Abstract
Background:
The association of obesity and lean mass (LM) has not been examined well in children and adolescents, and it remains controversial.
Objective:
The objective of this study was to evaluate the relationship of body mass index (BMI) categories and regional obesity with total and regional LM in children and adolescents.
Methods:
A total of 1408 children and adolescents (boys 58.9%; girls 41.1%) divided according to BMI (normal weight 79.5%, overweight 16.0%, and obese 4.5%) were included in this cross-sectional study. Total and regional LM and fat mass were measured by DXA. Leg and arm fat-to-total fat ratio (LATR) indicative of subcutaneous fat and trunk fat-to-total fat ratio (TTR), an indicator of visceral fat, were calculated.
Results:
Mean age of the study population was 13.2 ± 2.7 years (boys - 13.0 ± 2.7; girls - 13.4 ± 2.8 years). Total LM (TLM) and its regional distribution were higher in overweight and obese groups when compared with those with normal BMI in both genders. TLM was comparable between overweight and obese in both genders. TLM per unit of fat progressively decreased from normal to obese categories. The difference in LM per unit fat between BMI categories persisted after adjustment for age, height, and sexual maturity score. TLM increased across the quartiles of TTR, but decreased with an increment in subcutaneous fat (quartiles of LATR).
Conclusions:
Obese children and adolescents apparently have higher LM than normal BMI children, but have lower LM per unit of fat. Subcutaneous fat had a negative impact and visceral fat had a positive impact on TLM.
Key words: Lean mass, obesity, subcutaneous fat, visceral fat
INTRODUCTION
The obesity epidemic is affecting all age groups and could decrease prospects for an active life expectancy. The prevalence of overweight and obesity is increasing in children and adolescents.[1] The prevalence of overweight among children and adolescents in the India has doubled from 11.7% to 22.1% from 2006 to 2011.[1] Although the body mass index (BMI) is widely used as a surrogate measure of adiposity, it is a measure of excess weight relative to height, rather than excess body fat. The interpretation of BMI among children and adolescents is further complicated by the changes that occur in weight, height, and body composition during growth.[2,3] BMI levels among adults are highly correlated with the percentage of body fat among various race, sex, and age.[4,5] However, associations among children and adolescents have been variable and relatively weak.[6,7,8,9,10,11] These weaker associations among children and adolescents may be attributable to the asynchronous changes that occur in the levels of fat mass (FM) and lean mass (LM) during growth.[11]
It is suggested that the “obesity paradox” is merely based on the inadequacy of BMI to differentiate between LM and FM. Against the background of obesity, it is perceived that adipose tissue is a plain risk factor and LM is the protective element. It is argued that the favorable impact of elevated BMI results from the beneficial effects of lean tissue, overriding the adverse effects of excess body fat.[12] However, the association of obesity and LM has not been studied well in literature. Hence, we designed this cross-sectional population-based study to (i) evaluate the relationship between BMI categories and LM and (ii) to analyze the effect of regional obesity (subcutaneous and visceral) on total LM (TLM) in children and adolescents.
MATERIALS AND METHODS
This study was an extension of the analysis from our earlier study.[13] Adolescents were recruited from different schools in the city of New Delhi as a part of the project to generate normative data for BMD. There were 1829 apparently healthy children and adolescents who underwent health examination (clinical, biochemical, and densitometric) on a voluntary basis. The data on LM and FM and its distribution were available from 1403 children and adolescents for the present study. Children and adolescents with clinically overt hepatic, renal, neoplastic, gastrointestinal, dermatological, endocrine, and systemic infective disorders, steroid intake, or alcoholism were excluded. Demographic, anthropometric, and clinical data were ascertained, and a detailed physical examination was conducted.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving the children and adolescents were approved by the Institutional Ethics Committee of the Institute of Nuclear Medicine and Allied Sciences. Administrative approval was taken from the school authorities, written informed consent from parents/guardians, while verbal assent was taken from the children who participated in the study.
Height was measured to the nearest 0.1 cm using a portable wall-mounted stadiometer (200 cm/78 inches) Model WS045 (Narang Medical Limited, New Delhi, India) with the participant standing straight with head held in the Frankfurt horizontal plane. Weight, without shoes and while wearing light clothes, was measured to the nearest 0.1 kg, using an electronic scale (EQUINOX Digital weighing machine, Model EB6171, Equinox Overseas Private Limited, New Delhi, India). BMI was calculated by dividing weight with the square of height in meters. The study population was divided according to BMI-based criteria proposed by Cole et al.[14] as normal, overweight, and obese.
Pubertal staging was carried out by trained professionals of the same sex based on Tanner criteria.[15] Testicular volume was determined by comparative palpation with Prader Orchidometer (Pharmacia and Upjohn, Uppsala, Sweden). Based on the testicular volume, participants were divided into four stages. Stage 1 (prepubertal) included participants with testicular volume <4 ml, Stage 2 (early puberty) – volume ≥4 but ≤8 ml, Stage 3 – volume ≥10 but ≤15, and Stage 4 (fully mature) – testicular volume >15 ml. A testicular volume of 4 ml or greater was considered as the onset of puberty. If there was a discrepancy in the testicular volumes of two sides, the larger one was taken as the final volume.
LM was measured using the Prodigy Oracle (GE Lunar Corp., Madison, WI) according to a standard protocol. Quality control procedures were carried out in accordance with the manufacturer's recommendations. Instrument variation was determined regularly using a phantom supplied by the manufacturer, and the mean coefficient of variation was <0.5%. For in vivo measurements, mean coefficients of variation for all sites were <1%. Body FM and regional distribution were measured using the same DXA machine. Instrument variation was determined by measuring total FM and regional FM in twenty healthy adults twice, and the mean coefficient of variation was <0.5%. Leg and arm fat-to-total fat ratio (LATR) was considered as indicative of subcutaneous fat and trunk fat-to-total fat ratio (TTR) was indicative of visceral fat.[16] TLM was calculated according to the quartiles of LATR and TTR separately for genders. Interquartile range for LATR and TTR was 0.05 and 0.06 and 0.06 and 0.08 for girls and boys, respectively. Appendicular skeletal muscle mass index (ASMI) was calculated by LM at arms and leg in kilogram divided by square of height in meters.
Statistical analysis was carried out using SPSS software version 20.0 (Chicago, IL, USA). Data were presented as mean ± standard deviation or number (%) unless specified. One-way analysis of variance was used to test the differences between BMI groups. Post hoc analysis was used to compare the significance level between two groups within each parameter. P <0.05 was considered statistically significant.
RESULTS
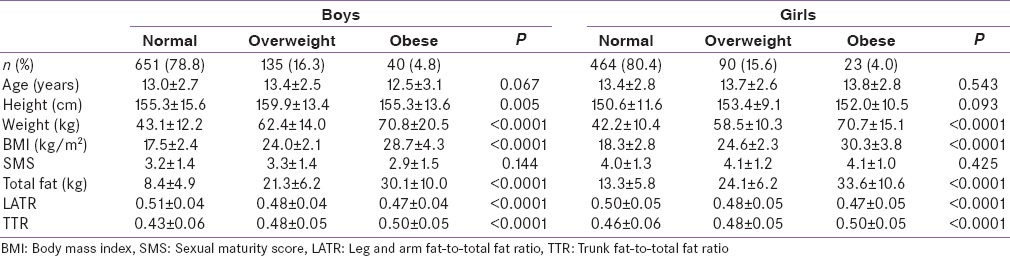
Mean age of the study participants was 13.2 ± 2.7 years (boys - 13.0 ± 2.7; girls - 13.4 ± 2.8 years). Nearly, 79.5% of the children and adolescents were normal weight, 16.0% were overweight, and 4.5% were obese. Age and pubertal status were comparable in all groups in both genders [Table 1].
Table 1.
Basic characteristics of the population
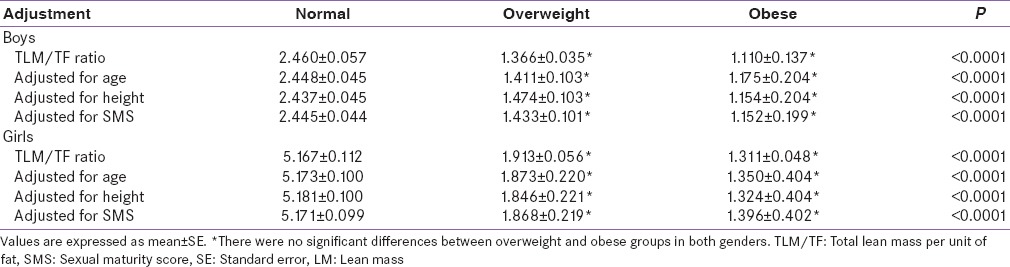
ASMI, TLM, and its regional distribution were higher in overweight and obese compared to normal weight children and adolescents, but were comparable between overweight and obese boys (LM: P =0.828; ASMI: P =0.982) and girls (LM: P =0.268; ASMI: P =0.096) [Table 2]. TLM per unit of fat (TLM/TF ratio) showed a significant decrease from normal weight group to obese group, but was comparable between overweight and obese groups, even after adjustment with age, height, and SMS in both genders [Table 3].
Table 2.
Lean mass according to weight categories among boys and girls
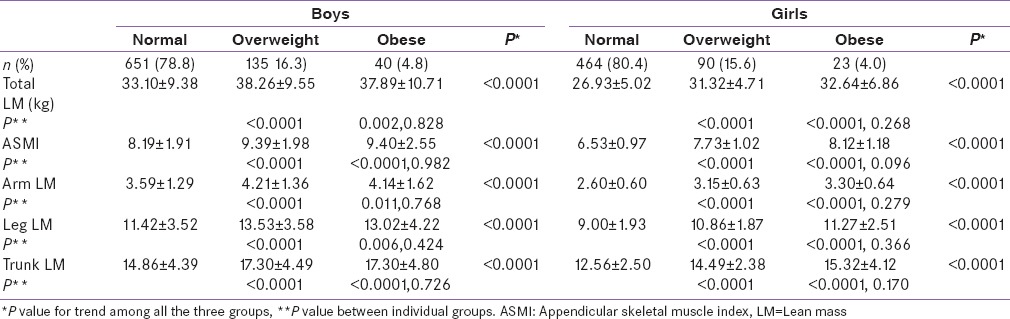
Table 3.
Total lean mass versus total fat ratio in weight categories according to interaction with age, height, and sexual maturity score
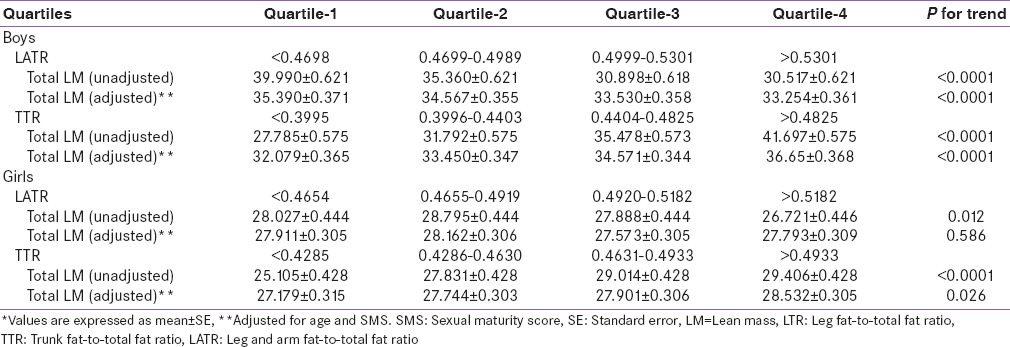
Unadjusted TLM decreased with the quartiles of LATR (subcutaneous fat) and increased with the quartiles of TTR (visceral fat) in both gender. However, when adjusted for age and SMS, the same pattern persisted among boys, but the pattern of TLM became nonsignificant with LATR in girls [Table 4].
Table 4.
Effect of subcutaneous fat (leg arm fat-to-total fat ratio) and visceral fat (Trunk-to-total fat ratio) on total lean mass*
DISCUSSION
In the present study, we observed that TLM and regional LM were more in overweight and obese categories when compared to the normal weight category, but were comparable between overweight and obese category. Overweight and obesity are associated with an increase in fat and bone mass.[8,9,10] Consistent with this, we expect that LM should show a similar pattern of increase. Surprisingly, TLM/TF decreased after adjustment with age, height, and SMS in both genders. This suggests that overweight and obese children and adolescents have lower TLM/TF when compared to normal BMI category. Studies have shown that LM is increased when weight gain is observed in early childhood, but FM increases when weight gain is seen in late childhood.[17,18] Hence, weight gain in later childhood will have relatively lower LM when compared to early childhood.
This has an implication in the management of obesity. Diet therapy is known to decrease fat as well as LM.[19] Physical activity increases LM and decreases FM with decreasing weight and obesity in children and adolescents.[20] Pharmacological therapy (sibutramine) is associated with a decrease in fat as well as LM.[21] Bariatric surgery in adults leads to more loss of LM compared to FM.[22] Hence, an increase in the physical activity is the best mode of therapy to achieve weight loss. Increase in LM may help in improving the health status by decreasing obesity while a decrease in LM is associated with an adverse outcome.[23]
The relationship between regional lean and FM was influenced by age and gender. With an increasing adiposity, skeletal muscle to adipose tissue ratio declined faster at the trunk in men and at the extremities in women.[24] We observed a negative effect of subcutaneous fat and a positive effect of visceral fat on LM which was influenced by age and pubertal status in girls, indicating the importance of pubertal status for the effect. Level of physical activity may be an important factor showing this effect. A study has shown that the change in the pattern of subcutaneous fat distribution could be due to a varied response of different sites toward the loss of subcutaneous fat due to increased levels of activity.[25] Furthermore, increase in physical activity will result in a decrease in subcutaneous fat and increase in LM,[26] and this can explain the inverse relationship. Visceral fat due to its gravitational effect will put physical strain on appendicular system and will increase TLM.[27]
The main limitation of the study was the absence of longitudinal data, which could have assessed the change in BMI categories and its effect on LM.[28] Another limitation was the absence of measurement of adipokines and myokines, which could have highlighted the mechanism of association between body fat and LM.[29] However, the strength of the study attributes to the large sample size from healthy Indian population.
CONCLUSION
Obese children and adolescents have higher LM than normal weight children, but when calculated as per the unit of FM, they have lower LM. Subcutaneous fat had a negative impact and visceral fat had a positive impact on TLM which is influenced by age and pubertal status.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta N, Goel K, Shah P, Misra A. Childhood obesity in developing countries: Epidemiology, determinants, and prevention. Endocr Rev. 2012;33:48–70. doi: 10.1210/er.2010-0028. [DOI] [PubMed] [Google Scholar]
- 2.Franklin M. Comparison of weight and height relations in boys from 4 countries. Am J Clin Nutr. 1999;70:157S–62S. doi: 10.1093/ajcn/70.1.157s. [DOI] [PubMed] [Google Scholar]
- 3.Horlick M. Body mass index in childhood – Measuring a moving target. J Clin Endocrinol Metab. 2001;86:4059–60. doi: 10.1210/jcem.86.9.7948. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 5.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26:789–96. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, DeLany JP, Harsha DW, Volaufova J, Champagne CC. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: The Baton Rouge Children's Study. Am J Clin Nutr. 2001;73:687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 7.Mast M, Langnäse K, Labitzke K, Bruse U, Preuss U, Müller MJ. Use of BMI as a measure of overweight and obesity in a field study on 5-7 year old children. Eur J Nutr. 2002;41:61–7. doi: 10.1007/s003940200009. [DOI] [PubMed] [Google Scholar]
- 8.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics. 2001;107:344–50. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 9.Widhalm K, Schönegger K, Huemer C, Auterith A. Does the BMI reflect body fat in obese children and adolescents? A study using the TOBEC method. Int J Obes Relat Metab Disord. 2001;25:279–85. doi: 10.1038/sj.ijo.0801511. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay RS, Hanson RL, Roumain J, Ravussin E, Knowler WC, Tataranni PA. Body mass index as a measure of adiposity in children and adolescents: Relationship to adiposity by dual energy x-ray absorptiometry and to cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86:4061–7. doi: 10.1210/jcem.86.9.7760. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RW, Jones IE, Williams SM, Goulding A. Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3-18 y. Am J Clin Nutr. 2002;76:1416–21. doi: 10.1093/ajcn/76.6.1416. [DOI] [PubMed] [Google Scholar]
- 12.Szabó T, von Haehling S, Doehner W. Differentiating between body fat and lean mass – how should we measure obesity? Nat Clin Pract Endocrinol Metab. 2008;4:E1. doi: 10.1038/ncpendmet0999. [DOI] [PubMed] [Google Scholar]
- 13.Shivaprasad C, Marwaha RK, Tandon N, Kanwar R, Mani K, Narang A, et al. Correlation between bone mineral density measured by peripheral and central dual energy X-ray absorptiometry in healthy Indian children and adolescents aged 10-18 years. J Pediatr Endocrinol Metab. 2013;26:695–702. doi: 10.1515/jpem-2012-0359. [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner JM. Growth at Adolescence: With a General Consideration of the Effects of Hereditary and Environmental Factors Upon Growth and Maturation from Birth to Maturity. Oxford: Blackwell Scientific Publications; 1969. [Google Scholar]
- 16.Marwaha RK, Garg MK, Tandon N, Mehan N, Sastry A, Bhadra K. Relationship of body fat and its distribution with bone mineral density in Indian population. J Clin Densitom. 2013;16:353–9. doi: 10.1016/j.jocd.2012.08.074. [DOI] [PubMed] [Google Scholar]
- 17.Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: A link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–30. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- 18.Bann D, Wills A, Cooper R, Hardy R, Aihie Sayer A, Adams J, et al. Birth weight and growth from infancy to late adolescence in relation to fat and lean mass in early old age: Findings from the MRC National Survey of Health and Development. Int J Obes (Lond) 2014;38:69–75. doi: 10.1038/ijo.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: A randomized clinical trial. J Obes 2011. 2011:pii: 516576. doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: Results from the POUNDS LOST trial. Am J Clin Nutr. 2012;95:614–25. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parks EP, Zemel B, Moore RH, Berkowitz RI. Change in body composition during a weight loss trial in obese adolescents. Pediatr Obes. 2014;9:26–35. doi: 10.1111/j.2047-6310.2012.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huettner F, Rammos CK, Dynda DI, Lange ML, Marshall JS, Rossi TR, et al. Body composition analysis in bariatric surgery: Use of air displacement plethysmograph. Am Surg. 2012;78:698–701. [PubMed] [Google Scholar]
- 23.den Hoed MA, Pluijm SM, de Groot-Kruseman HA, te Winkel ML, Fiocco M, van den Akker EL, et al. The negative impact of being underweight and weight loss on survival of children with acute lymphoblastic leukemia. Haematologica. 2015;100:62–9. doi: 10.3324/haematol.2014.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schautz B, Later W, Heller M, Müller MJ, Bosy-Westphal A. Total and regional relationship between lean and fat mass with increasing adiposity – Impact for the diagnosis of sarcopenic obesity. Eur J Clin Nutr. 2012;66:1356–61. doi: 10.1038/ejcn.2012.138. [DOI] [PubMed] [Google Scholar]
- 25.Bhalla R, Satwanti, Kapoor AK, Singh IP. The distribution pattern of subcutaneous fat with reference to level of physical activity in adult females. Z Morphol Anthropol. 1983;74:191–7. [PubMed] [Google Scholar]
- 26.Marwaha RK, Garg MK, Tandon N, Mahalle N. Comparison of body composition between professional sportswomen and apparently healthy age- and sex-matched controls. Indian J Endocrinol Metab. 2015;19:288–91. doi: 10.4103/2230-8210.149323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki Y. Influences of gravitational stress and total muscle mass on human cardiovascular adjustments during prolonged dynamic exercise. Ann Physiol Anthropol. 1990;9:139–51. doi: 10.2114/ahs1983.9.139. [DOI] [PubMed] [Google Scholar]
- 28.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006;117:e487–95. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
- 29.Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle – Adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem. 2011;117:47–56. doi: 10.3109/13813455.2010.535835. [DOI] [PubMed] [Google Scholar]


