae of head injury.[6] This explains the delayed diagnosis of hypopituitarism in most of the patients, leading to serious consequences. The management of patients with TBI requires a close liaison between the endocrinologists, neurosurgeons, and intensive care physicians. There are limited data regarding the prevalence of hypopituitarism after TBI from India.[7] Hence, we conducted this study to identify the prevalence of hypothalamo-pituitary dysfunction in patients with TBI and its association with the severity of trauma. We also analyzed the risk factors predicting the long-term hypopituitarism after TBI.
MATERIALS AND METHODS
We conducted this prospective study over a period of 2 years at a tertiary care referral center, and the study protocol is shown in Figure 1.
Figure 1.
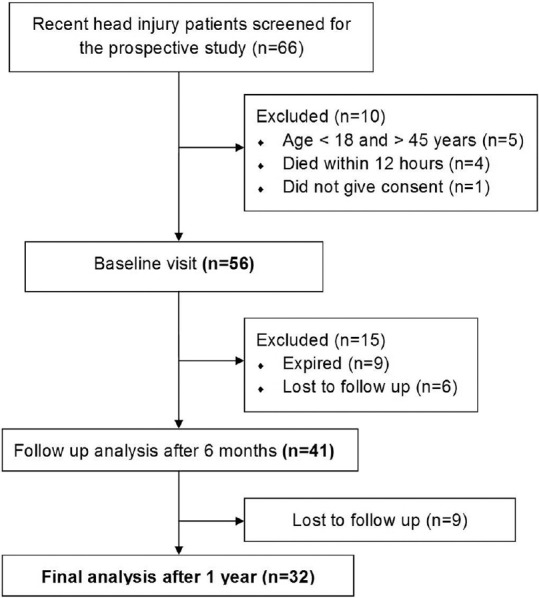
Flow diagram of the study
Study participants
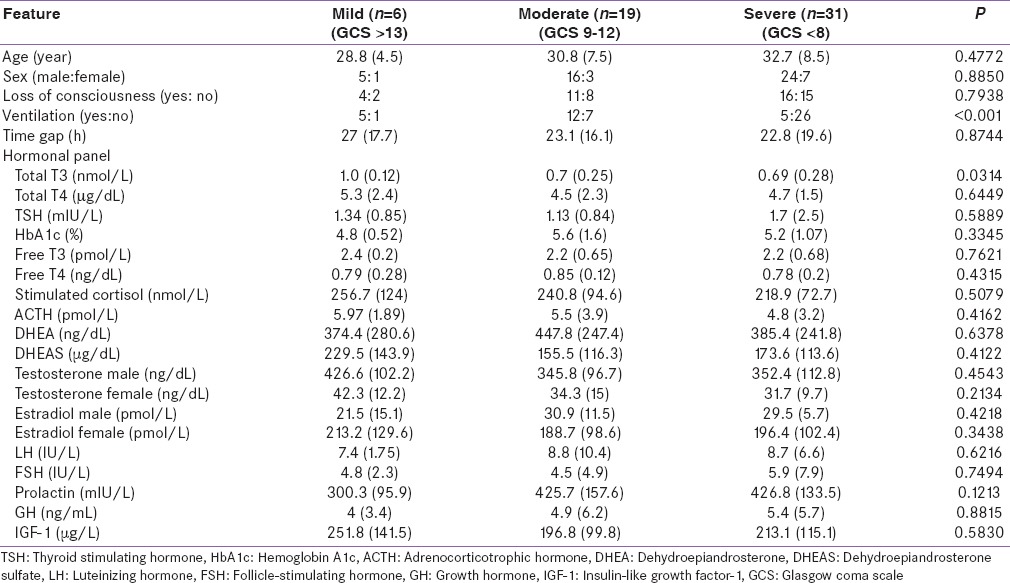
All consecutive patients with head injury aged 18–45 years admitted to surgical Intensive Care Unit (ICU) with a diagnosis of head injury between January and December 2012 were included in the study. The patient who sustained a head injury at a peripheral location and was transferred to our center also was included in the study provided the date of admission in our hospital is within 7 days from the date of injury. We excluded patients with a history of thyroid or endocrine disorders, long-term drug intake affecting the hypothalamo-pituitary axis, radiation exposure, and known diagnosis of alcohol dependence syndrome. The patients were subdivided into three groups based on the documented initial Glasgow coma scale (GCS). They include mild (GCS 13–15), moderate (GCS 9–12), and severe (GCS ≤ 8).
Tools and techniques
Detailed history, physical examination, including neurological deficit were assessed in all patients at admission and after 12 months of observation. The patients were specifically asked about the features to suggest hormonal dysfunction during the follow-up. All the patients underwent evaluation for hormonal dysfunction three times (baseline, at 6, and 12 months) during the study. Baseline evaluation was done between 0 and 10 days after sustaining the head injury.
Interventions
A fasting blood sample was collected between 0800 and 0900 h for all patients from an indwelling venous catheter. The samples from admitted patients in the immediate postinjury phase were collected in the 1st day morning after admission to the ICU. The hormonal dysfunction was assessed in the basal sample only in majority and dynamic testing (insulin tolerance test, adrenocorticotropic hormone [ACTH] stimulation test) was done in selected cases. All the samples were analyzed for thyroid hormones (total triiodothyronine [TT3], total thyroxine, thyroid stimulating hormone [TSH], free triiodothyronine [FT3], Free thyroxine [FT4]), gonadal hormones (luteinizing hormone (LH), follicle-stimulating hormone [FSH], testosterone in males, estradiol in females), adrenal (cortisol, ACTH, dehydroepiandrosterone [DHEA], DHEA sulfate [DHEAS]), GH [insulin like growth factor-1 (IGF-1)], prolactin, and HbA1c.
Assay techniques
The collected blood samples were centrifuged immediately, and the plasma samples were stored at −80°C before the final analysis. The samples were labeled and analyzed simultaneously by a single laboratory technician to minimize the sampling errors. The entire hormonal panel was evaluated using an electrochemiluminescence assay except for IGF-1 and testosterone, which were measured by the enzyme immuno assay method.
Diagnostic criteria
Secondary hypothyroidism was defined as low FT4 (normal, 0.8–2.1 ng/ml) with normal or low TSH (normal 0.5–4.5 µIU/ml). Hypogonadism was defined in males with testosterone <300 ng/dL (normal 310–1200 ng/dL) and amenorrhea along with estradiol <30 pg/mL in females with inappropriately normal or low LH and FSH levels. Adrenal insufficiency was diagnosed when 8 AM cortisol was < 100 nmol/L and stimulated cortisol is below 500 nmol/L. We did not study the relative adrenal insufficiency in the subjects and performed either insulin tolerance test or post-ACTH cortisol to assess for adrenal function. An IGF-1 level below the range specific for the age is considered as diagnostic of GH deficiency (GHD), and we did not do GH stimulation test. The antidiuretic hormone (ADH) deficiency and diabetes insipidus (DI) was considered in cases of symptomatic polyuria and polydipsia and is confirmed with the help of water deprivation test. A diagnosis of complete hypopituitarism was made with dysfunction of more than or equal to three hormonal axes and partial hypopituitarism otherwise.
Statistical analysis
Date are presented as mean and standard deviation and comparison between the groups was done by one-way ANOVA and Chi-square test. Follow-up data were analyzed by repeated measures ANOVA test at the end of 1 year of observation (n = 32). To identify the associations with pituitary insufficiency, univariate logistic regression analysis was conducted with hormone deficiency (Yes/No) as a dependent variable and others (age, GCS at onset, duration of ICU stay, ventilator requirement, single, or multiple hormonal dysfunction) as independent continuous variables. The statistical analysis was done using GraphPad Prism Software, Version 6 (GraphPad Prism Software, USA) and a P < 0.05 was considered as statistically significant in all the tests.
RESULTS
A total of 56 patients (44 males, 12 females) aged (mean 31.7 ± 7.8 years, range 18–45 years) participated in our study. The detailed demographic characteristics of the study population are given in Table 1. Patients were hospitalized for mean 12.4 days (4–132), and the duration of stay in the ICU is 3.2 days. The gap between the occurrence of the head injury and the admission to the service hospital was 23.8 ± 18 h (range between 3 and 96 h). At the time of testing early posttraumatically, 14 patients received noradrenaline, and 46 patients were treated with antibiotics.
Table 1.
Baseline characteristics of the study population
The details of the hormonal axes dysfunction identified are shown in Table 2. Thirty-nine (70%) patients had biochemical changes in the early posttraumatic phase and the numbers decreased to 7 and 8 at the end of 6 months and 1-year follow-up, respectively.
Table 2.
Hormonal axes dysfunction in the study population
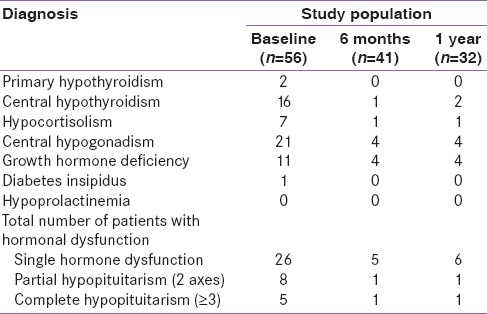
Thirty-one (67%) patients had hypogonadotropic hypogonadism and 15 (33%) patients had central hypothyroidism. Two patients had elevated TSH with low T3 and T4 suggestive of primary hypothyroidism, which is unrelated to the TBI. The patients were grouped as per the initial GCS into 3 groups, and the hormonal parameters are given in Table 3. All the hormonal results were the same between all 3 groups except for the TT3, which was less in patients with severe TBI.
Table 3.
Clinical and hormonal features as per the initial severity of traumatic brain injury
Serial evaluation
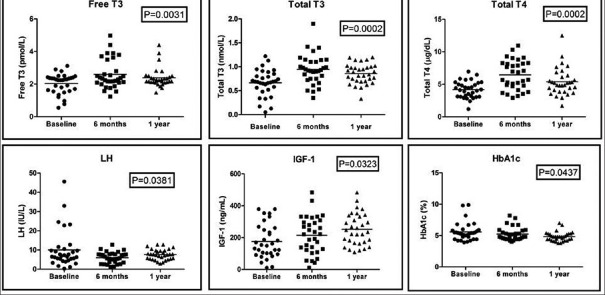
The observed change in the hormonal panel during the follow-up is given in Table 4. Thyroid hormones and IGF-1 showed a gradual rise serially after initial suppression as shown in Figure 2. HbA1c and LH showed a negative trend during the follow-up. Other adrenogonadal hormones did not show any significant changes during follow-up. The number of patients with hormonal dysfunction at different time intervals is shown in Figure 3. In the initial evaluation, a total of 39 out of 56 patients had evidence of hormonal dysfunction. Eight patients had two axes disturbances which include hypocortisolism with GHD (n = 3), GHD with hypogonadism (n = 3), hypocortisolism with DI (n = 1), and hypogonadism with central hypothyroidism (n = 1). Five patients had biochemical changes mimicking complete hypopituitarism in the early posttraumatic phase. Three patients had hypogonadism, GHD, and hypocortisolism while the remaining two had secondary hypothyroidism, GHD, and hypogonadism. None of the patients had altered prolactin levels in our study.
Table 4.
Serial changes in hormone levels after traumatic brain injury (n=32)*
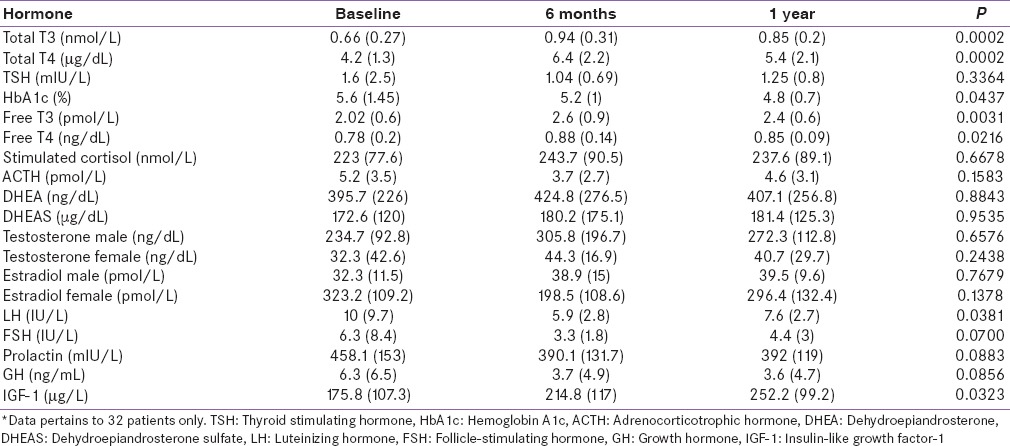
Figure 2.
Serial changes in selected hormones
Figure 3.
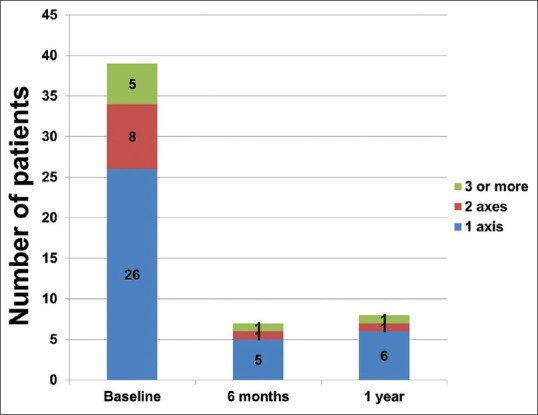
Number of Patients with hormonal dysfunction
Six months posttraumatically, anterior pituitary deficiencies were confirmed by repeated testing in 7 of the 41 (13%) patients. Out of the seven patients, only 1 patient had complete hypopituitarism (hypogonadism, GHD, and hypothyroidism), 1 had partial hypopituitarism (hypogonadism and GHD), and the remaining 5 had single hormonal dysfunction. At 12 months, 1 patient persisted with multiple hormone deficits, and 1 additional patient developed isolated central hypothyroidism.
High-risk factors for hormonal dysfunction
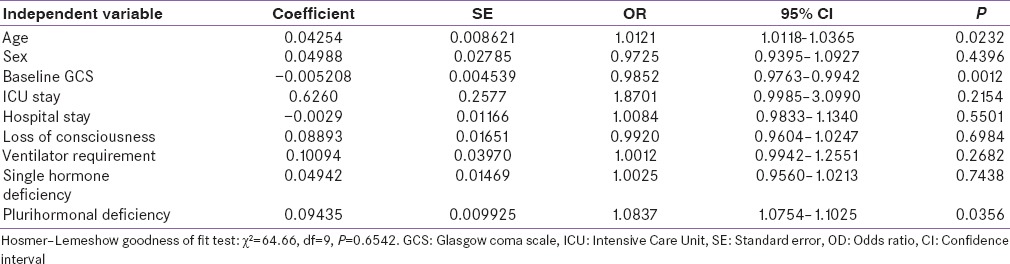
Old age (P = 0.0232), low GCS score at onset (P = 0.0012), and plurihormonal disturbance at baseline (P = 0.0356) are the risk factors for the persistent hormonal dysfunction at the end of 1 year as shown in Table 5. Other parameters such as sex of the patient, the nature of the injury, ventilator requirement, duration of stay in the hospital, and loss of consciousness did not predict the hypopituitarism during follow-up (P > 0.05).
Table 5.
Logistic regression model for hormone deficiency at the end of one year
DISCUSSION
Our findings have important implications in the clinical practice, especially for the armed forces. We showed that the hypothalamo-pituitary dysfunction was seen in 70% of the patients during initial evaluation with spontaneous improvement in the majority. Long-term dysfunction is seen in 15% (8 out of 56) of the patients. There is an unmet need to rehabilitate these soldiers of TBI, and it is mandatory to screen for the endocrine dysfunction to prevent the long-term morbidity. Old age, the severity of TBI, and the presence of multiple hormonal axes disturbances in the initial stages predicted the long-term hypopituitarism. Our data and previous reports suggest that the severity of the TBI correlates well with the permanent hypothalamic pituitary dysfunction.[2,3,7] Hypopituitarism is overlooked in survivors of TBI, due to lack of protocol-based follow-up, and incoordination between the concerned specialists.[8,9]
Our data suggest that isolated pituitary deficiency was common when compared with multiple hormone deficiencies. The previous reports suggest that the multiple hormonal deficiency is common over the isolated hormonal axis involvement.[10,11] This could be due to the differences in the study cohort, number of performing tests, assay problems, and the use of different diagnostic tests and criteria. The results of the dynamic tests pertaining to the GH and ACTH axes vary widely, thereby prompting a confirmatory test in the majority of the cases.[12,13,14] None of the patients with hormonal abnormality at the end of 6 months recovered during the follow-up period. This suggests that the recovery phase after brain injury is limited to the initial 3–6 months only. In acute phase after the head injury, there is a reduction in the thyroid, gonadal, ADH hormones, and hyperprolactinemia.[15,16] Severe TBI patients show these compensatory changes in the hormonal milieu, which is mediated by the release of inflammatory cytokines.[17]
Central hypogonadism and hypothyroidism are common during the acute phase after the injury, and hence alterations of these hormonal axes did not predict the long-term hypopituitarism.[18] Few reports suggest that the level of free cortisol is a useful marker for identifying the patients at risk for long-term hypopituitarism in TBI patients.[19] Hypothalamo-pituitary lesions were observed in up to two-thirds of patients on autopsy studies of TBI patients.[20,21] The previous reports also suggest the role of GCS as a predictive factor as observed in our study.[22] Increased intracranial pressure (ICP) is a strong indicator of increased TBI severity. We were unable to measure the ICP, precluding the evaluation of the same as a prognostic factor.
The presence of structural changes did not correlate well with underlying hormonal dysfunction in patients after TBI.[20,22] Neuroimaging was done as per the relevant clinical indications and MRI was not done in all the study participants. Hence, we did not include the structural changes as an important variable in our study. The strength of our study is the single-center, prospective design, and the limitations include small sample size for carrying the primary and subgroup analysis, hormonal evaluation in a single sample and nonconduct of dynamic testing in all the study participants. We could not localize the etiology of hypopituitarism, either to pituitary, or the hypothalamus, for the lack of facilities to test for specific trophic hormones.
CONCLUSION
High prevalence of anterior pituitary hormone dysfunction is observed in the immediate phase after TBI. The majority of the patients recover spontaneously and persisting abnormalities are seen in one-sixth of the patients. Screening for occult endocrine dysfunction is essential in TBI patients to improve the long-term rehabilitation.
Financial support and sponsorship
The authors wish to thank the office of the DGAFMS for the research grant under AFMRC project number 4264/2012 for the conduct of this study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors sincerely acknowledge the help rendered by Nb/Sub JBS Yadava in conduct of the study.
REFERENCES
- 1.Gururaj G. Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24–8. doi: 10.1179/016164102101199503. [DOI] [PubMed] [Google Scholar]
- 2.Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA. 2007;298:1429–38. doi: 10.1001/jama.298.12.1429. [DOI] [PubMed] [Google Scholar]
- 3.Tanriverdi F, Ulutabanca H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. Three years prospective investigation of anterior pituitary function after traumatic brain injury: A pilot study. Clin Endocrinol (Oxf) 2008;68:573–9. doi: 10.1111/j.1365-2265.2007.03070.x. [DOI] [PubMed] [Google Scholar]
- 4.van Baalen B, Odding E, Maas AI, Ribbers GM, Bergen MP, Stam HJ. Traumatic brain injury: Classification of initial severity and determination of functional outcome. Disabil Rehabil. 2003;25:9–18. doi: 10.1080/dre.25.1.9.18. [DOI] [PubMed] [Google Scholar]
- 5.Gulf War and Health, Volume 7: Long-term Consequences of Traumatic Brain Injury. Washington, DC: The National Academies Press; 2009. Institute of Medicine. [PubMed] [Google Scholar]
- 6.Agha A, Phillips J, O’Kelly P, Tormey W, Thompson CJ. The natural history of post-traumatic hypopituitarism: Implications for assessment and treatment. Am J Med. 2005;118:1416. doi: 10.1016/j.amjmed.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Prasanna KL, Mittal RS, Gandhi A. Neuroendocrine dysfunction in acute phase of moderate-to-severe traumatic brain injury: A prospective study. Brain Inj. 2015;29:336–42. doi: 10.3109/02699052.2014.955882. [DOI] [PubMed] [Google Scholar]
- 8.van der Eerden AW, Twickler MT, Sweep FC, Beems T, Hendricks HT, Hermus AR, et al. Should anterior pituitary function be tested during follow-up of all patients presenting at the emergency department because of traumatic brain injury? Eur J Endocrinol. 2010;162:19–28. doi: 10.1530/EJE-09-0436. [DOI] [PubMed] [Google Scholar]
- 9.Klose M, Juul A, Poulsgaard L, Kosteljanetz M, Brennum J, Feldt-Rasmussen U. Prevalence and predictive factors of post-traumatic hypopituitarism. Clin Endocrinol (Oxf) 2007;67:193–201. doi: 10.1111/j.1365-2265.2007.02860.x. [DOI] [PubMed] [Google Scholar]
- 10.Bondanelli M, De Marinis L, Ambrosio MR, Monesi M, Valle D, Zatelli MC, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685–96. doi: 10.1089/0897715041269713. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman SA, Oberoi AL, Gilkison CR, Masel BE, Urban RJ. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J Clin Endocrinol Metab. 2001;86:2752–6. doi: 10.1210/jcem.86.6.7592. [DOI] [PubMed] [Google Scholar]
- 12.Leal-Cerro A, Flores JM, Rincon M, Murillo F, Pujol M, Garcia-Pesquera F, et al. Prevalence of hypopituitarism and growth hormone deficiency in adults long-term after severe traumatic brain injury. Clin Endocrinol (Oxf) 2005;62:525–32. doi: 10.1111/j.1365-2265.2005.02250.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoeck HC, Jakobsen PE, Vestergaard P, Falhof J, Laurberg P. Differences in reproducibility and peak growth hormone responses to repeated testing with various stimulators in healthy adults. Growth Horm IGF Res. 1999;9:18–24. doi: 10.1054/ghir.1998.0085. [DOI] [PubMed] [Google Scholar]
- 14.Harris EK, Kanofsky P, Shakarji G, Cotlove E. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. II. Estimating biological components of variation. Clin Chem. 1970;16:1022–7. [PubMed] [Google Scholar]
- 15.Agha A, Rogers B, Sherlock M, O’Kelly P, Tormey W, Phillips J, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab. 2004;89:4929–36. doi: 10.1210/jc.2004-0511. [DOI] [PubMed] [Google Scholar]
- 16.Bavisetty S, Bavisetty S, McArthur DL, Dusick JR, Wang C, Cohan P, et al. Chronic hypopituitarism after traumatic brain injury: Risk assessment and relationship to outcome. Neurosurgery. 2008;62:1080–93. doi: 10.1227/01.neu.0000325870.60129.6a. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berghe G, de Zegher F, Bouillon R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83:1827–34. doi: 10.1210/jcem.83.6.4763. [DOI] [PubMed] [Google Scholar]
- 18.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–78. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 19.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–38. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 20.Kornblum RN, Fisher RS. Pituitary lesions in craniocerebral injuries. Arch Pathol. 1969;88:242–8. [PubMed] [Google Scholar]
- 21.Schneider M, Schneider HJ, Yassouridis A, Saller B, von Rosen F, Stalla GK. Predictors of anterior pituitary insufficiency after traumatic brain injury. Clin Endocrinol (Oxf) 2008;68:206–12. doi: 10.1111/j.1365-2265.2007.03020.x. [DOI] [PubMed] [Google Scholar]
- 22.Aimaretti G, Ambrosio MR, Di Somma C, Gasperi M, Cannavò S, Scaroni C, et al. Residual pituitary function after brain injury-induced hypopituitarism: A prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085–92. doi: 10.1210/jc.2005-0504. [DOI] [PubMed] [Google Scholar]